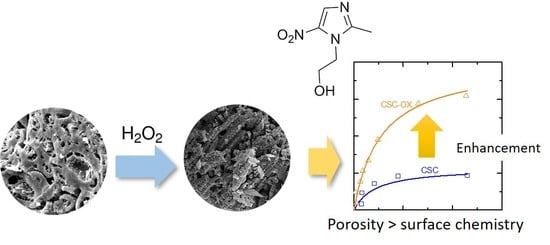
Modifying Nanoporous Carbon through Hydrogen Peroxide Oxidation for Removal of Metronidazole Antibiotics from Simulated Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Oxidation of Nanoporous Carbon
2.3. Characterization of Nanoporous Carbon
2.4. Adsorption of Antibiotic Substances
2.5. Adsorption Model of Antibiotic Substances
3. Results and Discussion
3.1. Pore Structure of Carbon Material
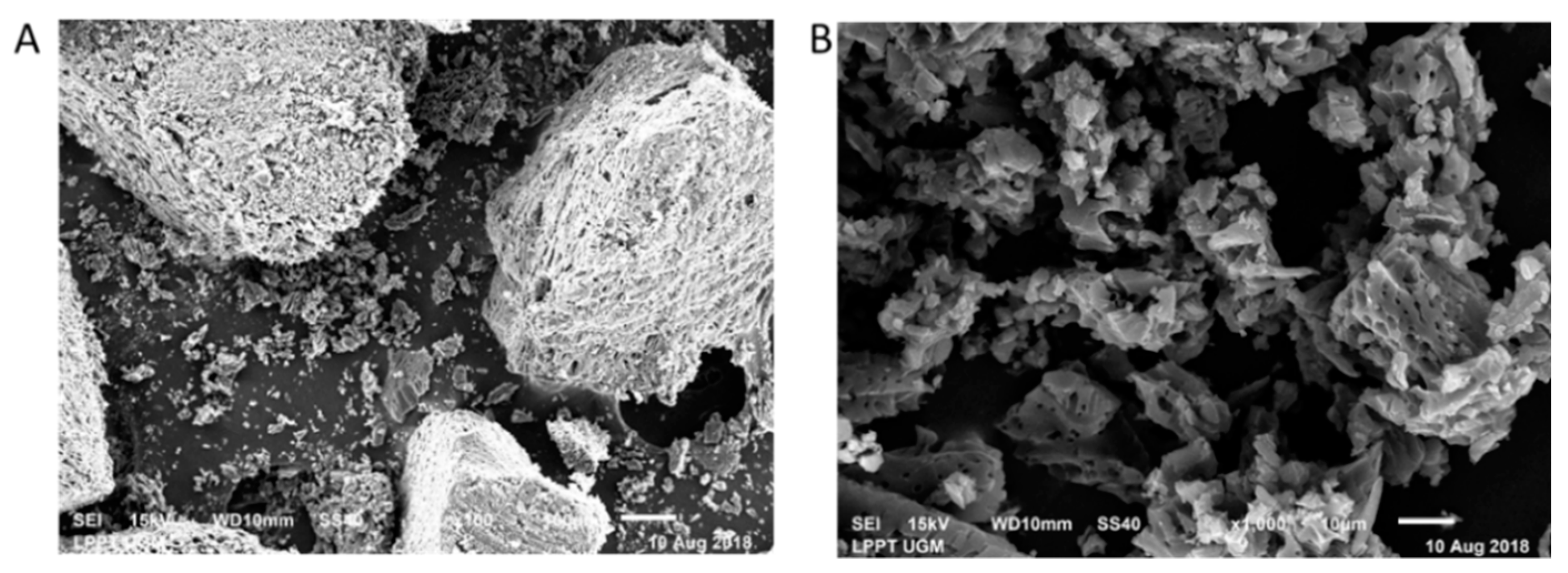
3.2. Surface Morphology
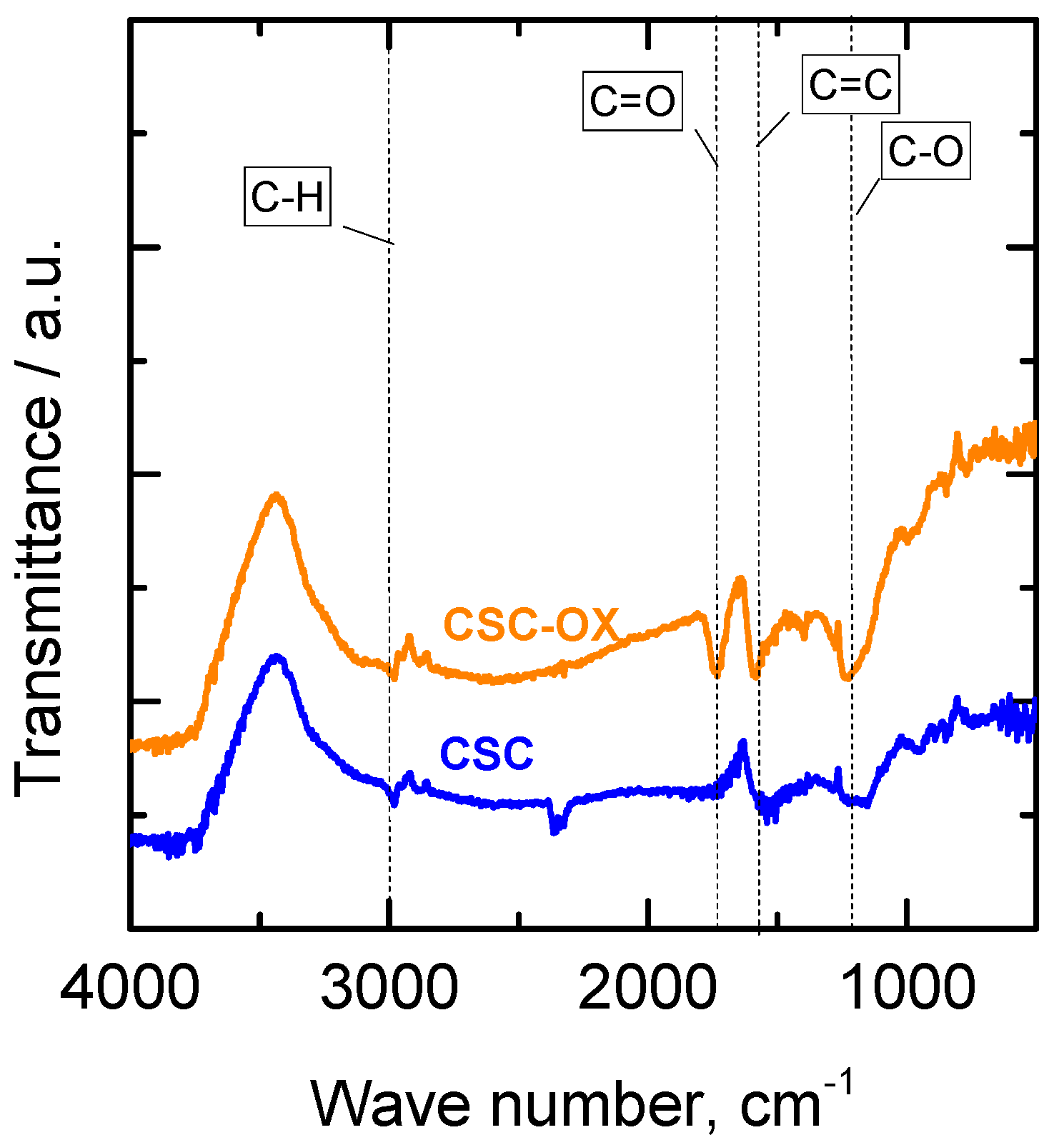
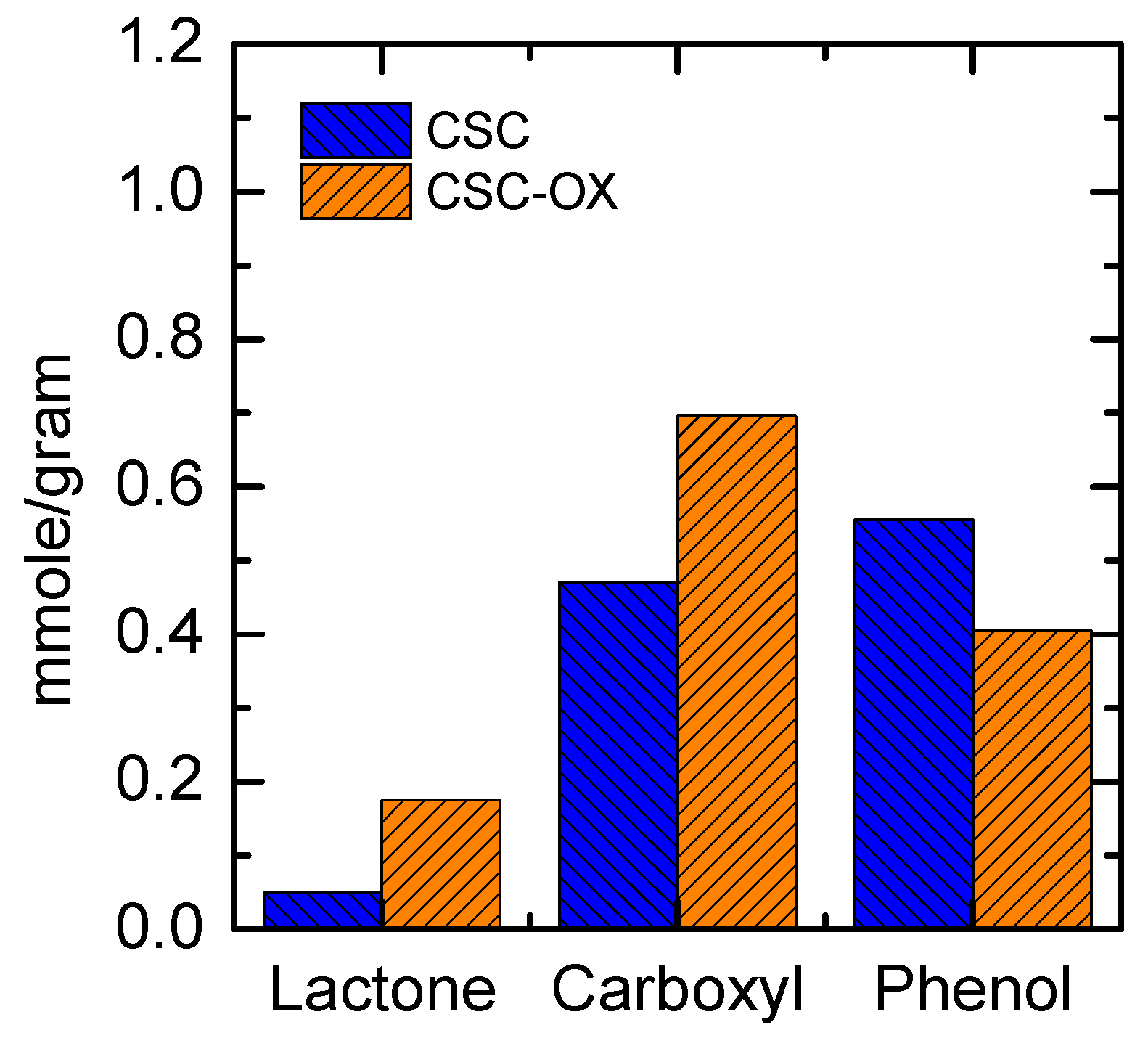
3.3. Oxygen Functional Groups
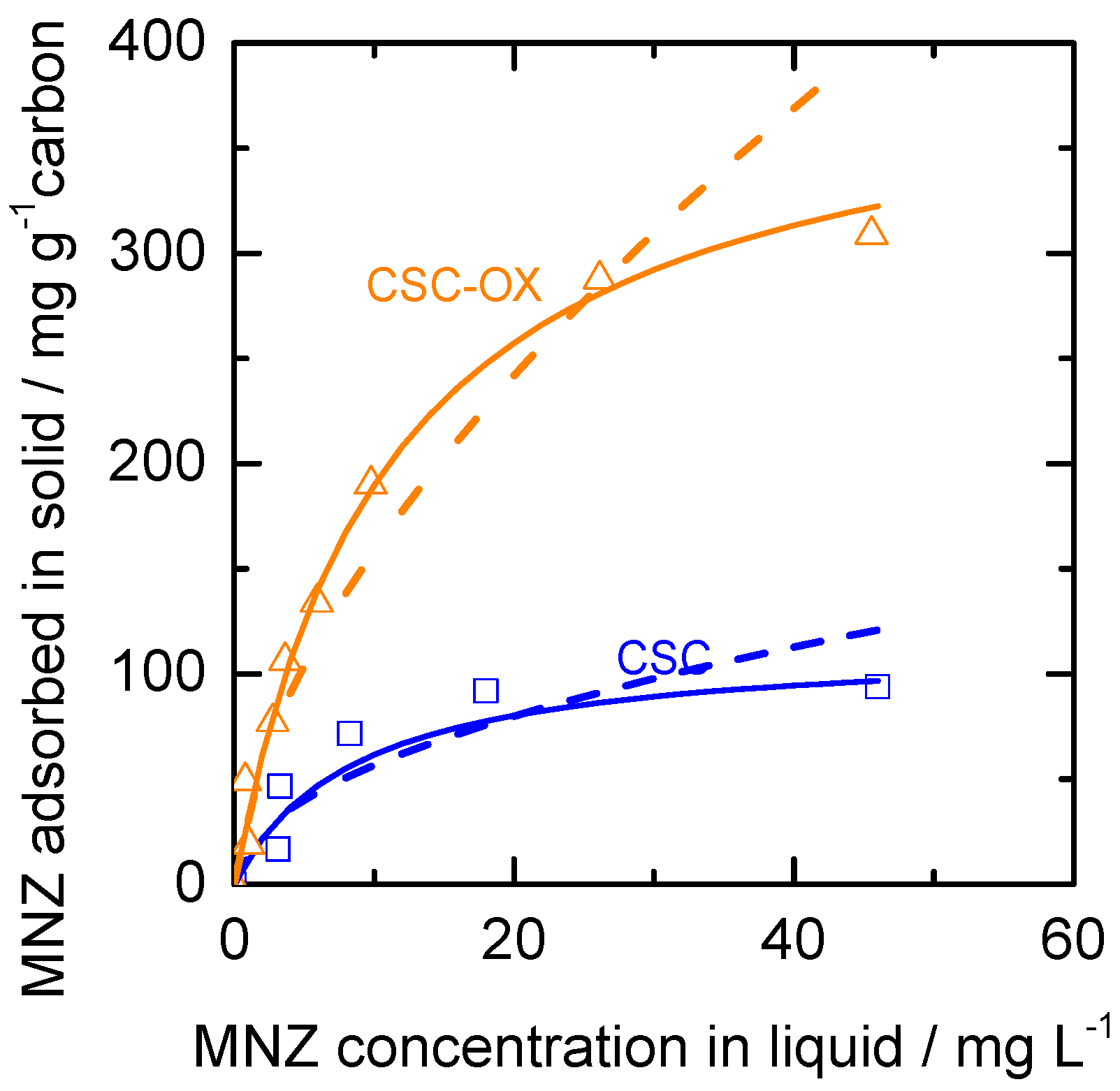
3.4. Metronidazole Adsorption Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, O.H. Pharmaceutical wastewater effluent-source of contaminants of emerging concern: Phytotoxicity of metronidazole to soybean (Glycine max). Toxics 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Garcia-Muñoz, P.; Pliego, G.; Pedro, Z.M.D.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J. Application of intensified Fenton oxidation to the treatment of hospital wastewater: Kinetics, ecotoxicity and disinfection. J. Environ. Chem. Eng. 2016, 4, 4107–4112. [Google Scholar] [CrossRef]
- Goolsby, T.A.; Jakeman, B.; Gaynes, R.P. Clinical relevance of metronidazole and peripheral neuropathy: A systematic review of the literature. Int. J. Antimicrob. Agents 2018, 51, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lanzky, P.F.; Halting-Sørensen, B. The toxic effect of the antibiotic metronidazole on aquatic organisms. Chemosphere 1997, 35, 2553–2561. [Google Scholar] [CrossRef]
- Prasetyo, I.; Rochmadi, R.; Wahyono, E.; Ariyanto, T. Controlling synthesis of polymer-derived carbon molecular sieve and its performance for CO2/CH4 separation. Eng. J. 2017, 21, 83–94. [Google Scholar] [CrossRef]
- Amelia, S.; Sediawan, W.B.; Prasetyo, I.; Munoz, M.; Ariyanto, T. Role of the pore structure of Fe/C catalysts on heterogeneous Fenton oxidation. J. Environ. Chem. Eng. 2019, 102921. [Google Scholar] [CrossRef]
- Ariyanto, T.; Kurniasari, M.; Laksmana, W.T.; Prasetyo, I. Pore size control of polymer-derived carbon adsorbent and its application for dye removal. Int. J. Environ. Sci. Technol. 2019, 16, 4631–4636. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Ocampo-Pérez, R.; Leyva-Ramos, R.; Rivera-Utrilla, J. Removal of the antibiotic metronidazole by adsorption on various carbon materials from aqueous phase. J. Colloid Interface Sci. 2014, 436, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Çalişkan, E.; Göktürk, S. Adsorption characteristics of sulfamethoxazole and metronidazole on activated carbon. Sep. Sci. Technol. 2010, 45, 244–255. [Google Scholar] [CrossRef]
- Ilomuanya, M.; Nashiru, B.; Ifudu, N.; Igwilo, C. Effect of pore size and morphology of activated charcoal prepared from midribs of Elaeis guineensis on adsorption of poisons using metronidazole and Escherichia coli O157:H7 as a case study. J. Microsc. Ultrastruct. 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, X.; Campos, L.C. Role of the functional groups in the adsorption of bisphenol A onto activated carbon: Thermal modification and mechanism. J. Water Supply Res. Technol. AQUA 2017, 66, 105–115. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, H. Effects of oxidation by hydrogen peroxide on the structures of multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2006, 45, 6483–6488. [Google Scholar] [CrossRef]
- Rehman, A.; Park, M.; Park, S.-J. Current progress on the surface chemical modification of carbonaceous materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Ferro-Garcia, M.A.; Joly, J.P.; Bautista-Toledo, I.; Carrasco-Marin, F.; Rivera-Utrilla, J. Activated carbon surface modifications by nitric acid, hydrogen peroxide, and ammonium peroxydisulfate treatments. Langmuir 1995, 11, 4386–4392. [Google Scholar] [CrossRef]
- Ros, T.G.; Van Dillen, A.J.; Geus, J.W.; Koningsberger, D.C. Surface oxidation of carbon nanofibres. Chem. A Eur. J. 2002, 8, 1151–1162. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics, 2nd ed.; Imperial College Press: London, UK, 1998; ISBN 1860941303. [Google Scholar]
- Allwar, A. Characteristics of pore structures and surface chemistry of activated carbons by physisorption, FTIR And Boehm methods. IOSR J. Appl. Chem. 2012, 2, 9–15. [Google Scholar] [CrossRef]
- Saffaj, T.; Charrouf, M.; Abourrche, A.; Aboud, Y.; Bennamara, A.; Berrada, M. Spectrophotometric determination of metronidazole and secnidazole in pharmaceutical preparations based on the formation of dyes. Dyes Pigments 2006, 70, 259–262. [Google Scholar] [CrossRef]
- Sarwendah, R.A.G. Oksidasi Karbon Berpori dari dari Tempurung Kelapa dengan Hidrogen Peroksida serta Pengaruhnya untuk Adsorpsi Metronidazol. Bachelor’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2018. [Google Scholar]
- Amimmal, Y.M.N. Oksidasi Karbon Berpori dari Kayu Mlanding dengan Hidrogen Peroksida serta Pengaruhnya untuk Adsorpsi Metronidazol. Bachelor’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2018. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Hasse, B.; Glasel, J.; Kern, A.M.; Murzin, D.Y.; Etzold, B.J.M. Preparation of carbide-derived carbon supported platinum catalysts. Catal. Today 2015, 249, 30–37. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Ni, T.; Li, S. Adsorption of methylene blue on natural cotton based flexible carbon fiber aerogels activated by novel air-limited carbonization method. J. Mol. Liq. 2017, 242, 747–756. [Google Scholar] [CrossRef]
- Ania, C.O.; Parra, J.B.; Pis, J.J. Influence of oxygen-containing functional groups on active carbon adsorption of selected organic compounds. Fuel Process. Technol. 2002, 79, 265–271. [Google Scholar] [CrossRef]

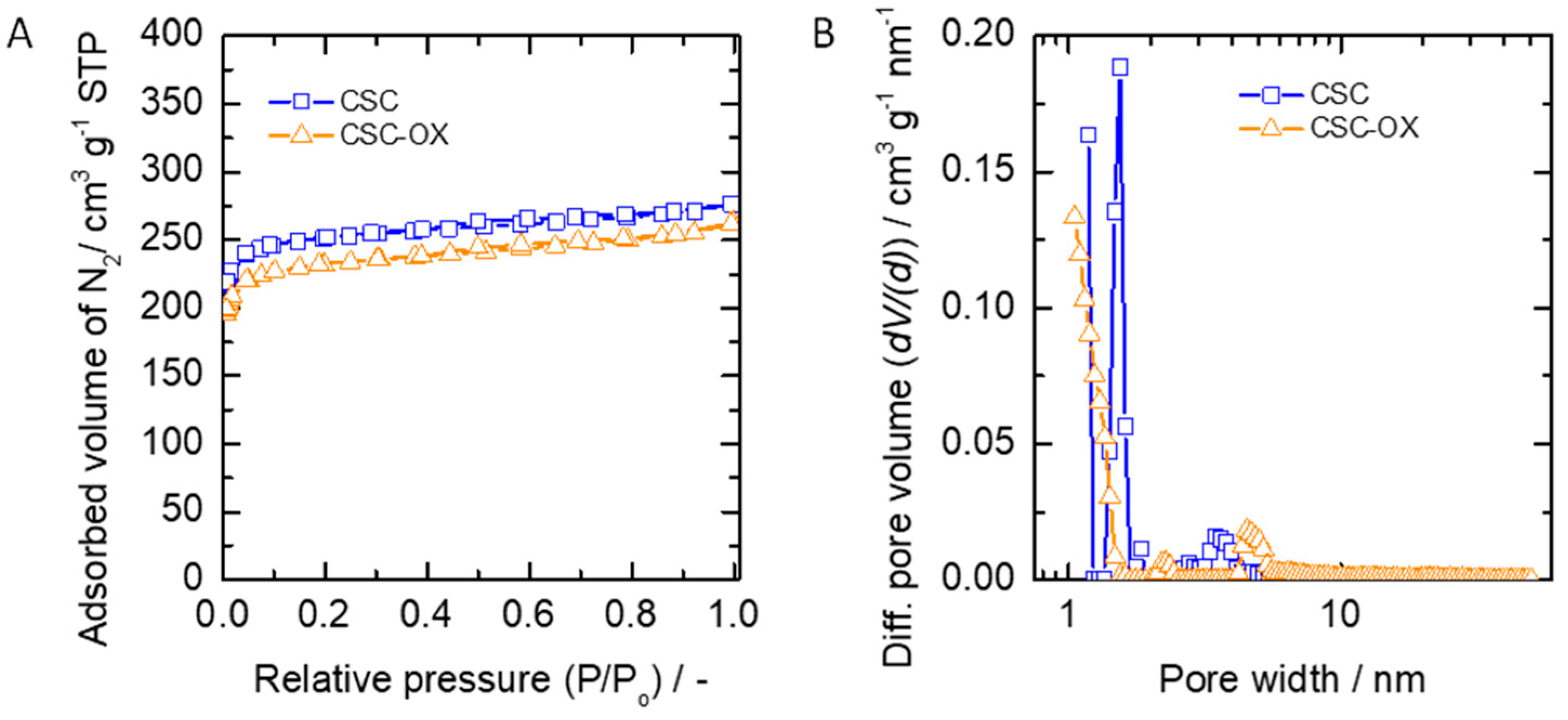

| No. | Carbon Type | SSA a (m2/g) | Total Pore Volume b (cm3/g) | Percentage of Micropore SSA c (%) | Percentage of Micropore Volume c (%) |
|---|---|---|---|---|---|
| 1. | CSC | 973 | 0.43 | 95 | 84 |
| 2. | CSC-OX | 895 | 0.41 | 94 | 80 |
| No. | Material | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| KL, L/mol | Qm, mg Adsorbate/g Adsorbent | R2 | KF mg Adsorbate/(g Adsorbent (mol/L)n) | n | R2 | ||
| 1. | CSC | 0.12 | 114.94 | 0.90 | 17.99 | 2.00 | 0.64 |
| 2. | CSC-OX | 0.09 | 400.00 | 0.95 | 39.03 | 1.64 | 0.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyanto, T.; Sarwendah, R.A.G.; Amimmal, Y.M.N.; Laksmana, W.T.; Prasetyo, I. Modifying Nanoporous Carbon through Hydrogen Peroxide Oxidation for Removal of Metronidazole Antibiotics from Simulated Wastewater. Processes 2019, 7, 835. https://doi.org/10.3390/pr7110835
Ariyanto T, Sarwendah RAG, Amimmal YMN, Laksmana WT, Prasetyo I. Modifying Nanoporous Carbon through Hydrogen Peroxide Oxidation for Removal of Metronidazole Antibiotics from Simulated Wastewater. Processes. 2019; 7(11):835. https://doi.org/10.3390/pr7110835
Chicago/Turabian StyleAriyanto, Teguh, Rut Aprillia Galuh Sarwendah, Yove Maulana Novirdaus Amimmal, William Teja Laksmana, and Imam Prasetyo. 2019. "Modifying Nanoporous Carbon through Hydrogen Peroxide Oxidation for Removal of Metronidazole Antibiotics from Simulated Wastewater" Processes 7, no. 11: 835. https://doi.org/10.3390/pr7110835
APA StyleAriyanto, T., Sarwendah, R. A. G., Amimmal, Y. M. N., Laksmana, W. T., & Prasetyo, I. (2019). Modifying Nanoporous Carbon through Hydrogen Peroxide Oxidation for Removal of Metronidazole Antibiotics from Simulated Wastewater. Processes, 7(11), 835. https://doi.org/10.3390/pr7110835