1. Introduction
Glioblastoma multiforme (GBM), also known as grade IV astrocytoma, is the deadliest type of brain cancer. Almost 90% of the GBM cases develop de novo (primary glioblastoma) from normal glial cells via multistep tumorigenesis within a short period of three months [
1]. The rapid tumour growth is mainly related to deregulation of the G1/S checkpoint in the cell cycle and the occurrence of multiple genetic abnormalities of tumor cells [
2,
3]. However, the actual causes of these brain tumors in adults remain largely unknown [
4]. At present, standard clinical treatment for GBM relies on surgery and chemo-radiation therapies [
5].
Total surgical resection of the tumour is by far the best therapeutic option for GBM patients; however, complete removal of the extensive primary tumor mass without damaging its adjacent healthy neural tissue is extremely difficult due to its invasive and seamlessly migratory nature into surrounding tissue. As a consequence, infiltrating tumor cells remain within the surrounding tissue, leading to poor prognosis and high recurrence incidence after the surgical treatment. In order to address to this problem, follow-up treatments such as radiotherapy, chemotherapy, or a combination of both are commonly associated with the surgical procedure for GBM treatment remedy. Nevertheless, chemo-radiation therapy is notorious for causing adverse effects on patients due to its non-selectivity nature [
6,
7]. It is a double-edged sword that while damaging cancer cells, it also damages healthy cells. The non-selective killing effect of chemo-radiation therapy places side effects on the patients and could reduce their compliance to the treatment. As a result, the tumor cannot be completely healed, leaving resistant cancerous cell populations to grow more aggressively and finally attributing to drug resistance and cancer recurrence.
Due to the aggressive nature of GBM and the complexity of targeting the cancerous cells by chemo-radiation therapy, effective treatment for GBM is still a major unmet medical need. In order to improve current therapeutic options, studies to identify and to validate potential alternative treatment for GBM are imperative. Recently, there has been increased scientific interest in the study of phytochemicals from natural plant resources as complementary anticancer compounds due to their high bioavailability, minimal side effects (compared to the chemodrugs) and, most importantly, cost effectiveness. Several studies have validated the role of medicinal plants in prevention and treatment of various cancer models [
8,
9,
10], setting new possibilities for alternative cancer therapies. In this study, the anti-cancer therapeutic role of baicalein, an active compound found abundantly in
Oroxylum indicum (L.) Benth. ex Kurz, was investigated. This is the first study to determine the anti-GBM role exerted by a baicalein-enriched compound obtained from
O. indicum plant extract.
O. indicum is a medicinal herb that is commonly found in many regions of Southeast Asia. This medium-sized plant is also known as “beko” in Malaysia. The leaves and fruits of beko are traditionally consumed as raw salad by the local populations as “ulam”* (*Ulam refers to healthy vegetables that are eaten raw as salad in the Malaysian multiracial culture), which has been reported to exert anti-aging properties and improve one’s health [
11], making this plant a potential natural resource for complementary therapy. In fact, different parts of
O. indicum, such as root, bark, leaves and fruits, have already been applied in complementary medicine to cure bronchitis, dyspepsia, asthma, gastropathy, inflammation and microbial infection [
12]. The major phytochemical presents in
O. indicum that confers it the medicinal benefits is baicalein (5,6,7-trihydroxyflavone), a flavonoid compound that primarily act through interaction with cancer cell membrane proteins leading to the release of cytochrome c, which activated the caspase cascade and thus increase apoptosis [
13,
14]. Baicalein also can be found in other plants such as
Scutellaria baicalensis [
15] and
Scutellaria lateriflora [
16]; it also can be synthesized pharmacologically. Different sources of baicalein has been listed as active constituents undergoing clinical trials for anticancer activity for breast cancer, liver cancer, hepatocellular carcinoma, prostate cancer and gallbladder cancer [
17]. However, the scientific description regarding the anti-GBM medicinal potential of baicalein enriched from
O. indicum plants is yet to be done.
The goal of this study was to produce an optimized O. indicum extract with enriched baicalein content to enhance its bioactivity. To achieve this aim, we tested the baicalein extraction efficacy of three solvent systems, namely, petroleum ether/methanol (PM); methanol or aqueous; and followed by a preliminary fractionation procedure using an ion-exchange resin column and gradient elution with methanol–water to further concentrate the baicalein, as well as to reduce other unknown phenolic compounds which may exert adverse effects related to cancer metastasis. In order to determine the advantage of the enrichment procedure developed in this study, we evaluated the antioxidant property before and after the fractionation and tested the inhibitory effects of the enriched extract against a GBM cell line (DBTRG-05MG) along with a clinically used anti-GBM chemodrug, Temozolomide. Moreover, the comparison of the biological activities exerted by the enriched baicalein crude extract obtained from this study and a pure synthetic baicalein compound available commercially was also the significance of this study.
2. Materials and Methods
2.1. Plant Material and Extraction Methods
Fresh leaves of
O. indicum were collected from Kampung Pasir Parit, Pasir Mas (LGPS coordinate: latitude 5.905471, longitude 102.1884469). The plant material was verified by Dr. Rahmad Zakaria and the voucher specimen (USM. Herbarium 11751) was deposited in the Universiti Sains Malaysia Herbarium by Mr. V. Shunmugam (
Appendix A). The leaves were cleaned with distilled water and oven-dried at 50 °C before crumbled into powder. A total of 25 g of the
O. indicum leaf powder was transferred into a thimble and a plug of glass wool was placed on top of the thimble to prevent spillage during extraction procedure. The thimble was carefully loaded into a Soxhlet extractor and subjected to three different extraction procedures, respectively, as described below.
2.1.1. Petroleum Ether and Methanol (PM) Extraction
A total of 300 mL of petroleum ether was added to a distillation flask and the flask was placed on top of a heating mantle. The Soxhlet extractor with thimble containing the O. indicum leaf powder was placed atop the flask. The heating mantle was turned on and maintained at the gentle boiling point of petroleum ether (42–62 °C) until the color of the solvent turned dark green. The heating procedure must be carefully monitored to avoid rolling boil. Then the solvent was discarded and replaced with fresh 300 mL petroleum ether. The gentle boil procedure was continued until the color of the solvent in the Soxhlet extractor turned clear. Again, the solvent was discarded, and the thimble was air dried to remove residues of petroleum ether solvent. The purpose of the petroleum ether extraction was to remove undesired non-polar lipid components from the leaf powder before subjecting the plant material to the subsequent extraction procedure using methanol. For methanol extraction, 500 mL of methanol was added to the Soxhlet extractor and gently boiled at the methanol boiling point (62–65 °C) until the color of solvent turned dark green. Then, the solvent was collected into a glass bottle and the extraction was continued with another 500 mL of methanol until the solvent in the extractor turned colorless. All solvents were collected into the same glass bottle and dried using a rotary evaporator (Buchi AG, Flavil, Switzerland). Dried PM crude extract powder was weighed and stored at 4 °C until future use.
2.1.2. Methanol Extraction
A total of 500 mL of methanol was added to a Soxhlet extractor as previously described. The heating mantle was turned on and maintained at the gentle boiling point of methanol (62–65 °C) until the color of solvent turned dark green. Then, the dark green solvent was collected into a glass bottle and the extraction was continued with another 500 mL of methanol until the solvent in the extractor turned colorless. The solvent was collected into the same glass bottle and dried using a rotary evaporator (Buchi AG, Flavil, Switzerland). Dried methanol crude extract powder was weighed and stored at 4 °C until future use.
2.1.3. Aqueous Extraction
A total of 500 mL of distilled water was added to a Soxhlet extractor as previously described. Extraction was performed by gently boiling the solvent at water boiling point (100 °C) until the color of the solvent turned dark green. Then, the dark green solvent was collected into a glass bottle and the extraction was continued with another 500 mL of distilled water until the solvent in the extractor turned colorless. The clear solvent was collected into same bottle and dried using a rotary evaporator (Buchi AG, Flavil, Switzerland). Dried aqueous crude extract powder was weighed and stored at 4 °C until future use.
2.2. Fractionation
The fractionation protocol was developed based on a procedure described previously with modifications [
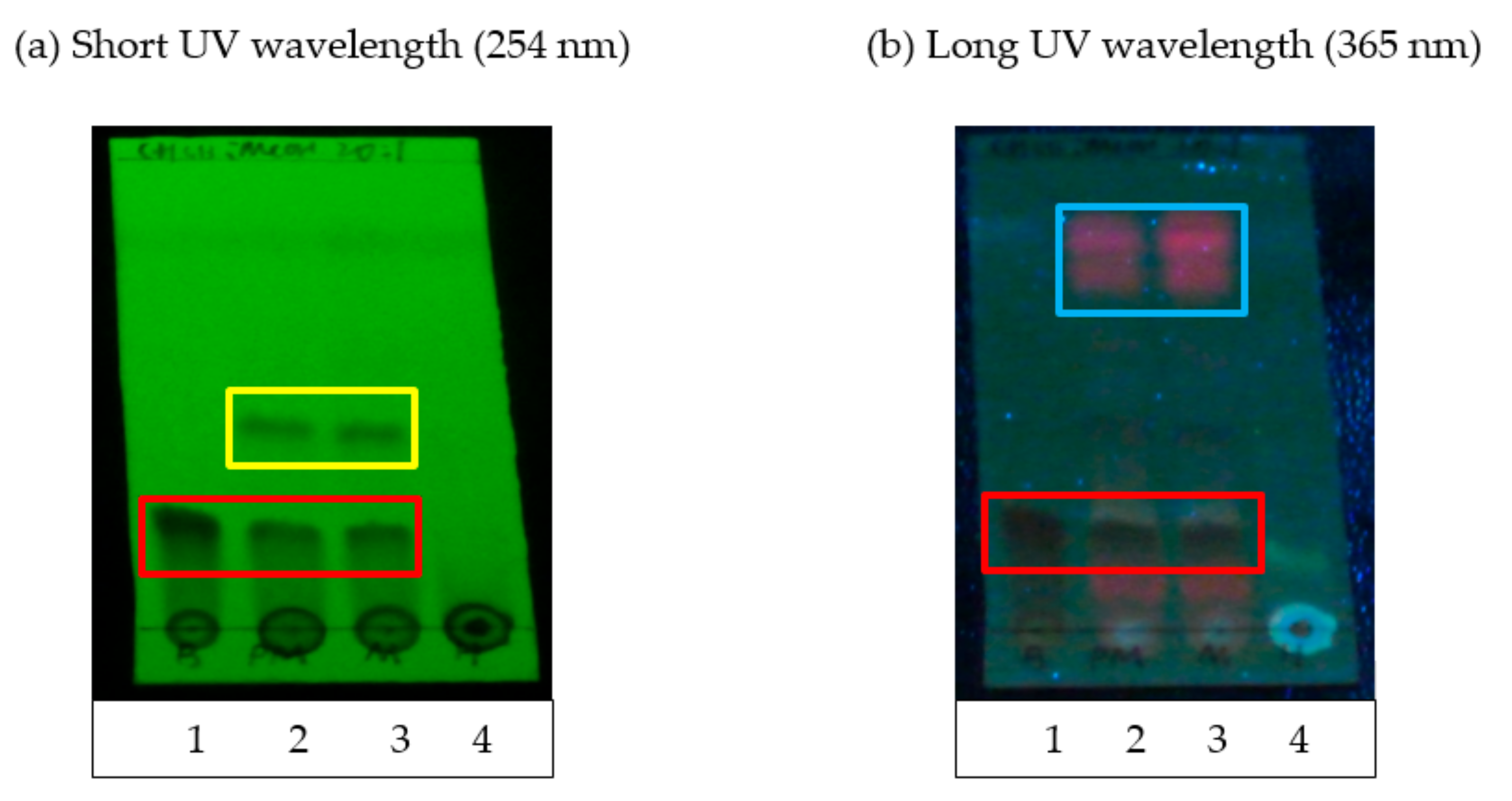
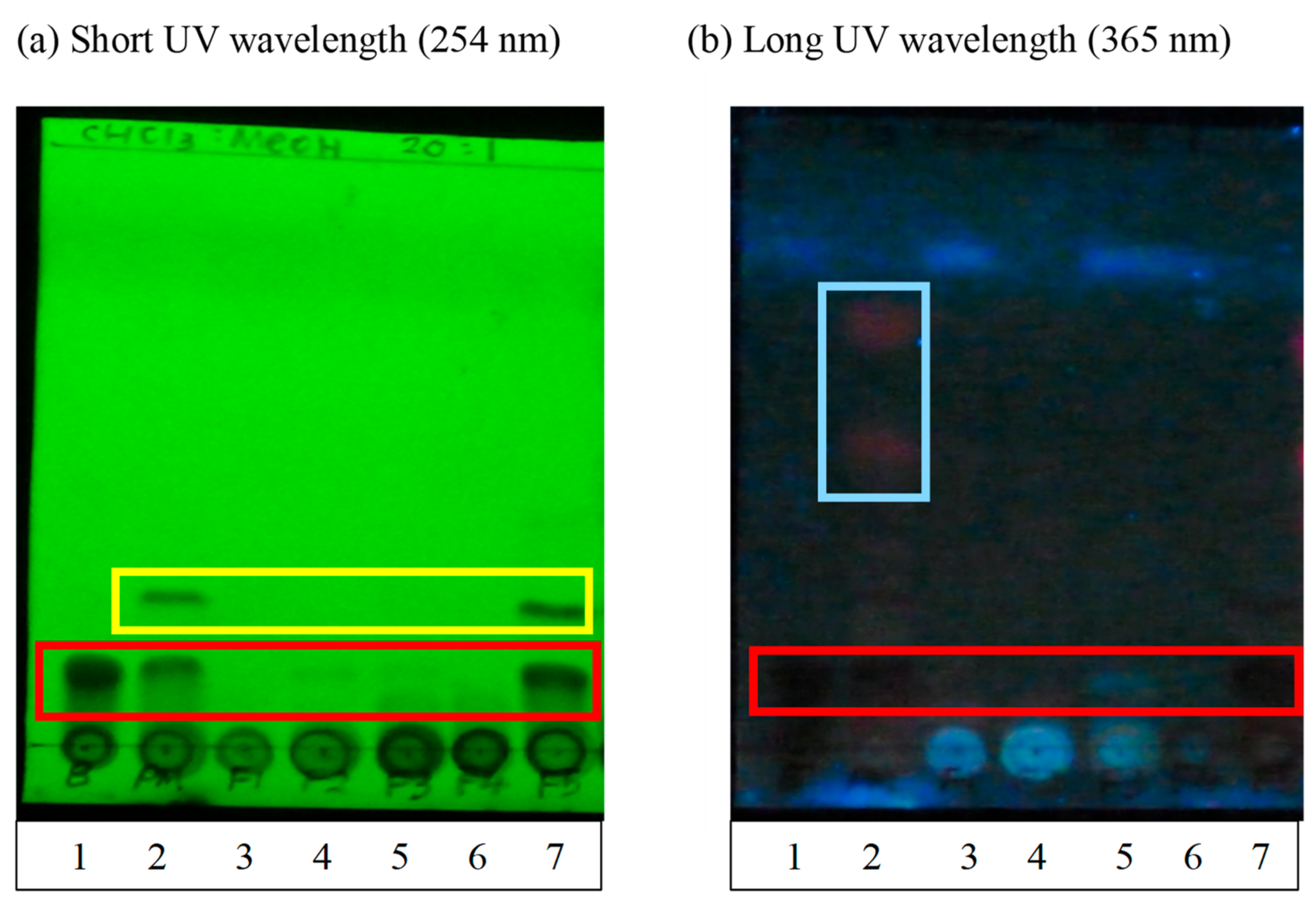
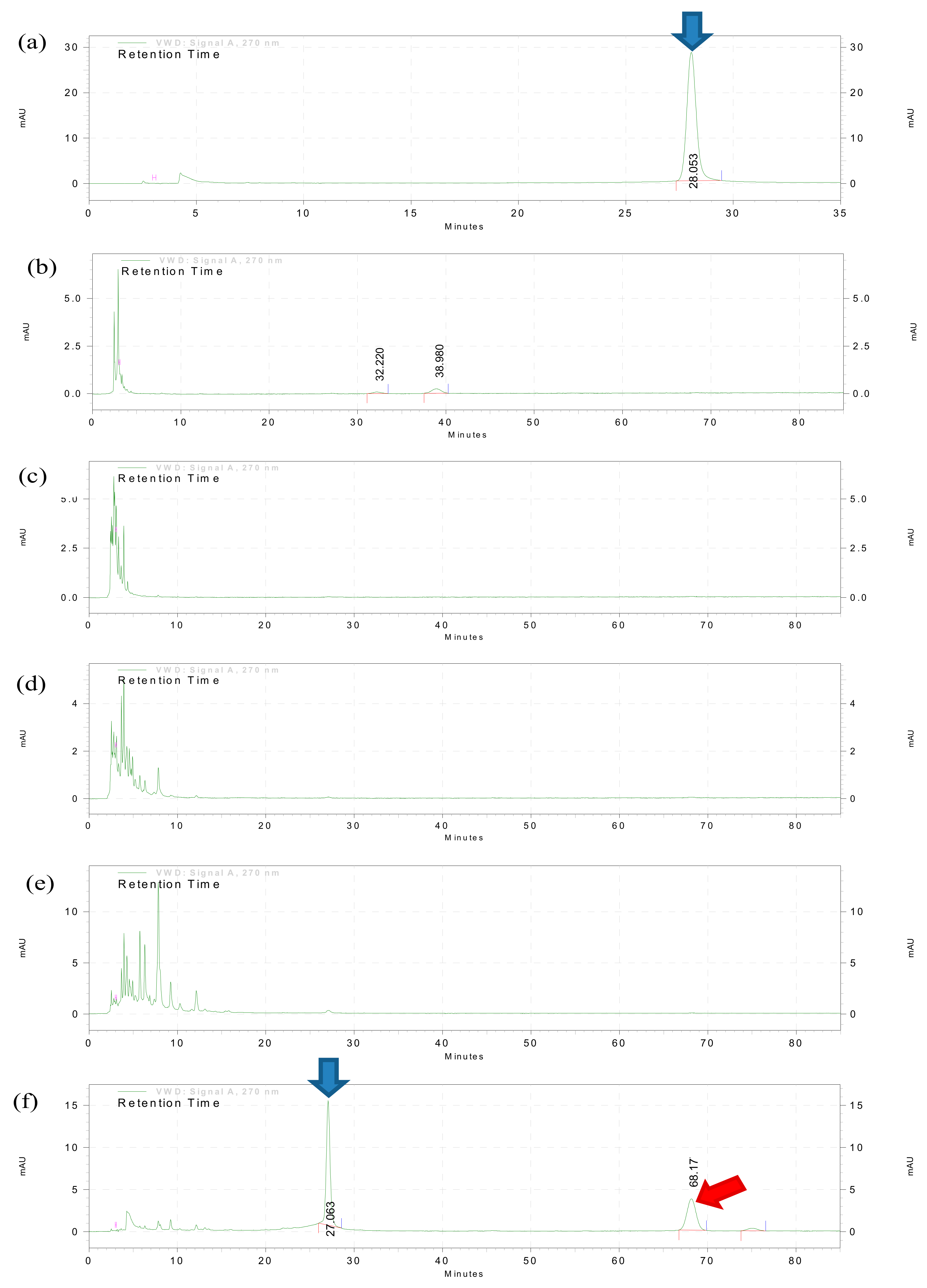
18]. In brief, a Diaion HP20 resin column (Mitsubishi Chemical Corporation, Tokyo, Japan) was prepared and rinsed with a 400 mL mobile phase (10% methanol) to remove any resin residue remaining on the surface and to equilibrate the column. Then, 5 g of crude extract powder obtained from the previous extraction procedures was dissolved in 5 mL of methanol to form a slurry suspension before carefully being loaded onto the top of the resin column without disturbing the surface. The suspension was eluted with gradient mixtures of distilled water and methanol of the ratio of 1:9, 3:7, 5:5, 7:3 and 9:1 to obtain 5 fractions (F1–F5). The five fractions were collected and dried using a rotary evaporator. The presence of baicalein in each fractions and crude extracts were determined using thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC) analysis.
2.3. Thin Layer Chromatography (TLC) Analysis
TLC separation was performed using aluminum sheets coated with silica gel 60 F254 (Merck KGaA, Darmstadt, Germany). Crude extracts and fractions F1–F5 with a final concentration of 1 mg/mL were prepared and spotted on the TLC plate. Synthetic baicalein compound at a concentration of 1 mg/mL was also spotted on the same TLC plate as the positive control/standard marker in this assay. The spotted TLC plate was dried and placed in a developing chamber with a mobile phase consisting of chloroform:methanol 20:1 (v/v). The plate was removed from the chamber when the solvent front almost reached the top of the plate. Once the plate was dried, the TLC sheet was immediately examined under short wavelength UV (254 nm) and long wavelength UV (365 nm) light. UV light is useful for visualizing aromatic and polycyclic compounds such as baicalein because such compounds strongly absorb UV. The plate background would appear green under short-waved UV light, and the UV-active compounds would appear dark. On the other hand, the plate background would appear dark blue under long-waved UV light, and the UV-active compounds would appear dark. Other functional groups that do not absorb UV light at the wavelengths used would not appear; thus, TLC can be used to detect the presence of baicalein.
2.4. High Performance Liquid Chromatography (HPLC)
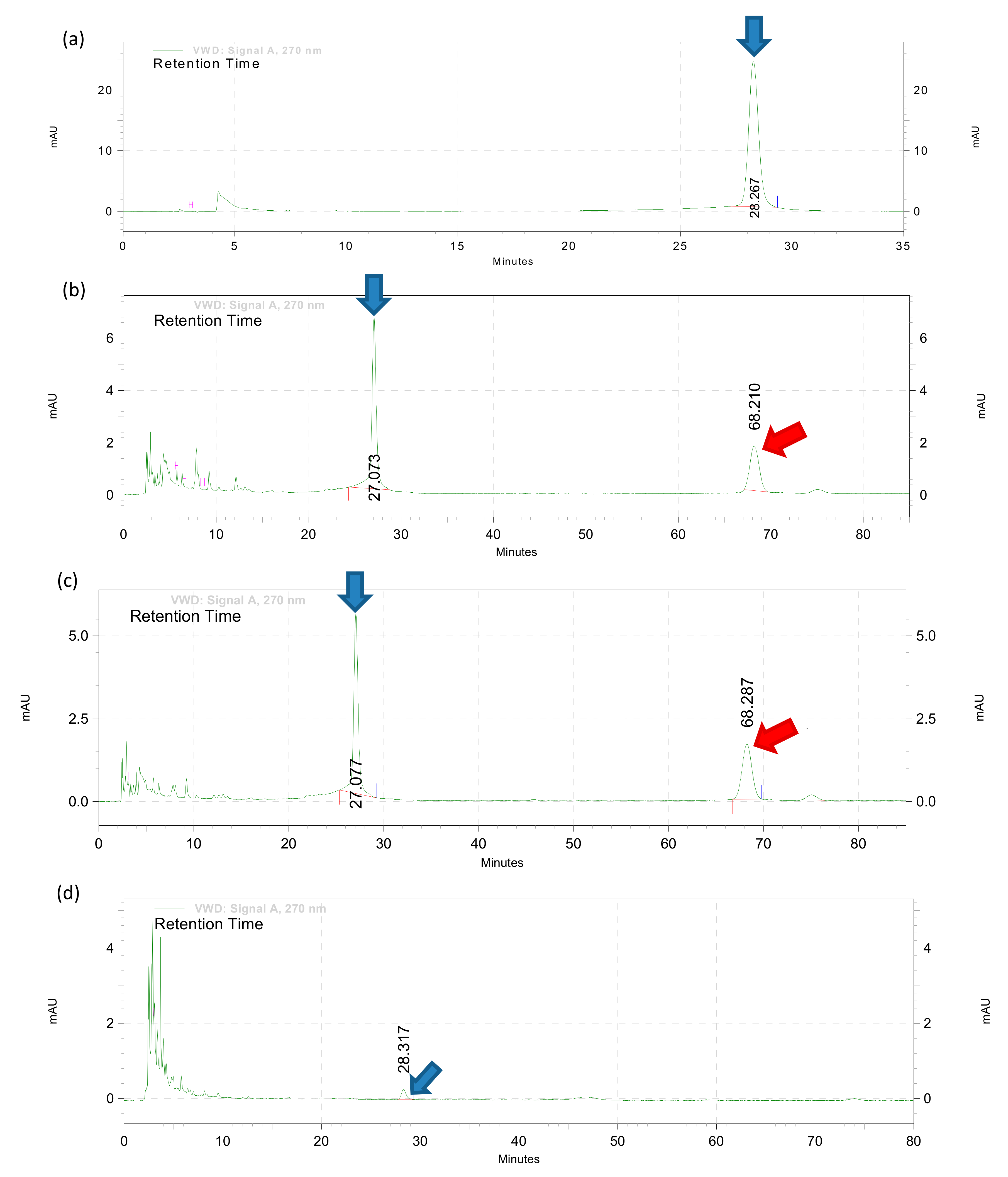
HPLC analysis was performed on crude extracts and fractions F1–F5 to detect and quantify baicalein content based on a previously described method with modification [
19]. Briefly, 0.5 µg samples were analyzed by an Agilent Technologies 1120 Compact LC System with a TC-C18 column of 4.6 mm i.d. × 250 mm, 5 µm which was pre-connected with a guard column, Zorbax SB-C18 of 4.6 mm i.d. × 12.5 mm, 5 µm (Agilent Technologies, Santa Clara, CA, USA). Samples were dissolved in 20 µL mobile phase consisting of 0.1% formic acid:acetonitrile 70:30 (
v/
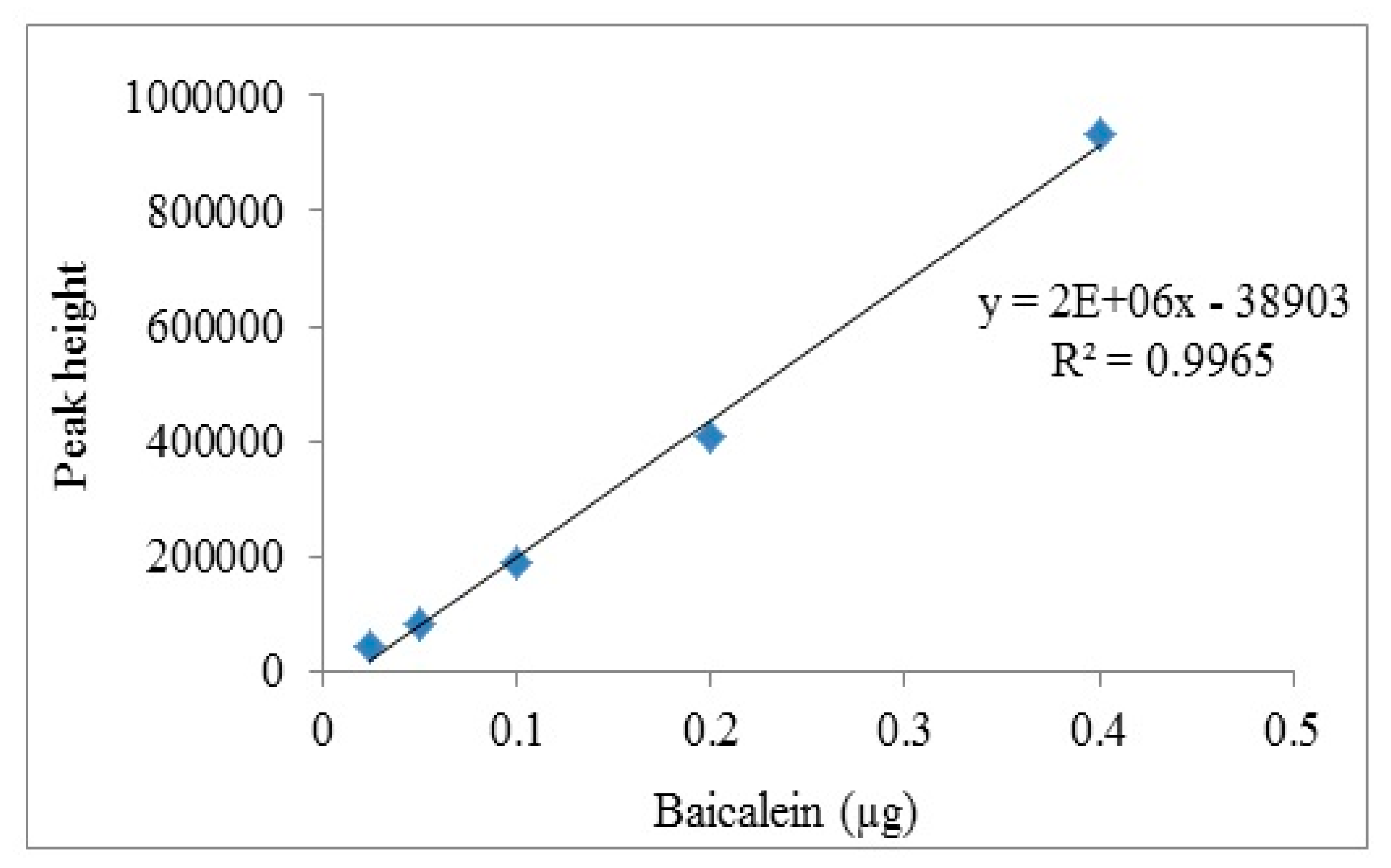
v) and injected with a flow rate of 1 mL/min at ambient room temperature. Acetonitrile was selected as the mobile phase due to the advantage of lower viscosity which gave lower back pressure and minimum peak broadening. A small amount of formic acid (0.1%) was added to sharpen the peak shapes of chromatograms for better resolution. UV detection for baicalein was carried out at a wavelength of 270 nm. The isocratic method was run for 80 min. To construct the standard calibration curves, a synthetic baicalein compound (used as the standard marker/positive control) was serially diluted with the mobile phase to a gradient concentration range between 0.025 and 0.4 µg. The calibration curve was plotted using the peak heights which were generated by EZChrom Elite software against the weight of the synthetic baicalein compound loaded. The chromatographic peaks of samples were identified. Then the relative peak height of each samples was compared to the calibration curve to determine the baicalein concentration.
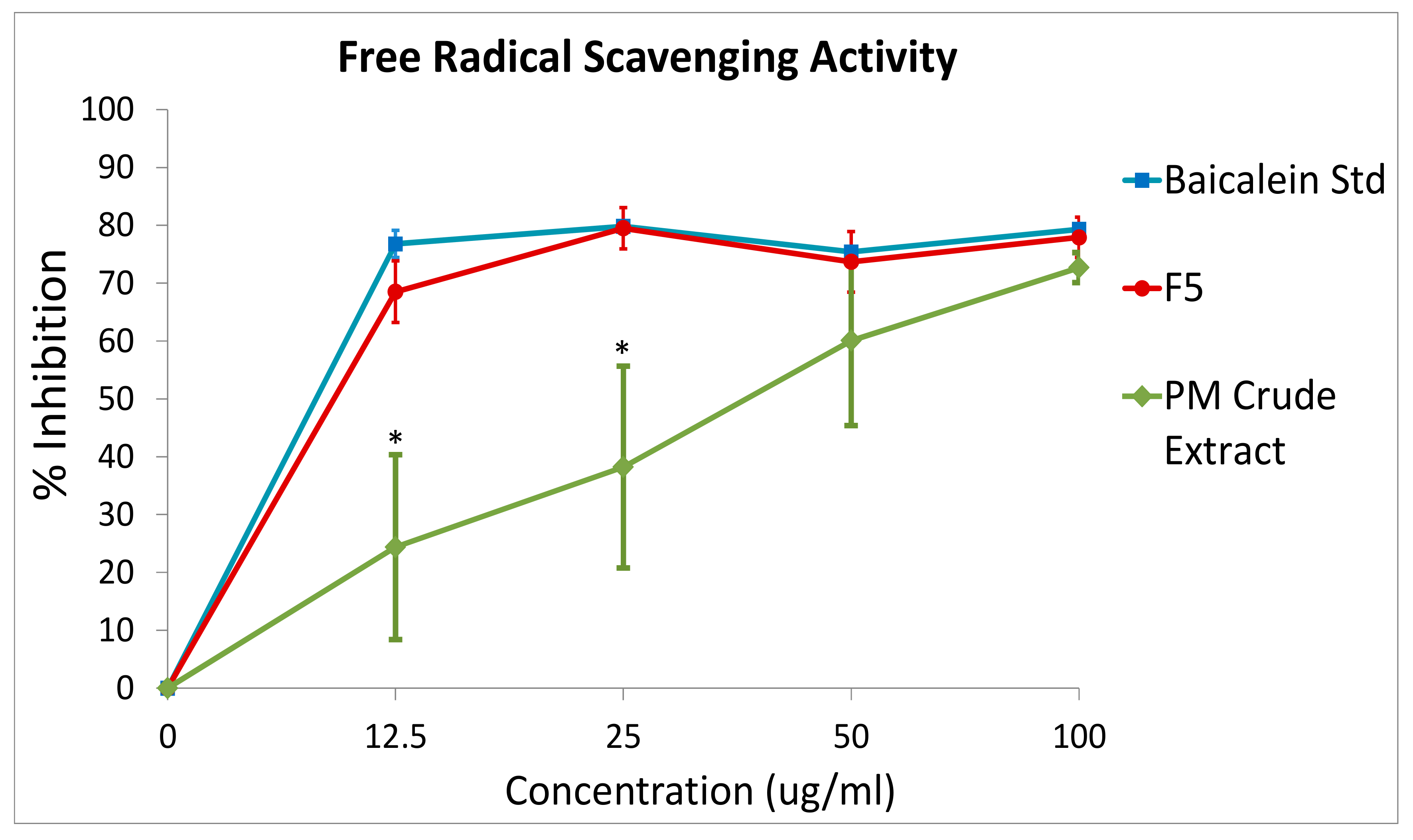
2.5. DPPH Free Radical Scavenging Activity
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) was used to evaluate the antioxidant potential of the PM crude extract and fraction F5. Synthetic baicalein pure compound was used as the positive control. First, the samples were serially diluted with methanol to 0, 12.5, 25, 50 and 100 µg/mL, and added to 1 mL of 0.1 mM DPPH (Merck KGaA, Darmstadt, Germany), respectively. Then, the mixtures were incubated at room temperature in the dark for 30 min. During the incubation period, antioxidant compounds in samples could reduce the in situ formed DPPH radical into DPPHH, resulting in a change of color from purple to yellow, which has a maximum absorption wavelength at 517 nm. The higher the yellow color intensity, the higher the radical savaging activity in the samples. After 30 min of incubation time, the optical density of the samples (OD) were determined at 517 nm. The free radical scavenging activity was calculated using the following formula:
2.6. Cell Culture
The DBTRG-05MG cell line (ATCC CRL-2020) established by the Denver Brain Tumor Group from a 59-year-old female patient with GBM was purchased from ATCC, USA. The DBTRG-05MG cells were cultured in complete growth medium Roswell Park Memorial Institute (RPMI) 1640 (GIBCO, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (GIBCO, Waltham, MA, USA) and 1% Penicillin–Streptomycin (GIBCO, Waltham, MA, USA). The cells were cultivated in a humidified incubator under 5% CO2, at 37 °C until the cells were confluent.
2.7. Cell Viability Assay
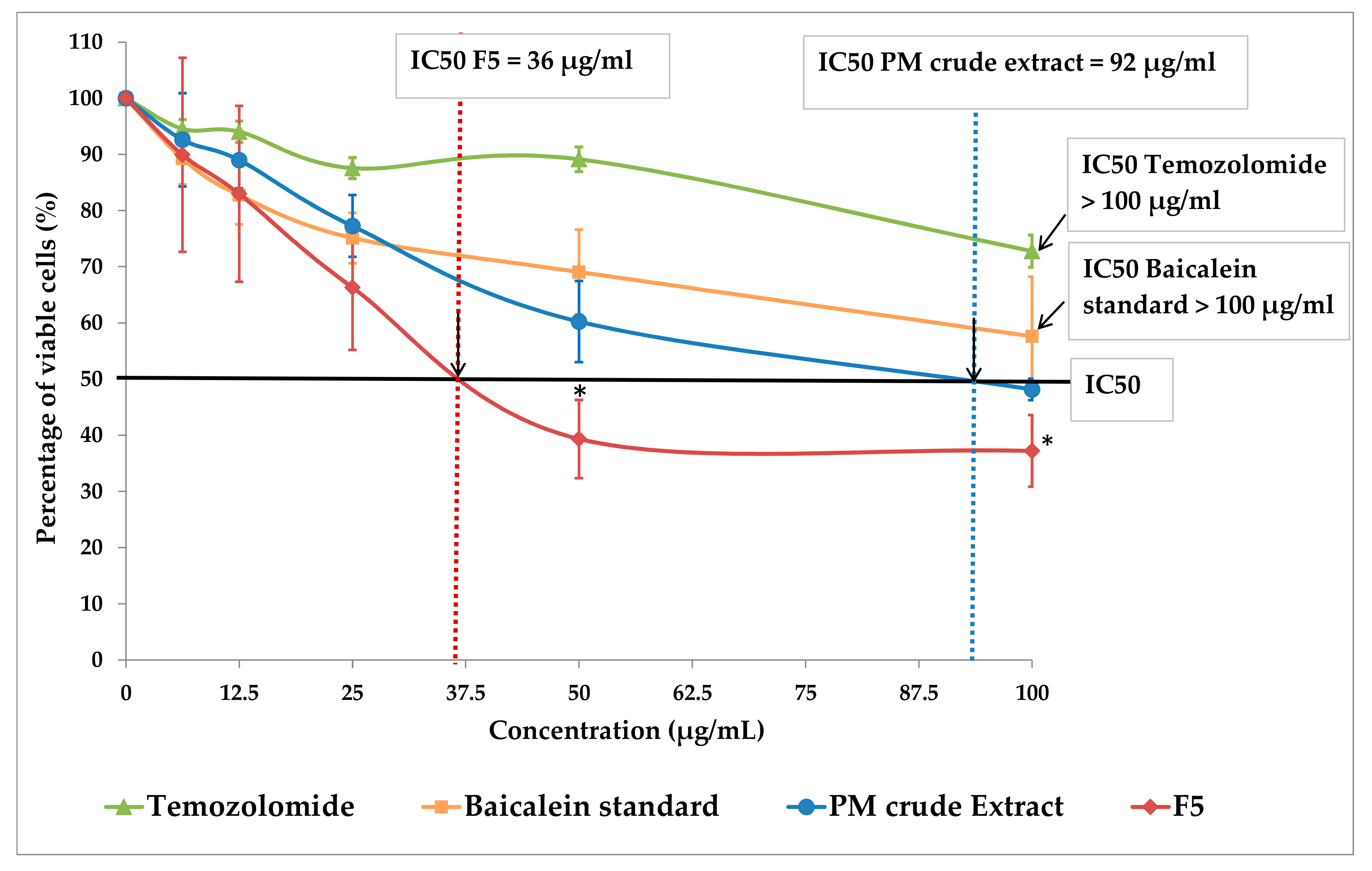
DBTRG-05MG cells were cultured into a 96-well plate at 20,000 cells per well. Extracts were added to the cells at concentrations of 0, 6.25, 12.5, 25, 50 and 100 µg/mL respectively. DMSO of 1% was used as the negative control. Temozolomide (TEMODAL, Merck, Kenilworth, NJ, USA) and the synthetic baicalein compound (Sigma, Welwyn Garden City, UK) was used as the positive controls. All experiments were carried out in triplicate. After 3 days, 10 µL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (5 mg/mL) was added to each well and then incubated at 37 °C for 4 h. The medium was discarded and 100 µL of DMSO were added into each well. The absorbance was measured at 570 nm using a microplate reader (Bio-Rad, USA). The percentage of viable cells was determined using the following equation:
4. Discussion
This study pioneered the development of a two-phase extraction processes to enrich a baicalein natural compound from the leaves of O. indicum. Moreover, we are the first to describe the effectiveness of this baicalein-enriched fraction in combating GBM cancerous cells in vitro as compared to the clinically used chemodrug for GBM, Temozolomide, as well as the synthetic pure baicalein compound.
Medicinal plants are important sources for bioactive natural products in drug development. However, the amount of these natural herbal medicines is always fairly low in plants. Therefore, extraction processes play a crucial role in the maximum recovery of the bioactive compounds from natural samples. Nonetheless, there is no universal extraction procedure that is applicable to all plant types due to the huge diversity in plant structures and materials, as well as the complexity and uniqueness of the physical properties of each bioactive compound’s presence in a particular plant. Therefore, an extraction process for natural products must be carefully optimized to maximize the yield. In the present study, baicalein extraction from
O. indicum leaves was initially performed using different solvents based on polarity. Pure solvents of different polarity (methanol vs. aqueous), as well as binary solvents consisting of a combination of non-polar petroleum ether and polar methanol were selected in this study. Pure water solvent was selected because aqueous extraction closely imitates the manner by which herbal medicines are traditionally prepared. However, the aqueous extract was found to have no appreciable yield of the desired baicalein metabolites compared to methanol. The extremely low yield of metabolites in the pure water solvent suggests their inability to extract the flavonoid compounds effectively. This is mainly because baicalein is a member of the flavones class of compounds with a structure based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one) [
17]. Thus, baicalein is considered to be a flavonoid lipid molecule and therefore could be practically insoluble in water. This is in agreement with a previous study that stated that water could dissolve alkaloid and glycoside compounds, but on the other hand, methanolic extract could yield the maximum amount of flavonoids and phenols [
21].
Furthermore, the binary solvent system (petroleum ether followed by methanol) was found superior to the mono-solvent system (pure methanol only) in baicalein extraction yield. The is due to the function of the non-polar petroleum ether solvent to remove undesired lipid components from the O. indicum leaves before subjecting the plant material to second phase extraction using pure polar methanol. A defatting procedure prior to extraction may eliminate several low polarity fatty constituents and produce a relatively purer natural product to proceed to subsequent extraction or fractionation procedures. In this study, fractionation was carried out to further purify the PM crude using methanol concentrations increasing in a step-wise manner from 10% to 100%; this is because compounds with different polarity will be eluted at a different proportion of methanol. In this study, baicalein was found eluted by 100% methanol (F5), and successfully enriched baicalein 2-fold as compared to the crude extracts as well as the other fractions. With this significant enrichment, the bioactivity brought forth by the compound would be enhanced.
Total flavonoid of
O. indicum leaves could be related to antioxidant activity [
22]. Our finding highlighted that there was a significant increase in antioxidant property in the F5 baicalein-enriched fraction as compared to its crude extract. Moreover, the antioxidant property exerted by the F5 was found to mimic the pure baicalein compound, indicating the effectiveness of the extraction processes described in this study. Many researchers had shown antioxidant activity of baicalein extract either by direct scavenging of superoxide radicals and hydroxyl radicals [
23], or via the activation of anti-oxidant enzymes, such as Nrf2 transcription factor, which resulted in the protection of cells against oxidative stress [
24]. In either ways, the capacity of natural phytochemical compounds in scavenging free radical could help to protect against chemotherapy-induced oxidative stress, especially in some cancer patients who have an impaired capacity to deal with oxidative insult [
25].
Moreover, the F5 displayed a superior inhibitory capability on cancerous GBM cells when compared to its crude extract. The inhibition of cancerous GBM cell proliferation by F5 was found nearly 2.5-fold more efficient than the crude extract. This indicated that the
O. indicum baicalein extraction and enrichment processes developed in this study was successful, providing a potential natural product which serves as an important controlling point in anti-cancer therapy. The fascinating effects of this baicalein-enriched F5 on GBM cell inhibitory could be due to the its major capacities to inhibit complexes of cyclins to regulate the cell cycle [
26], to induce apoptosis by activating caspase-9/-3 [
27] and to inhibit tumor invasion and metastasis by reducing the expression of matrix metalloproteinase-2/-9 (MMP-2/-9) [
28]. In addition, the ability of baicalein to cross the blood–brain barrier (BBB) [
29,
30] and distribute over a wide range of brain area after the intravenous or oral administration [
31,
32] has significantly supported its therapeutic potential for brain and central nervous system (CNS) diseases, including the brain cancer as discussed in this study. However, further detailed studies regarding the mechanism of baicalein-enriched F5 permeability to BBB and its inhibitory effects on cancerous brain cells are imperative to confirm the efficacy of this compound for treatment in clinical practice.
Last but not least, to our surprise, the inhibitory effects of F5 against the GBM cell line was found to be significantly more effective than the synthetic baicalein pure compound. Although the full characterization profile of F5 is yet to be elucidated, we proposed that the observed effect is probably due to the synergistic effects of other phenolic compounds present in the O. indicum plant that share a similar polarity as baicalein, such as baicalein-7-O-glucoside, baicalein-7-O-diglucoside or baicalin. These compounds may be present in the F5 extract and aid in inhibiting cancerous cell growth, making F5 a better anti-GBM drug as compared to the single pure baicalein compound.