RETRACTED: Physical and Thermal Studies of Carbon-Enriched Silicon Oxycarbide Synthesized from Floating Plants
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Materials
2.2. Characterization of Carbon-Enriched Silicon Oxycarbide
3. Results and Discussion
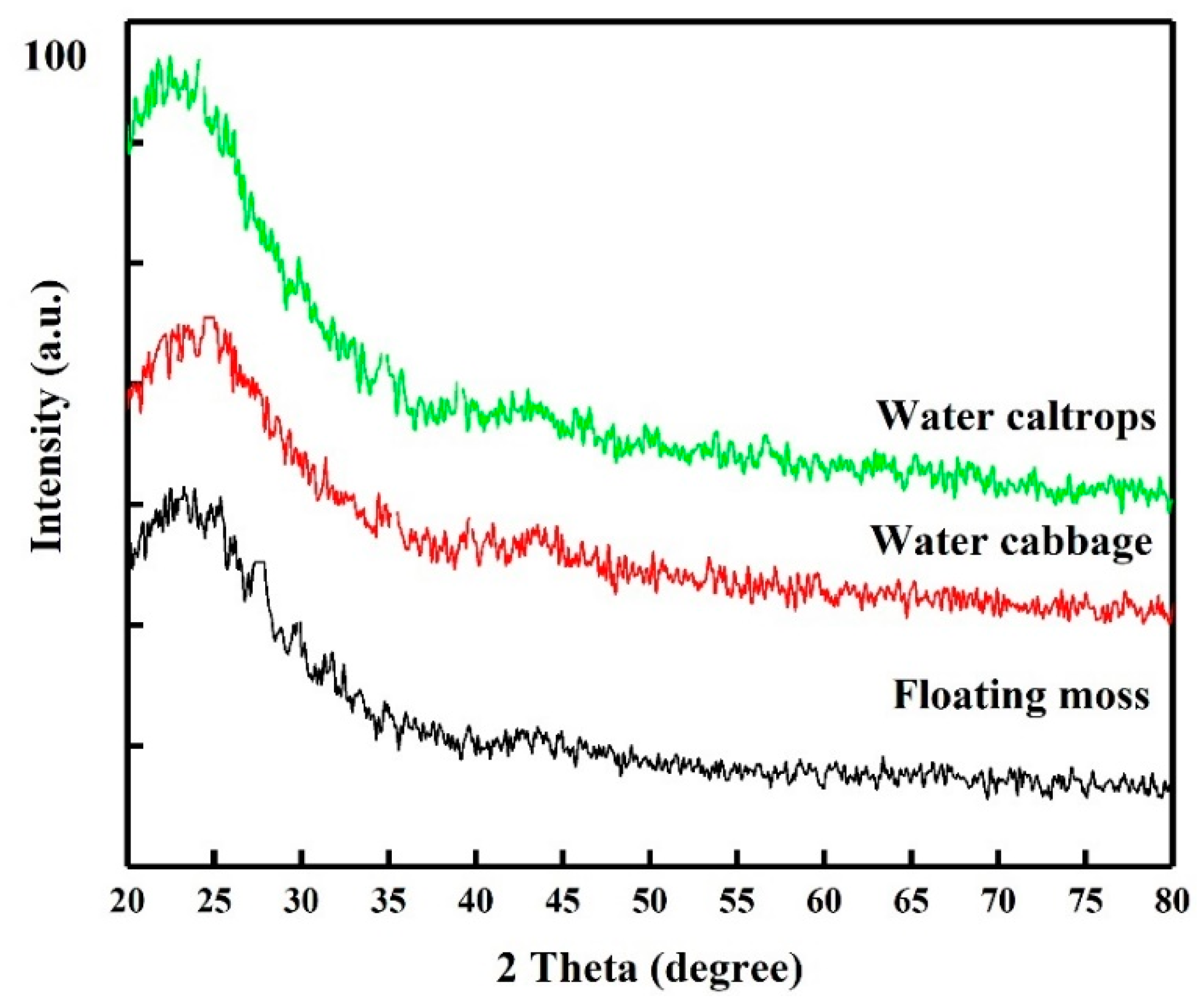
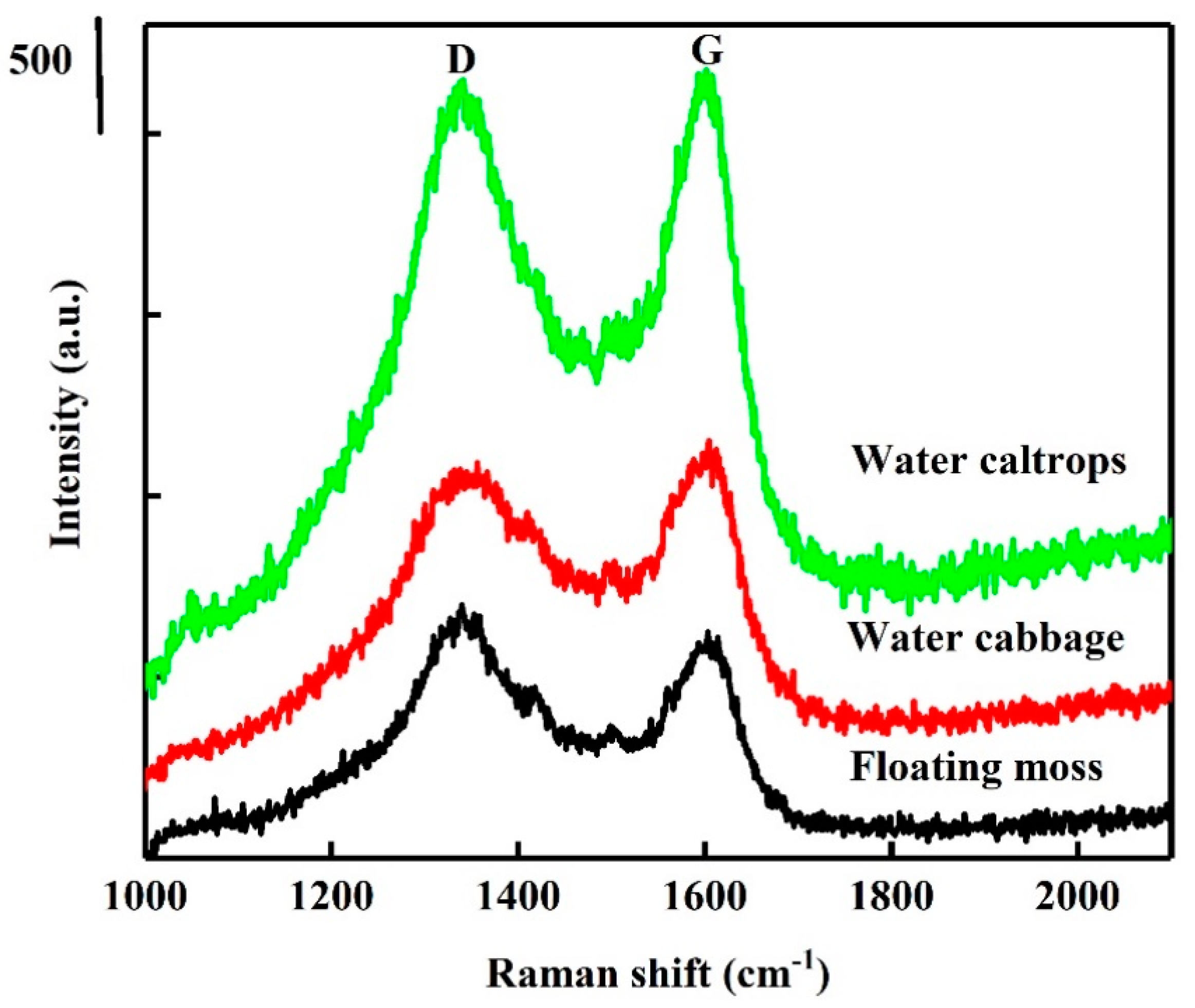
3.1. Structural Analysis
3.2. Morphology and Composition Analysis
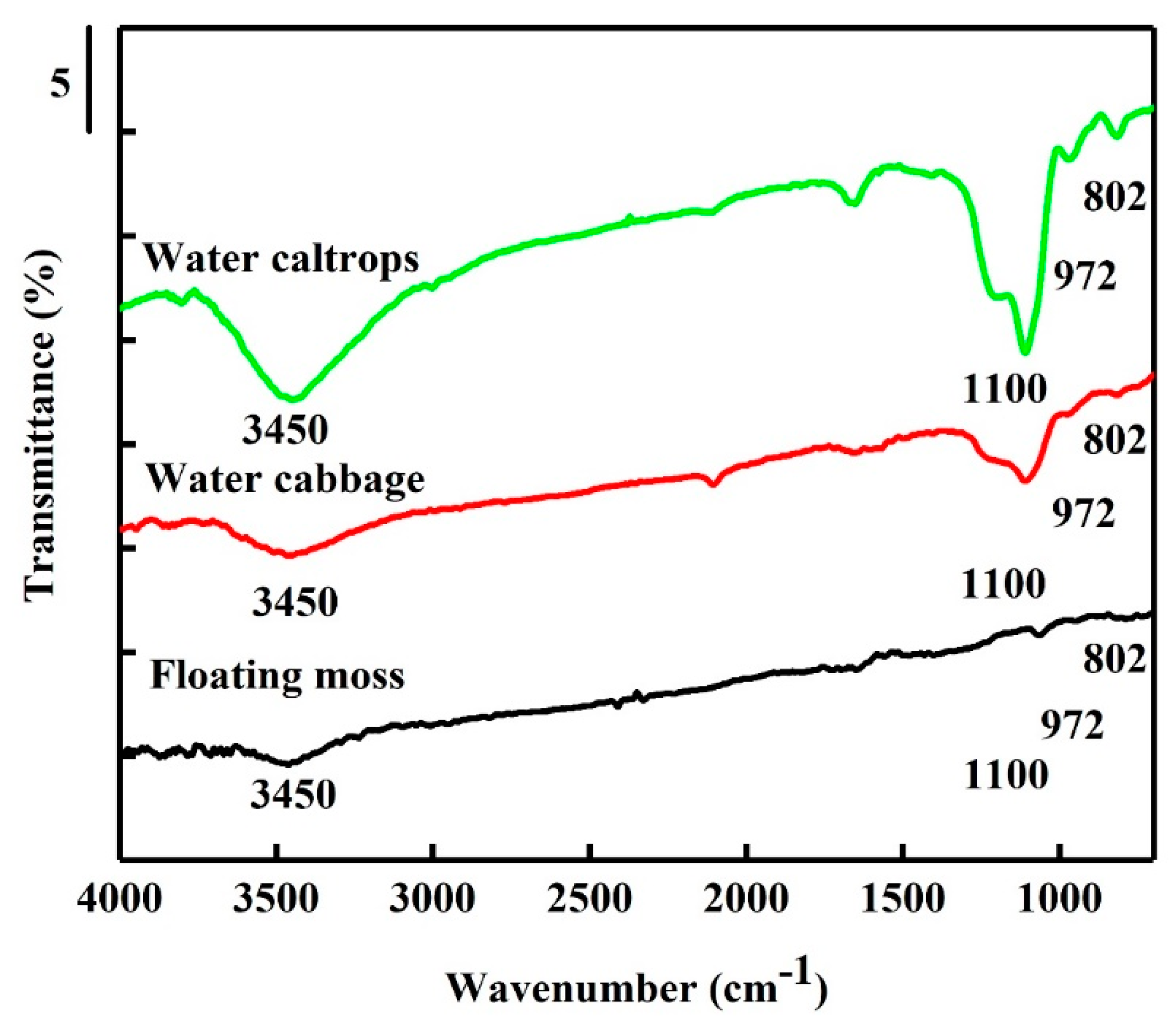
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Spectra
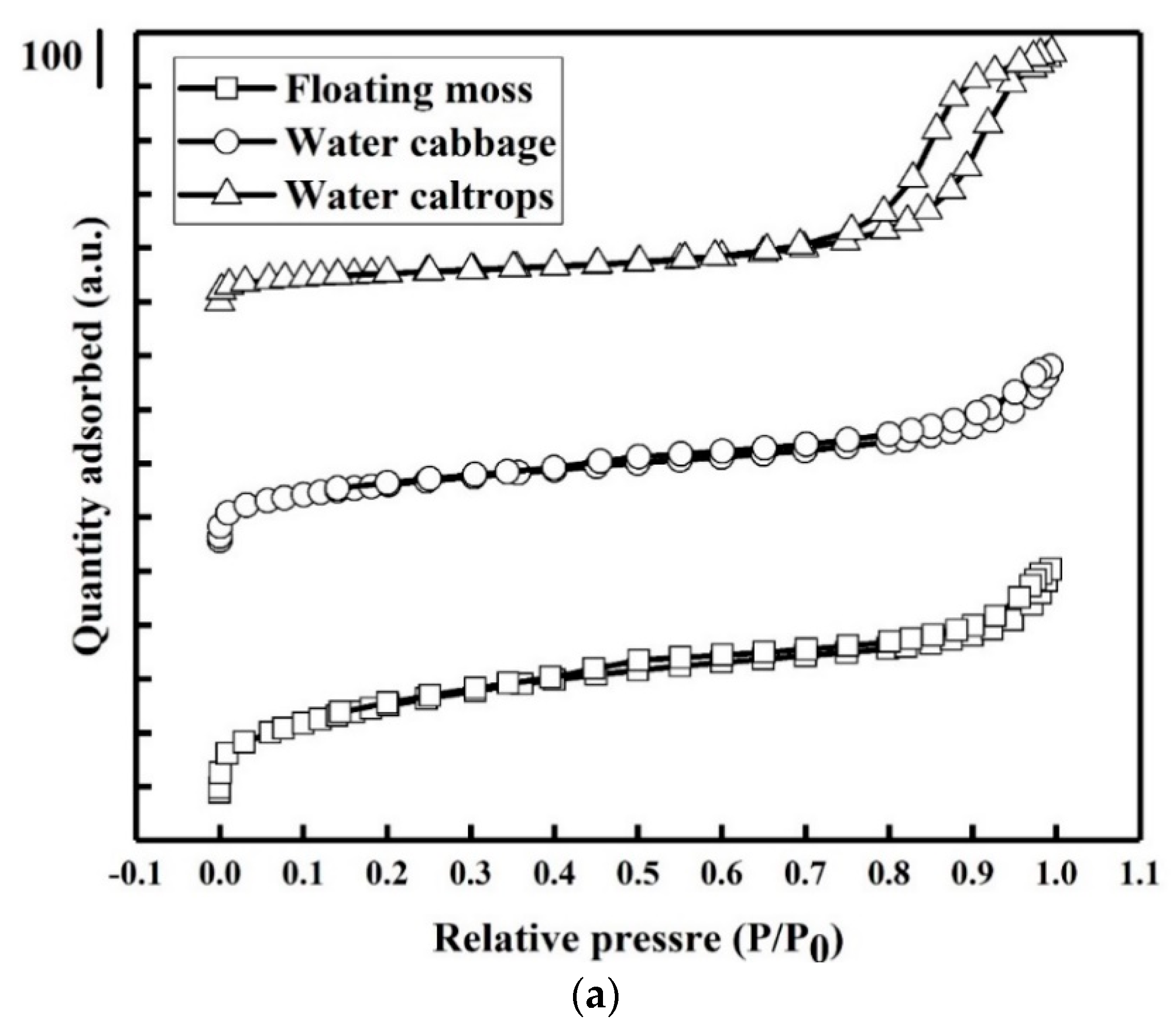
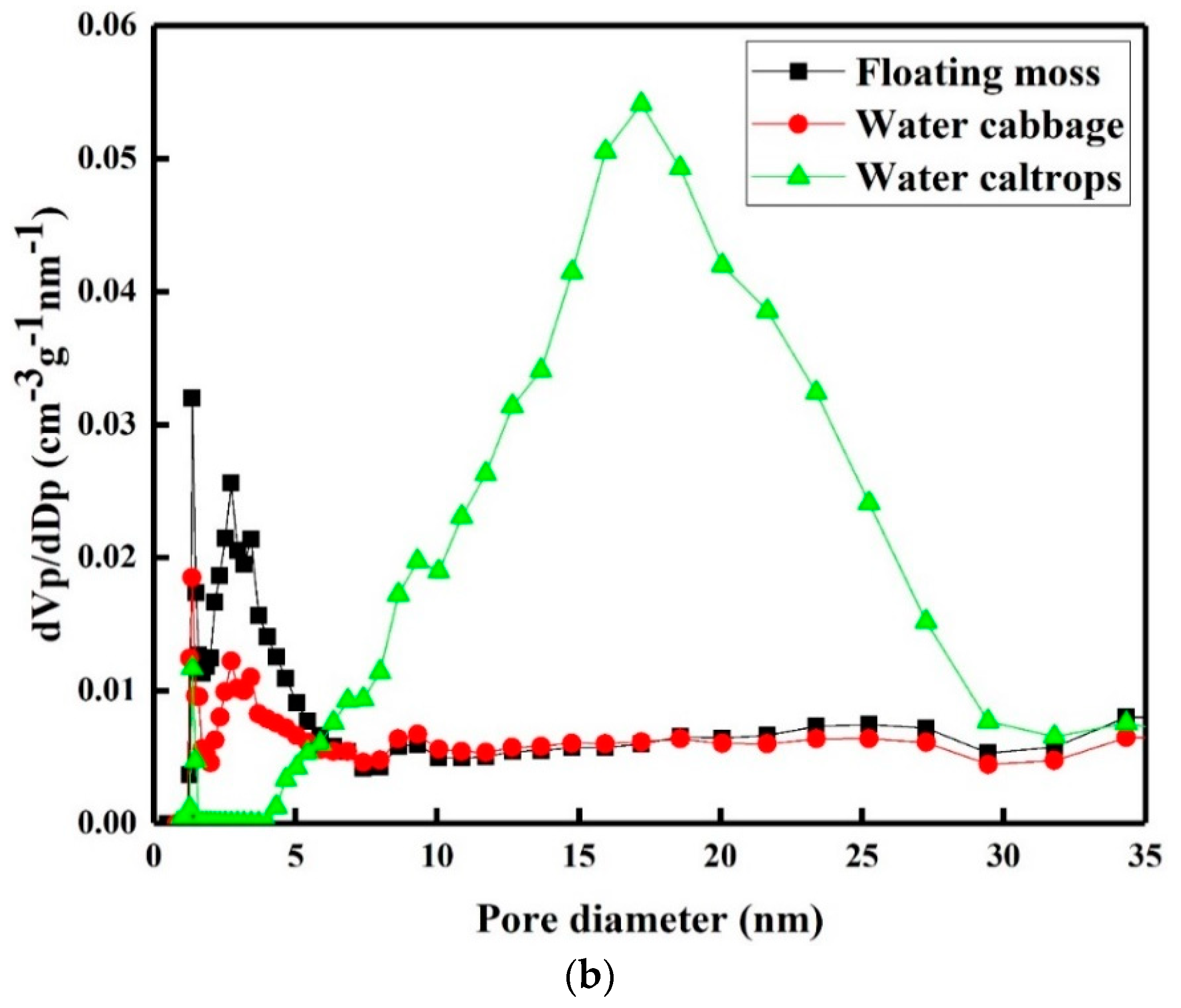
3.4. Porosity and Surface Area Study
3.5. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyajima, H.; Masuda, H.; Watanabe, K.; Ishikawa, K.; Sekine, M.; Hori, M. Chemical bonding structure in porous SiOC films (k < 2.4) with high plasma-induced damage resistance. Micro Nano Eng. 2019, 3, 1–6. [Google Scholar]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Wu, Z.; Lv, W.; Cheng, X.; Gao, J.; Qian, Z.; Tian, D.; Li, J.; He, W.; Yang, C. A Nanostructured Si/SiOC Composite Anode with Volume-Change-Buffering Microstructure for Lithium-Ion Batteries. Chem. A Eur. J. 2019, 25, 2604–2609. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, X.; Tian, D.; Gao, T.; He, W.; Yang, C. SiOC nanolayers directly-embedded in graphite as stable anode for high-rate lithium ion batteries. Chem. Eng. J. 2019, 375, 121997. [Google Scholar] [CrossRef]
- Duan, L.; Ma, Q.; Mei, L.; Chen, Z. Fabrication and electrochemical performance of nanoporous carbon derived from silicon oxycarbide. Microporous Mesoporous Mater. 2015, 202, 97–105. [Google Scholar] [CrossRef]
- Roth, F.; Schmerbauch, C.; Ionescu, E.; Nicoloso, N.; Guillon, O.; Riedel, R. High-temperature piezoresistive C/SiOC sensors. J. Sens. Sens. Syst. 2015, 4, 133–136. [Google Scholar] [CrossRef]
- Bik, M.; Stygar, M.; Jeleń, P.; Dąbrowa, J.; Leśniak, M.; Brylewski, T.; Sitarz, M. Protective-conducting coatings based on black glasses (SiOC) for application in solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 27298–27307. [Google Scholar] [CrossRef]
- Tolosa, A.; Krüner, B.; Jäckel, N.; Aslan, M.; Vakifahmetoglu, C.; Presser, V. Electrospinning and electrospraying of silicon oxycarbide-derived nanoporous carbon for supercapacitor electrodes. J. Power Source 2016, 313, 178–188. [Google Scholar] [CrossRef]
- Wu, N.; Wang, B.; Wang, Y. Enhanced mechanical properties of amorphous Si OC nanofibrous membrane through in situ embedding nanoparticles. J. Am. Ceram. Soc. 2018, 101, 4763–4772. [Google Scholar] [CrossRef]
- Sorarù, G.D.; Modena, S.; Guadagnino, E.; Colombo, P.; Egan, J.; Pantano, C. Chemical durability of silicon oxycarbide glasses. J. Am. Ceram. Soc. 2002, 85, 1529–1536. [Google Scholar] [CrossRef]
- Saha, A.; Raj, R. Crystallization maps for SiCO amorphous ceramics. J. Am. Ceram. Soc. 2007, 90, 578–583. [Google Scholar] [CrossRef]
- Stabler, C.; Reitz, A.; Stein, P.; Albert, B.; Riedel, R.; Ionescu, E. Thermal properties of SiOC glasses and glass ceramics at elevated temperatures. Materials 2018, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, Y.; Zheng, X.; Zhu, J.; Tang, D.; Wu, J.; Xu, C. Thermal-conductivity studies of macro-porous polymer-derived SiOC ceramics. Int. J. Thermophys. 2014, 35, 76–89. [Google Scholar] [CrossRef]
- Santana, J.A.C.; Mora, E.E.; Price, L.; Balerio, R.; Shao, L.; Nastasi, M. Synthesis, thermal stability and the effects of ion irradiation in amorphous Si–O–C alloys. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 350, 6–13. [Google Scholar] [CrossRef]
- Nastasi, M.; Su, Q.; Price, L.; Santana, J.A.C.; Chen, T.; Balerio, R.; Shao, L. Superior radiation tolerant materials: Amorphous silicon oxycarbide. J. Nucl. Mater. 2015, 461, 200–205. [Google Scholar] [CrossRef]
- Gurlo, A.; Ionescu, E.; Riedel, R.; Clarke, D.R. The Thermal Conductivity of Polymer-Derived Amorphous Si–O–C Compounds and Nano-Composites. J. Am. Ceram. Soc. 2016, 99, 281–285. [Google Scholar] [CrossRef]
- Mazo, M.A.; Palencia, C.; Nistal, A.; Rubio, F.; Rubio, J.; Oteo, J.L. Dense bulk silicon oxycarbide glasses obtained by spark plasma sintering. J. Eur. Ceram. Soc. 2012, 32, 3369–3378. [Google Scholar] [CrossRef]
- Eom, J.-H.; Kim, Y.-W.; Kim, K.J.; Seo, W.-S. Improved electrical and thermal conductivities of polysiloxane-derived silicon oxycarbide ceramics by barium addition. J. Eur. Ceram. Soc. 2018, 38, 487–493. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Hattar, K.; Nastasi, M.; Cui, B. Novel amorphous SiOC dispersion-strengthened austenitic steels. Materialia 2019, 6, 100345. [Google Scholar] [CrossRef]
- Zare, A.; Su, Q.; Gigax, J.; Shojaee, S.; Harriman, T.; Nastasi, M.; Shao, L.; Materer, N.; Lucca, D. Effects of ion irradiation on chemical and mechanical properties of magnetron sputtered amorphous SiOC. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 446, 10–14. [Google Scholar] [CrossRef]
- Zare, M.; Niroumand, B.; Maleki, A.; Allafchian, A.R. Sol-gel synthesis of amorphous SiOC nanoparticles from BS290 silicone precursor. Ceram. Int. 2017, 43, 12898–12903. [Google Scholar] [CrossRef]
- Liu, C.; Meng, X.; Zhang, X.; Hong, C.; Han, J.; Han, W.; Xu, B.; Dong, S.; Du, S. High temperature structure evolution of macroporous SiOC ceramics prepared by a sol–gel method. Ceram. Int. 2015, 41, 11091–11096. [Google Scholar] [CrossRef]
- Yu, S.; Tu, R.; Goto, T. Preparation of SiOC nanocomposite films by laser chemical vapor deposition. J. Eur. Ceram. Soc. 2016, 36, 403–409. [Google Scholar] [CrossRef]
- Zollfrank, C.; Kladny, R.; Sieber, H.; Greil, P. Biomorphous SiOC/C-ceramic composites from chemically modified wood templates. J. Eur. Ceram. Soc. 2004, 24, 479–487. [Google Scholar] [CrossRef]
- Pan, J.; Pan, J.; Cheng, X.; Yan, X.; Lu, Q.; Zhang, C. Synthesis of hierarchical porous silicon oxycarbide ceramics from preceramic polymer and wood biomass composites. J. Eur. Ceram. Soc. 2014, 34, 249–256. [Google Scholar] [CrossRef]
- Wang, M.; Xia, Y.; Wang, X.; Xiao, Y.; Liu, R.; Wu, Q.; Qiu, B.; Metwalli, E.; Xia, S.; Yao, Y. Silicon oxycarbide/carbon nanohybrids with tiny silicon oxycarbide particles embedded in free carbon matrix based on photoactive dental methacrylates. ACS Appl. Mater. Interfaces 2016, 8, 13982–13992. [Google Scholar] [CrossRef]
- Xue, J.; Myrtle, K.; Dahn, J. An Epoxy-Silane Approach to Prepare Anode Materials for Rechargeable Lithium Ion Batteries. J. Electrochem. Soc. 1995, 142, 2927–2935. [Google Scholar] [CrossRef]
- Dibandjo, P.; Graczyk-Zajac, M.; Riedel, R.; Pradeep, V.; Soraru, G. Lithium insertion into dense and porous carbon-rich polymer-derived SiOC ceramics. J. Eur. Ceram. Soc. 2012, 32, 2495–2503. [Google Scholar] [CrossRef]
- Fukui, H.; Ohsuka, H.; Hino, T.; Kanamura, K. A Si–O–C composite anode: High capability and proposed mechanism of lithium storage associated with microstructural characteristics. ACS Appl. Mater. Interfaces 2010, 2, 998–1008. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, L.; Wang, K.; Miao, L.; Lan, Q.; Jiang, K.; Lu, H.; Li, M.; Li, Y.; Shen, B. Analysis of oxidation degree of graphite oxide and chemical structure of corresponding reduced graphite oxide by selecting different-sized original graphite. RSC Adv. 2018, 8, 17209–17217. [Google Scholar] [CrossRef]
- Amutha, K.; Ravibaskar, R.; Sivakumar, G. Extraction, synthesis and characterization of nanosilica from rice husk ash. Int. J. Nanotechnol. Appl. 2010, 4, 61–66. [Google Scholar]
- Witoon, T.; Chareonpanich, M.; Limtrakul, J. Synthesis of bimodal porous silica from rice husk ash via sol–gel process using chitosan as template. Mater. Lett. 2008, 62, 1476–1479. [Google Scholar] [CrossRef]
- Rida, M.A.; Harb, F. Synthesis and characterization of amorphous silica nanoparitcles from aqueous silicates uisng cationic surfactants. J. Met. Mater. Miner. 2014, 24, 37–42. [Google Scholar]
- Chapter 3 Surface chemistry of porous silica. J. Chromatogr. Libr. 1979, 16, 57–146. [CrossRef]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic–Inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Yao, W.; Guangsheng, G.; Fei, W.; Jun, W. Fluidization and agglomerate structure of SiO2 nanoparticles. Powder Technol. 2002, 124, 152–159. [Google Scholar] [CrossRef]
- Pan, G.-T.; Chong, S.; Yang, T.C.-K.; Yang, Y.-L.; Arjun, N. Surface modification of amorphous SiO2 nanoparticles by oxygen-plasma and nitrogen-plasma treatments. Chem. Eng. Commun. 2016, 203, 1666–1670. [Google Scholar] [CrossRef]
- Eom, J.-H.; Kim, Y.-W.; Raju, S. Processing and properties of macroporous silicon carbide ceramics: A review. J. Asian Ceram. Soc. 2013, 1, 220–242. [Google Scholar] [CrossRef]
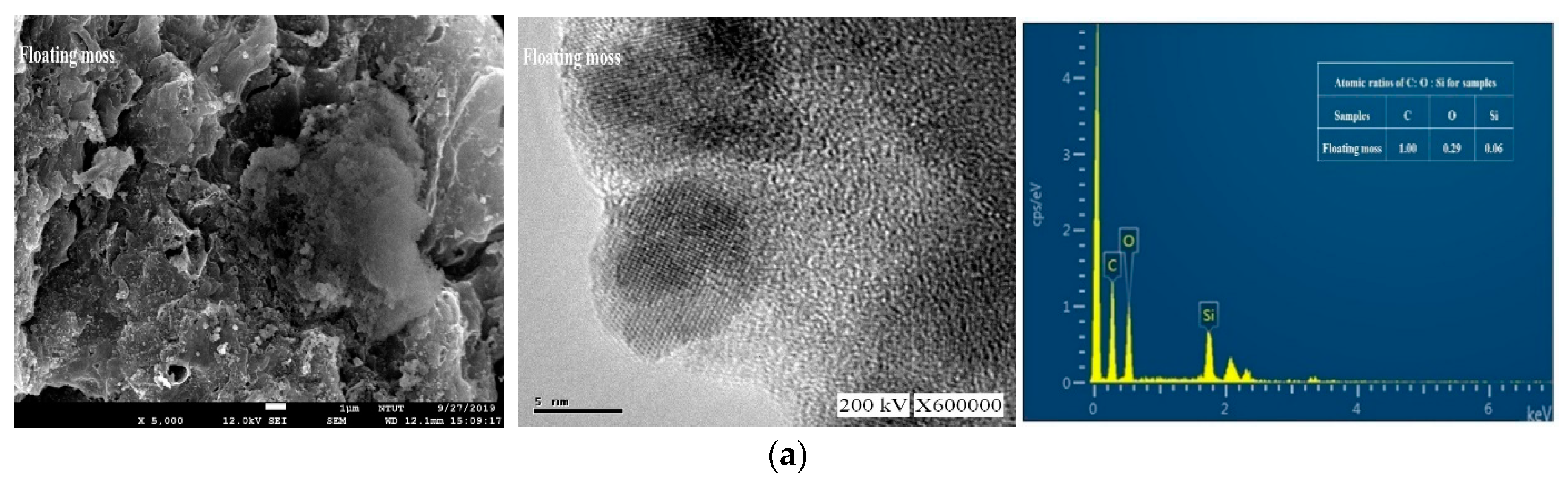
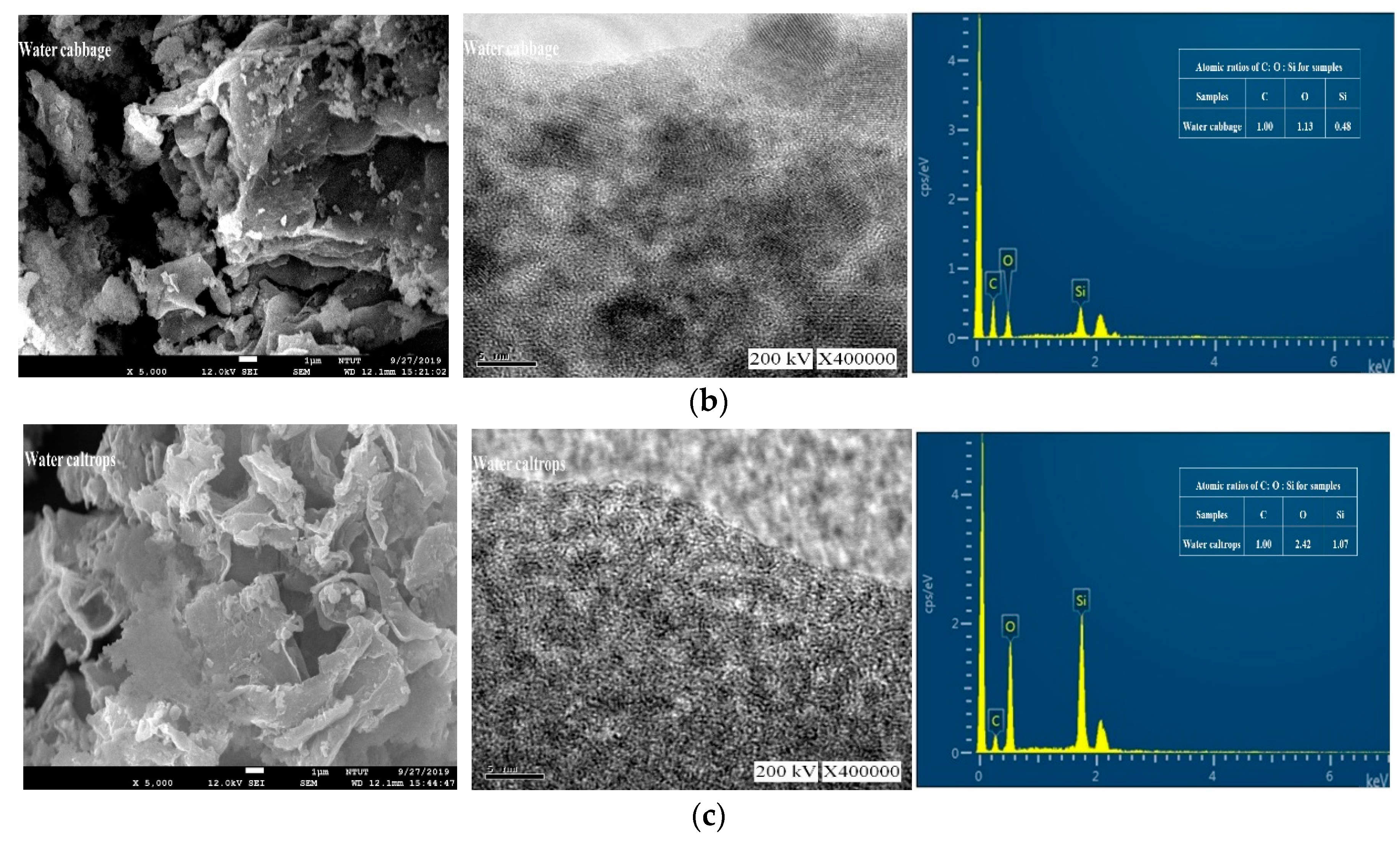
| Atomic Ratios | |||
|---|---|---|---|
| Sample | C | O | Si |
| Floating moss | 1 | 0.29 | 0.06 |
| Water cabbage | 1 | 1.13 | 0.48 |
| Water caltrops | 1 | 2.42 | 1.07 |
| Sample | Vpore (cm3 g−1) | SBET (m2 g−1) | Dp (nm) | Thermal Conductivity (Wm−1 K−1) |
|---|---|---|---|---|
| Floating moss | 0.40 | 894.81 | 7.00 | 6.55 |
| Water cabbage | 0.49 | 565.21 | 6.04 | 2.46 |
| Water caltrops | 0.63 | 213.00 | 4.75 | 1.14 |
| No | Precursors | Method | Thermal Conductivity (Wm−1 K−1) | References |
|---|---|---|---|---|
| 1 | Silicon oxide carbide powders | Pyrolysis | 1.8~2.7 | [12] |
| 2 | Alkoxy silane | Sol–gel | 0.041~0.078 | [13] |
| 3 | Polysiloxane pyrolysis residue, and barium isopropoxide | Pyrolysis | 1.8~5.6 | [18] |
| 4 | Commercial poly(methylsilsesquioxane) starting polymer and zirconium acetylacetonate | Pyrolysis | 2 | [16] |
| 5 | Silicon oxycarbide (SiOC) powders | Spark plasma sintering | 1.38 | [17] |
| 6 | Floating plants | Pyrolysis | 1.14~6.55 | This paper |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, G.-T.; Chong, S.; Chan, Y.J.; Tiong, T.J.; Lim, J.W.; Huang, C.-M.; Shukla, P.; Yang, T.C.-K. RETRACTED: Physical and Thermal Studies of Carbon-Enriched Silicon Oxycarbide Synthesized from Floating Plants. Processes 2019, 7, 794. https://doi.org/10.3390/pr7110794
Pan G-T, Chong S, Chan YJ, Tiong TJ, Lim JW, Huang C-M, Shukla P, Yang TC-K. RETRACTED: Physical and Thermal Studies of Carbon-Enriched Silicon Oxycarbide Synthesized from Floating Plants. Processes. 2019; 7(11):794. https://doi.org/10.3390/pr7110794
Chicago/Turabian StylePan, Guan-Ting, Siewhui Chong, Yi Jing Chan, Timm Joyce Tiong, Jun Wei Lim, Chao-Ming Huang, Pradeep Shukla, and Thomas Chung-Kuang Yang. 2019. "RETRACTED: Physical and Thermal Studies of Carbon-Enriched Silicon Oxycarbide Synthesized from Floating Plants" Processes 7, no. 11: 794. https://doi.org/10.3390/pr7110794
APA StylePan, G.-T., Chong, S., Chan, Y. J., Tiong, T. J., Lim, J. W., Huang, C.-M., Shukla, P., & Yang, T. C.-K. (2019). RETRACTED: Physical and Thermal Studies of Carbon-Enriched Silicon Oxycarbide Synthesized from Floating Plants. Processes, 7(11), 794. https://doi.org/10.3390/pr7110794












