Isosteric Heat: Comparative Study between Clausius–Clapeyron, CSK and Adsorption Calorimetry Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Porous Solids
2.1.1. Synthesis of MOF-199
2.1.2. Synthesis of Mesostructured Silica, MCM-41
2.1.3. Synthesis of SBA-15
2.1.4. Preparation of Activated Carbons from Corn Cobs
2.1.5. Graphite
2.2. Measurement and Characterization
2.2.1. Nitrogen Adsorption-Desorption Isotherms at −196 °C
2.2.2. Thermogravimetric Analysis (TGA)
2.2.3. Infrared Spectroscopy (FTIR) and XRD of Samples used in this Research
2.2.4. SEM of Adsorbents
2.2.5. Differential Enthalpy Measurement by Adsorption Calorimetry
2.2.6. Evaluation of Isosteric Heat of Adsorption by Clausius–Clapeyron (C-C)
2.2.7. Evaluation of the Isosteric Heats of Adsorption by Chakraborty-Saha-Koyama (CSK)
- Vg: Specific volume of the gas
- T: Temperature
- P: Pressure
- ma: Adsorbate mass
- ΔHads: Conventional form of Clausius–Clapeyron isosteric heat (C-C)
3. Results and Discussion
3.1. Porous Texture Analysis
3.2. Thermogravimetric (TG) Analysis of Samples
3.3. XRD and FTIR Analysis of Samples
3.4. SEM-EDX Analysis
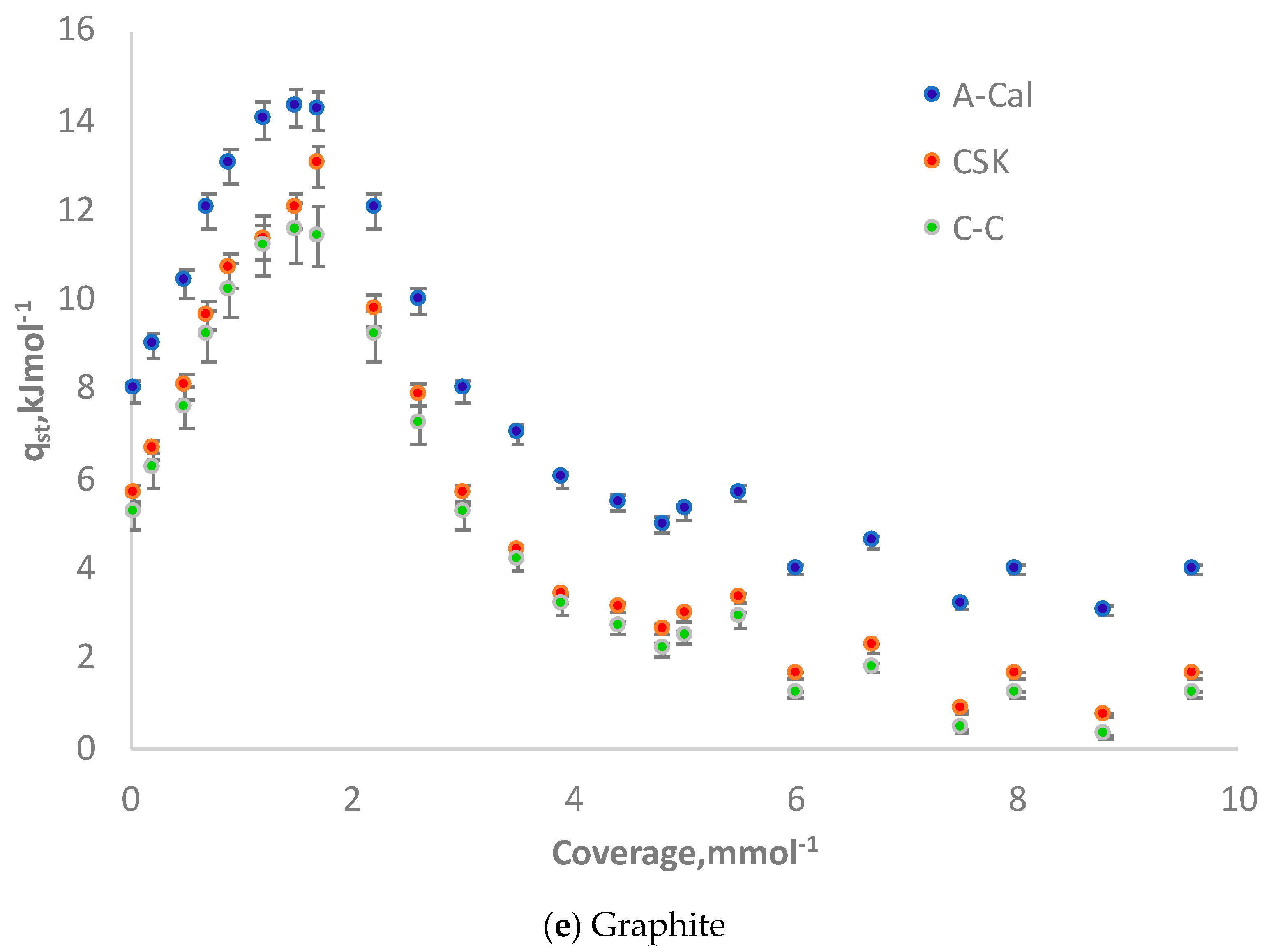
3.5. Isosteric Heat: Comparison of CSK, C-C and Adsorption Calorimetry (A-Cal) Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Myers, A.L. Thermodynamics of adsorption in porous materials. AIChE J. 2002, 48, 145–160. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Myers, A.L. Optimum conditions for adsorptive storage. Langmuir 2006, 22, 1688–1700. [Google Scholar] [CrossRef]
- Lee, S.-J.; Bae, Y.-S. Can Metal-Organic Frameworks attain new DOE targets for on-board methane storage by increasing methane heat of adsorption? J. Phys. Chem. C 2014, 118, 19833–19841. [Google Scholar] [CrossRef]
- Amrouche, H.; Creton, B.; Siperstein, F.; Nieto-Draghi, C. Prediction of thermodynamic properties of adsorbed gases in zeolitic imidazolate frameworks. RSC Adv. 2012, 2, 6028–6035. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, J. Differential heat of adsorption and isosteres. Langmuir 2017, 33, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Sircar, S. Basic research needs for design of adsorptive gas separation processes. Ind. Eng. Chem. Res. 2006, 45, 5435–5448. [Google Scholar] [CrossRef]
- Vuong, T.; Monson, P.A. Monte Carlo simulation studies of heats of adsorption in heterogeneous solids. Langmuir 1996, 12, 5425–5432. [Google Scholar] [CrossRef]
- Shen, D.; Bülow, M.; Siperstein, F.; Engelhard, M.; Myers, A.L. Comparison of experimental techniques for measuring isosteric heat of adsorption. Adsorption 2000, 6, 275–286. [Google Scholar] [CrossRef]
- Zimmermann, W.; Keller, J.U. A new calorimeter for simultaneous measurement of isotherms and heats of adsorption. Thermochim. Acta 2003, 405, 31–41. [Google Scholar] [CrossRef]
- Siperstein, F.; Gorte, R.J.; Myers, A.L. A new calorimeter for simultaneous measurements of loading and heats of adsorption from gaseous mixtures. Langmuir 1999, 15, 1570–1576. [Google Scholar] [CrossRef]
- Pinto, M.L.; Pires, J.; Carvalho, A.P.; de Carvalho, M.B. On the difficulties of predicting the adsorption of volatile organic compounds at low pressures in microporous solid: The example of ethyl benzene. J. Phys. Chem. B. 2006, 110, 250–257. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Mathias, P.M.; Kumar, R.; Moyer, J.D.; Schork, J.M.; Srinivasan, S.R.; Auvil, S.R.; Talu, O. Correlation of multicomponent gas adsorption by the dual-site langmuir model. application to nitrogen/oxygen adsorption on 5A-zeolite. Ind. Eng. Chem. Res. 1996, 35, 2477–2483. [Google Scholar] [CrossRef]
- Bimbo, N.; Ting, V.P.; Hruzewicz-Kolodziejczyk, A.; Mays, T.J. Analysis of hydrogen storage in nanoporous materials for low carbon energy applications. Faraday Discuss. 2011, 151, 59–74. [Google Scholar] [CrossRef]
- Bimbo, N.; Sharpe, J.E.; Ting, V.P.; Noguera-Díaz, A.; Mays, T.J. Isosteric enthalpies for hydrogen adsorbed on nanoporous materials at high pressures. Adsorption. 2014, 20, 373–384. [Google Scholar] [CrossRef]
- Whittaker, P.B.; Wang, X.; Regenauer-Lieb, K.; Chua, H.T. Predicting isosteric heats for gas adsorption. Phys. Chem. Chem. Phys. 2013, 15, 473–482. [Google Scholar] [CrossRef]
- Czepirski, L.; Jagiello, J. Virial-type thermal equation of gas-solid adsorption. Chem. Eng. Sci. 1989, 44, 797–801. [Google Scholar] [CrossRef]
- Chowdhury, P.; Mekala, S.; Dreisbach, F.; Gumma, S. Adsorption of CO, CO2 and CH4 on Cu-BTC and MIL-101 metal organic frameworks: Effect of open metal sites and adsorbate polarity. Microporous Mesoporous Mater. 2012, 152, 246–252. [Google Scholar] [CrossRef]
- Mishra, P.; Mekala, S.; Dreisbach, F.; Mandal, B.; Gumma, S. Adsorption of CO2, CO, CH4 and N2 on a zinc based metal organic framework. Sep. Purif. Technol. 2012, 94, 124–130. [Google Scholar] [CrossRef]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Myers, A.L.; Monson, P.A. Adsorption in porous materials at high pressure: Theory and experiment. Langmuir 2002, 18, 10261–10273. [Google Scholar] [CrossRef]
- Cimino, R.T.; Kowalczyk, P.; Ravikovitch, P.I.; Neimark, A.V. Determination of isosteric heat of adsorption by quenched solid density functional theory. Langmuir 2017, 33, 1769–1779. [Google Scholar] [CrossRef]
- Askalany, A.A.; Saha, B.B. Towards an accurate estimation of the isosteric heat of adsorption – A correlation with the potential theory. J. Colloid Interface Sci. 2017, 490, 59–63. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, B.B.; Koyama, S. On the thermodynamic modeling of the isosteric heat of adsorption and comparison with experiments. Appl. Phys. Lett. 2006, 89, 171901. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, B.B.; Koyama, S.; Ng, K.C.; Srinivasan, K. Adsorption thermodynamics of silica gel-water systems. J. Chem. Eng. Data 2009, 54, 448–452. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, B.B.; Koyama, S. Specific heat capacity of a single component adsorbent—Adsorbate system. Appl. Phys. Lett. 2007, 90, 171902. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 1st ed.; Academic Press: London, UK, 1998; pp. 86–102. [Google Scholar]
- Beebe, R.A.; Young, D.M. Heats of adsorption of argon. J. Phys. Chem. 1954, 58, 93–96. [Google Scholar] [CrossRef]
- Joyner, L.G.; Emmett, P.H. Differential heats of adsorption of nitrogen on carbon blacks. J. Am. Chem. Soc. 1948, 70, 2353–2359. [Google Scholar] [CrossRef]
- Walker, P.L., Jr. Chemistry and Physics of Carbon; Marcel Dekker Inc.: New York, NY, USA, 1970; Volume 6, pp. 1–124. [Google Scholar]
- Rouquerol, J.; Partyka, S.; Rouquerol, F. Calorimetric evidence for a bidimensional phase change in the monolayer of nitrogen or argon adsorbed on graphite at 77 K. J. Chem. Soc. Faraday Trans. 1977, 73, 306–314. [Google Scholar] [CrossRef]
- Do, D.D.; Nicholson, D.; Do, H.D. On the anatomy of the adsorption heat versus loading as a function of temperature and adsorbate for a graphitic surface. J. Colloid Interface Sci. 2008, 325, 7–22. [Google Scholar] [CrossRef]
- Grillet, Y.; Rouquerol, F.; Rouquerol, J. Two-dimensional freezing of nitrogen or argon on differently graphitized carbons. J. Colloid Interface Sci. 1979, 70, 239–244. [Google Scholar] [CrossRef]
- Inaba, A.; Koga, Y.; Morrison, J.A. Multilayers of methane adsorbed on graphite. J. Chem. Soc. Faraday Trans. 1986, 82, 1635–1646. [Google Scholar] [CrossRef]
- He, Y.; Seaton, N.A. Monte Carlo Simulation of the isosteric heats—Implications for the characterization of porous materials. Stud. Surf. Sci. Catal. 2007, 160, 511–518. [Google Scholar] [CrossRef]
- Wang, Y.; Do, D.D.; Nicholson, D. Study of heat of adsorption across the capillary condensation in cylindrical pores. Colloids Surf. A. Physicochem. Eng. Asp. 2011, 380, 66–78. [Google Scholar] [CrossRef]
- Wang, Y.; Do, D.D.; Herrera, L.F.; Nicholson, D. On the condensation/evaporation pressures and isosteric heats for argon adsorption in pores of different cross-sections. Colloids Surf. A Physicochem. Eng. Asp. 2013, 420, 96–102. [Google Scholar] [CrossRef]
- Bergaoui, M.; Nakhli, A.; Al-Muhtaseb, S.; Khalfaoui, M. Adsorption process of n-alkanes onto BAX-1100 activated carbon: Theoretical estimation of isosteric heat of adsorption and energy distribution of heterogeneous surfaces. J. Mol. Liq. 2018, 252, 399–407. [Google Scholar] [CrossRef]
- Pan, H.; Ritter, J.A.; Balbuena, P.B. Examination of the approximations used in determining the isosteric heat of adsorption from the clausius-clapeyron equation. Langmuir 1998, 14, 6323–6327. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. USA 2008, 33, 11623–11627. [Google Scholar] [CrossRef]
- Nguyen, L.T.L.; Nguyen, T.T.; Nguyen, K.D.; Phan, N.T.S. Metal–organic framework MOF-199 as an efficient heterogeneous catalyst for the aza-Michael reaction. Appl. Catal. A Gen. 2012, 425, 44–52. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal-Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef]
- George, J.; Shylesh, S.; Singh, A.P. Vanadium-containing ordered mesoporous silicas: Synthesis, characterization and catalytic activity in the hydroxylation of biphenyl. Appl. Catal. A Gen. 2005, 290, 148–158. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Mercado-Silva, A.; García-Cerda, L.A.; Castruita, G.; Perera-Mercado, Y.A. Hydrothermal synthesis of mesoporous silica MCM-41 using commercial sodium silicate. J. Mex. Chem. Soc. 2013, 57, 73–79. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblockcopolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Garcia-Cuello, V.S.; Giraldo, L.; Moreno-Piraján, J.C. Synthesis, Characterization, and Application in the CO Oxidation over a Copper Nanocatalyst Confined in SBA-15. J. Chem. Eng. Data. 2011, 56, 1167–1173. [Google Scholar] [CrossRef]
- Fonseca-Correa, R.A.; Giraldo, L.; Moreno-Piraján, J.C. Trivalent chromium removal from aqueous solution with physically and chemically modified corncob waste. J. Anal. Appl. Pyrolysis 2013, 101, 132–141. [Google Scholar] [CrossRef]
- Giraldo, L.; Rodríguez-Estupiñán, P.; Moreno-Piraján, J.C. A microcalorimetric study of methane adsorption on activated carbons obtained from mangosteen peel at different conditions. J. Therm. Anal. Calorim. 2017, 132, 525–541. [Google Scholar] [CrossRef]
- Garcia-Cuello, V.; Moreno-Piraján, J.C.; Giraldo, L.; Sapag, K.; Zgrablich, G. A new microcalorimeter of adsorption for the determination of differential enthalpies. Micro Mesoporous Mat. 2009, 120, 239–245. [Google Scholar] [CrossRef]
- Giraldo, L.; Bastidas-Barranco, M.; Moreno-Piraján, J.C. Adsorption calorimetry: Energetic characterisation of the surface of mesoporous silicas and their adsorption capacity of non-linear chain alcohols. Colloids Surf. A Physicochem. Eng. Asp. 2016, 496, 100–113. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Llewellyn, P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Seaton, N. Characterization of Porous Solids VII; Elsevier: Amsterdam, The Netherlands, 2005; Volume 160, pp. 89–112. [Google Scholar]
- Rodríguez-Reinoso, F.; Garrido, J.; Martín-Martínez, J.; Molina-Sabio, M.; Torregrosa, R. The combined use of different approaches in the characterization of microporous carbons. Carbon 1989, 27, 23–32. [Google Scholar] [CrossRef]
- Llewellyn, P. Recent Advances in Gas Separation by Microporous Ceramic Membranes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents e characteristics of their adsorption properties and microporous structures. Carbon 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982; pp. 62–89. [Google Scholar]
- Tarazona, P. Solid-fluid transition and interfaces with density functional approaches. Surf Sci. 1995, 331, 989–994. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Stoeckli, F.; Slasli, A.; Hugi-Cleary, D.; Guillot, A. The characterization of microporosity in carbons with molecular sieve effects. Microporous Mesoporous Mater. 2002, 51, 197–202. [Google Scholar] [CrossRef]
- Matthias, T.; Katsumi, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhang, W.X.; Feng, F.Y.; Zhang, J.P.; Chen, X.M. A highly connected porous coordination polymer with unusual channel structure and sorption properties. Angew. Chem. Int. Ed. Engl. 2009, 48, 5287–5290. [Google Scholar] [CrossRef]
- Lin, X.; Telepeni, I.; Blake, A.J.; Dailly, A.; Brown, C.M.; Simmons, J.M.; Zoppi, M.; Walker, G.S.; Thomas, K.M.; Mays, T.J.; et al. High capacity hydrogen adsorption in Cu(II) tetracarboxylate framework materials: The role of pore size, ligand functionalization, and exposed metal sites. J. Am. Chem. Soc. 2009, 131, 2159–2171. [Google Scholar] [CrossRef]
- Alsmail, N.H.; Suyetin, M.; Yan, Y.; Cabot, R.; Krap, C.P.; Lü, J.; Easun, T.L.; Bichoutskaia, E.; Lewis, W.; Blake, A.J.; et al. Analysis of high and selective uptake of CO2 in an oxamide containing {Cu2(OOCR)4}-based metal–organic framework. Chem. Eur. J. 2014, 20, 7317–7324. [Google Scholar] [CrossRef]
- Banerjeea, A.; Singha, U.; Aravindan, V.; Srinivasan, M.; Ogale, S. Synthesis of CuO nanostructures from Cu-based metal organic framework (MOF-199) for application as anode for Li-ion batteries. Nano Energy 2013, 2, 1158–1163. [Google Scholar] [CrossRef]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Micropor. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Micro Mesporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q.; Whittaker, A.K.; Millar, G.J.; Zhu, H.Y. Comprehensive Study of Surface Chemistry of MCM-41 Using 29Si CP/MAS NMR, FTIR, Pyridine-TPD, and TGA. J. Phys. Chem. B. 1997, 101, 6525–6531. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Micro Mesporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Marx, S.; Kleist, W.; Baiker, A. Synthesis, structural properties, and catalytic behavior of Cu-BTC and mixed-linker Cu-BTC-PyDC in the oxidation of benzene derivatives. J. Catal. 2011, 281, 76–87. [Google Scholar] [CrossRef]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Wang, F.; Guo, H.; Chai, Y.; Li, Y.; Liu, C. The controlled regulation of morphology and size of HKUST-1 by “coordination modulation method”. Microporous Mesoporous Mater. 2013, 173, 181–188. [Google Scholar] [CrossRef]
- Nakamoto, K.; Martell, A.E. Infrared spectra of metal-chelate compounds. I. A normal coordinate treatment on Bis-(Acetylacetonato)-Cu (II). J. Chem. Phys. 1960, 32, 588–597. [Google Scholar] [CrossRef]
- Myers, A.L.; Valenzuela, D.P. Adsorption Equilibrium Data Handbook; Prentice Hall: Englewood Cliffs, NJ, USA, 1989. [Google Scholar]
- Ahemd Saidd, A.A.; Abd El-Wahab, M.M.M.; Alian, A.M. Catalytic performance of Brønsted acid sites during esterification of acetic acid with ethyl alcohol over phosphotungestic acid supported on silica. J. Chem. Tech. Biotechnol. 2007, 82, 513–523. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Videla, M.; Calvo, A.; Requejo, F.G.; Soler-Illia, G.J.A.A. Amino-propyl-modified mesoporous silica SBA-15 as recovery agents of Cu (II)-sulfate solutions: Adsorption efficiency, functional stability and reusability aspects. J. Hazard. Mater. 2012, 223, 53–62. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Wang, P.; Liu, J.; Yang, Q. An efficient solid acid catalyst: Poly-p-styrenesulfonic acid supported on SBA-15 via surface-initiated ATRP. Microporous Mesoporous Mater. 2009, 123, 228–233. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Qi, Z.; Fang, Y.; Richards, R. Gold nanoparticles intercalated into the walls of mesoporous silica as a versatile redox catalyst. Ind. Eng. Chem. Res. 2011, 50, 13642–13649. [Google Scholar] [CrossRef]
- Karnan, M.; Subramania, K.; Srividhya, P.K.; Sathish, M. Electrochemical Studies on Corncob Derived Activated Porous Carbon for Supercapacitors Application in Aqueous and Non-aqueous Electrolytes. Electrochim. Acta 2017, 228, 586–596. [Google Scholar] [CrossRef]
- Chen, W.-F.; Yan, L.-F.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Bai, H.; Lu, G.-W.; Li, C.; Shi, G.-Q. Flexible graphene films via the filtration of water-Soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Jiaoa, X.; Zhang, L.; Qiua, Y.; Guan, J. Comparison of the adsorption of cationic blue onto graphene oxides prepared from natural graphites with different graphitization degrees. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 292–301. [Google Scholar] [CrossRef]
- Gaydhankar, T.R.; Samuel, V.; Joshi, P.N. Hydrothermal synthesis of MCM-41 using differently manufactured amorphous dioxosilicon sources. Mater. Lett. 2005, 60, 957–961. [Google Scholar] [CrossRef]
- Guthrie, C.P.; Reardon, E.J. Metastability of MCM-41 and Al-MCM-41. J. Phys. Chem. A 2008, 112, 3386–3390. [Google Scholar] [CrossRef]
- Wongkoblap, A.; Do, D.D.; Nicholson, D. Explanation of the unusual peak of calorimetric heat in the adsorption of nitrogen, argon and methane on graphitized thermal carbon black. Phys. Chem. Chem. Phys. 2008, 10, 1106–1113. [Google Scholar] [CrossRef]
- Sircar, S.; Mohr, R.; Ristic, C.; Rao, M.B. Isosteric heat of adsorption: Theory and experiment. J. Phys. Chem. B 1999, 103, 6539–6546. [Google Scholar] [CrossRef]
- Ray, M.S. Adsorptive and membrane-type separations: A bibliographical update 2000. Adsorp. Sci. Technol. 2001, 19, 821–849. [Google Scholar] [CrossRef]
- Groszek, A.J. Adsorption and Its Applications in Industry and Environmental Protection, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 1–2. [Google Scholar]
- Kast, W. Adsorption aus der Gasphase. Ingenieurwissen-Schaftliche Grundlagen und Technische Verfahren; Verlag Chemie: Weinheim, Germany, 1988. [Google Scholar]
- Moreno-Piraján, J.C.; Giraldo, L. Setups for simultaneous measurement of isotherms and adsorption heats. Rev. Sci. Instrum. 2005, 76, 103–115. [Google Scholar] [CrossRef]
- Fatin, H.M.; Azahara, S.M.; Akihiro, Y.; Akira, H.; Bidyut, B.; Sahad, K.T. Improved model for the isosteric heat of adsorption and impacts on the T performance of heat pump cycles. Appl. Therm. Eng. 2008, 143, 688–700. [Google Scholar] [CrossRef]
- Rahman, K.A. Experimental and Theoretical Studies on Adsorbed Natural Gas Storage System Using Activated Carbons. Ph.D. Thesis, National University of Singapore, Singapore, 2011. [Google Scholar]
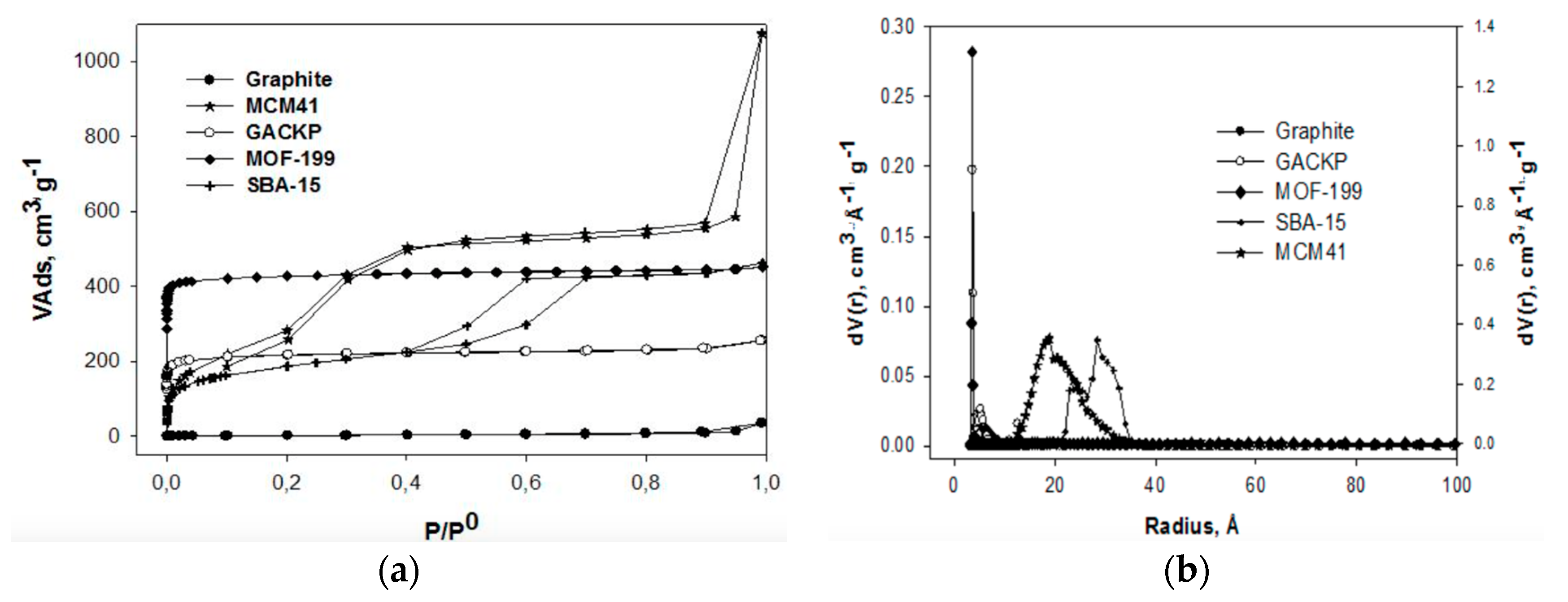


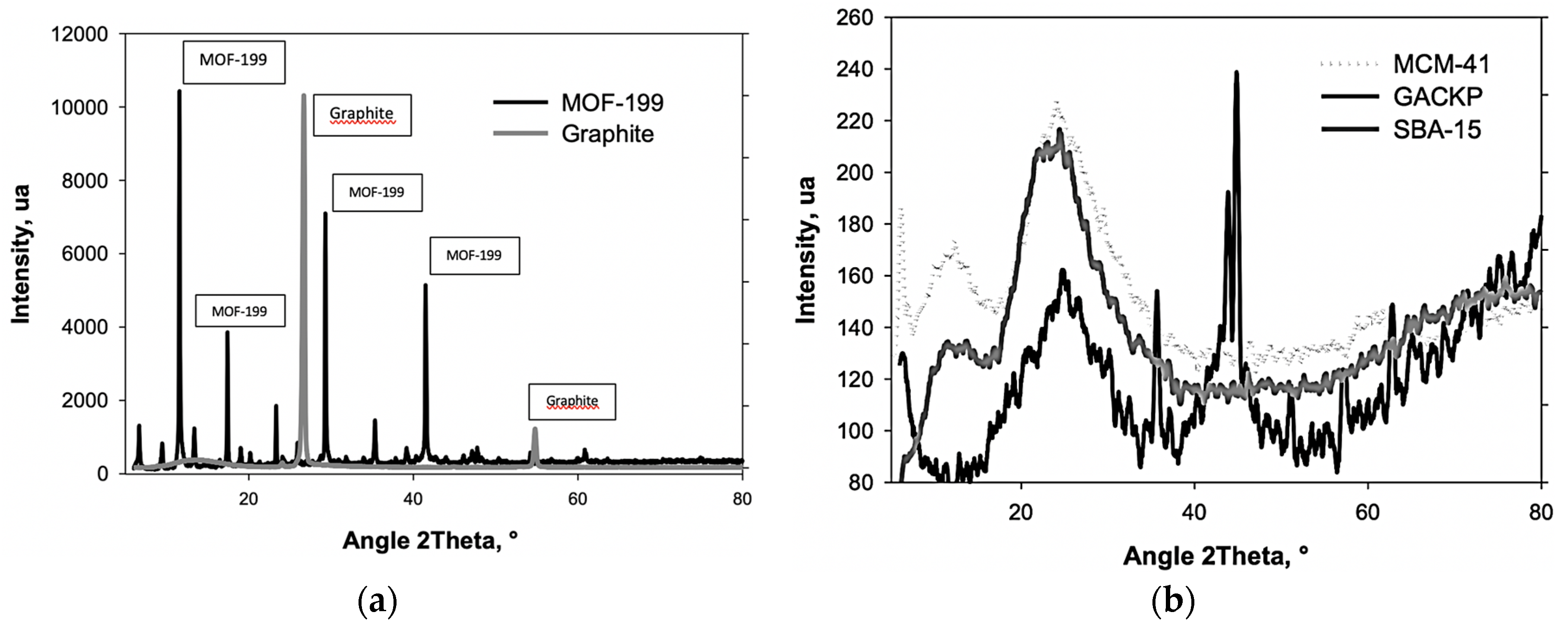



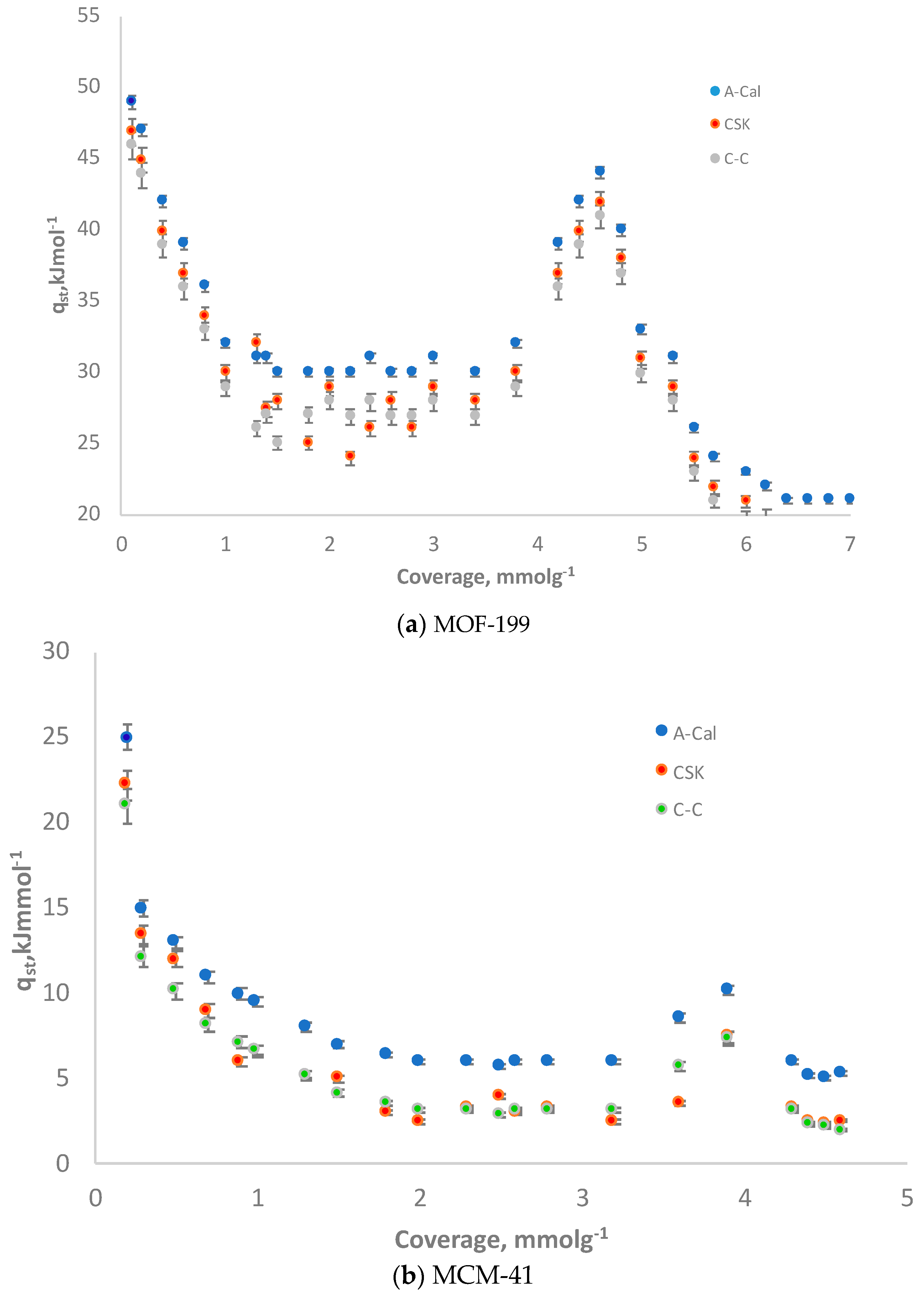
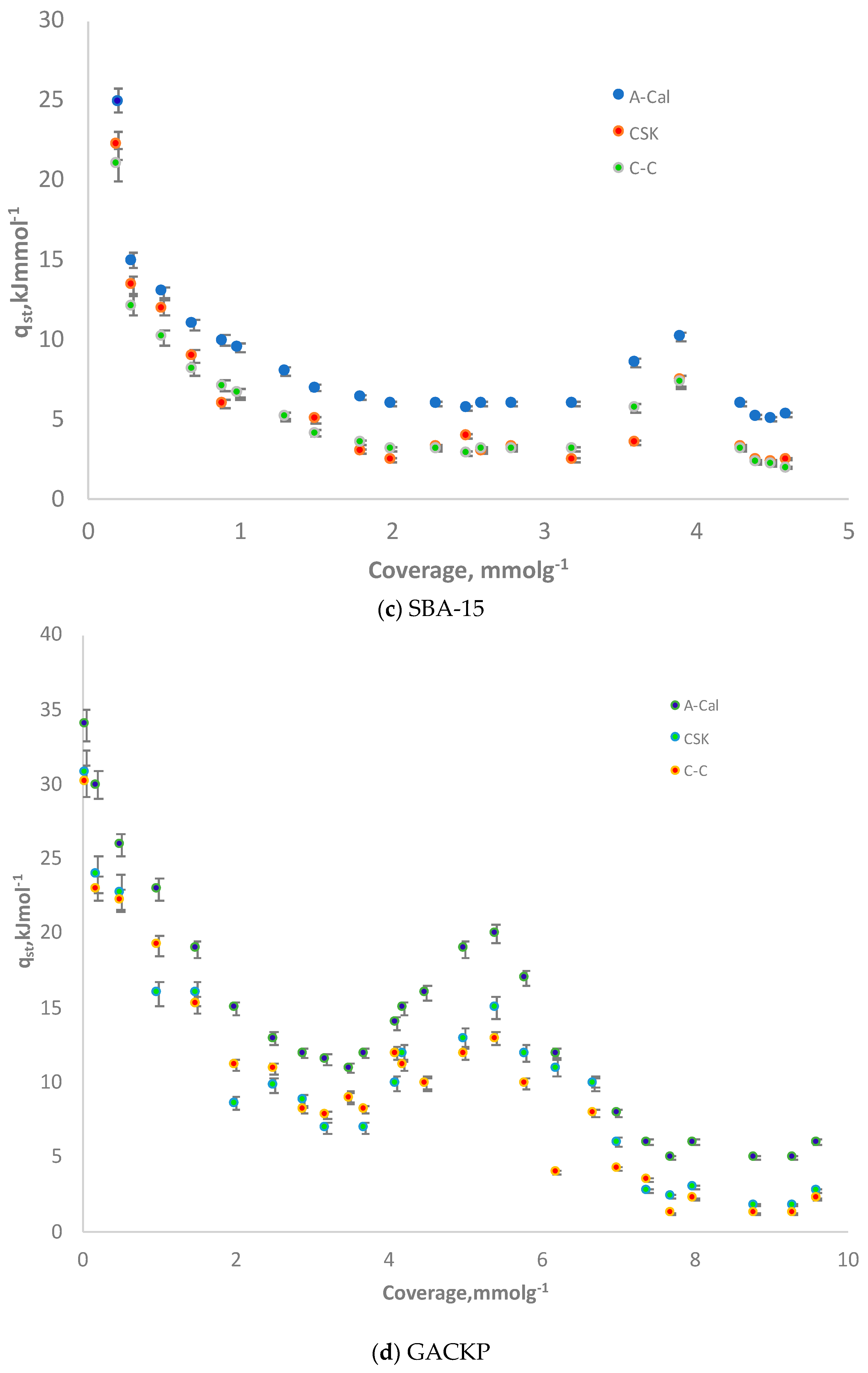
| Samples | SBET (m2·g−1) | V0.99 (cm3·g−1) | VDR (cm3·g−1) | Eo (kJ·mol−1) | Lo (nm) | VDR/V0.99 | VMeso (cm3·g−1) |
|---|---|---|---|---|---|---|---|
| MOF-199 | 2271 | 0.66 | 0.62 | 8.47 | 7.3 | 0.94 | 0.04 |
| MCM-41 | 1274 | 0.44 | 0.40 | 3.58 | 8.4 | 0.91 | 0.04 |
| SBA-15 | 663 | 0.24 | 0.17 | 5.41 | 6.2 | 0.83 | 0.07 |
| GACKP | 856 | 0.33 | 0.28 | 9.32 | 7.1 | 0.85 | 0.05 |
| Graphite | 5.2 | 0.10 | - | 7.25 | 0.3 | - | 0.001 |
| Sample | NLDFT | QSDFT | ||||
|---|---|---|---|---|---|---|
| Fitting Error (Slit Pore) (%) | Fitting Error (Cyl. Pore) (%) | Fitting Error (Combined) (%) | Fitting Error (Slit Pore) (%) | Fitting Error (Cyl. Pore) (%) | Fitting Error (Combined) (%) | |
| Graphite | 5.296 | 6.819 | 6.819 | 4.364 | 7.510 | 7.510 |
| MOF-199 | 0.600 | 0.140 | 0.135 | 0.840 | 0.700 | 0.698 |
| SBA-15 | - | 0.513 | - | - | - | - |
| GACKP | 0.499 | 0.335 | 0.300 | 0.138 | 0.057 | 0.030 |
| MCM-41 | - | 3.100 | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo, L.; Rodriguez-Estupiñán, P.; Moreno-Piraján, J.C. Isosteric Heat: Comparative Study between Clausius–Clapeyron, CSK and Adsorption Calorimetry Methods. Processes 2019, 7, 203. https://doi.org/10.3390/pr7040203
Giraldo L, Rodriguez-Estupiñán P, Moreno-Piraján JC. Isosteric Heat: Comparative Study between Clausius–Clapeyron, CSK and Adsorption Calorimetry Methods. Processes. 2019; 7(4):203. https://doi.org/10.3390/pr7040203
Chicago/Turabian StyleGiraldo, Liliana, Paola Rodriguez-Estupiñán, and Juan Carlos Moreno-Piraján. 2019. "Isosteric Heat: Comparative Study between Clausius–Clapeyron, CSK and Adsorption Calorimetry Methods" Processes 7, no. 4: 203. https://doi.org/10.3390/pr7040203
APA StyleGiraldo, L., Rodriguez-Estupiñán, P., & Moreno-Piraján, J. C. (2019). Isosteric Heat: Comparative Study between Clausius–Clapeyron, CSK and Adsorption Calorimetry Methods. Processes, 7(4), 203. https://doi.org/10.3390/pr7040203






