4.2. Simulation Results
Table 2 shows the optimum set of stoichiometric numbers (a to q) generated from the simulation program for the five different biomasses studied. It should be noted that the char formula, CH
0.22O
0.51, was estimated from the ultimate analysis data of the produced solid char and was assumed constant for all the simulation conditions.
Table 3 shows the corresponding kinetic parameters (A and E) as a function of pyrolysis temperature over the range from 300 to 800 °C, along with the comparison between experimental product yields and those predicted from the simulation program. The designated symbols P01, P02, P03, P04 and P05 indicate the set of simulation parameters (stoichiometry and kinetic parameters) pertaining to palm shell (PS), coconut shell (CS), palm kernel cake (PK), cassava pulp residue (CP) and longan fruit seed (LS), respectively. As
Table 2 shows, the stoichiometry of the pyrolysis reaction is strongly dependent on the type of biomass. In terms of gas components, the order of increasing stoichiometric number is as follows, H
2(a) < CH
4(d) << CO(c) < CO
2(b). The stoichiometric number of CO
2 is highest among the gas products and the following order of increasing CO
2 stoichiometric number is noted for different biomass types, PS < CS < LS, PK < CP. This observation may indicate the highest percentage composition of CO
2 in the pyrolysis gas products and cassava pulp residue could produce the highest percentage yield of CO
2. The stoichiometric numbers of liquid water and phenol are much higher than the other liquid-phase chemical components, possibly an indication of large proportions of water and oxygenated compounds present in the liquid bio-oil product.
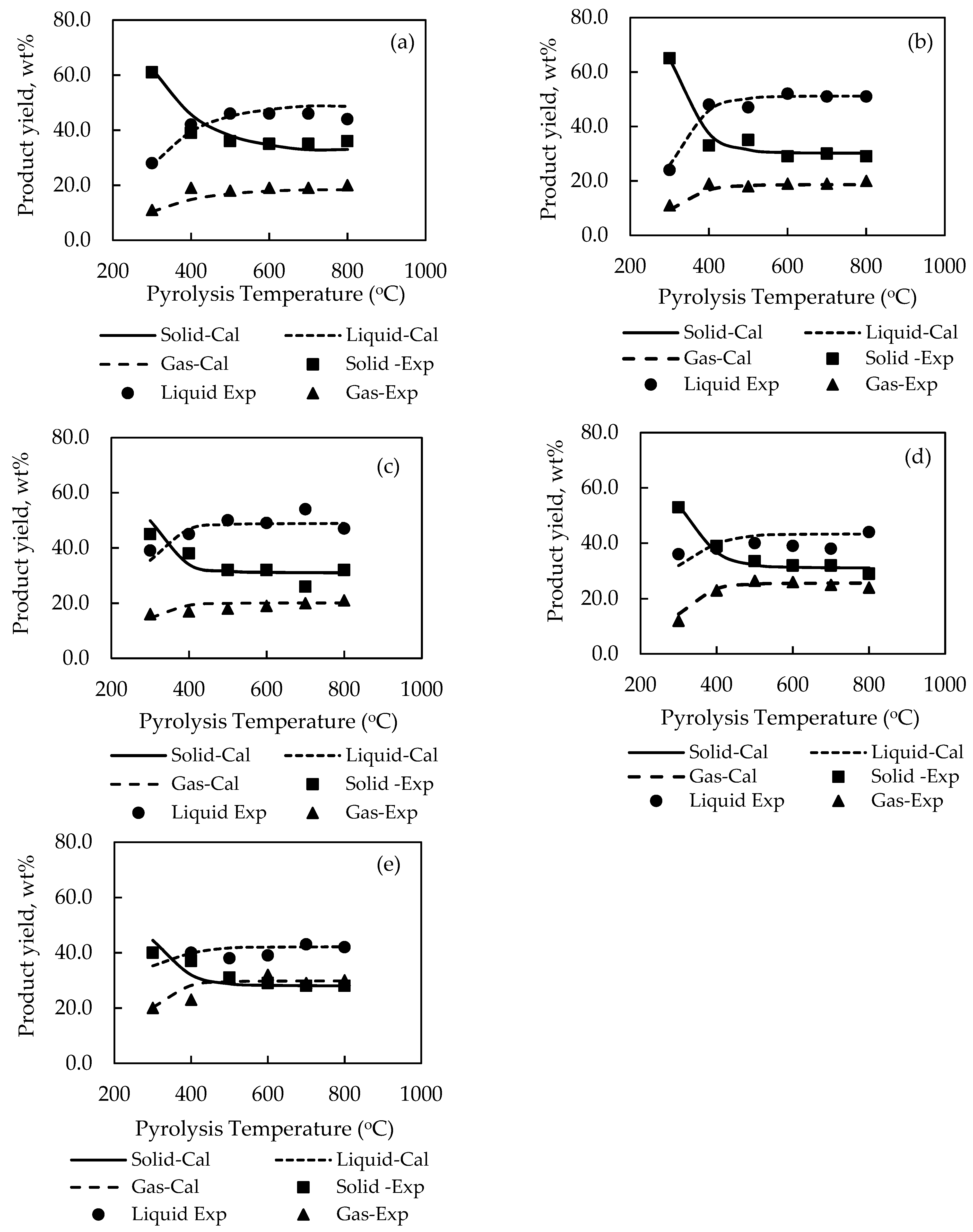
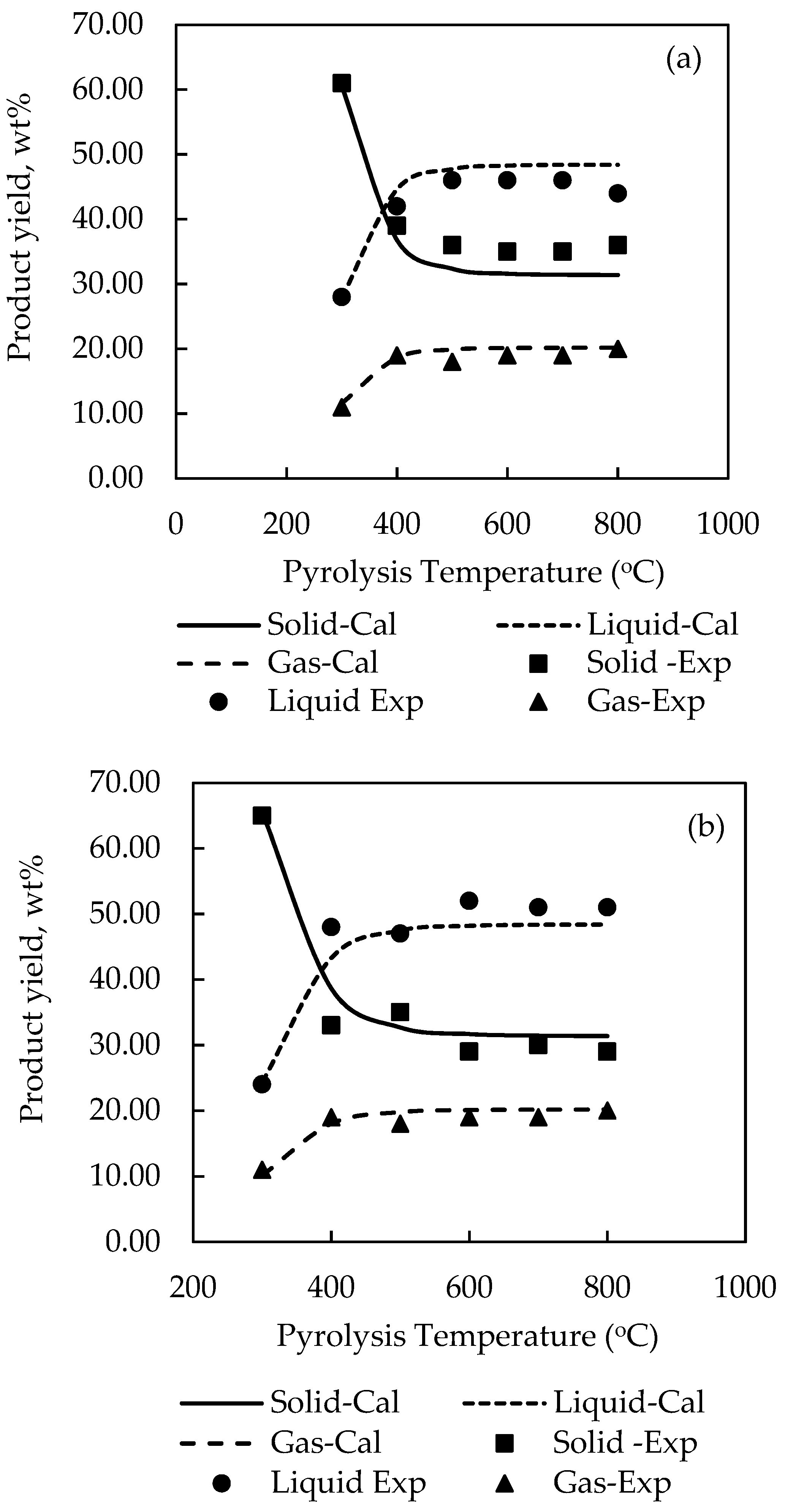
For the sake of clarity, the effect of pyrolysis temperature on the product yields is shown plotted in
Figure 2. It is seen that there is a general tendency for the yield of solid char to abruptly decreased over a narrow range of temperature from 300 to 500 °C, caused by the release of low-molecular weight volatiles, and gradually decreased at higher temperatures before attaining a constant average value of about 32 wt % solid at 800 °C for all biomasses. On the contrary, the yields of liquid and gas products tended to increase steadily from 300 to 400 °C and became relatively constant at higher temperatures. It is noted that the maximum liquid yields of all biomasses varied in the range from around 42.0 to 54.0 wt % with palm kernel cake giving the highest liquid yield. The optimum temperature giving the maximum liquid yield depended on the type of biomass, being 700 °C for cassava pulp residue and palm kernel cake, and 500, 600 and 800 °C for palm shell, coconut shell and longan fruit seed, respectively. Previous works have shown that this optimum temperature was in the range of 500–800 °C for the slow pyrolysis of different biomasses [
32,
33,
34]. It is further observed that the liquid yield was higher than the gas yield with the difference being higher for palm shell and coconut shell. Visual observation shows that the simulation program was able to predict the experimental product yields reasonably well over the temperature range studied, e.g., for biomass, etc.
Closer examination of reveals that the maximum prediction errors by the simulation are 19.5% for the solid char of PK biomass, 13.9% for the liquid products of LS biomass and 22.5% for the gas products of CP biomass. The overall average error for predicting the yields of solid, liquid, and gas products was 6.02%, 5.45%, and 7.03%, respectively. Overall, the relatively low values of sum-square-error (SSE) in the range of 0.0887–0.1258 and with about 78% of the total data having an error less than 10% substantiate that the simulation model proposed in this study was capable of describing the thermal decomposition characteristics of the tested biomass in a packed-bed reactor within acceptable accuracy.
The pre-exponential factor (A), shown in
Table 3, was found to be insensitive to the type of biomass, and the pyrolysis temperature and the best constant value of 7 × 10
6 s
−1 was, thus, employed. The activation energy € derived from the simulation had the values of 8.0 × 10
4, 7.9 × 10
4, 7.4 × 10
4, 7.6 × 10
4 and 7.8 × 10
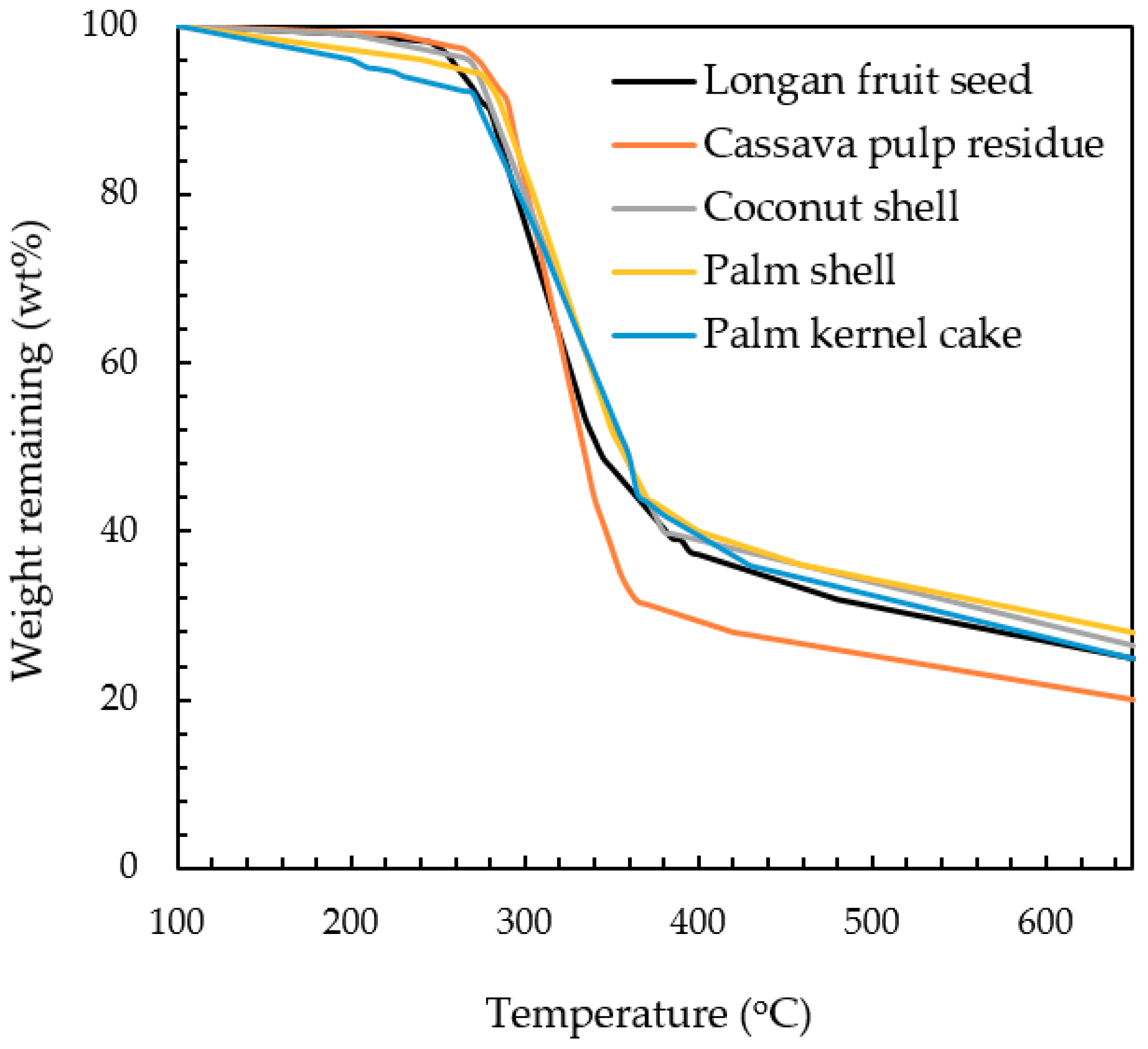
4 J/mol for palm shell, coconut shell, palm kernel, cassava pulp residue and longan fruit seed, respectively. The value of activation energy indicates the minimum energy barrier required for the initiation of a reaction, or it is a measure of chemical reactivity of a substance. Based on this argument, palm kernel cake is considered to be most reactive (lowest activation energy), while the palm shell is the least reactive. Another evidence concerning the biomass reactivity can be observed from TGA curves as displayed in
Figure 3. The minimum temperature at which the biomass starts to decompose can be defined here as the temperature corresponding to the weight remaining of 90% or designated as T
d. The lower this threshold temperature is, the easier for the reaction to occur. The determination of T
d gave the following order: T
d,PK (270 °C) < T
d,PS (280 °C) < T
d,LS (285 °C) < T
d,CS (287 °C) < T
d,CP (290 °C). Although this temperature order does not exactly match that of the activation energy (PK < CP < LS < CS < PS), it can be qualitatively inferred that palm kernel is most reactive with reference to the decomposition reaction.
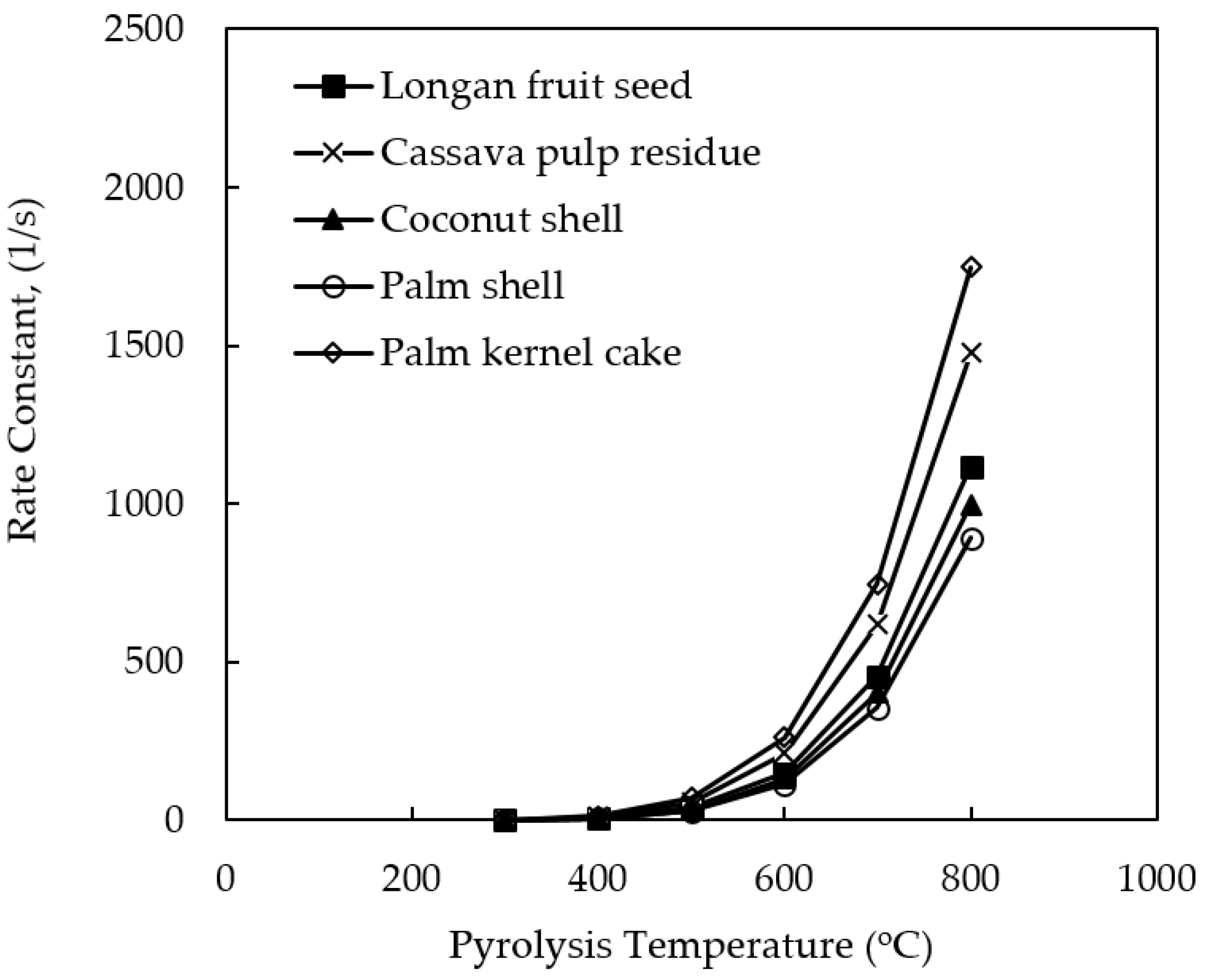
Figure 4 shows the effect of pyrolysis temperature on the rate constant (k) for the decomposition reaction of all biomasses studied. The rate constant (k) increased in an exponential fashion with the increase in the pyrolysis temperature as expected. This is because the decomposition reaction is an endothermic process. It is further noticed that k
PK > k
CP > k
LS > k
CS > k
PS which is in reverse order as compared to that of the activation energy of the pyrolysis reaction. That is, the chemical reaction of a low-activation energy process can proceed at a faster rate. The values of the rate constant (k) can be compared with those of previous investigators at 500 °C, although the actual pyrolysis conditions were not the same. For example, k for CP = 1.12 s
−1 [
25], k for PS = 3.59 s
−1 [
35], and k for PK = 1303 s
−1 [
35], calculated based on their reported values of the pre-exponential factor (A) and the activation ener€(E). It is noted that these numbers fall within the range of k values for each biomass, as reported in
Table 3.
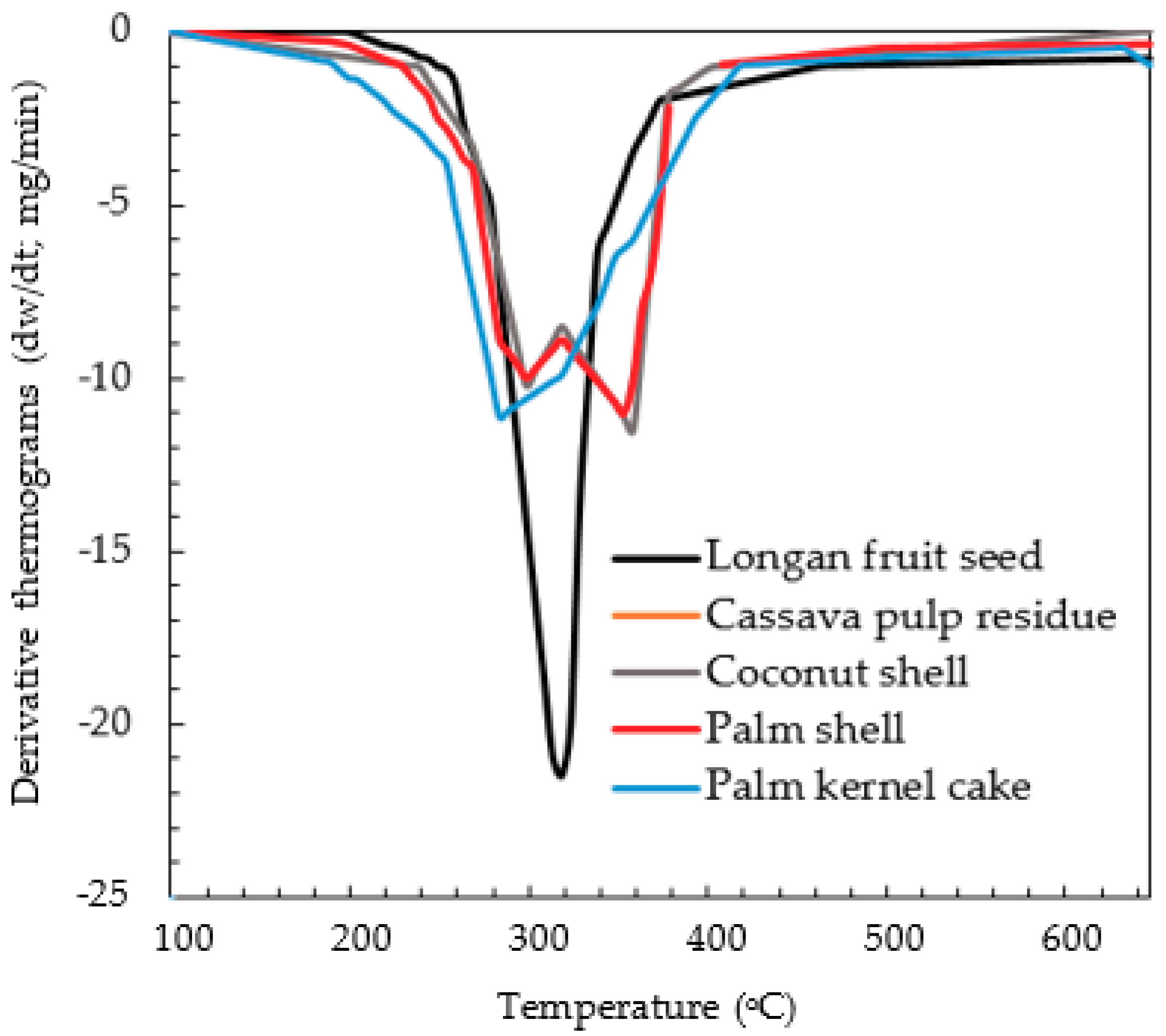
It is generally known that the pyrolysis behavior of biomass is highly complex, resulting from different decomposition rates and behavior of its cellulosic components, as well as a large number of secondary reactions among the released products [
36,
37]. The difference in the decomposition behavior of different biomasses is manifested in the derivative thermogravimetry (DTG) curves, as shown in
Figure 5. Palm shell and coconut shell, which are hard and dense, show a distinct two-peak curve, while cassava pulp residue, palm kernel cake and longan seed, which are much softer, exhibit a single-peak characteristic curve. Based on this distinct decomposition behavior, it was, thus, decided to run the simulation program by using two separate chemical formulae (biomass structure) for these two groups of biomasses. They are C
4.33H
6.26O
2.56 for palm shell and coconut shell (model G01) and C
3.63H
6.18O
3.04 for palm kernel cake, cassava pulp residue and longan fruit seed (model G02). The atomic ratio numbers for carbon, hydrogen and oxygen representing the chemical formulae for G01 and G02 were estimated by averaging the atomic ratio numbers of the respective biomasses in each biomass group. For example, the number 4.33 for the carbon of model G01 was calculated from (4.11 + 4.55)/2 = 4.33, where the numbers 4.11 and 4.55 are the atomic mole numbers of palm shell (PS) and coconut shell (CS) from
Table 1, respectively. The reason for trying this simulation approach is an attempt to generalize the simulation of biomass pyrolysis based on the differences in the thermal decomposition behavior of biomass, as reflected in the DTG curves.
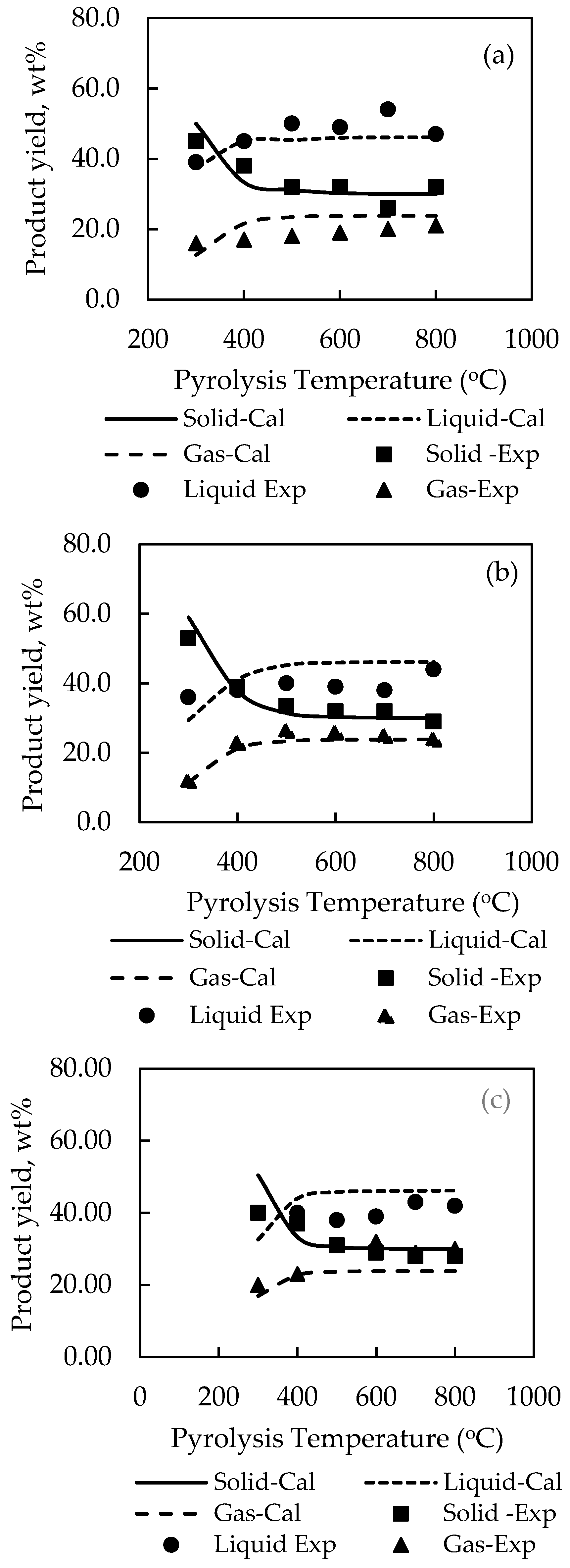
Table 4 shows the optimum set of stoichiometry derived for model G01 and model G02 and
Figure 6;
Figure 7 compares the experimental and computed product yields. It is visually observed that the simulation program is able to predict the correct effect of temperature on the product yields. The general agreement between the simulated and experimental results is satisfactory, although relatively large errors exist for the prediction of liquid yields for palm kernel cake and cassava pulp residue. The SSE were found for model G01 to be 0.1120 for palm shell (cf. 0.1065 for P01) and 0.0983 for coconut shell (cf. 0.0877 for P02), and for model G02 to be 0.4215 for palm kernel cake (cf. 0.1253 for P03), 0.4427 for cassava pulp residue (cf. 0.1258 for P04) and 0.1093 for longan fruit seed (cf. 0.1117 for P05). The pre-exponential factor (A) was kept constant as 7 × 10
6 (s
−1) as in the previous cases. The optimum values of activation energy were 7.8 × 10
4, 8.0 × 10
4, 7.9 × 10
4, 7.45 × 10
4 and 8.0 × 10
4 J/mol for palm shell, coconut shell, palm kernel cake, cassava pulp residue and longan fruit seed, respectively.
Next, the simulation scheme proposed in this work for the modelling of biomass pyrolysis using the COCO simulation program was extended to the analysis of previous works on the slow pyrolysis of various biomasses. The systems selected were based on the same mode of slow pyrolysis and same types of biomass used in the present study.
Table 5 summarizes the results using models P01–P04, with the respective stoichiometric numbers and kinetic parameters being obtained from
Table 2 and
Table 3, respectively.
Table 6 presents the analysis of error of simulated results in
Table 5. Overall, the simulation does not give good predictive results for all pyrolysis products, although
Table 5 shows the correct trend for the effect of temperature on the product yields. The sum of squared errors (SSE) of estimation for P01–P04 is much larger than those reported in
Table 3. The percentage error for predicting the yield of each pyrolysis product (solid, liquid and gas) varies rather widely, and the maximum error is relatively large, particularly for the gas products. However, the prediction of liquid and solid yields by the simulation program seems to be reasonable, considering the overall average error of around 10.4% and with about 89% of the simulated results being within ±20% error range. In view of the biomass precursors (P01–P04) and from the values of average percentage error, the approximate order of decreasing predictive accuracy for the yields of solid and liquid products (excluding gas yields) is as follows: P01 (PS) > P02 (CS) > P03 (PK) > P04 (CP). The relatively large discrepancies between the experimental and simulated product yields could be due to the differences in the pyrolysis conditions, for example, heating rate, particle size, chemical properties of the biomass, etc.
To improve the simulation accuracy, it is proposed that there should be an optimum set of kinetic parameters (A and E) and stoichiometry numbers (a to q) pertaining to a specific pyrolysis condition. To test this hypothesis, the pyrolysis data of Abdul Rahman (2016) [
40] in
Table 5 were directly used in the simulation to search for the optimum set of model parameters and the results on product yields calculated. The calculated optimum stoichiometric numbers are presented in
Table 7, while the computed rate constants and the product yields are listed in
Table 8. As compared with the results in
Table 5, it is seen that the simulation capability improves considerably, notably for the gas product yields. The average percentage error of model prediction on product yields over the temperature range of 300–500 °C decreases for the solid, liquid and gas products from 8.1 to 5.3%, 3.8 to 3.1% and 45.4 to 20.6% for the solid, liquid and gas products, respectively. It is, therefore, concluded that the prediction of product yields from the slow pyrolysis of biomass using the COCO simulator is most accurate for a particular set of pyrolysis condition. However, the simulation program can still be applied for the prediction of pyrolysis behavior of the biomass under pyrolysis conditions differing from those used in the simulation, but with a certain loss of prediction accuracy.
Next, the results on liquid and gas product compositions generated from the simulation program are presented.
Table 9 shows the typical comparison of experimental and simulated compositions of gas and liquid products from the pyrolysis of cassava pulp residue and palm kernel cake at 500 °C. The simulation program could predict the compositions of the four gas products fairly well with an overall average error of about 55%, as compared to the experimental results. Furthermore, the simulation could predict the correct proportions of the gas contents as in the following order, CO
2 > CO > CH
4 > H
2. It is further observed that the prediction accuracy by the simulation for the liquid product compositions is not as good as that for the gas compositions, but the error of prediction is quite random. To improve the model capability for better prediction of both the product yields and compositions, the program algorithm needs to be modified that involves the minimization of the objective function of SSE for both the product yields and compositions. Due to a large number of chemicals presenting in the liquid bio-oil products, it may be necessary to limit the liquid compositions to some major compounds, possibly the group of oxygenated compounds.
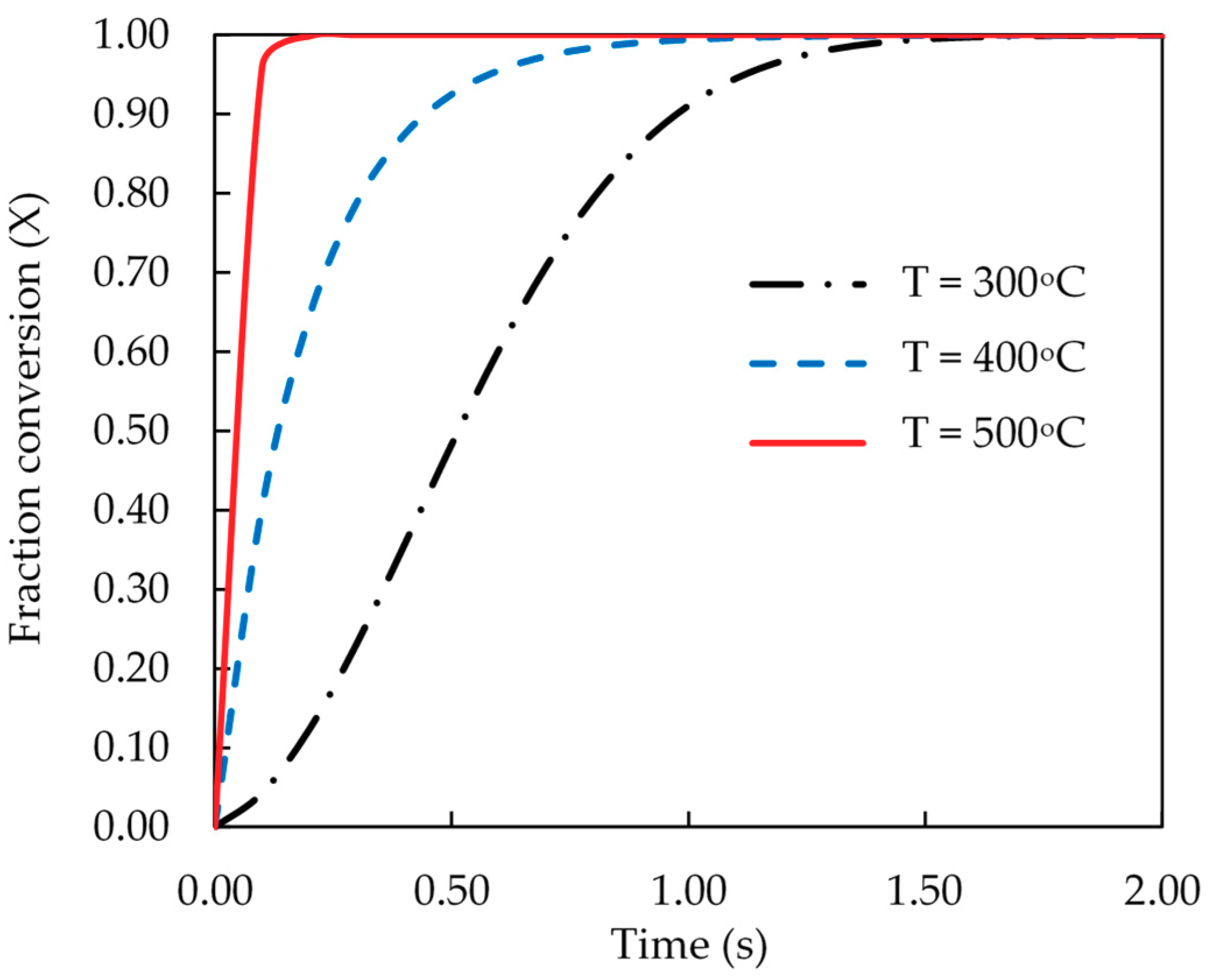
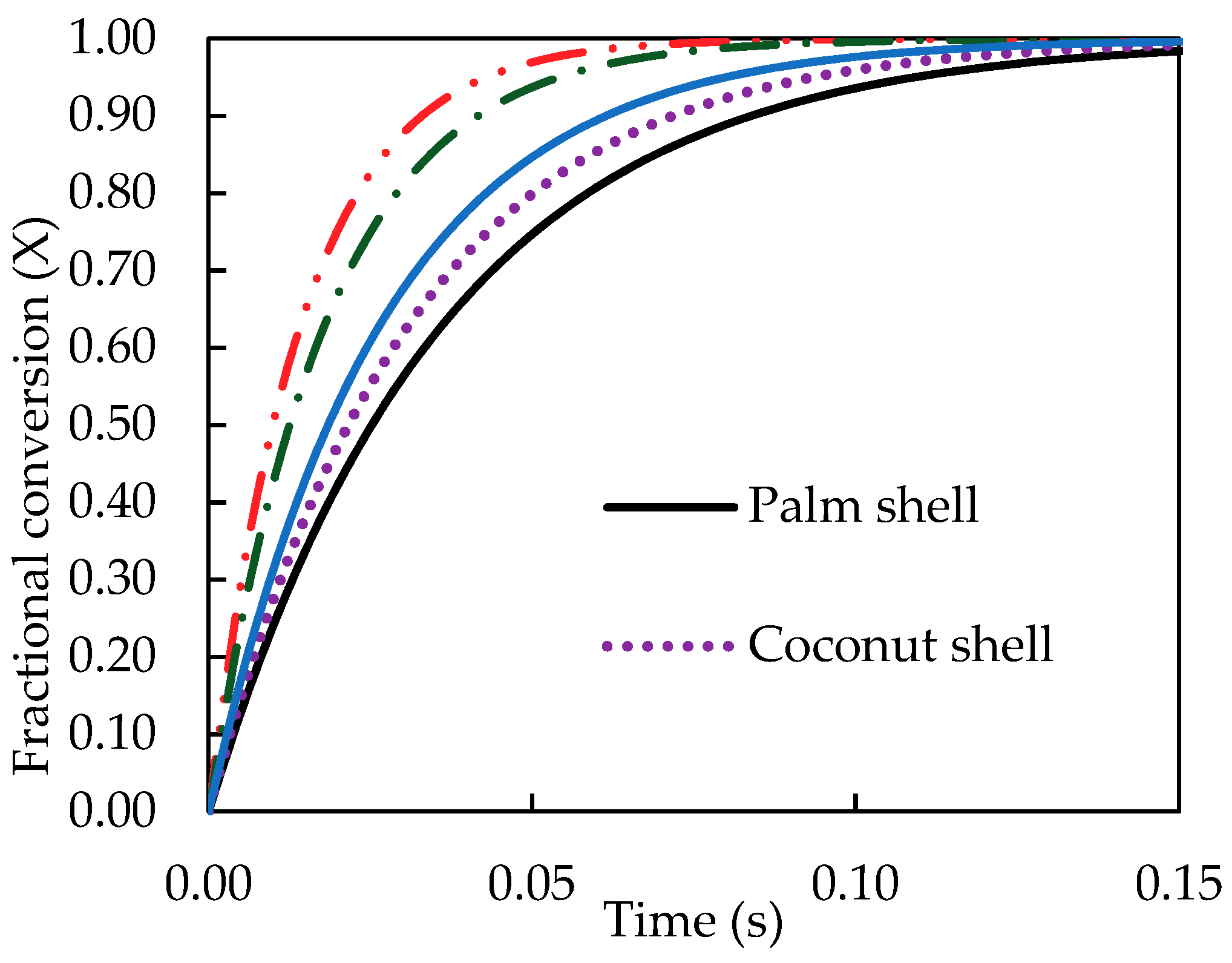
Finally, the kinetic data of pyrolysis reaction was computed and presented as the fractional conversion of biomass versus pyrolysis time.
Figure 8 and
Figure 9 show the results for the effects of pyrolysis temperature (for coconut shell) and biomass type (at 500 °C), respectively. It is seen that the pyrolysis kinetics of coconut shell is strongly dependent on the temperature. The initial conversion rates (dX/dt) were estimated to be 1.07, 3.33 and 10.10 s
−1 for the pyrolysis temperatures of 300, 400 and 500 °C, respectively. A tenfold increase in the conversion rate is noticed for the increasing of pyrolysis temperature from 300 to 500 °C. As to the biomass type, palm kernel cake shows the highest conversion rate and palm shell gives the lowest one, in accord with the results of the rate constant (k) previously reported.
In summary, the simulation method proposed in the present study was able to provide complete information on the yields and compositions of pyrolysis products, as well as the pyrolysis kinetic data for different biomasses over the range of pyrolysis temperature from 300 to 800 °C. It should be noted that a similar but more sophisticated approach was proposed [
23] for modelling the fast pyrolysis of beech wood biomass over the temperature range from 470 to 570 °C by incorporating the kinetic reaction model for the decomposition of the three lignocellulosic components into the Aspen Plus program.