Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Leaves Collection, Preparation and Carbonization
2.3. Reliability of Results
2.4. Batch Adsorption Studies
2.5. Control Experiments
2.6. Reactivation of AAJPL
3. Results and Discussion
3.1. Reliability of the Measurements
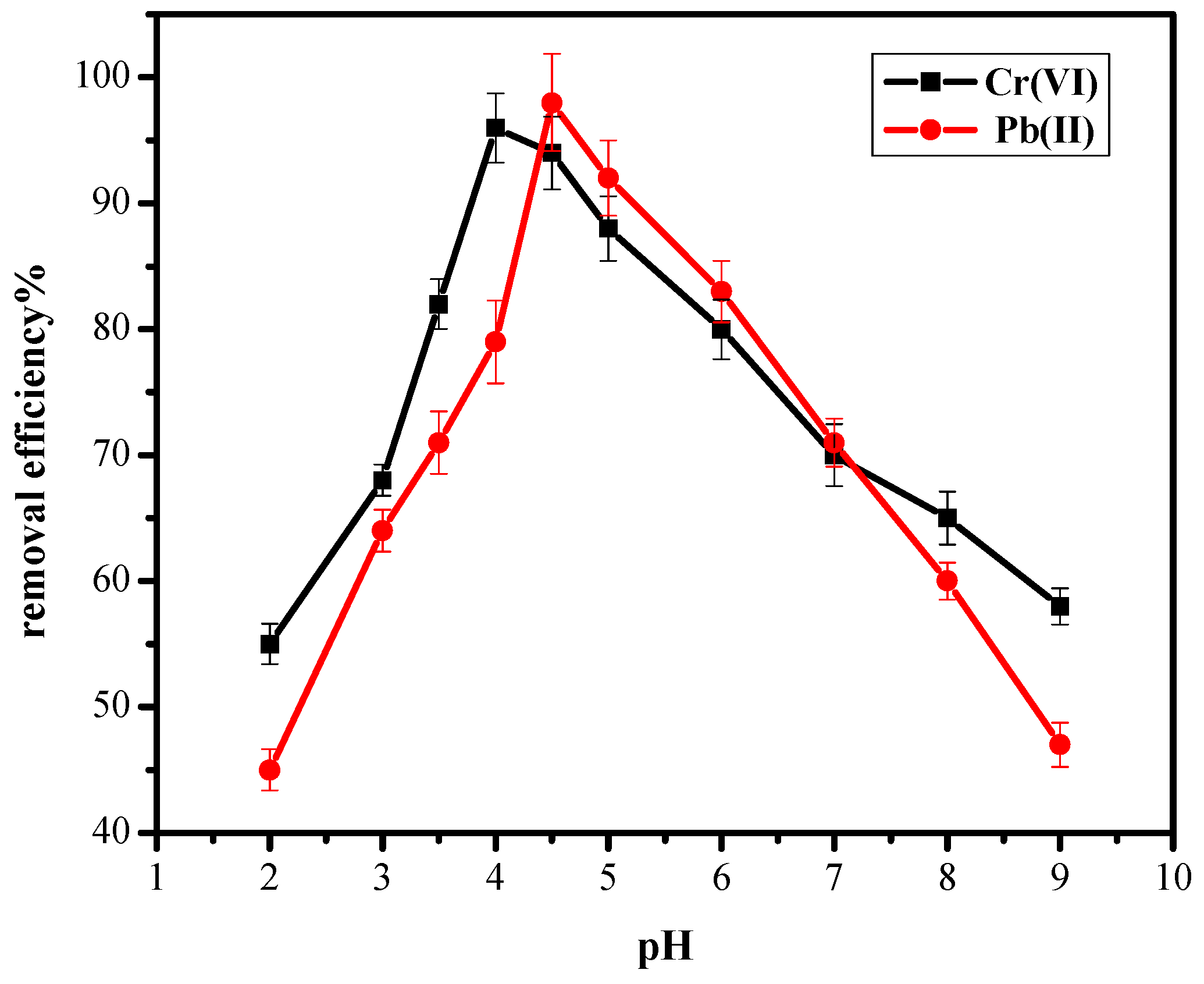
3.2. Effect of pH
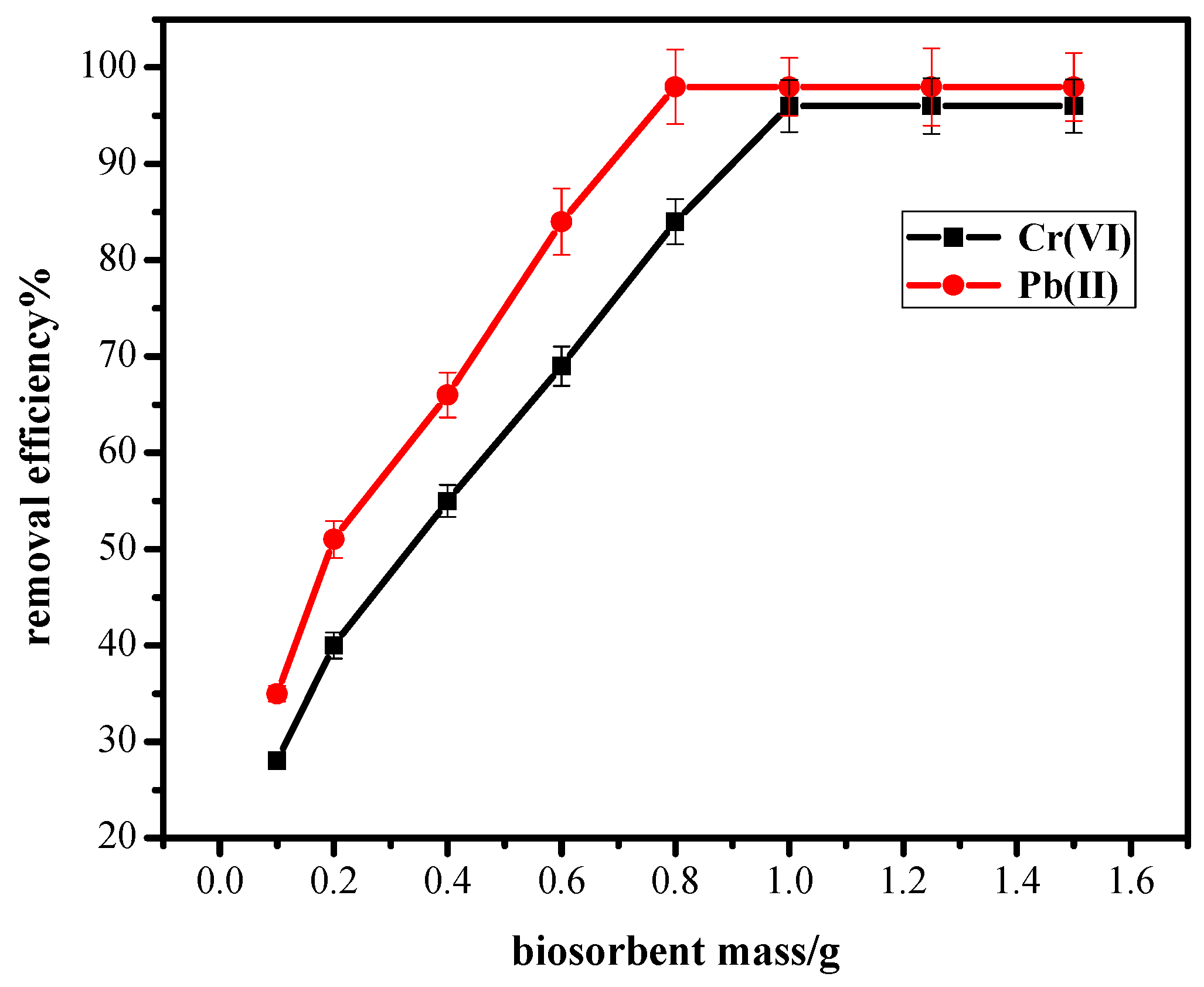
3.3. Effect of Biosorbent Mass
3.4. Effect of Metal Ion Concentration
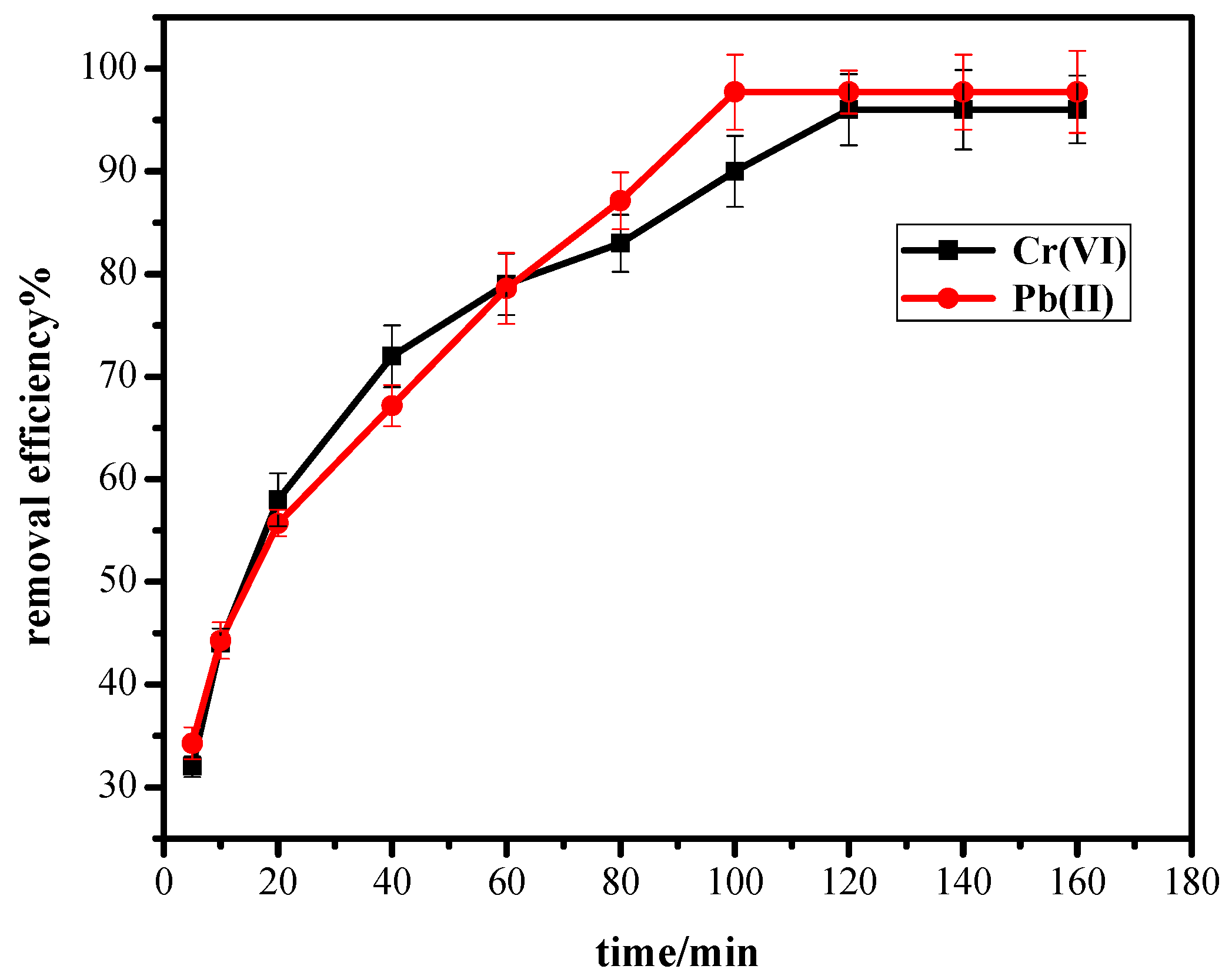
3.5. Effect of Contact Time
3.6. Biosorption Isotherms
3.6.1. Langmuir Isotherm
3.6.2. Freundlich Isotherm
3.6.3. Temkin Isotherm
3.6.4. Dubinin–Radushkevich (D-R) Isotherm
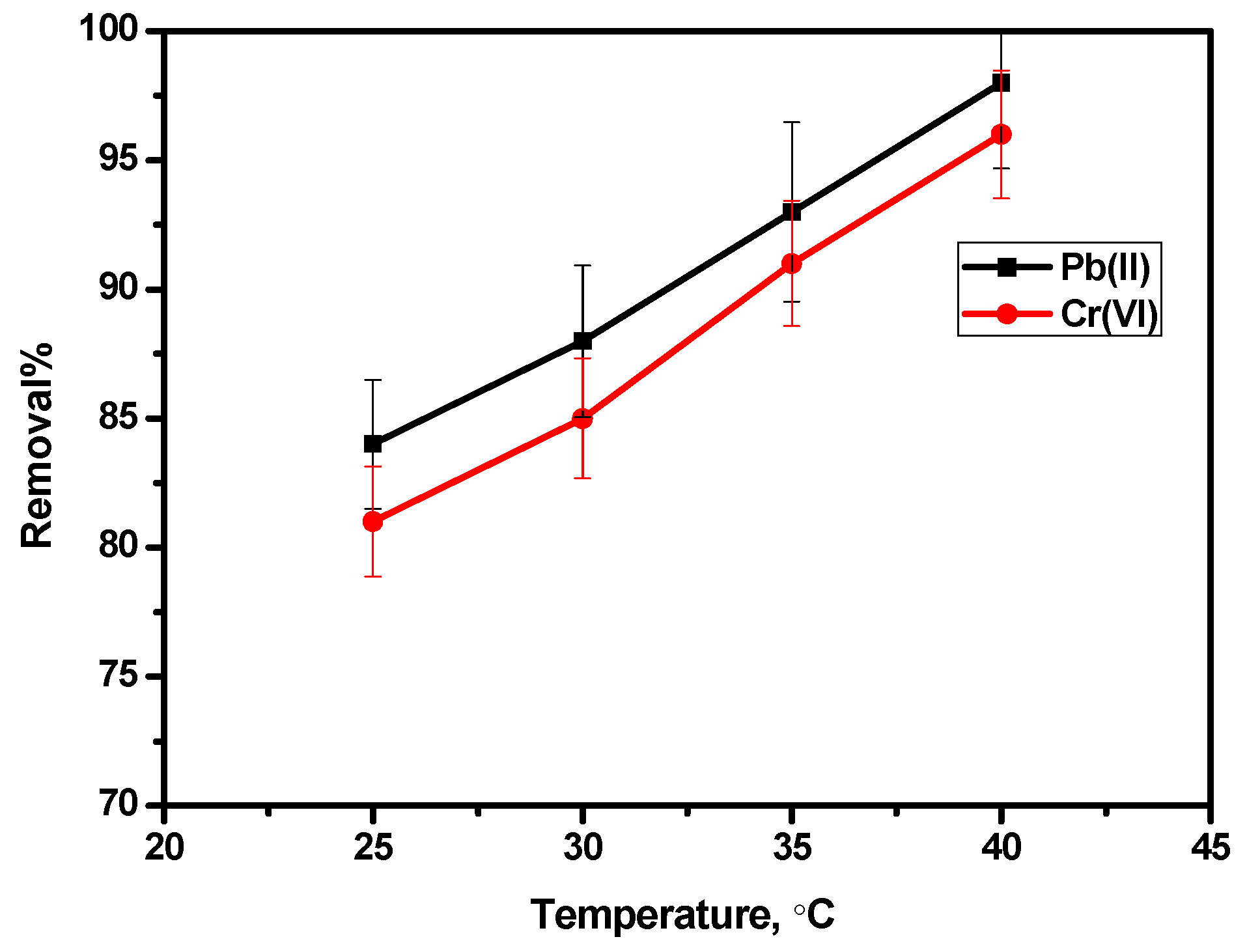
3.7. Effect of Temperature
Thermodynamic Parameters
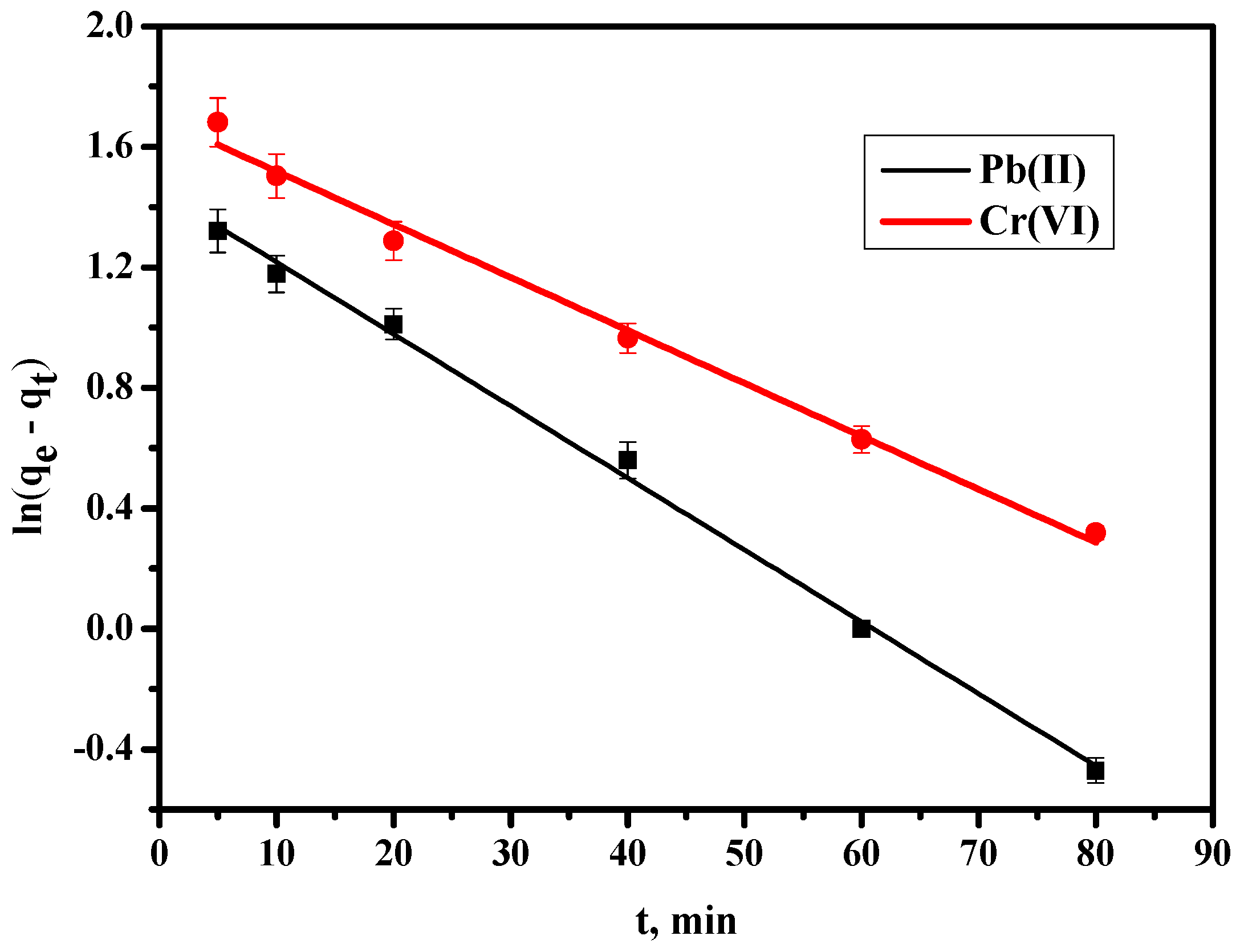
3.8. Biosorption Kinetics
3.9. Control Experiments
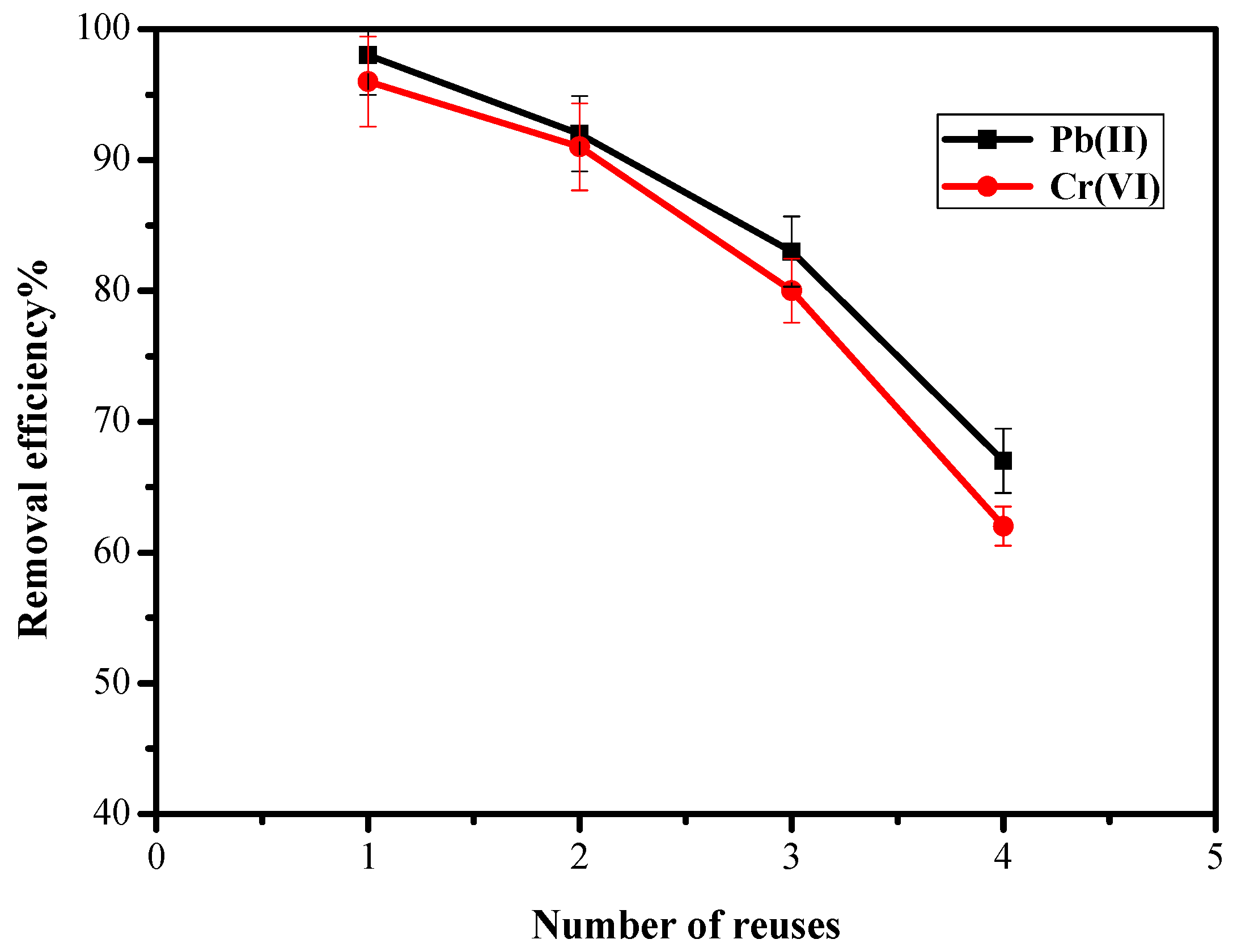
3.10. Reactivation and Reusability of AAJPL
3.11. Comparison of AAJPL with other Sorbents
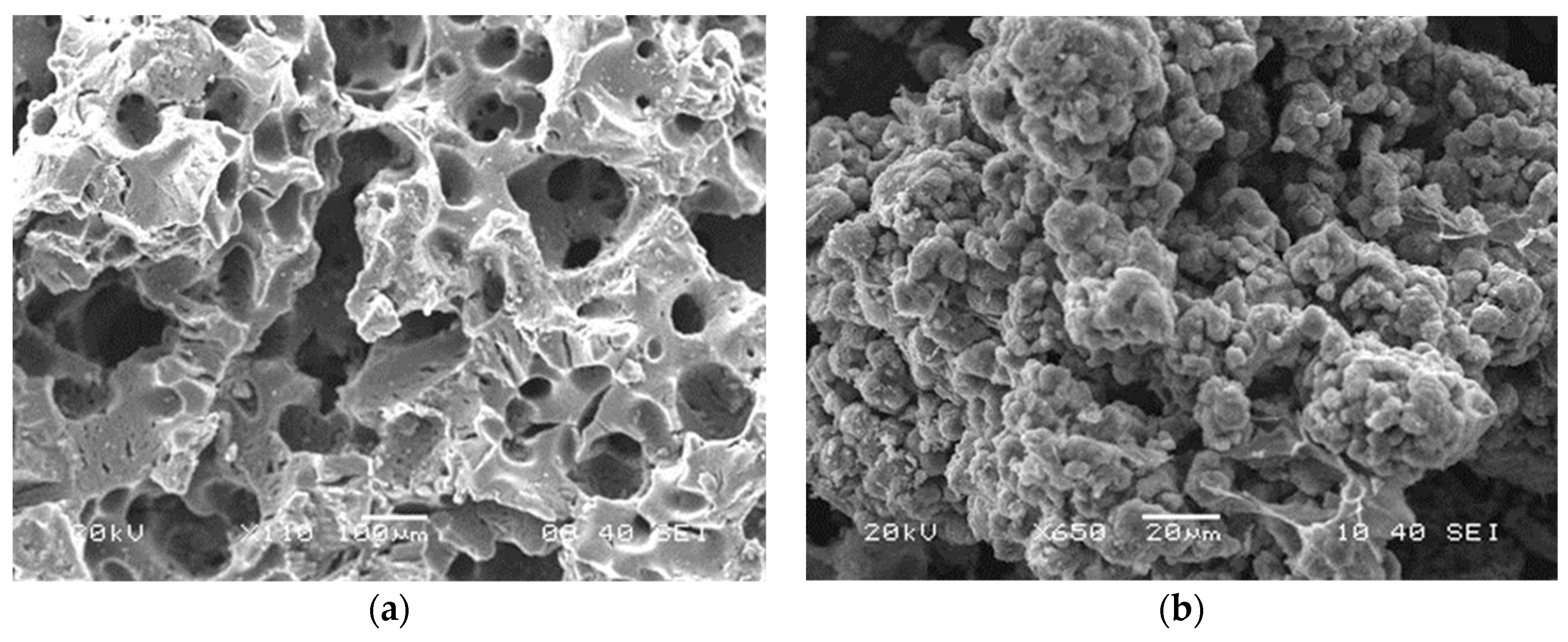
3.12. Morphology of AAJPL Surface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ali, I.H.; Ateeg, A. Study of SoilPollutants inOmdurman IndustrialArea, Sudan, Using X-ray Fluorescence Technique. Int. J. Environ. Res. 2015, 9, 291–294. [Google Scholar]
- Ali, I.H.; Sulfab, A. Concurrent two one-electron transfer in the oxidation of chromium (III) complexes with trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetate and diethylenetriaminepentaacetate ligands by periodate ion. Int. J. Chem. Kinet. 2012, 44, 729–735. [Google Scholar] [CrossRef]
- Kumar, R.; Arya, D.K.; Singh, N.; Vats, H.K. Removal of Cr (VI) Using Low Cost Activated Carbon Developed by Agricultural Waste. J. Appl. Chem. 2017, 10, 76–79. [Google Scholar] [CrossRef]
- Krishna, D.; Sree, R.P. Removal of chromium from aqueous solution by Custard apple (Annona squamosa) peel powder as adsorbent. Int. J. Appl. Sci. Eng. 2013, 11, 171–194. [Google Scholar]
- Mohanty, K.; Jha, M.; Meikap, B.C.; Biswas, M.N. Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. Chem. Eng. Sci. 2005, 60, 3049–3059. [Google Scholar] [CrossRef]
- Kobya, M. Removal of Cr (VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: Kinetic and equilibrium studies. Bioresour. Technol. 2004, 91, 317–321. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Removal of heavy metals from aqueous solution by sawdust adsorption. J. Environ. Sci. 2007, 19, 160–166. [Google Scholar] [CrossRef]
- Honnannavar, S.M.; Hampannavar, U.; Hegde, P. Removal of Hexavalent chromium from wastewater by using activated sugarcane bagasse as an adsorbent. J. Environ. Sci. Sustain. 2013, 1, 120–123. [Google Scholar]
- Rai, M.K.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.S.; Rai, B.N. Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour.-Effic. Technol. 2016, 2, S63–S70. [Google Scholar] [CrossRef]
- Elabbas, S.; Mandi, L.; Berrekhis, F.; Pons, M.N.; Leclerc, J.P.; Ouazzani, N. Removal of Cr (III) from chrome tanning wastewater by adsorption using two natural carbonaceous materials: Eggshell and powdered marble. J. Environ. Manag. 2016, 166, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Attar, S.; Parande, M. Removal of Hexavalent Chromium (Cr (VI)) from Industrial Wastewater by Using Biomass Adsorbent (Rice Husk Carbone). Int. J. Adv. Eng. Stud. 2012, 1, 92–94. [Google Scholar]
- Meunier, N.; Laroulandie, J.; Blais, J.F.; Tyagi, R.D. Cocoa shells for heavy metal removal from acidic solutions. Bioresour. Technol. 2003, 90, 255–263. [Google Scholar] [CrossRef]
- Moyo, M.; Chikazaza, M.; Nyamunda, B.C.; Guyo, U. Adsorption Batch Studies on the Removal of Pb(II) Using Maize Tassel Based Activated Carbon. J. Chem. 2013, 2013, 508934. [Google Scholar] [CrossRef]
- Bernard, E.; Jimoh, A.; Odigure, J. Heavy metals removal from industrial wastewater by activated carbon prepared from coconut shell. Res. J. Chem. Sci. 2013, 3, 3–9. [Google Scholar]
- Badmus, M.; Audu, T.; Anyata, B. Removal of lead ion from industrial wastewaters by activated carbon prepared from periwinkle shells (Typanotonus fuscatus). Turk. J. Eng. Environ. Sci. 2007, 31, 251–263. [Google Scholar]
- Khan, M.A.; Alemayehu, A.; Duraisamy, R.; Berekete, A.K. Removal of Lead ion from aqueous solution by Bamboo activated Carbon. Int. J. Water Res. 2015, 5, 33–46. [Google Scholar]
- Li, M.; Zhang, Z.; Li, R.; Wang, J.J.; Ali, A. Removal of Pb(II) and Cd(II) ions from aqueous solution by thiosemicarbazide modified chitosan. Int. J. Biol. Macromol. 2016, 86, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, P. Adsorption of lead (II) ions from simulated wastewater using natural waste: A kinetic, thermodynamic and equilibrium study. Environ. Prog. Sustain. Energy 2014, 33, 55–64. [Google Scholar] [CrossRef]
- Rahman, M.M.; Adil, M.; Yusof, M.A.; Kamaruzzaman, B.Y.; Ansary, H.R. Removal of Heavy Metal Ions with Acid Activated Carbons Derived from Oil Palm and Coconut Shells. Materials 2014, 7, 3634–3650. [Google Scholar] [CrossRef]
- Tahiruddin, N.S.M.; Ab Rahman, S.Z. Adsorption of lead in aqueous solution by a mixture of activated charcoal and peanut shell. World J. Sci. Technol. Res. 2013, 1, 102–109. [Google Scholar]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Rao, M.M.; Wang, M.C. Biosorption of Pb21 from aqueous solutions by Moringa oleifera bark: Equilibrium and kinetic studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef]
- Okoye, A.; Ejikeme, P.; Onukwuli, O. Lead removal from wastewater using fluted pumpkin seed shell activated carbon: Adsorption modeling and kinetics. Int. J. Environ. Sci. Technol. 2010, 7, 793–800. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, J.; Shen, D.; Xiao, R.; Gu, S.; Zhao, M.; Liang, J. Removal of Pb(II) from water by the activated carbon modified by nitric acid under microwave heating. J. Colloid Interface Sci. 2016, 436, 118–127. [Google Scholar] [CrossRef]
- Taha, M.F.; Kiat, C.F.; Shaharun, M.S.; Ramli, A. Removal of Ni(II), Zn(II) and Pb(II) ions from single metal aqueous solution using activated carbon prepared from rice husk. Int. J. Environ. Ecol. Eng. 2011, 5, 855–860. [Google Scholar]
- Azouaou, N.; Sadaoui, Z.; Mokaddem, H. Adsorption of Lead from Aqueous Solution onto Untreated Orange Barks: Equilibrium, Kinetics and Thermodynamics. In Proceedings of the E3S Web of Conferences, Guizhou, China, 22–24 September 2013. [Google Scholar]
- Singanan, M. Removal of lead (II) and cadmium (II) ions from wastewater using activated biocarbon. Sci. Asia 2011, 37, 115–119. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C. Adsorption of heavy metals by biomaterials derived from the marine alga Ecklonia maxima. Hydrometallurgy 2004, 73, 1–10. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Eldesoky, E.G.; AbdulKhaleque, M.; Rahman, M.M.; Naushad, M. Facile mercury detection and removal from aqueous media involving ligand impregnated conjugate nanomaterials. Chem. Eng. J. 2016, 290, 243–251. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, C. Synthesis, characterization and application of triethylenetetraamine modified polystyrene resin in removal of mercury, cadmium and lead from aqueous solutions. Chem. Eng. J. 2009, 155, 844–850. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, C. Preparation and application of acrylic acid grafted polytetraflouroethylene fiber as a weak acid cation exchanger for adsorption of Er(III). J. Hazard. Mater. 2009, 170, 1125–1132. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, Z.Z.; Tao, S.R. Removal of chromium (VI) from aqueous solution using walnut hull. J. Environ. Manag. 2009, 90, 721–729. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical basis for the Dubinin-Radushkevitch (DR) adsorption isotherm equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- El-Wakil, A.M.; Abou El-Maaty, W.M.; Awad, F.S. Removal of lead from aqueous solution on activated carbon and modified activated carbon prepared from Dried Water hyacinth plant. J. Anal. Bioanal. Tech. 2014, 5, 1–14. [Google Scholar]
- Ali, I.H.; Alrafai, H.A. Kinetic, isotherm and thermodynamic studies on biosorption of chromium(VI) by using activated carbon from leaves of Ficus nitida. Chem. Cent. J. 2016, 10, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.A.; Khedr, S.A.; Elkholy, S.A. Adsorption of chromium ion (VI) by acid activated carbon. Braz. J. Chem. Eng. 2010, 27, 183–193. [Google Scholar] [CrossRef]
- Erhayem, M.; Al-Tohami, F.; Mohamed, R.; Ahmida, K. Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. Am. J. Anal. Chem. 2015, 6, 1–10. [Google Scholar] [CrossRef]

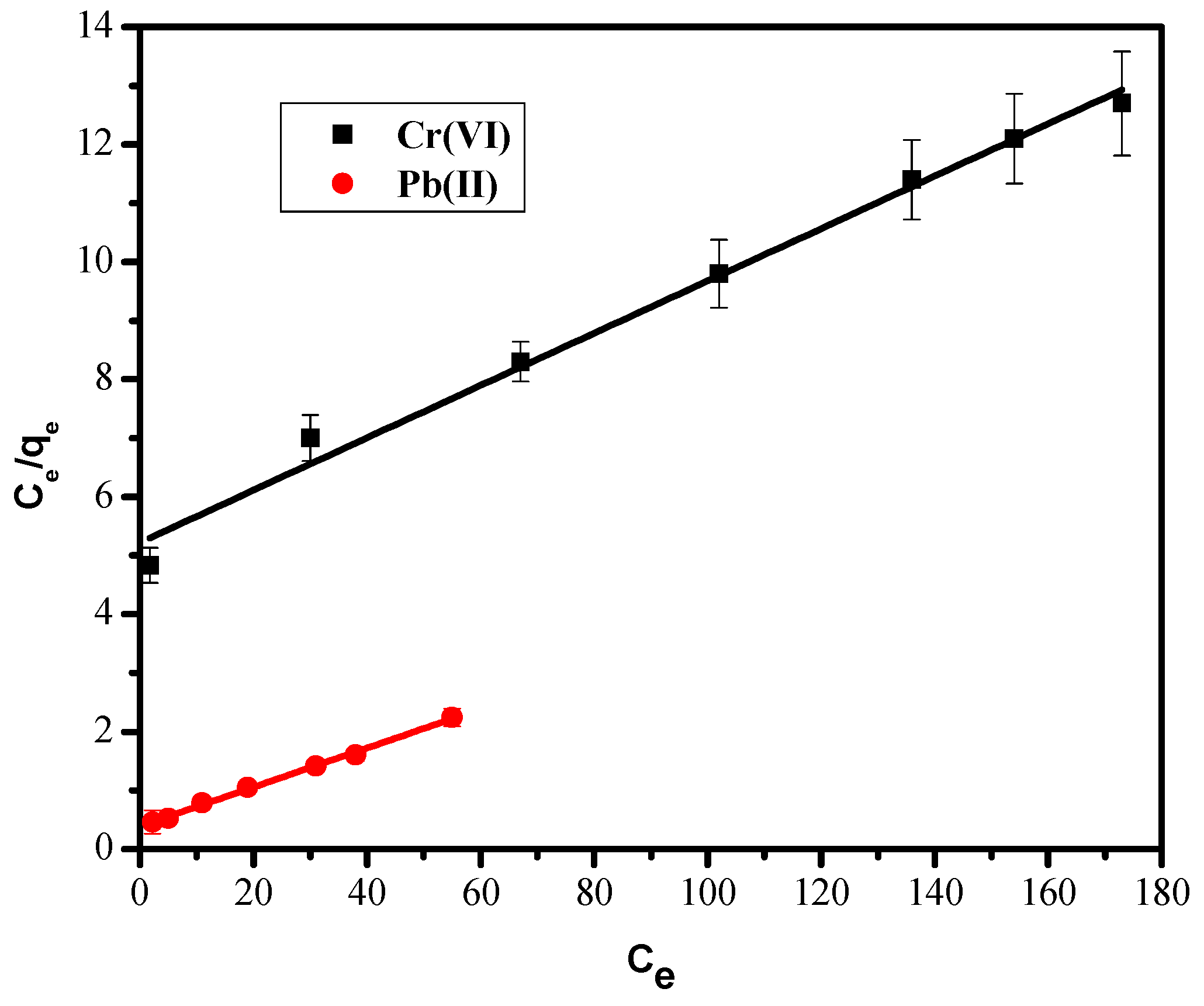

| Langmuir Isotherm | |||
| qmax, mg/g | KL, L/g | R2 | |
| Pb(II) | 30.3 | 0.08 | 0.997 |
| Cr(VI) | 23.0 | 0.01 | 0.991 |
| Freundlich Isotherm | |||
| n | Kf, (mg/g)/(mg/L)n | R2 | |
| Pb(II) | 0.50 | 21.23 | 0.965 |
| Cr(VI) | 0.20 | 24.45 | 0.920 |
| Temkin isotherm | |||
| A, L/g | B | R2 | |
| Pb(II) | 0.89 | 6.45 | 0.992 |
| Cr(VI) | 0.23 | 3.29 | 0.935 |
| D-R isotherm | |||
| β | qmax, mg/g | R2 | |
| Pb(II) | 2.0 × 10−6 | 20.1 | 0.851 |
| Cr(VI) | 8.0 × 10−5 | 13.5 | 0.712 |
| T, K | KD | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) |
|---|---|---|---|---|
| Pb(II) | ||||
| 298 | 22.83 | −2.5 | 107.58 | 369.3 |
| 303 | 5.36 | −4.3 | ||
| 313 | 2.50 | −8.0 | ||
| 323 | 1.41 | −11.7 | ||
| Cr(VI) | ||||
| 298 | 22.86 | −4.2 | 78.59 | 274.4 |
| 303 | 9.78 | −5.5 | ||
| 313 | 5.60 | −8.3 | ||
| 323 | 2.94 | −11.0 |
| Adsorbent | qm | Kinetics | Isotherm model | Adsorption Type | References |
|---|---|---|---|---|---|
| Pb(II) | |||||
| Juniperus procera | 30.3 | 2nd order | Langmuir | physical | This study |
| Tridax procumbens | 4.5 | 1st order | Langmuir | physical | [28] |
| Pumpkin seed shell | 14.3 | 2nd order | Langmuir | ** | [22] |
| Water Hyacinth | 33.4 | 2nd order | Langmuir | physical | [33] |
| Cr(VI) | |||||
| Juniperus procera | 23.0 | 2nd order | Langmuir | physical | This study |
| Ficus nitida | 21.0 | 2nd order | Langmuir | physical | [34] |
| Annona squamosal | 7.9 | 2nd order | Freundlich | chemical | [4] |
| Olive stones | 25.6 | 1st order | Langmuir | ** | [35] |
| Rosmarinus officinalis | 1.0 | ** | Langmuir | ** | [36] |
| Walnut hull | 98.1 | 2nd order | Langmuir | ** | [31] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.H.; Al Mesfer, M.K.; Khan, M.I.; Danish, M.; Alghamdi, M.M. Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves. Processes 2019, 7, 217. https://doi.org/10.3390/pr7040217
Ali IH, Al Mesfer MK, Khan MI, Danish M, Alghamdi MM. Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves. Processes. 2019; 7(4):217. https://doi.org/10.3390/pr7040217
Chicago/Turabian StyleAli, Ismat H., Mohammed K. Al Mesfer, Mohammad I. Khan, Mohd Danish, and Majed M. Alghamdi. 2019. "Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves" Processes 7, no. 4: 217. https://doi.org/10.3390/pr7040217
APA StyleAli, I. H., Al Mesfer, M. K., Khan, M. I., Danish, M., & Alghamdi, M. M. (2019). Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves. Processes, 7(4), 217. https://doi.org/10.3390/pr7040217







