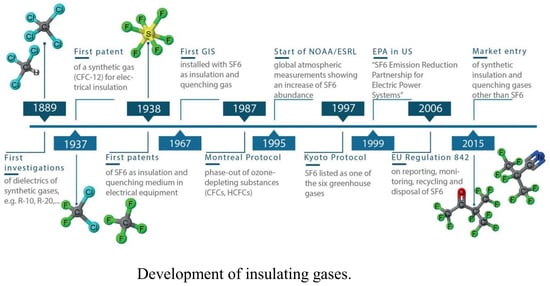
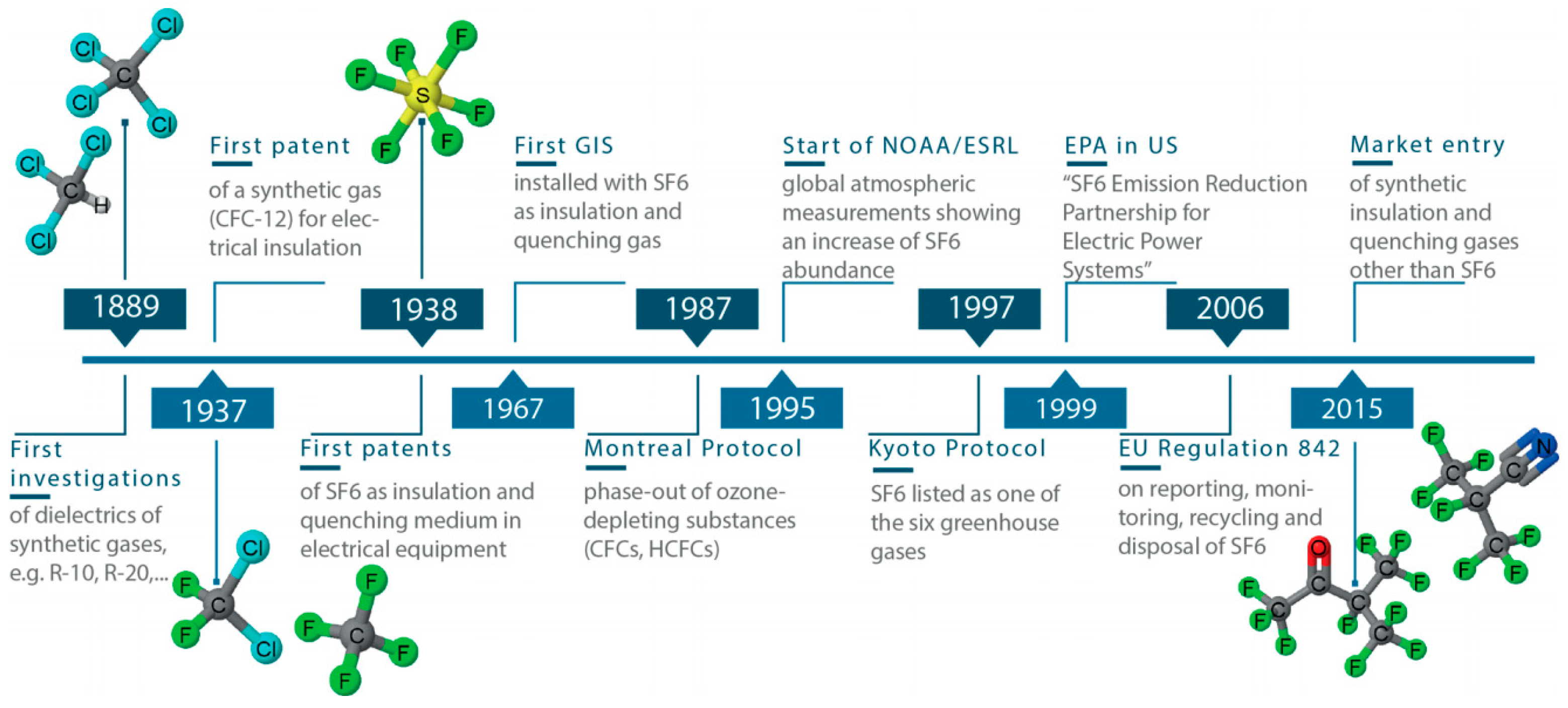
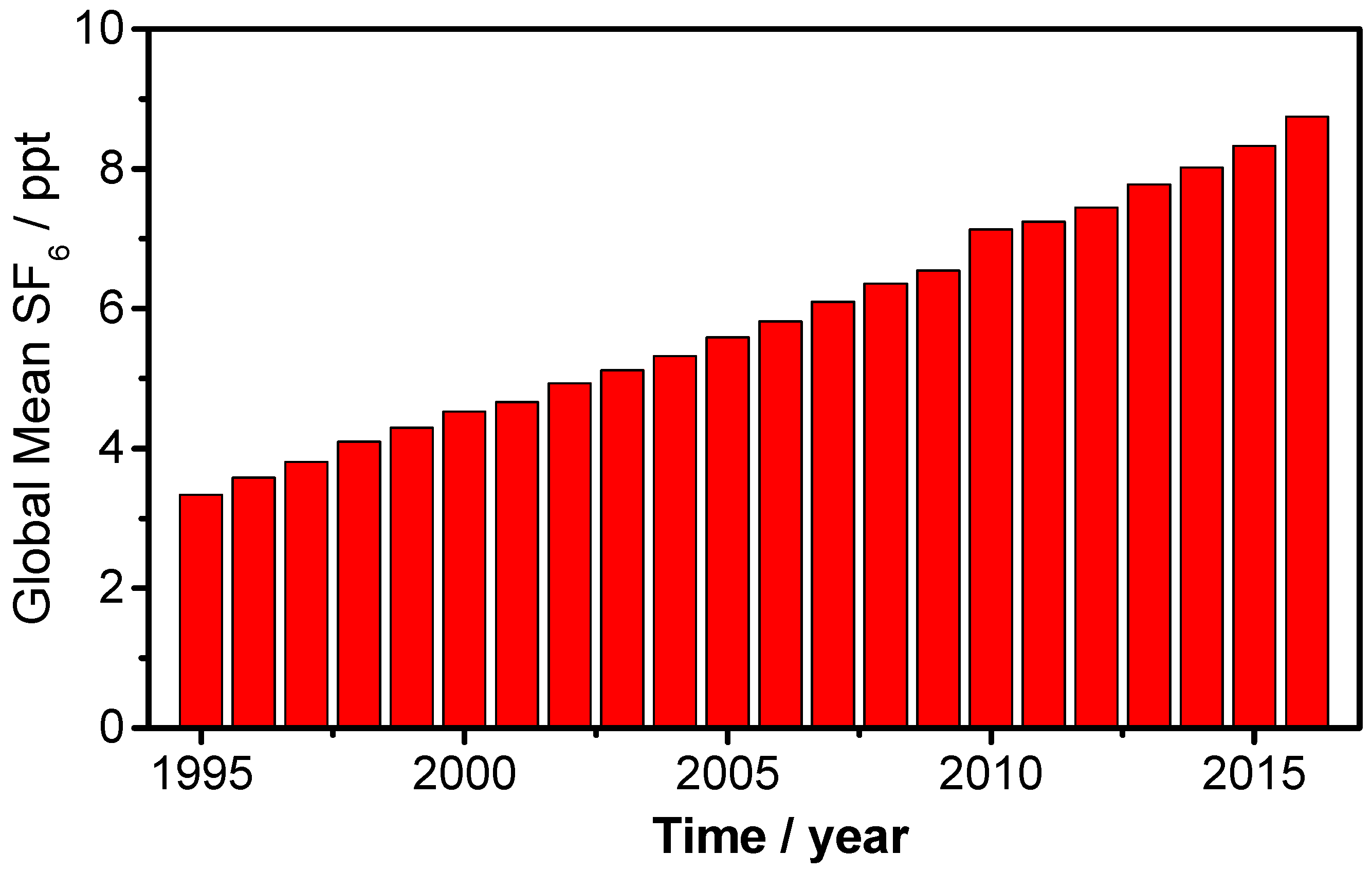
Alternative Environmentally Friendly Insulating Gases for SF6
Abstract
1. Introduction
2. Perfluorocarbons
2.1. Perfluoromethane (CF4), Perfluoroethane (C2F6), and Perfluoropropane (C3F8)
2.2. Perfluorocyclobutane (c-C4F8)
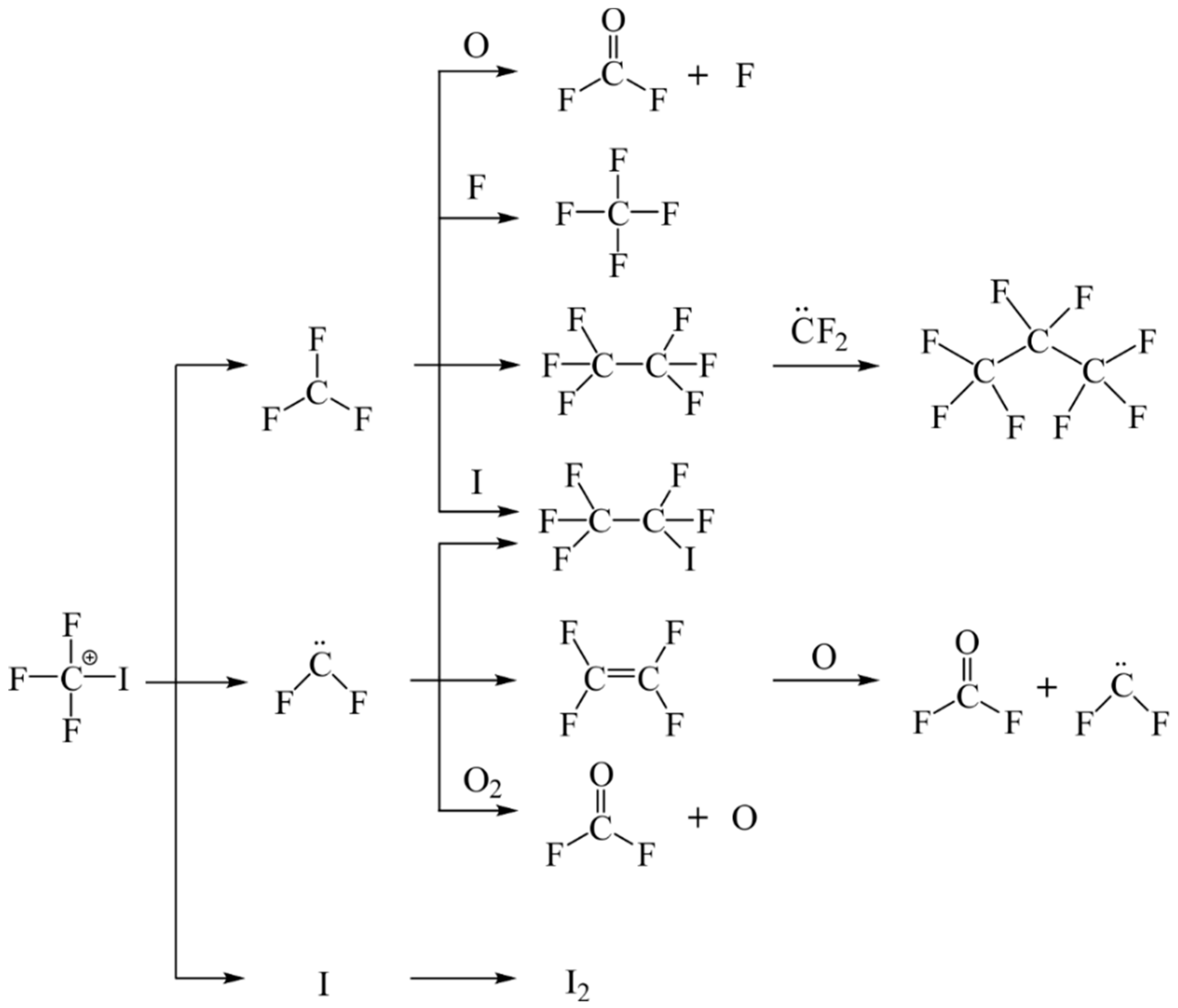
3. Trifluoroiodomethane (CF3I)
4. Perfluorinated Ketones (C5F10O and C6F12O)
4.1. Perfluoropentanone (C5F10O)
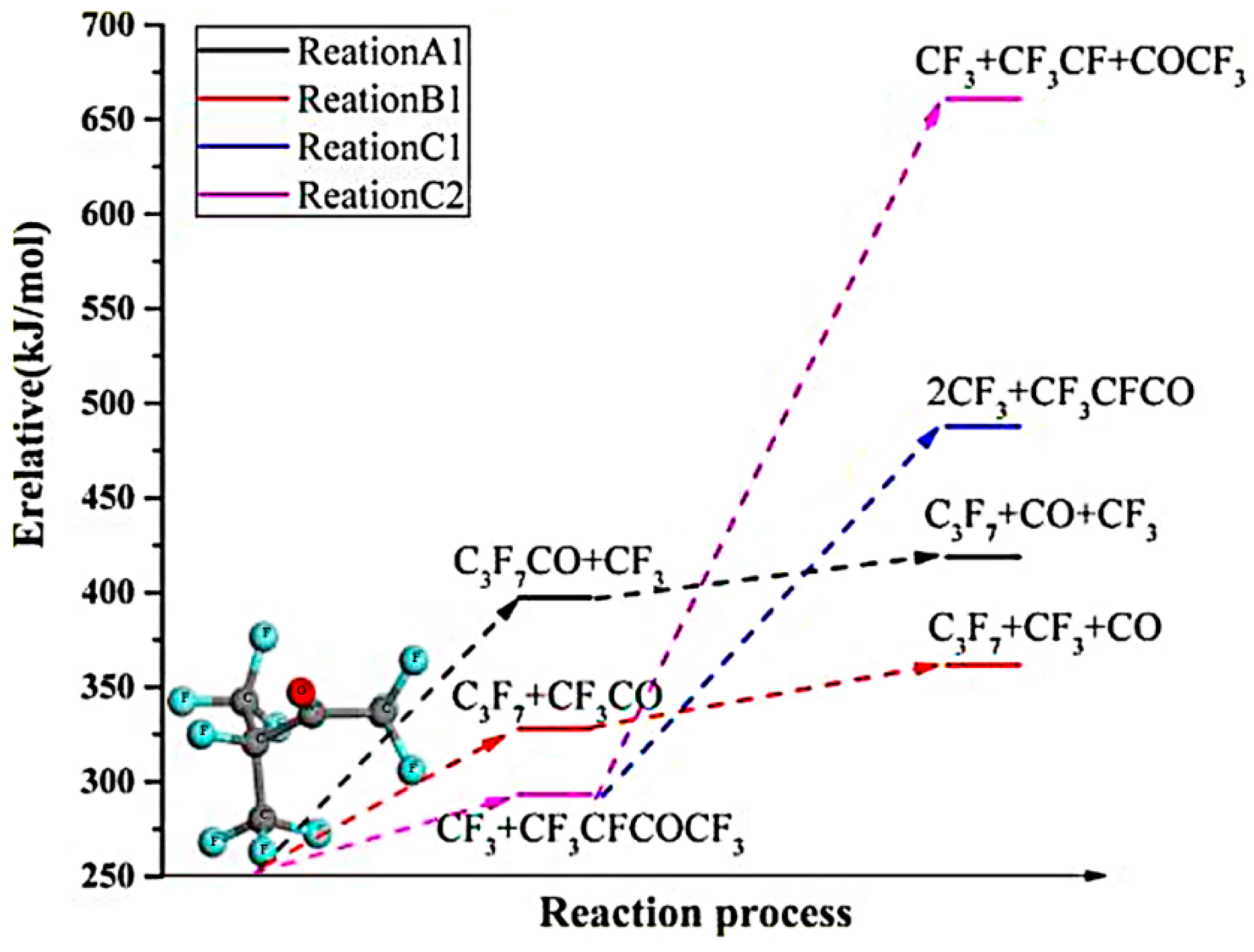
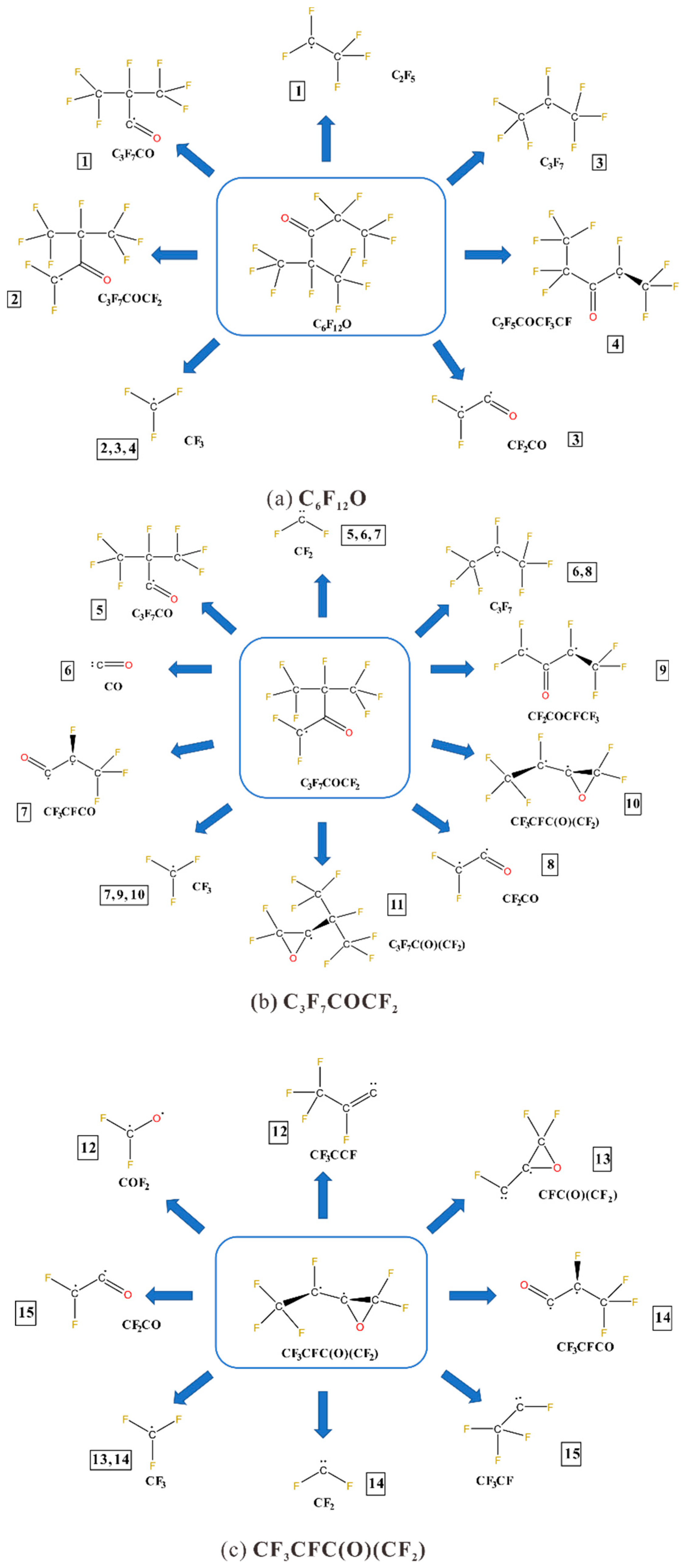
4.2. Perfluorohexanone(C6F12O)
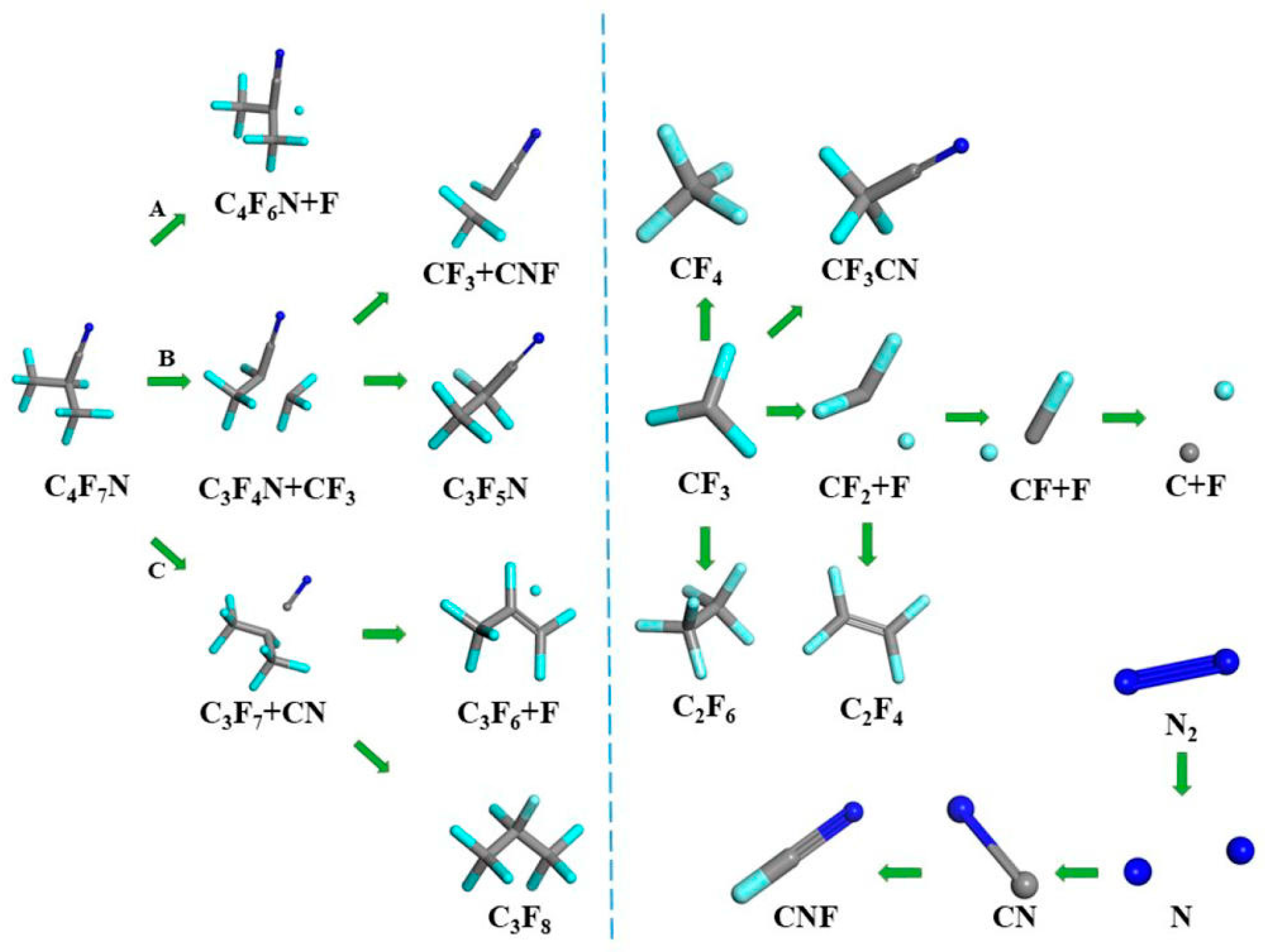
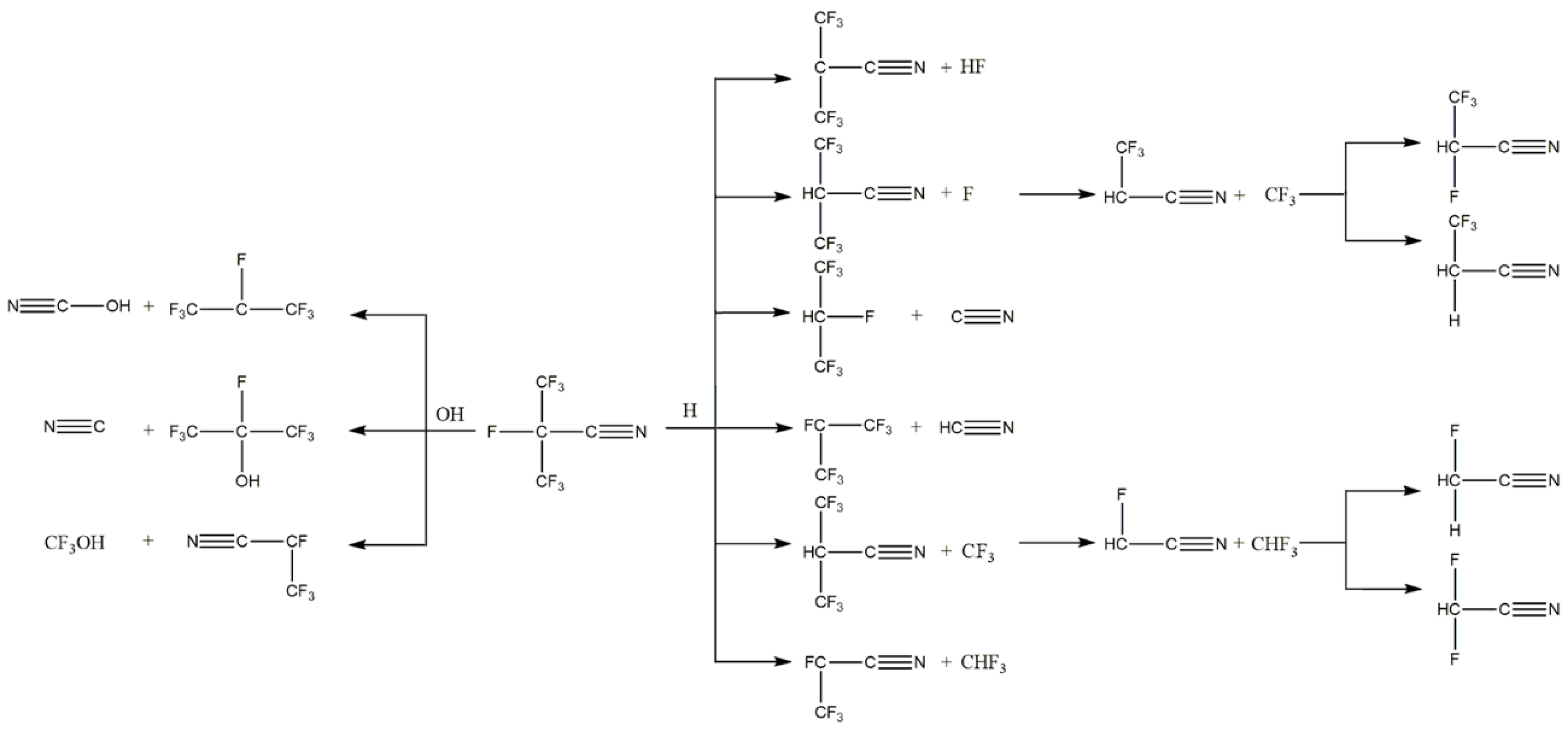
5. Fluoronitrile (C4F7N)
6. Challenges and Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodine, M.T.; Herb, R.G. Effect of CCl4 vapor on the dielectric strength of Air. Phys. Rev. 1937, 51, 508–511. [Google Scholar] [CrossRef]
- Charlton, E.; Cooper, F. Dielectric strength of insulating fluids I. Gases and gas-vapour mixtures. Gen. Electr. Rev. 1937, 40, 438–442. [Google Scholar]
- Cooper, F.S. Gas-Insulated Electric Device. U.S. Patent 2221670, 27 July 1937. [Google Scholar]
- Pollock, H.C.; Cooper, F.S. The effect of pressure on the positive point-to-plane discharge in N2, O2, CO2, SO2, SF6, CCl2F2, A, He, and H2. Phys. Rev. 1939, 56, 170–175. [Google Scholar] [CrossRef]
- Beroual, A.; Haddad, A. Recent advances in the quest for a new insulation gas with a low impact on the environment to replace sulfur hexafluoride (SF6) gas in high-voltage power network applications. Energies 2017, 10, 1216. [Google Scholar] [CrossRef]
- Maiss, M.; Brenninkmeijer, C.A.M. Atmospheric SF6: Trends, sources, and prospects. Environ. Sci. Technol. 1998, 32, 3077–3086. [Google Scholar] [CrossRef]
- Rabie, M.; Franck, C.M. Assessment of eco-friendly gases for electrical insulation to replace the most potent industrial greenhouse gas SF6. Environ. Sci. Technol. 2018, 52, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Ray, E.A.; Moore, F.L.; Elkins, J.W.; Rosenlof, K.H.; Laube, J.C.; Röckmann, T.; Marsh, D.R.; Andrews, A.E. Quantification of the SF6 lifetime based on mesospheric loss measured in the stratospheric polar vortex. J. Geophys. Res. Atmos. 2017, 122, 4626–4638. [Google Scholar] [CrossRef]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green gas to replace SF6 in electrical grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Fang, X.; Hu, X.; Janssens, M.G.; Wu, J.; Han, J.; Su, S.; Zhang, J.; Hu, J. Sulfur hexafluoride (SF6) emission estimates for China: An inventory for 1990-2010 and a projection to 2020. Environ. Sci. Technol. 2013, 47, 3848–3855. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.M.; Friedman, J.F.; Caples, C.M.; Shuman, N.S.; Van Doren, J.M.; Bardaro, M.F., Jr.; Nguyen, P.; Zweiben, C.; Campbell, M.J.; Viggiano, A.A. Electron attachment to sulfur oxyhalides: SOF2, SOCl2, SO2F2, SO2Cl2, and SO2FCl attachment rate coefficients, 300-900 K. J. Chem. Phys. 2010, 132, 214302. [Google Scholar] [CrossRef]
- Okabe, S.; Wada, J.; Ueta, G. Dielectric properties of gas mixtures with C3F8/C2F6 and N2/CO2. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2108–2116. [Google Scholar] [CrossRef]
- Deng, Y.; Li, B.; Xiao, D. Analysis of the insulation characteristics of C3F8 gas mixtures with N2 and CO2 using boltzmann equation method. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3253–3259. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, J.; Wang, X.; Rong, M. Comparison of dielectric breakdown properties for different carbon-fluoride insulating gases as SF6 alternatives. AIP Adv. 2018, 8, 085122. [Google Scholar] [CrossRef]
- Yamamoto, O.; Takuma, T.; Hamada, S.; Yamakawa, Y. Applying a gas mixtures containing c-C4F8 as an insulation medium. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 1075–1081. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, Y.; Zhang, Z.; Xiao, D. Analysis of the insulation characteristics of c-C4F8/CO2 gas mixtures by the Monte Carlo method. J. Phys. D Appl. Phys. 2008, 41, 015206. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Jia, S.; Murphy, A.B. Prediction of the dielectric strength for c-C4F8 mixtures with CF4, CO2, N2, O2 and air by boltzmann equation analysis. J. Phys. D Appl. Phys. 2014, 47, 425204. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhang, X.; Xiao, S.; Tang, J. Insights on decomposition process of c-C4F8 and c-C4F8/N2 mixture as substitutes for SF6. R. Soc. Open Sci. 2018, 5, 181104. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, S.; Cressault, Y.; Zhang, X.; Tang, J.; Li, Y.; Deng, Z. Study on the influence of O2 on the breakdown voltage and self-recovery characteristics of c-C4F8/N2 mixture. AIP Adv. 2018, 8, 085121. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, S.; Zhang, X.; Cressault, Y.; Tang, J.; Deng, Z.; Li, Y. The influence of O2 on decomposition characteristics of c-C4F8/N2 environmental friendly insulating gas. Processes 2018, 6, 174. [Google Scholar] [CrossRef]
- Solomon, S.; Burkholder, J.B.; Ravishankara, A.R.; Garcia, R.R. Ozone depletion and global warming potentials of CF3I. J. Geophys. Res. 1994, 99, 20929–20935. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, D. Analysis of the insulation characteristics of CF3I gas mixtures with Ar, Xe, He, N2, and CO2 using Boltzmann equation method. Jpn. J. Appl. Phys. 2014, 53, 096201. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Wu, J.; Jia, S. Analysis of the insulation characteristics of CF3I mixtures with CF4, CO2, N2, O2 and air. J. Phys. D Appl. Phys. 2013, 46, 345203. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Lin, H. Insulation characteristics of c-C4F8-N2 and CF3I-N2 mixtures as possible substitutes for SF6. IEEE Trans. Power Deliv. 2017, 32, 254–262. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Zhou, J.; Tang, J. Experimental analysis of the feasibility of CF3I/CO2 substituting SF6 as insulation medium using needle-plate electrodes. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1895–1900. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, S.; Xiao, S.; Li, Y.; Deng, Z.; Tang, J. Experimental studies on the power–frequency breakdown voltage of CF3I/N2/CO2 gas mixture. J. Appl. Phys. 2017, 121, 103303. [Google Scholar] [CrossRef]
- Zhao, S.; Xiao, D.; Xue, P.; Zhong, R.; Deng, Y. Analysis of insulation performance and polar effect of CF3I/CO2 mixtures. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1364–1370. [Google Scholar] [CrossRef]
- Chen, L.; Griffiths, H.; Haddad, A.; Kamarudin, M.S. Breakdown of CF3I gas and its mixtures under lightning impulse in coaxial-gIL geometry. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1959–1967. [Google Scholar] [CrossRef]
- Jamil, M.K.M.; Ohtsuka, S.; Hikita, M.; Saitoh, H.; Sakaki, M. Gas by-products of CF3I under AC partial discharge. J. Electrost. 2011, 69, 611–617. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Han, Y.; Dai, Q. Analysis of the feasibility of CF3I/CO2 used in c-GIS by partial discharge inception voltages in positive half cycle and breakdown voltages. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3234–3243. [Google Scholar] [CrossRef]
- Song, X.; Yi, L.; Xiaoxing, Z.; Ran, Z.; Dibo, W.; Shuangshuang, T. Discharge decomposition components forming mechanism of CF3I under micro-aerobic condition. High Volt. Eng. 2017, 43, 727–735. [Google Scholar]
- Xiao, S.; Li, Y.; Zhang, X.; Tang, J.; Tian, S.; Deng, Z. Formation mechanism of CF3I discharge components and effect of oxygen on decomposition. J. Phys. D Appl. Phys. 2017, 50, 155601. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Zhang, J.; Li, C.; Dai, Q.; Han, Y. Influence of humidity on the decomposition products and insulating characteristics of CF3I. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 819–828. [Google Scholar] [CrossRef]
- Xiaoxing, Z.; Qiwei, D.; Yefei, H.; Song, X. Investigation towards the influence of trace water on CF3I decomposition components under discharge. High Volt. Eng. 2016, 42, 172–178. [Google Scholar]
- Xiao, S.; Cressault, Y.; Zhang, X.; Teulet, P. The influence of Cu, Al, or Fe on the insulating capacity of CF3I. Phys. Plasmas 2016, 23, 123505. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, S.; Han, Y.; Cressault, Y. Experimental studies on power frequency breakdown voltage of CF3I/N2 mixed gas under different electric fields. Appl. Phys. Lett. 2016, 108, 092901. [Google Scholar] [CrossRef]
- Tan, D.; Zhou, B.; Xue, J.; Cai, F.; Xiao, D. Basic impulse performance of high-pressure CF3I-N2 gas mixture and its application for 126 kV GIL. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1380–1386. [Google Scholar]
- Pagliaro, J.L.; Linteris, G.T. Hydrocarbon flame inhibition by C6F12O (Novec 1230): Unstretched burning velocity measurements and predictions. Fire Saf. J. 2017, 87, 10–17. [Google Scholar] [CrossRef]
- Linteris, G.T.; Babushok, V.I.; Sunderland, P.B.; Takahashi, F.; Katta, V.R.; Meier, O. Unwanted combustion enhancement by C6F12O fire suppressant. Proc. Combust. Inst. 2013, 34, 2683–2690. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xiao, S.; Tang, J.; Tian, S.; Deng, Z. Decomposition mechanism of C5F10O: An environmentally friendly insulation medium. Environ. Sci. Technol. 2017, 51, 10127–10136. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xiao, S.; Huang, L.; Tang, J.; Deng, Z.; Tian, S. Study on the discharge characteristics of an environmental-friendly insulating medium C5F10O. Proc. CSEE 2018, 38, 4298–4306. [Google Scholar]
- Zhong, L.; Rong, M.; Wang, X.; Wu, J.; Han, G.; Han, G.; Lu, Y.; Yang, A.; Wu, Y. Compositions, thermodynamic properties, and transport coefficients of high-temperature C5F10O mixed with CO2 and O2 as substitutes for SF6 to reduce global warming potential. AIP Adv. 2017, 7, 075003. [Google Scholar] [CrossRef]
- Hyrenbach, M.; Paul, T.A.; Owens, J. Environmental and safety aspects of AirPlus insulated GIS. CIRED Open Access Proc. J. 2017, 2017, 132–135. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Wang, D. Decomposition characteristics of C5F10O/air mixture as substitutes for SF6 to reduce global warming. J. Fluor. Chem. 2018, 208, 65–72. [Google Scholar] [CrossRef]
- Tatarinov, A.V.; Bilera, I.V.; Avtaeva, S.V.; Shakhatov, V.A.; Solomakhin, P.V.; Maladen, R.; Prévé, C.; Piccoz, D. Dielectric barrier discharge processing of trans-CF3CH=CHF and CF3C(O)CF(CF3)2, their mixtures with Air, N2, CO2 and analysis of their decomposition products. Plasma Chem. Plasma Process. 2015, 35, 845–862. [Google Scholar] [CrossRef]
- Wang, X.; Fu, X.; Han, G.; Lu, Y.; Li, X.; Gao, Q.; Rong, M. Insulation performance of C5F10O/CO2 gas mixture. High Volt. Eng. 2017, 43, 715–720. [Google Scholar]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, D.; Chen, Q.; Wang, D. Theoretical evaluation of the interaction between C5-PFK molecule and Cu (1 1 1). J. Fluor. Chem. 2018, 208, 48–54. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, D.; Li, Y.; Zhang, J.; Cui, Z.; Xiao, S.; Tang, J. Theoretical study on the interaction between C5-PFK and Al (1 1 1), Ag (1 1 1): A comparative study. Appl. Surface Sci. 2019, 464, 586–596. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Han, D.; Han, X.C.; Yan, X.L.; Rong, W.Q.; Zhang, G.Q. Decomposition by-products of C6F12O/N2 and C6F12O/air mixed gases under AC 50Hz corona discharge. Adv. Technol. Electr. Eng. Energy 2018, 37, 1–8. [Google Scholar]
- Tian, S.; Zhang, X.; Xiao, S.; Zhuo, R.; Wang, D.; Deng, Z.; Li, Y. Breakdown characteristics and decomposition characteristics of C6F12O and N2 mixed gas under AC voltage. Proc. CSEE 2018, 38, 3125–3132. [Google Scholar]
- Zhang, X.; Tian, S.; Xiao, S.; Deng, Z.; Li, Y.; Tang, J. Insulation strength and decomposition characteristics of a C6F12O and N2 gas mixture. Energies 2017, 10, 1170. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Li, Y.; Chen, D. Insight into the decomposition mechanism of C6F12O-CO2 gas mixture. Chem. Eng. J. 2018, 360, 929–940. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Y.; Zhang, X.; Tian, S.; Deng, Z.; Tang, J. Effects of micro-water on decomposition of the environment-friendly insulating medium C5F10O. AIP Adv. 2017, 7, 065017. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, Q.; Fu, M.; Zhuo, R.; Xiao, S.; Chen, D.; Tang, J. Study on the dielectric properties of C4F7N/N2 mixture under highly non-uniform electric field. IEEE Access 2018, 6, 42868–42876. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Chen, Q.; Chen, D.; Cui, Z.; Tang, J. Insight into the compatibility between C6F12O and metal materials: Experiment and theory. IEEE Access 2018, 6, 58154–58160. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Tang, J.; Chen, D.; Wang, D. Decomposition properties of C4F7N/N2 gas mixture: An environmentally friendly gas to replace SF6. Ind. Eng. Chem. Res. 2018, 57, 5173–5182. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Li, Y.; Xiao, S.; Zhang, J.; Liu, C. Decomposition mechanism of environmental-friendly insulating medium C3F7CN/CO2. Proc. CSEE 2018, 38, 7174–7182. [Google Scholar]
- Zhang, X.; Li, Y.; Chen, D.; Xiao, S.; Tian, S.; Tang, J.; Zhuo, R. Reactive molecular dynamics study of the decomposition mechanism of the environmentally friendly insulating medium C3F7CN. RSC Adv. 2017, 7, 50663–50671. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xiao, S.; Tian, S.; Deng, Z.; Tang, J. Theoretical study of the decomposition mechanism of environmentally friendly insulating medium C3F7CN in the presence of H2O in a discharge. J. Phys. D Appl. Phys. 2017, 50, 325201. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, D.; Xiao, S.; Tian, S.; Tang, J.; Wang, D. Dissociative adsorption of environment-friendly insulating medium C3F7CN on Cu (1 1 1) and Al (1 1 1) surface: A theoretical evaluation. Appl. Surf. Sci. 2018, 434, 549–560. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, D.; Liu, C.; Shi, Y. Insights into the interaction between C4F7N decomposition products and Cu (1 1 1), Ag (1 1 1) surface. J. Fluor. Chem. 2018, 213, 24–30. [Google Scholar] [CrossRef]
| Chemical Formula | GWP/100-Years | Lifetime/Years | Dielectric Strength Relative to SF6 | Boiling Point/°C | Toxicity | ODP | Flammability |
|---|---|---|---|---|---|---|---|
| SF6 | 22,800 | 850 | 1 | −64 | Non-toxic | 0 | Non-flammable |
| CF4 | 9200 | 50,000 | 0.4 | −128 | Low-toxicity | 0 | Non-flammable |
| C2F6 | 12,200 | 10,000 | 0.76 | −78.1 | Non-toxic | 0 | Non-flammable |
| C3F8 | 8830 | 2600 | 1.01 | −36.7 | Non-toxic | 0 | Non-flammable |
| c-C4F8 | 8700 | 3200 | 1.3 | −8 | Non-toxic | 0 | Non-flammable |
| CF3I | 0.4 | 0.0055 | 1.23 | −22 | Non-toxic | 0 | Non-flammable |
| C5F10O | 1 | 0.044 | 1.5–2 | 27 | Non-toxic | 0 | Non-flammable |
| C6F12O | 1 | 0.014 | 2.7 | 49 | Non-toxic | 0 | Non-flammable |
| C4F7N | 2100 | 22 | 2 | −4.7 | Non-toxic | 0 | Non-flammable |
| CF4 | 6630 | 50,000 | 0.4 | −128 | - | - | Non-flammable |
| CO2 | 1 | - | 0.32–0.37 | −79 | Non-toxic | 0 | Non-flammable |
| N2 | - | - | 0.34–0.43 | −196 | Non-toxic | 0 | Non-flammable |
| air | - | - | 0.37–0.40 | −193 | Non-toxic | 0 | Non-flammable |
| He | - | - | 0.02–0.06 | −268.9 | Non-toxic | 0 | Non-flammable |
| Ar | - | - | 0.04–0.10 | −186 | Non-toxic | 0 | Non-flammable |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, D.; Liu, J.; Zhang, Y.; Zeng, L. Alternative Environmentally Friendly Insulating Gases for SF6. Processes 2019, 7, 216. https://doi.org/10.3390/pr7040216
Wang Y, Huang D, Liu J, Zhang Y, Zeng L. Alternative Environmentally Friendly Insulating Gases for SF6. Processes. 2019; 7(4):216. https://doi.org/10.3390/pr7040216
Chicago/Turabian StyleWang, Yong, Danqing Huang, Jing Liu, Yaru Zhang, and Lian Zeng. 2019. "Alternative Environmentally Friendly Insulating Gases for SF6" Processes 7, no. 4: 216. https://doi.org/10.3390/pr7040216
APA StyleWang, Y., Huang, D., Liu, J., Zhang, Y., & Zeng, L. (2019). Alternative Environmentally Friendly Insulating Gases for SF6. Processes, 7(4), 216. https://doi.org/10.3390/pr7040216