Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis
Abstract
1. Introduction
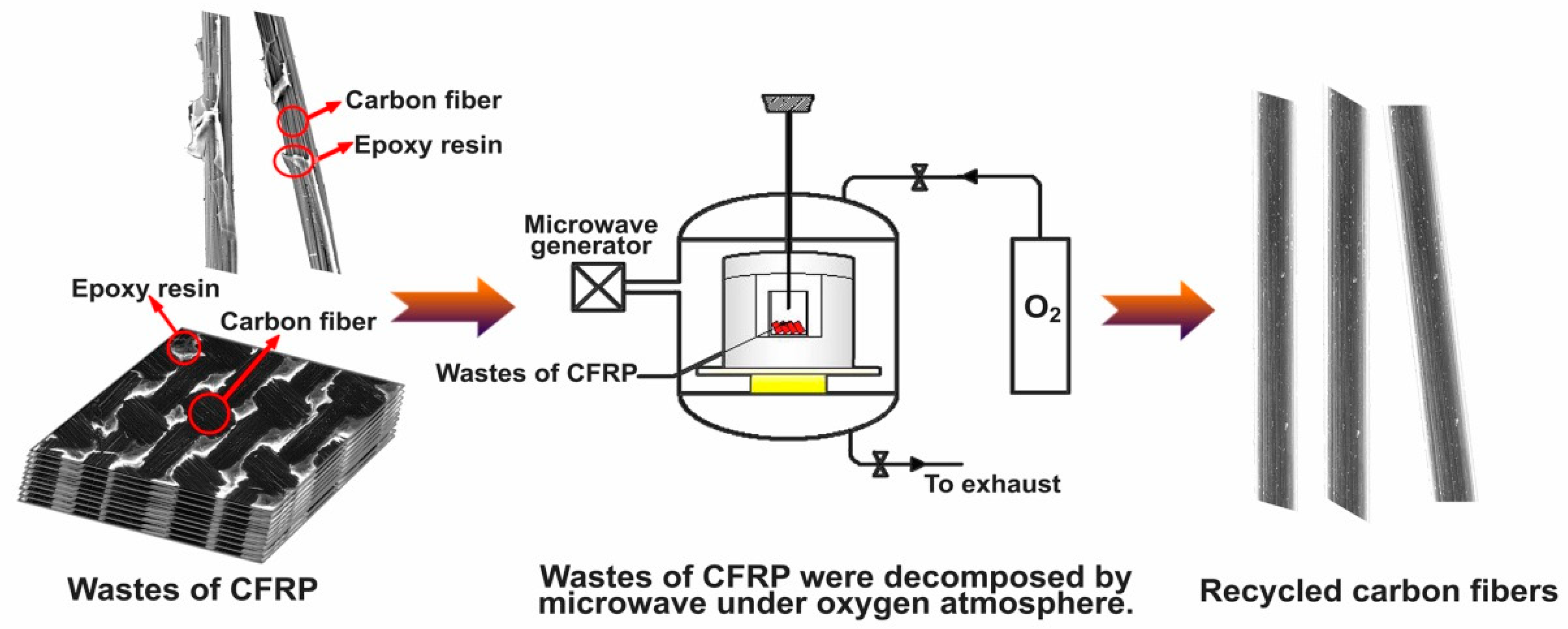
2. Materials and Methods
2.1. Materials and Experimental Procedure
2.2. Characterization
3. Results and Discussion
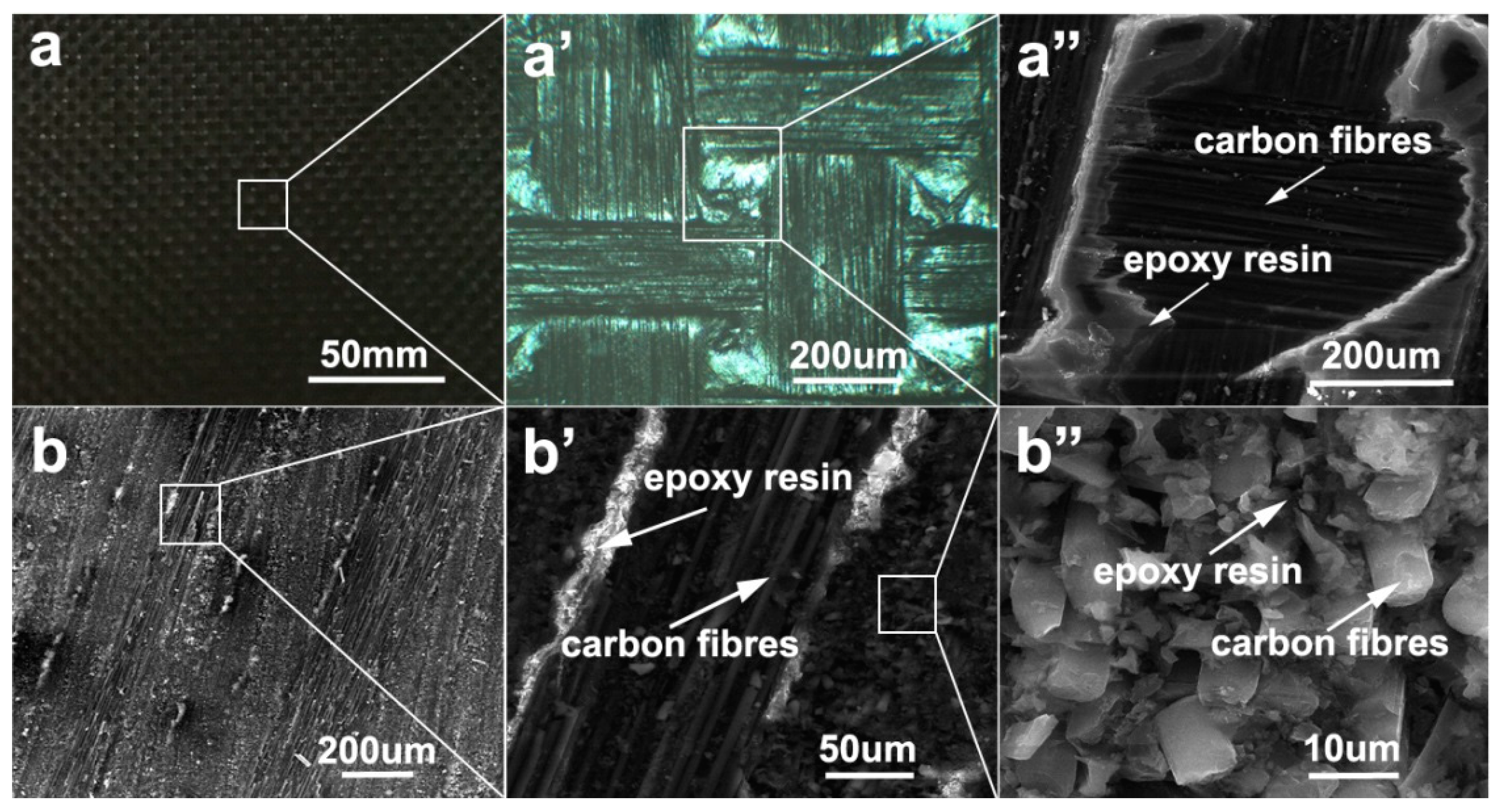
3.1. Characterization of Carbon Fiber-Reinforced Polymer (CFRP) by Metallographic Microscopy and Scanning Electron Microscopy (SEM)
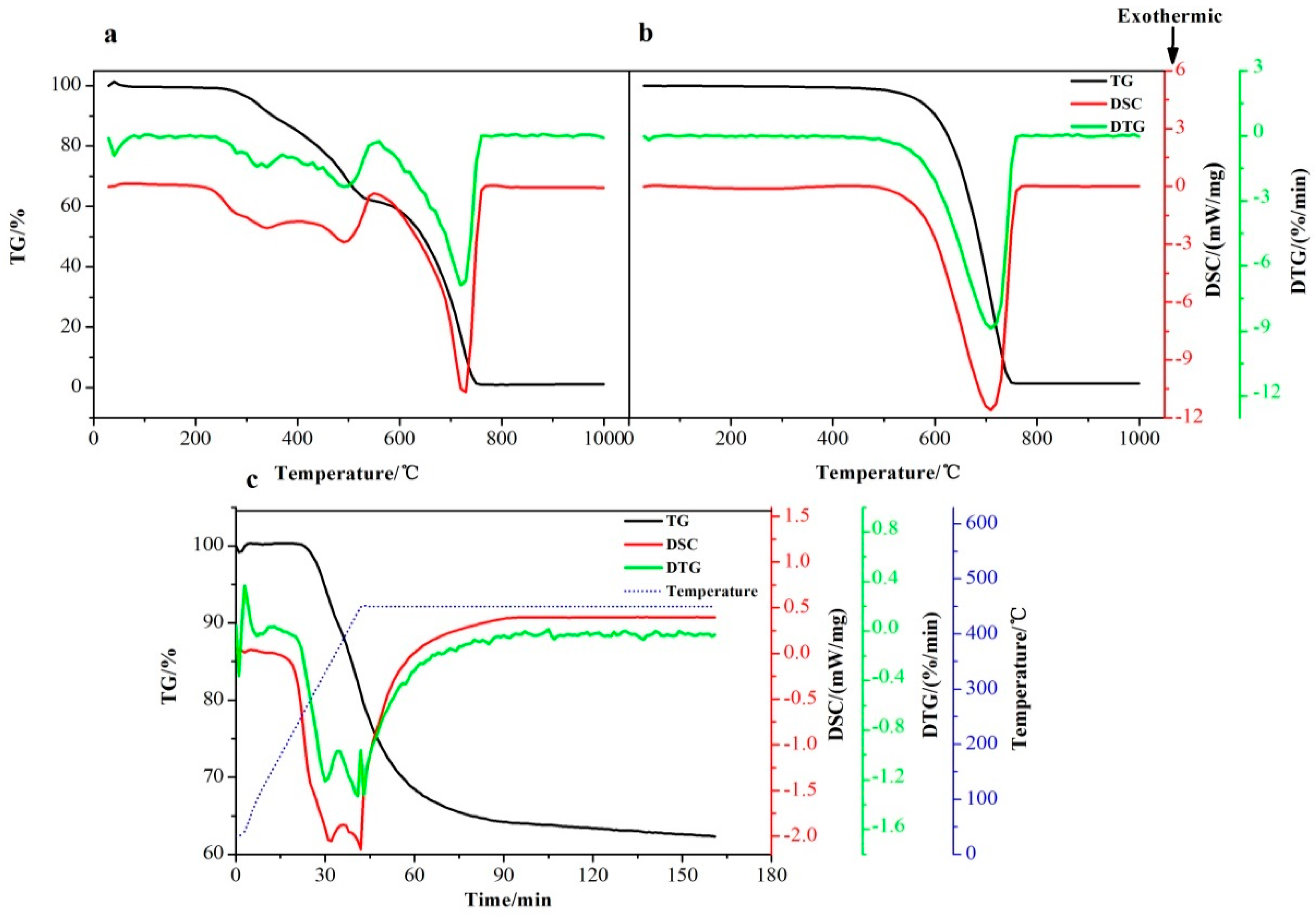
3.2. Thermal Decomposition Behavior of the CFRP Waste
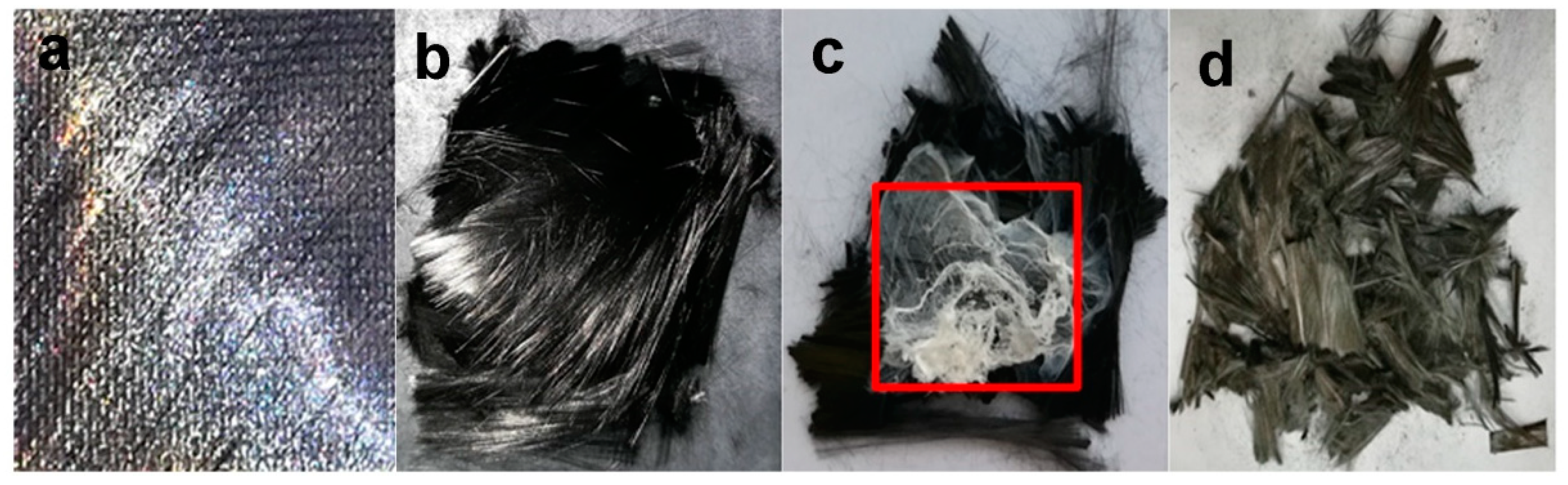
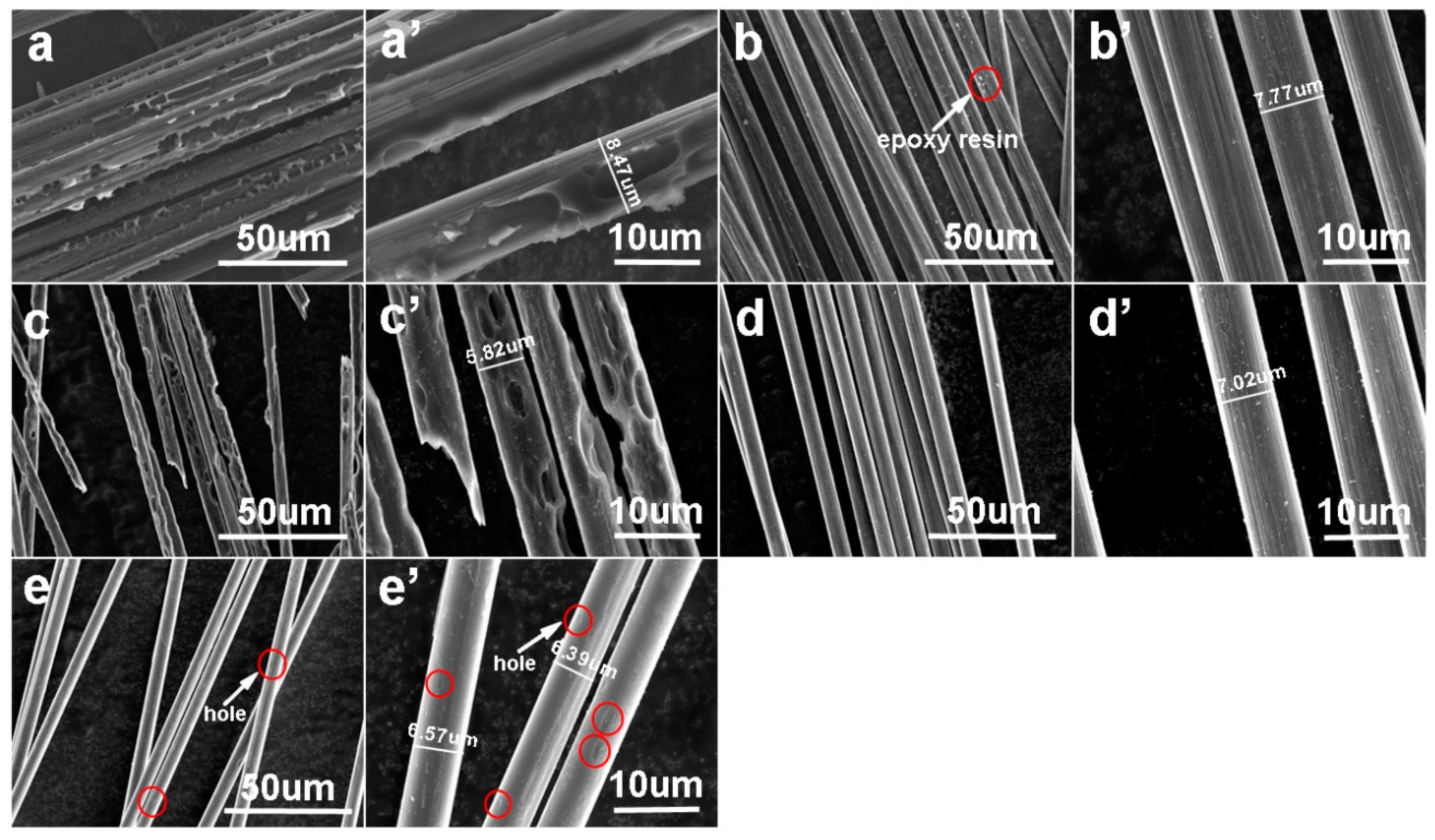
3.3. Morphology of the Recovered Carbon Fibers
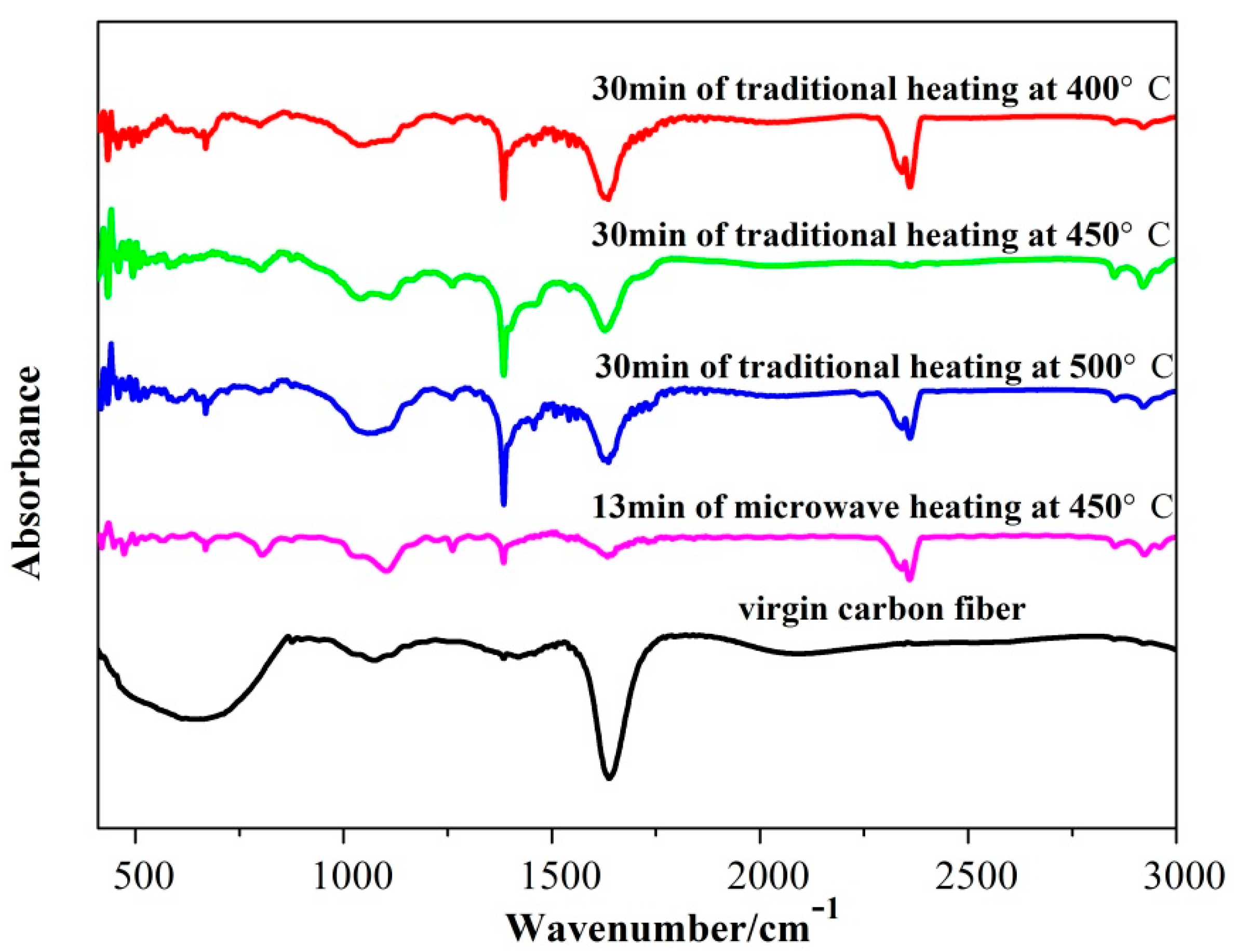
3.4. Fourier Transform Infrared (FT–IR) Analysis of the Recovered Carbon Fibers
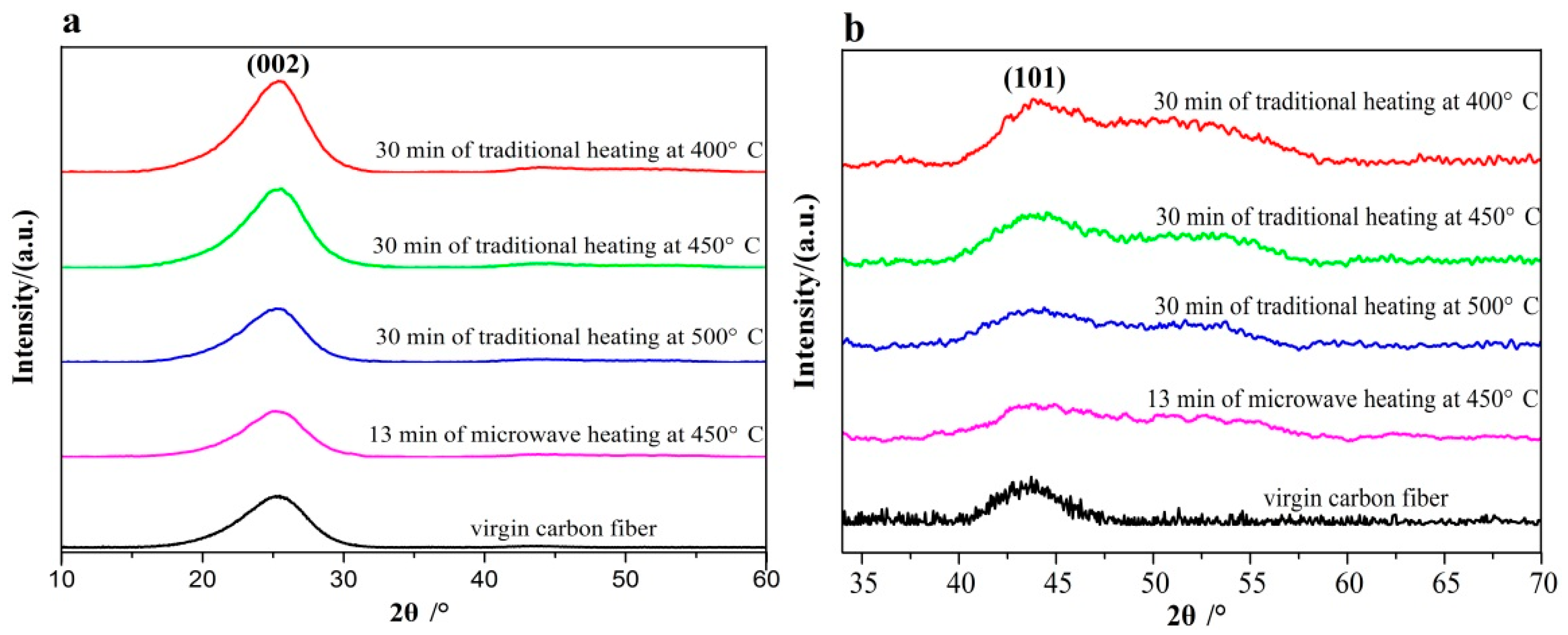
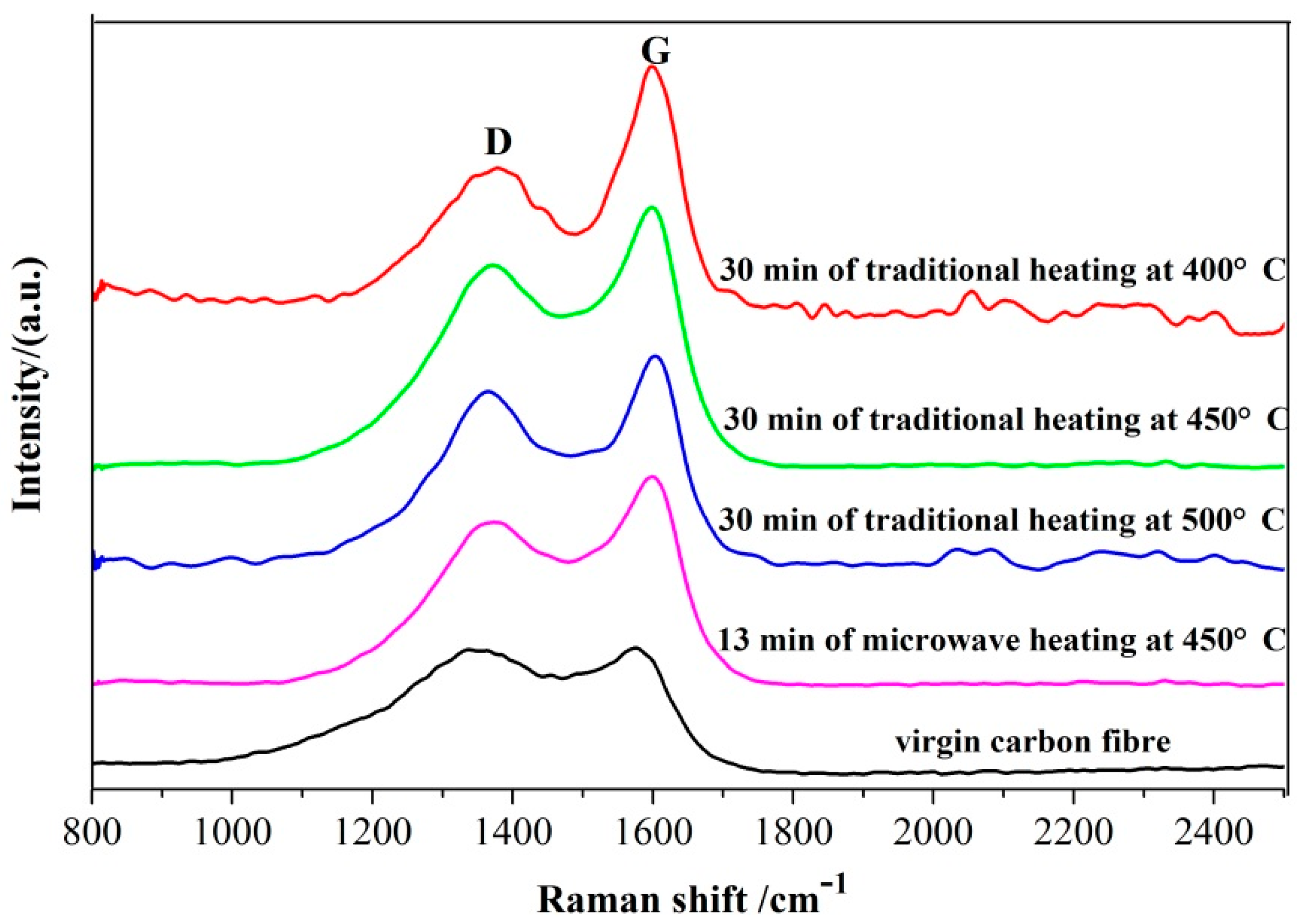
3.5. X-ray Diffraction (XRD) and Raman Analyses of the Recovered Carbon Fibers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.H.; Xing, F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method. Compos. Part A Appl. Sci. Manuf. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.L.; Gillet, A.; Mantaux, O.; Leeke, G.A. Recovery and reuse of discontinuous carbon fibres by solvolysis: Realignment and properties of remanufactured materials. Compos. Sci. Technol. 2017, 139, 99–108. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Liu, W.; Wang, J.; Tang, T. Recycling of carbon fibre reinforced epoxy resin composites under various oxygen concentrations in nitrogen–oxygen atmosphere. J. Anal. Appl. Pyrolysis 2015, 112, 253–261. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical recycling of carbon fibre/epoxy composites in a mixed solution of peroxide hydrogen and n,n-dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Frohs, W.; Jaeger, H. Carbon fiber & composite material—Landscape Germany. Carbon 2012, 50, 737. [Google Scholar]
- Kraus, T.; Kühnel, M.; Witten, E. Composites Market Report 2014 Market Developments, Trends, Challenges and Opportunities; Federation of Reinforced Plastics: Frankfurt, Germany, 2014. [Google Scholar]
- Roberts, T. The Carbon Fibre Industry: Global Strategic Market Evaluation 2006–2010; Materials Technology Publications: Watford, Hertfordshire, UK, 2006; Volume 10. [Google Scholar]
- Jiang, G.; Pickering, S.J.; Walker, G.S.; Wong, K.H.; Rudd, C.D. Surface characterisation of carbon fibre recycled using fluidised bed. Appl. Surf. Sci. 2008, 254, 2588–2593. [Google Scholar] [CrossRef]
- Marsh, G. Reclaiming value from post-use carbon composite. Reinf. Plast. 2008, 52, 36–39. [Google Scholar] [CrossRef]
- Mcconnell, V.P. Launching the carbon fibre recycling industry. Reinf. Plast. 2010, 54, 33–37. [Google Scholar] [CrossRef]
- Roberts, T. Rapid growth forecast for carbon fibre market. Reinf. Plast. 2007, 51, 10–13. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J.; Lester, E.H.; Turner, T.A.; Wong, K.H.; Warrior, N.A. Characterisation of carbon fibres recycled from carbon fibre/epoxy resin composites using supercritical n-propanol. Compos. Sci. Technol. 2009, 69, 192–198. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, G.; Meng, L. Recycling of carbon fibre reinforced composites using water in subcritical conditions. Mater. Sci. Eng. A 2009, 520, 179–183. [Google Scholar]
- Kouparitsas, C.E.; Kartalis, C.N.; Varelidis, P.C.; Tsenoglou, C.J.; Papaspyrides, C.D. Recycling of the fibrous fraction of reinforced thermoset composites. Polym. Compos. 2010, 23, 682–689. [Google Scholar] [CrossRef]
- Ogi, K.; Shinoda, T.; Mizui, M. Strength in concrete reinforced with recycled cfrp pieces. Compos. Part A Appl. Sci. Manuf. 2005, 36, 893–902. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Feng, L. Chemical recycling of carbon fibers reinforced epoxy resin composites in oxygen in supercritical water. Mater. Des. 2010, 31, 999–1002. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Jiang, Z.; Tang, T. Chemical recycling of carbon fibre reinforced epoxy resin composites in subcritical water: Synergistic effect of phenol and koh on the decomposition efficiency. Polym. Degrad. Stab. 2012, 97, 214–220. [Google Scholar] [CrossRef]
- Yan, H.; Lu, C.X.; Jing, D.Q.; Chang, C.B.; Liu, N.X.; Hou, X.L. Recycling of carbon fibers in epoxy resin composites using supercritical 1-propanol. Carbon 2016, 100, 710–711. [Google Scholar] [CrossRef]
- Yildirir, E.; Onwudili, J.A.; Williams, P.T. Recovery of carbon fibres and production of high quality fuel gas from the chemical recycling of carbon fibre reinforced plastic wastes. J. Supercrit. Fluids 2014, 92, 107–114. [Google Scholar] [CrossRef]
- Hyde, J.R.; Lester, E.; Kingman, S.; Pickering, S.; Wong, K.H. Supercritical propanol, a possible route to composite carbon fibre recovery: A viability study. Compos. Part A Appl. Sci. Manuf. 2006, 37, 2171–2175. [Google Scholar] [CrossRef]
- Yip, H.L.H.; Pickering, S.J.; Rudd, C.D. Characterisation of carbon fibres recycled from scrap composites using fluidised bed process. Plast. Rubber Compos. 2002, 31, 278–282. [Google Scholar] [CrossRef]
- López, F.A.; Rodríguez, O.; Alguacil, F.J.; García-Díaz, I.; Centeno, T.A.; García-Fierro, J.L.; González, C. Recovery of carbon fibres by the thermolysis and gasification of waste prepreg. J. Anal. Appl. Pyrolysis 2013, 104, 675–683. [Google Scholar] [CrossRef]
- Ye, S.Y.; Bounaceur, A.; Soudais, Y.; Barna, R. Parameter optimization of the steam thermolysis: A process to recover carbon fibers from polymer-matrix composites. Waste Biomass Valorization 2013, 4, 73–86. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; Dodds, C.; Hyde, J.; García-Serna, J.; Poliakoff, M.; Lester, E.; Cocero, M.J.; Kingman, S.; Pickering, S.; Wong, K.H. Chemical recycling of carbon fibre reinforced composites in nearcritical and supercritical water. Compos. Part A Appl. Sci. Manuf. 2008, 39, 454–461. [Google Scholar] [CrossRef]
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W.M.; Aymonier, C.; Horn, S. Semi-continuous flow recycling method for carbon fibre reinforced thermoset polymers by near- and supercritical solvolysis. Polym. Degrad. Stab. 2016, 133, 264–274. [Google Scholar] [CrossRef]
- Peng, Z.; Lin, X.; Li, Z.; Hwang, J.Y.; Kim, B.G.; Zhang, Y.; Li, G.; Jiang, T. Dielectric characterization of indonesian low-rank coal for microwave processing. Fuel Process. Technol. 2017, 156, 171–177. [Google Scholar] [CrossRef]
- Khaled, D.E.; Novas, N.; Gazquez, J.A.; Manzano-Agugliaro, F. Microwave dielectric heating: Applications on metals processing. Renew. Sustain. Energy Rev. 2018, 82, 2880–2892. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. Cheminform 2010, 41, 175–189. [Google Scholar] [CrossRef]
- Yadoji, P.; Peelamedu, R.; Agrawal, D.; Roy, R. Microwave sintering of ni–zn ferrites: Comparison with conventional sintering. Mater. Sci. Eng. B 2003, 98, 269–278. [Google Scholar] [CrossRef]
- Rybakov, K.I.; Buyanova, M.N. Microwave resonant sintering of powder metals. Scr. Mater. 2018, 149, 108–111. [Google Scholar] [CrossRef]
- Kumar, R.C.; Benal, M.M.; Prasad, B.D.; Krupashankara, M.S.; Kulkarni, R.S.; Siddaligaswamy, N.H. Microwave assisted extraction of oil from pongamia pinnata seeds. Mater. Today Proc. 2018, 5, 2960–2964. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Zhou, J.; Cheng, Q. Microwave curing of multidirectional carbon fiber reinforced polymer composites. Compos. Struct. 2019, 212, 83–93. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L.; Zhou, J. Curing multidirectional carbon fiber reinforced polymer composites with indirect microwave heating. Int. J. Adv. Manuf. Technol. 2018, 97, 1137–1147. [Google Scholar] [CrossRef]
- Long, J.; Ulven, C.A.; Gutschmidt, D.; Anderson, M.; Balo, S.; Lee, M.; Vigness, J. Recycling carbon fiber composites using microwave irradiation: Reinforcement study of the recycled fiber in new composites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Lester, E.; Kingman, S.; Wong, K.H.; Rudd, C.; Pickering, S.; Hilal, N. Microwave heating as a means for carbon fibre recovery from polymer composites: A technical feasibility study. Mater. Res. Bull. 2004, 39, 1549–1556. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Costa, C.; Santos, A.F.; Fortuny, M.; Araújo, P.H.H.; Sayer, C. Kinetic advantages of using microwaves in the emulsion polymerization of mma. Mater. Sci. Eng. C 2009, 29, 415–419. [Google Scholar] [CrossRef]
- Fariñas, J.C.; Moreno, R.; Pérez, A.; García, M.A.; García-Hernández, M.; Salvador, M.D.; Borrell, A. Microwave-assisted solution synthesis, microwave sintering and magnetic properties of cobalt ferrite. J. Eur. Ceram. Soc. 2018, 38, 2360–2368. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Okajima, I.; Hiramatsu, M.; Shimamura, Y.; Awaya, T.; Sako, T. Chemical recycling of carbon fiber reinforced plastic using supercritical methanol. J. Supercrit. Fluids 2014, 91, 68–76. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J.; Walker, G.S.; Bowering, N.; Wong, K.H.; Rudd, C.D. Soft ionisation analysis of evolved gas for oxidative decomposition of an epoxy resin/carbon fibre composite. Thermochim. Acta 2007, 454, 109–115. [Google Scholar] [CrossRef]
- Fei, J.; Duan, X.; Luo, L.; Zhang, C.; Qi, Y.; Li, H.; Feng, Y.; Huang, J. Grafting methyl acrylic onto carbon fiber via diels-alder reaction for excellent mechanical and tribological properties of phenolic composites. Appl. Surf. Sci. 2018, 433, 349–357. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-Ul-Haq, M.; Cayumil, R.; Rajarao, R.; Sahajwalla, V. Novel carbon micro fibers and foams from waste printed circuit boards. Fuel Process. Technol. 2015, 134, 473–479. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Characterization of graphite fiber surfaces with raman spectroscopy. J. Compos. Mater. 1970, 4, 492–499. [Google Scholar] [CrossRef]
| Sample | Temperature/(°C) | Time/min | Average Power/W | Heating Method | Weight-Loss Ratio/% |
|---|---|---|---|---|---|
| 1 | 400 | 30 | 850 | Traditional | 21.89 |
| 2 | 450 | 30 | 1060 | Traditional | 56.50 |
| 3 | 500 | 30 | 1300 | Traditional | 96.12 |
| 4 | 450 | 13 | 500 | Microwave | 47.03 |
| 5 | 450 | 30 | 500 | Microwave | 63.76 |
| Sample | 2θ (002)/° | D (002)/nm | 2θ (101)/° | D (101)/nm | β (rad) | La/nm | Lc/nm |
|---|---|---|---|---|---|---|---|
| 1 | 25.66 | 0.34684 | 43.52 | 0.20778 | 0.0898 | 3.06 | 1.57 |
| 2 | 25.60 | 0.34763 | 43.46 | 0.20804 | 0.0922 | 2.99 | 1.52 |
| 3 | 25.52 | 0.34879 | 43.42 | 0.20822 | 0.0963 | 2.73 | 1.46 |
| 4 | 25.54 | 0.34853 | 43.50 | 0.20789 | 0.0918 | 2.99 | 1.53 |
| Virgin carbon fiber | 25.36 | 0.35098 | 43.85 | 0.20631 | 0.0599 | 2.89 | 2.345 |
| Sample | WD/(cm−1) | WG/(cm−1) | R (ID/IG) | La/nm |
|---|---|---|---|---|
| 1 | 1378.24 | 1598.61 | 1.19 | 3.70 |
| 2 | 1371.17 | 1598.61 | 1.55 | 2.84 |
| 3 | 1365.52 | 1602.85 | 1.64 | 2.68 |
| 4 | 1372.59 | 1598.61 | 1.59 | 2.76 |
| Virgin carbon fiber | 1363.6 | 1576.39 | 1.61 | 2.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Xu, L.; Zhang, L.; Peng, J.; Guo, S.; Liu, J.; Koppala, S. Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis. Processes 2019, 7, 207. https://doi.org/10.3390/pr7040207
Deng J, Xu L, Zhang L, Peng J, Guo S, Liu J, Koppala S. Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis. Processes. 2019; 7(4):207. https://doi.org/10.3390/pr7040207
Chicago/Turabian StyleDeng, Jianying, Lei Xu, Libo Zhang, Jinhui Peng, Shenghui Guo, Jianhua Liu, and Sivasankar Koppala. 2019. "Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis" Processes 7, no. 4: 207. https://doi.org/10.3390/pr7040207
APA StyleDeng, J., Xu, L., Zhang, L., Peng, J., Guo, S., Liu, J., & Koppala, S. (2019). Recycling of Carbon Fibers from CFRP Waste by Microwave Thermolysis. Processes, 7(4), 207. https://doi.org/10.3390/pr7040207





