Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pine Cone Biosorbent
2.3. Batch Biosorption Studies
2.4. Normalized Standard Deviation
2.5. Chi-Square Test (χ2)
3. Results
3.1. Pine Cone Biomass Characterization
3.1.1. Characteristics of Native PCP and after Biosorption
3.1.2. SEM
3.1.3. XRD Analysis
3.2. Investigation of Solution pH
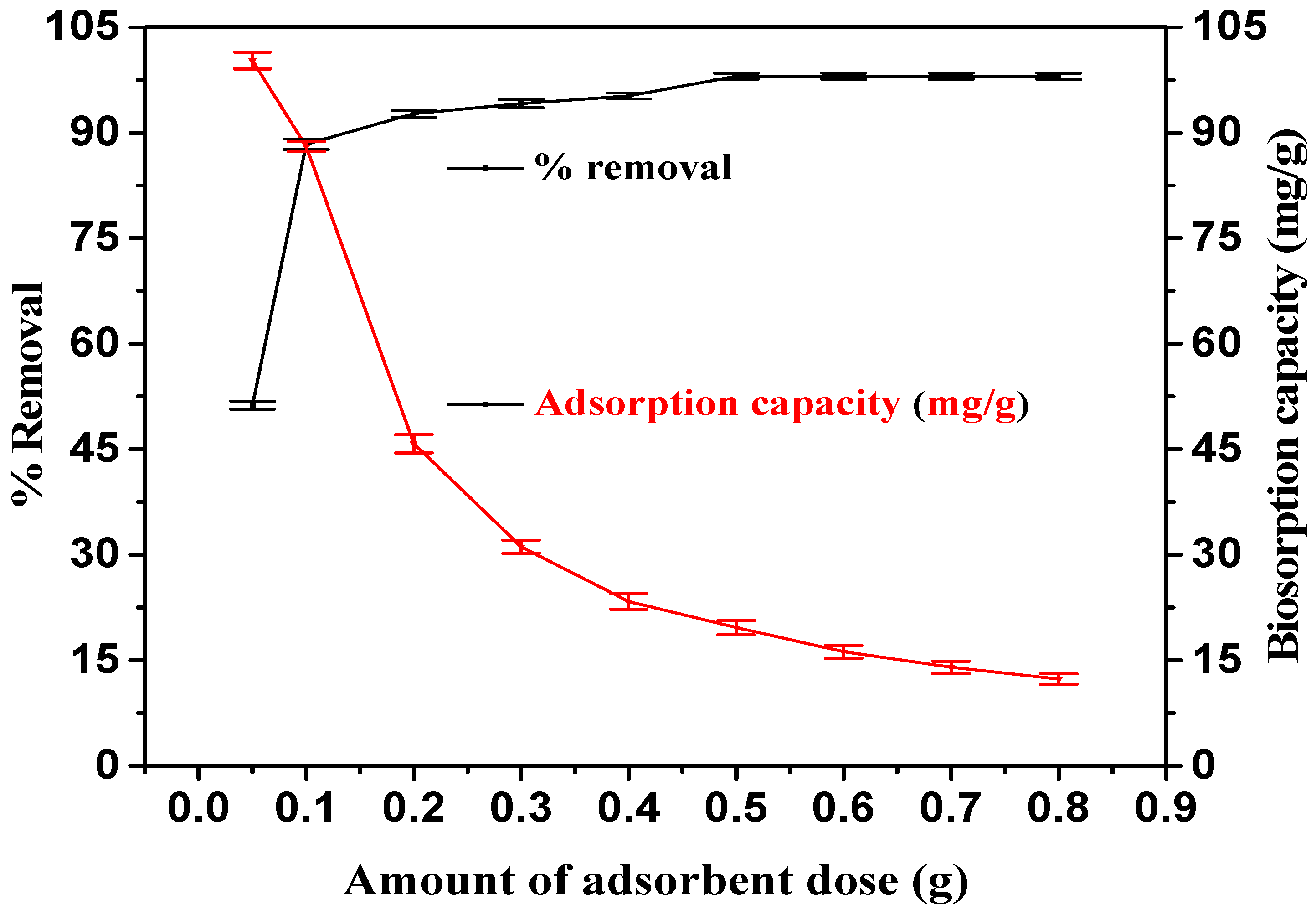
3.3. Influence of Sorbent Dosage
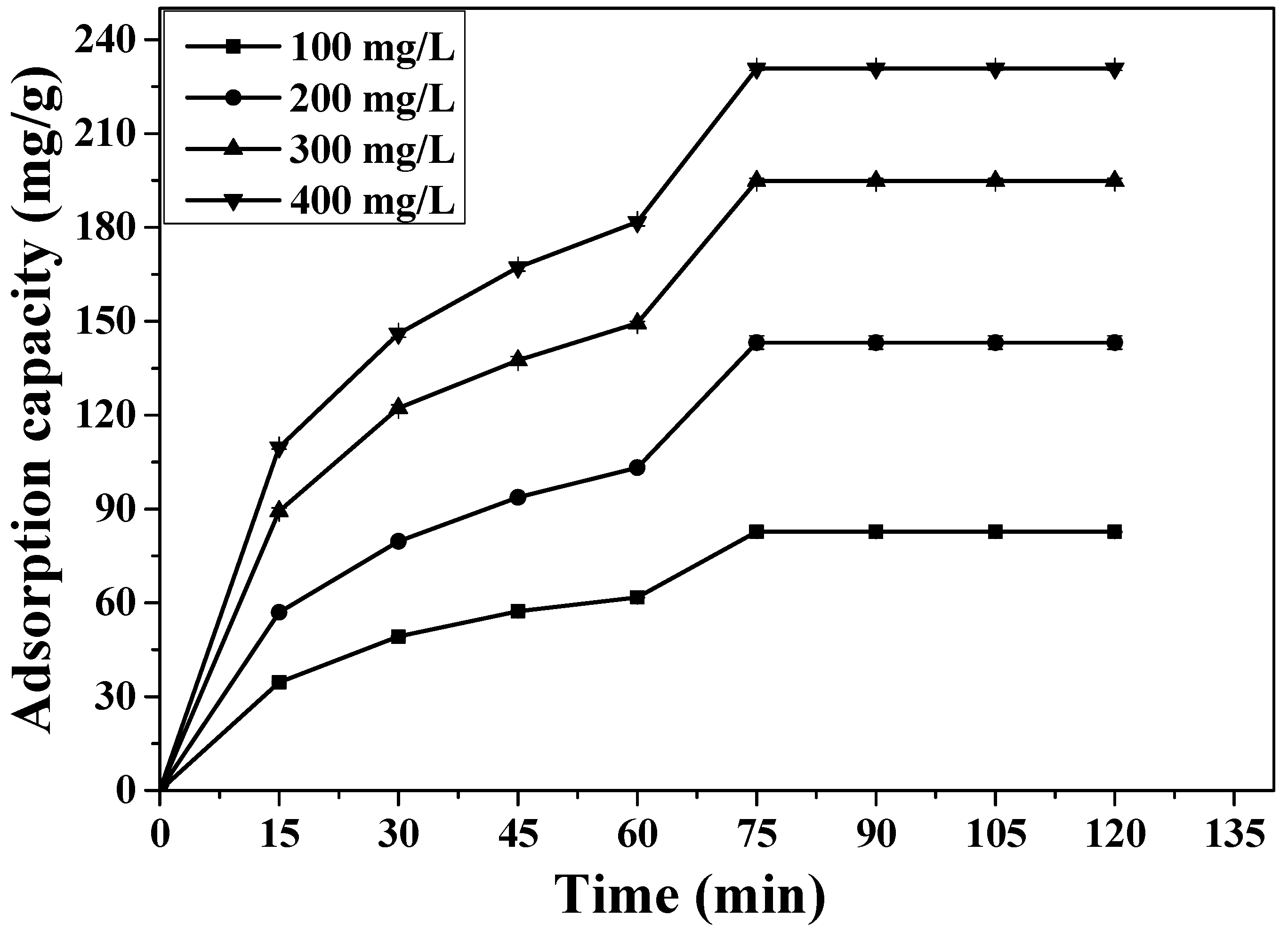
3.4. Effect of 2,4,6-TCP Initial Concentration and Contact Time
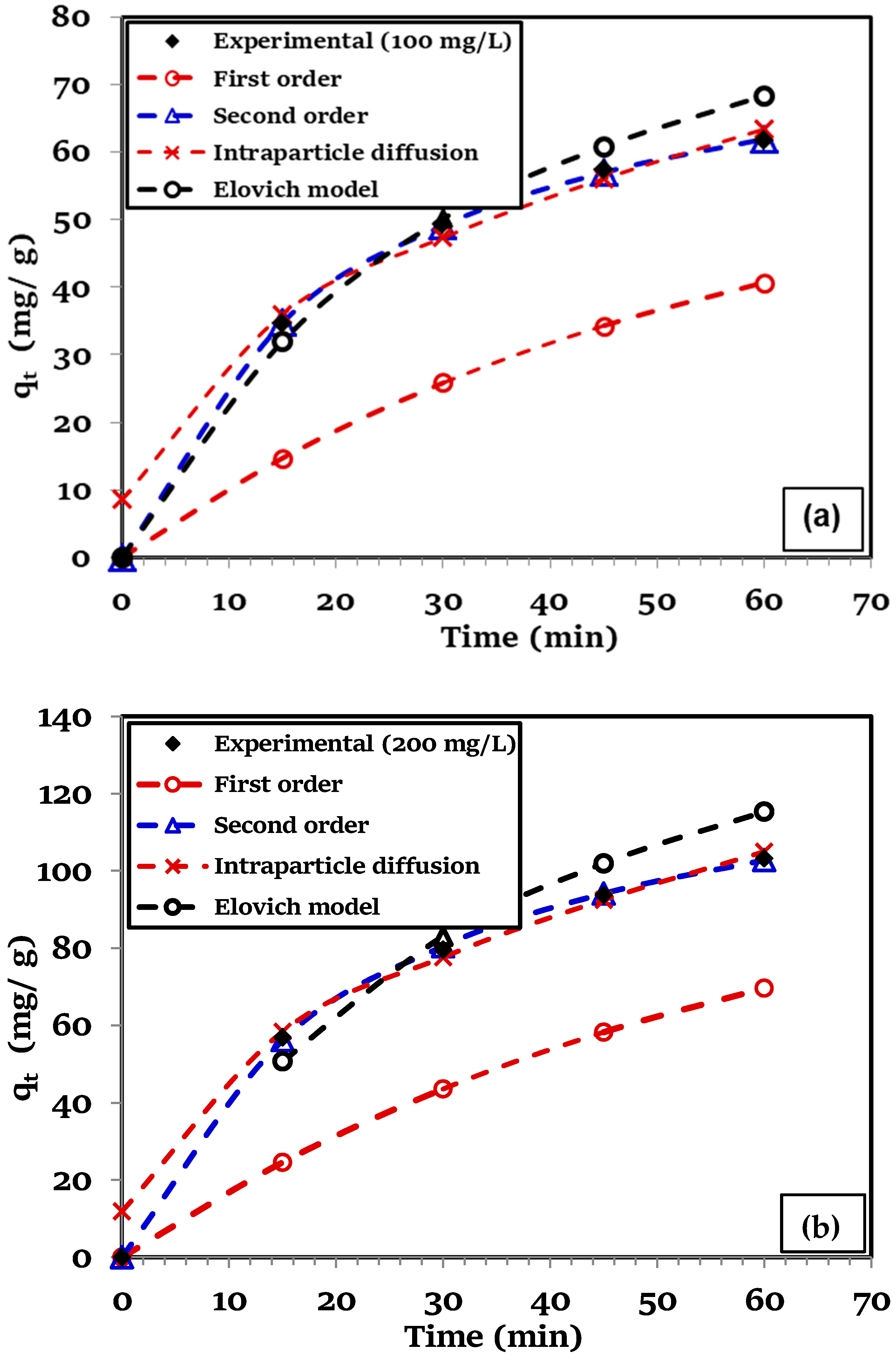
3.5. Biosorption Kinetics
3.6. Intraparticle Diffusion Model
3.7. Biosorption Isotherm Models
3.8. 2,4,6-TCP onto Pine Cone Powder (PCP) Biosorption Mechanism
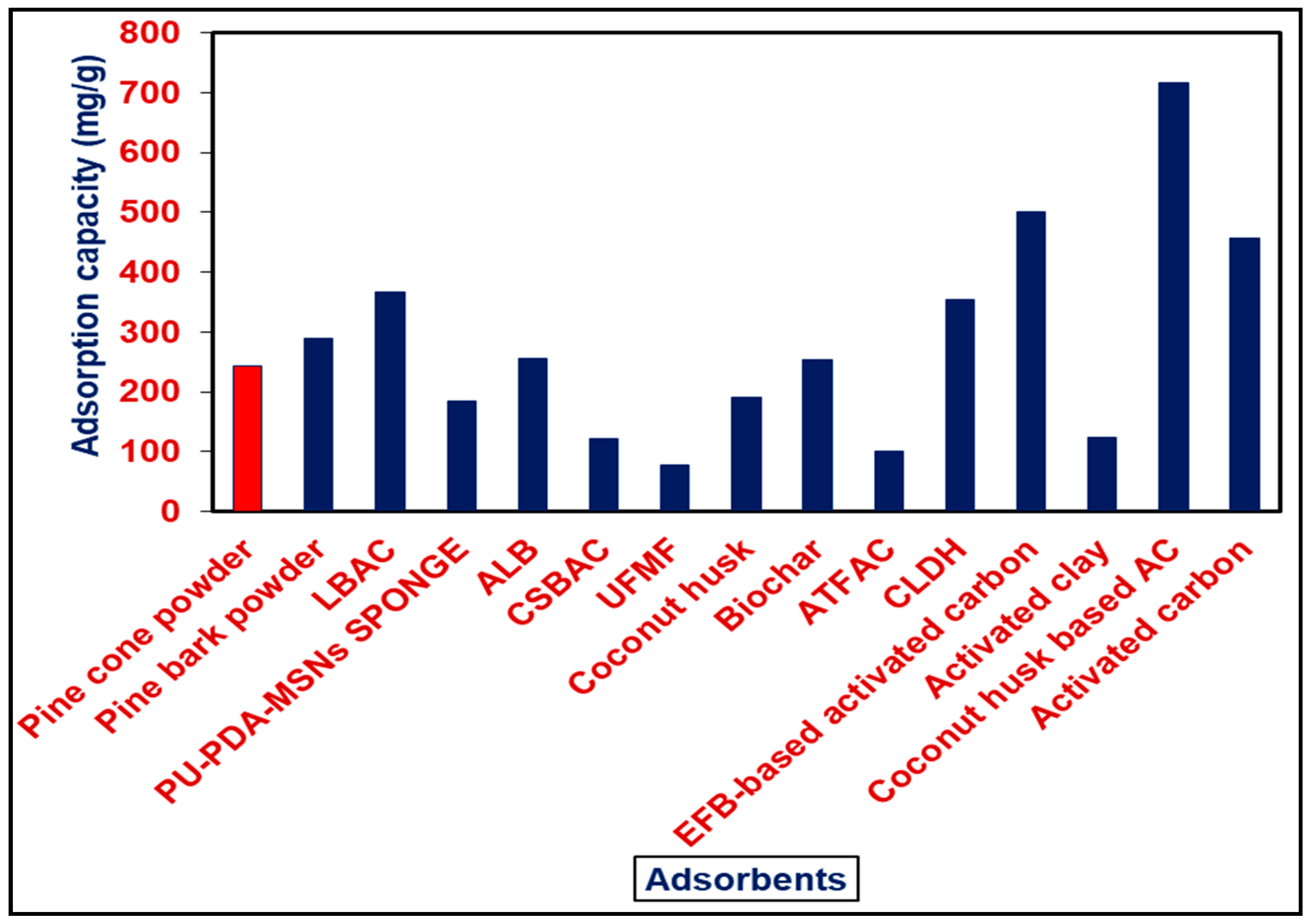
3.9. Comparison of PBP with other Adsorbents
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Achard, C.; Jaoui, M.; Schwing, M.; Rogalski, M. Aqueous solubilities of phenol derivatives by conductivity measurements. J. Chem. Eng. Data 1996, 41, 504–507. [Google Scholar] [CrossRef]
- Kumar, N.S.; Woo, H.S.; Min, K. Equilibrium and kinetic studies on biosorption of 2,4,6-trichlorophenol from aqueous solutions by Acacia leucocephala bark. Colloids Surf. B Biointerfaces 2012, 94, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, R.; Fu, G.; Xu, B.; Zhu, P.; Qiao, S.; Chen, X.; Xu, B.; Qin, Y.; Lu, C. Association of exposure to phenols and idiopathic male infertility. J. Hazard. Mater. 2013, 250, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Wang, D.; Xu, X. Phenol removal efficiencies of sewage treatment processes and ecological risks associated with phenols in effluents. J. Hazard. Mater. 2012, 217, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zgola-Grzeskowiak, A.; Grzeskowiak, T.; Rydlichowski, R.; Lukaszewski, Z. Determination of nonylphenol and short-chained nonylphenol ethoxylates in drain water from an agricultural area. Chemosphere 2009, 75, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Michalowicz, J.; Duda, W. Phenols-sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Marianna, C. Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ. 2004, 322, 21–39. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for 2,4,6-Trichlorophenol; U.S. Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 1990.
- Kurian, M.; Babu, R. Iron aluminium mixed pillared montmorillonite and the rare earth exchanged analogues as efficient catalysts for phenol oxidation. J. Environ. Chem. Eng. 2013, 1, 86–91. [Google Scholar] [CrossRef]
- McAllister, K.A.; Lee, H.; Trevors, J.T. Microbial degradation of pentachlorophenol. Biodegradation 1996, 7, 1–40. [Google Scholar] [CrossRef]
- Oh, C.G.; Ahn, J.H.; Ihm, S.K. Adsorptive removal of phenolic compounds by using hypercrosslinked polystyrenic beads with bimodal pore size distribution. Reac. Funct. Polym. 2003, 57, 103–111. [Google Scholar] [CrossRef]
- Kumar, N.S.; Subbaiah, M.V.; Reddy, A.S.; Krishnaiah, A. Biosorption of phenolic compounds from aqueous solutions onto chitosan–abrus precatorius blended beads. J. Chem. Tech. Biotechnol. 2009, 84, 972–981. [Google Scholar] [CrossRef]
- Rama Rao, K.; Sahu, J.N.; Jayakumar, N.S. Optimal isotherm parameters for phenol adsorption from aqueous solutions onto coconut shell based activated carbon: Error analysis of linear and non-linear methods. J. Taiwan Inst. Chem. Eng. 2017, 80, 472–487. [Google Scholar]
- Siva Kumar, N.; Boddu, V.M.; Krishnaiah, A. Biosorption of phenolic compounds by Trametes versicolor polyporus fungus. Adsorpt. Sci. Technol. 2009, 27, 31–46. [Google Scholar] [CrossRef]
- Garbaa, Z.N.; Rahima, A.A. Evaluation of optimal activated carbon from an agricultural waste for the removal of para-chlorophenol and 2,4-dichlorophenol. Process Saf. Environ. Prot. 2016, 102, 54–63. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Jyothi, R.K.; Chang, Y.Y.; Yang, J.K. Factors affect on bioremediation of Co (II) and Pb (II) onto Lonicera japonica flowers powder. Desalin. Water Treat. 2016, 57, 13066–13080. [Google Scholar] [CrossRef]
- Suguna, M.; Siva Kumar, N. Equilibrium, kinetic and thermodynamic studies on biosorption of lead (II) and cadmium (II) from aqueous solution by polypores biomass. Indian J. Chem. Technol. 2013, 20, 57–69. [Google Scholar]
- Nadavala, S.K.; Che Man, H.; Woo, H.S. Biosorption of phenolic compounds from aqueous solutions using pine (Pinus densiflora Sieb) bark powder. BioResources 2014, 9, 5155–5174. [Google Scholar] [CrossRef]
- Yildiz, D.; Keskin, F.; Demirak, A. Biosorption of 2,4 Dichlorophenol onto Turkish Sweetgum bark in a batch system: Equilibrium and kinetic study. Acta Chim. Slov. 2017, 64, 237–247. [Google Scholar] [CrossRef]
- Siva, K.N.; Asif, M.; Al-Hazzaa, M.I.; Ibrahim, A.A. Biosorption of 2,4,6-trichlorophenol from aqueous medium using agro-waste: Pine (Pinus densiflora Sieb) bark powder. Acta Chim. Slov. 2018, 65, 221–230. [Google Scholar] [CrossRef]
- Kusmierek, K.; Swiatkowski, A.; Dabek, L. Removal of 2,4,6-trichlorophenol from aqueous solutions using agricultural waste as low-cost adsorbents. Environ. Prot. Eng. 2017, 43, 149–163. [Google Scholar]
- Ucun, H.; Bayhan, Y.K.; Kaya, Y.; Cakici, A.; Algur, O.F. Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalination 2003, 154, 233–238. [Google Scholar] [CrossRef]
- Niyaz, M.M.; Hayati, B.; Arami, M.; Lan, C. Adsorption of textile dyes on Pine Cone from colored wastewater: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 268, 117–125. [Google Scholar]
- Ofomaja, A.E.; Naidoo, E.B. Biosorption of lead (II) onto pine cone powder: Studies on biosorption performance and process design to minimize biosorbent mass. Carbohydr. Polym. 2010, 82, 1031–1042. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Removal of copper (II) from aqueous solution by pine and base modified pine cone powder as biosorbent. J. Hazard. Mater. 2009, 168, 909–917. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Biosorption of copper (II) and lead (II) onto potassium hydroxide treated pine cone powder. J. Environ. Manag. 2010, 91, 1674–1685. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Pholosi, A.; Naidoo, E.B. Application of raw and modified pine biomass material for cesium removal from aqueous solution. Ecol. Eng. 2015, 82, 258–266. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Pholosi, A.; Naidoo, E.B. Kinetics and competitive modeling of cesium biosortion onto chemically modified pine cone powder. J. Taiwan Inst. Chem. Eng. 2013, 44, 943–951. [Google Scholar] [CrossRef]
- Kumar, N.S.; Asif, M.; Al-Hazzaa, M.I. Adsorptive removal of phenolic compounds from aqueous solutions using pine cone biomass: Kinetics and equilibrium studies. Environ. Sci. Pollut. Res. 2018, 25, 21949–21960. [Google Scholar] [CrossRef]
- Nassar, N.N.; Arar, L.A.; Marei, N.N.; Abu Ghanim, M.M.; Dwekat, M.S.; Sawalha, S.H. Treatment of olive mill based wastewater by means of magnetic nanoparticles: Decolourization, dephenolization and COD removal. Environ Nanotechnol. Monit. Manag. 2014, 1–2, 14–23. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Guduri, B.R.; Varada, R.A. Characterization of new natural cellulosic fabric Grewia tilifolia. Carbohydr. Polym. 2010, 79, 847–855. [Google Scholar] [CrossRef]
- Mas Haris, M.R.H.; Sathasivam, K. The removal of methyl red from aqueous solutions using banana pseudo stem fibers. Am. J. Appl. Sci. 2009, 6, 1690–1700. [Google Scholar]
- Malkoc, E. Ni (II) removal from aqueous solutions using cone biomass of Thujaorientalis. J. Hazard. Mater. 2006, 137, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Barka, N.; Abdennouri, M.; El Makhfouk, M.; Qourzal, S. Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficusindica) Cladodes. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Moon, S.H.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Agricultural solid waste for the removal of organics: Adsorption of phenol from water and wastewater by palm seed coat activated carbon. Waste Manag. 2002, 22, 543–548. [Google Scholar] [CrossRef]
- Hameed, B.H. Equilibrium and kinetics studies of 2,4,6-trichlorophenol adsorption onto activated clay. Coll. Surf. A Physicochem. Eng. Asp. 2007, 307, 45–52. [Google Scholar] [CrossRef]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Radhika, M.; Palanivelu, K. Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent—Kinetics and isotherm analysis. J. Hazard. Mater. 2006, 138, 116–124. [Google Scholar] [CrossRef]
- Chakravarty, P.; Sarma, N.S.; Sarma, H.P. Removal of lead (II) from aqueous solution using heartwood of Areca catechu powder. Desalination 2010, 256, 16–21. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, G.; Yu, J.; Yan, T.; Zhu, M.; Jiao, F. Thermodynamic and kinetic studies of effective adsorption of 2,4,6-trichlorophenol onto calcine Mg/Al-CO3 layered double hydroxide. J. Wuhan Univ. Technol. Mater Sci. Ed. 2016, 31, 1211–1218. [Google Scholar] [CrossRef]
- Liu, S.; Pan, J.; Cao, J.; Dai, X.; Meng, M.; Wu, R.; Yao, J.; Yan, Y. Simultaneous removal of Pb (II) and 2,4,6-trichlorophenol by a hierarchical porous PU@PDA@MSNs sponge with reversible “shape memory” effect. Chem. Eng. J. 2016, 284, 10–20. [Google Scholar] [CrossRef]
- Denizli, A.; Cihangir, N.; Tuzmen, N.; Alsancak, G. Removal of chlorophenols from aquatic systems using the dried and dead fungus Pleurotus sajor caju. Bioresour. Technol. 2005, 96, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Preparation of activated carbon from coconut husk: Optimization study on removal of 2,4,6-trichlorophenol using response surface methodology. J. Hazard. Mater. 2008, 153, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.S.A.; Manaf, L.A.; Man, H.C.; Kumar, N.S. Equilibrium studies and dynamic behavior of cadmium adsorption by palm oil boiler mill fly ash (POFA) as a natural low-cost adsorbent. Desalin. Water Treat. 2015, 54, 1956–1968. [Google Scholar] [CrossRef]
- Lagergren, S.Y. Zur Theorie der sogenannten adsorption geloster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Fakhri, A.; Rashidi, S.; Asif, M.; Ibrahim, A. Microwave-assisted synthesis of SiC Nanoparticles for the efficient adsorptive removal of Nitroimidazole Antibiotics from aqueous solution. Appl. Sci. 2017, 7, 205. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Suguna, M.; Kumar, N.S.; Sreenivasulu, V.; Krishnaiah, A. Removal of Pb (II) from aqueous solutions by using Chitosan coated Zero Valent Iron Nanoparticles. Sep. Sci. Technol. 2014, 49, 1613–1622. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Olu-Owolabi, B.I.; Alabi, A.H.; Diagboya, P.N.; Unuabonah, E.I.; During, R.A. Adsorptive removal of 2,4,6-trichlorophenol in aqueous solution using calcined kaolinite-biomass composites. J. Environ. Manag. 2017, 192, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, A.; Rashidi, S.; Asif, M.; Ibrahim, A. Synthesis and characterization of MnS2/reduced graphene oxide nanohybrid: An efficient adsorbent for pharmaceutical compound removal. Desalin. Water Treat. 2017, 68, 236–244. [Google Scholar] [CrossRef]
- Siva Kumar, N.; Subba Reddy, A.; Boddu, V.M.; Krishnaiah, A. Development of chitosan-alginate based biosorbent for the removal of p-chlorophenol from aqueous medium. Toxicol. Environ. Chem. 2009, 91, 1035–1054. [Google Scholar] [CrossRef]
- Freundlich, H.; Heller, W. The Adsorption of cis- and trans-azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Kinetics of the synthesis of ammonia on promoted iron catalysts. J. Phys. Chem. USSR 1939, 13, 851–867. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sec. 1947, 55, 331–337. [Google Scholar]
- Fan, J.; Zhang, J.; Zhang, C.; Ren, L.; Shi, Q. Adsorption of 2,4,6-trichlorophenol from aqueous solution onto activated carbon derived from loosestrife. Desalination 2011, 267, 139–146. [Google Scholar] [CrossRef]
- Pan, J.; Luo, J.; Cao, J.; Liu, J.; Huang, W.; Zhang, W.; Yang, L. Competitive adsorption of three phenolic compounds to hydrophilic urea-formaldehyde macroporous foams derived from lignin-based Pickering HIPEs template. RSC Adv. 2016, 6, 93894–93904. [Google Scholar] [CrossRef]
- Mubarik, S.; Saeed, A.; Athar, M.M.; Iqbal, M. Characterization and mechanism of the adsorptive removal of 2,4,6-trichlorophenol by biochar prepared from sugarcane baggase. J. Ind. Eng. Chem. 2016, 33, 115–121. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Anisuzzaman, S.M.; Bono, A.; Sarbatly, R. Adsorption of 2,4,6-trichlorophenol (TCP) onto activated carbon. J. King Saud Univ. Sci. 2013, 25, 251–255. [Google Scholar] [CrossRef]





| Property | Value |
|---|---|
| Color | Brown |
| Carbon (%) | 76.58 |
| Hydrogen (%) | 4.84 |
| Nitrogen (%) | 6.52 |
| Odor | None |
| Weight loss (%) | 48.8 |
| Apparent (bulk) density (g/cm3) | 0.42 |
| Moisture content (%) | 3.14 |
| Pore diameter (A0) | 15.73 |
| Ash content (%) | 4.89 |
| BET surface area (m2/g) | 7.108 |
| Parameter | Initial Concentration of TCP (mg/L) | |||
|---|---|---|---|---|
| 100 | 200 | 300 | 400 | |
| Pseudo-first-order-kinetic model | ||||
| q(e, exp) (mg/g) | 82.69 ± 0.35 | 143.20 ± 3.71 | 194.84 ± 1.51 | 230.83 ± 0.16 |
| q(e, cal) (mg/g) | 60.80 ± 0.89 | 108.80 ± 5.66 | 133.25 ± 1.84 | 159.22 ± 0.54 |
| k1 (1/min) | 0.018 ± 0.0004 | 0.017 ± 0.0004 | 0.018 ± 0.0001 | 0.019 ± 0.001 |
| R2 | 0.9812 | 0.9927 | 0.9815 | 0.9945 |
| Δqt (%) | 52.68 | 50.89 | 59.64 | 57.44 |
| χ2 | 74.21 | 110.23 | 270.71 | 292.10 |
| Pseudo-second-order-kinetic model | ||||
| q(e, cal) (mg/g) | 83.80 ± 1.06 | 142.28 ± 4.58 | 191.09 ± 2.09 | 232.64 ± 5.41 |
| k2 (g/mg/min) | 0.00056 ± 2.85 × 10−5 | 0.00030 ± 3.83 × 10−5 | 0.00030 ± 4.91 × 10−6 | 0.00024 ± 9.22 × 10−6 |
| R2 | 0.9994 | 0.9992 | 0.9998 | 0.9994 |
| Δqt (%) | 0.52 | 1.02 | 0.54 | 1.14 |
| χ2 | 0.01 | 0.02 | 0.01 | 0.05 |
| Elovich equation | ||||
| q(e, cal) (mg/g) | 100.01 ± 3.45 | 170.88 ± 12.30 | 203.64 ± 1.32 | 244.16 ± 4.31 |
| (1/b) ln(ab) (mg/g) | 18.79 ± 1.59 | 33.84 ± 5.65 | 26.72 ± 0.99 | 31.09 ± 1.03 |
| 1/b (mg/g) | 19.83 ± 0.45 | 33.47 ± 1.62 | 43.21 ± 0.09 | 52.03 ± 0.80 |
| R2 | 0.9963 | 0.9993 | 0.9943 | 0.9999 |
| Δqt (%) | 8.38 | 10.80 | 7.55 | 6.72 |
| χ2 | 1.07 | 2.82 | 2.14 | 2.04 |
| Weber–Morris | ||||
| q(e, cal) (mg/g) | 63.43 ± 0.33 | 105.01 ± 1.09 | 152.38 ± 0.83 | 184.73 ± 2.26 |
| kid | 7.07 ± 0.15 | 12.01 ± 0.58 | 15.42 ± 0.06 | 18.65 ± 0.27 |
| C | 8.56 ± 0.92 | 11.87 ± 3.43 | 32.80 ± 0.63 | 40.13 ± 0.14 |
| R2 | 0.9758 | 0.9907 | 0.9749 | 0.9885 |
| Δqt (%) | 47.49 | 49.58 | 40.35 | 40.23 |
| χ2 | 86.34 | 183.30 | 111.66 | 136.66 |
| Isotherms | Constants | R2 | Δqe (%) | χ2 | |
|---|---|---|---|---|---|
| Langmuir | qm (mg/g) | b (L/mg) | |||
| 243.90 | 0.009 | 0.9989 | 3.84 | 0.90 | |
| Freundlich | KF ((mg/g) (L/mg)1/n) | n | |||
| 3.229 | 1.245 | 0.9931 | 5.62 | 1.98 | |
| D-R model | qs (mg/g) | E (kJ/mol) | |||
| 8.22 × 10−3 | 8.28 | 0.9965 | 3.95 | 1.09 | |
| Temkin | A (L/g) | bT | |||
| 0.16 | 65.78 | 0.9451 | 32.21 | 112.34 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siva Kumar, N.; Asif, M.; Poulose, A.M.; Suguna, M.; Al-Hazza, M.I. Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass. Processes 2019, 7, 757. https://doi.org/10.3390/pr7100757
Siva Kumar N, Asif M, Poulose AM, Suguna M, Al-Hazza MI. Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass. Processes. 2019; 7(10):757. https://doi.org/10.3390/pr7100757
Chicago/Turabian StyleSiva Kumar, Nadavala, Mohammad Asif, Anesh Manjaly Poulose, Madala Suguna, and Mansour I. Al-Hazza. 2019. "Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass" Processes 7, no. 10: 757. https://doi.org/10.3390/pr7100757
APA StyleSiva Kumar, N., Asif, M., Poulose, A. M., Suguna, M., & Al-Hazza, M. I. (2019). Equilibrium and Kinetic Studies of Biosorptive Removal of 2,4,6-Trichlorophenol from Aqueous Solutions Using Untreated Agro-Waste Pine Cone Biomass. Processes, 7(10), 757. https://doi.org/10.3390/pr7100757







