Arsenic Removal from Arsenopyrite-Bearing Iron Ore and Arsenic Recovery from Dust Ash by Roasting Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment on Arsenic Removal from Roasting Iron Ore
2.2. Experiment on Recovery of Arsenic by Roasting Dust Ash
2.3. Sample Analysis and Testing
3. Results
4. Discussion
4.1. Thermodynamic Calculation of Mixed Ore Subjected to Roasting
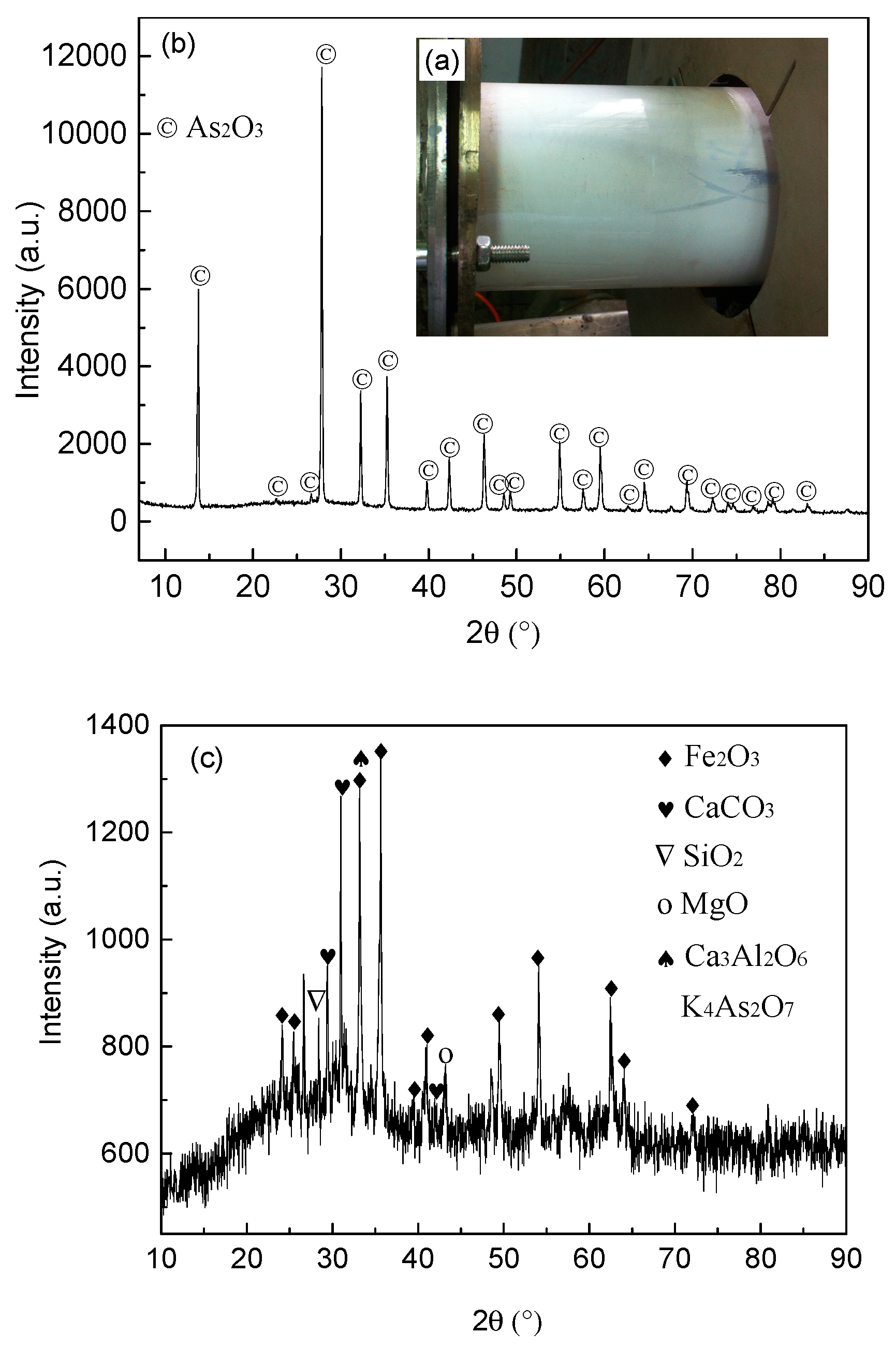
4.2. X-Ray Diffraction Analysis of the Roasted Ore and Dust in Different Atmospheres
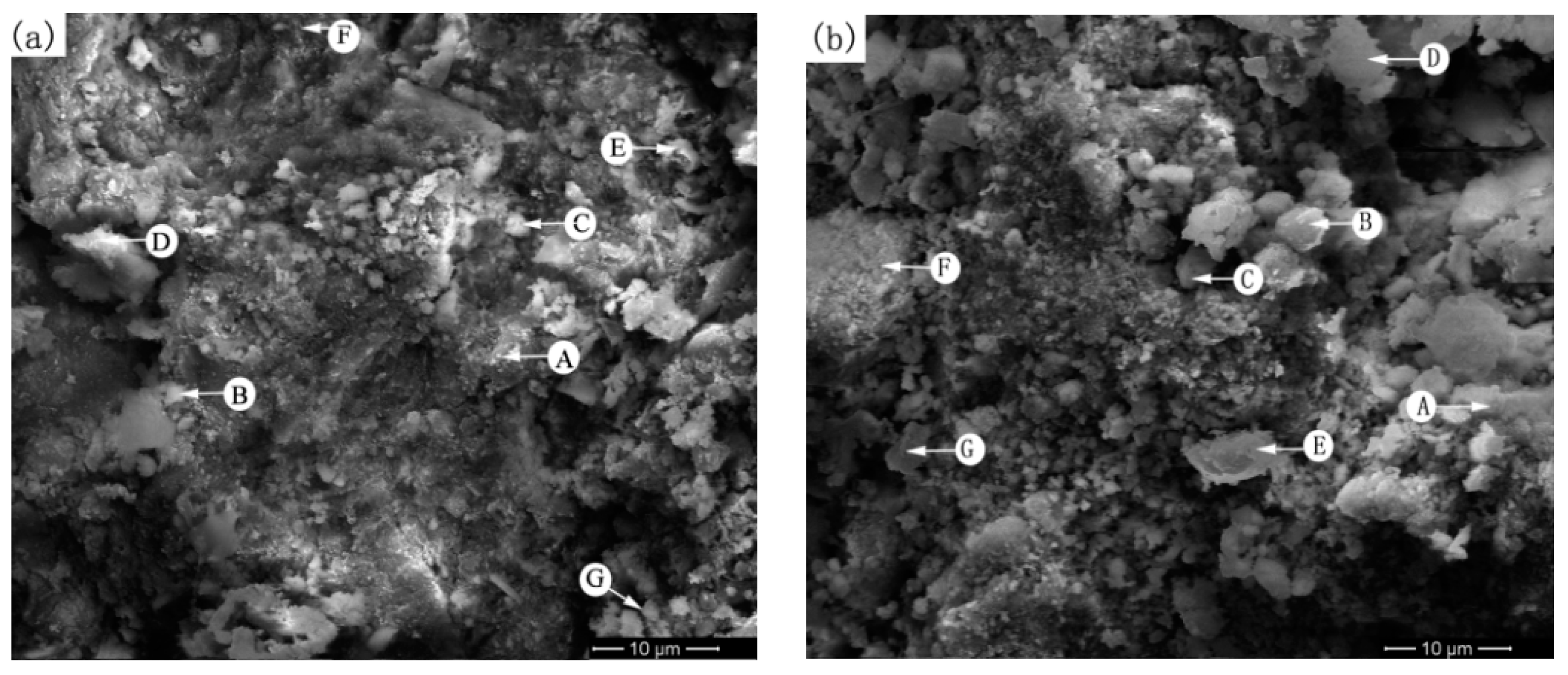
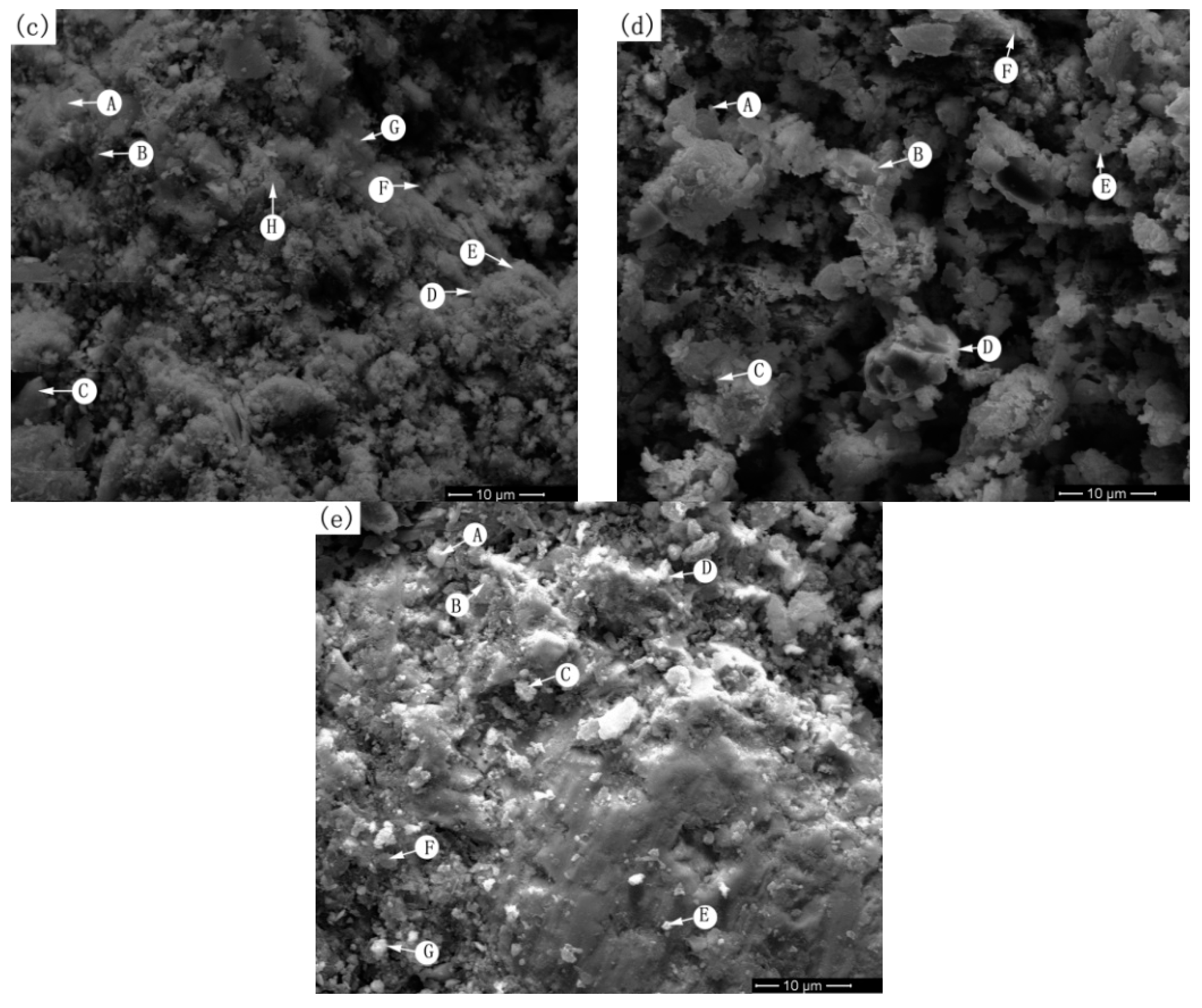
4.3. Mechanism Research on Arsenic Removal by Roasting Method and Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy Analysis
4.4. Route for the Recovery of Arsenic from Arsenic-Bearing Dust Ash
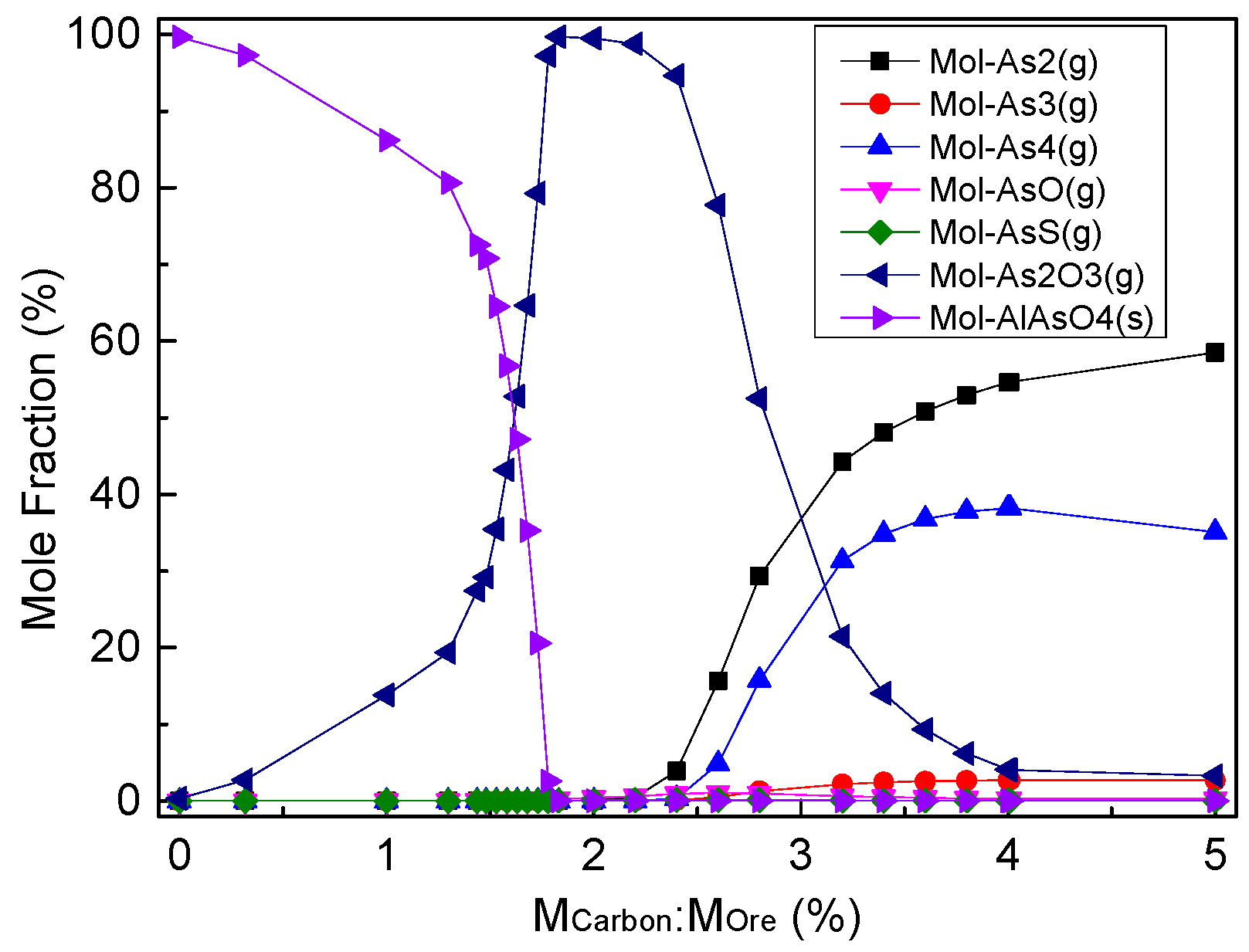
4.5. Effect of Carbon Mass Ratio on Arsenic Removal Products of Dust Ash by Roasting
5. Conclusions
- (1)
- Arsenic in arsenopyite-bearing iron ore can be removed by roasting method in an air or nitrogen atmosphere;
- (2)
- The mechanism of arsenic removal by roasting method indicates that the efficiency of arsenic removal by roasting in air is less than that in nitrogen atmosphere. The poor arsenic removal efficiency at low temperature and in an air atmosphere is due to the formation of arsenates by the reaction of As2O3(g) with other oxides in the strong oxidizing atmosphere. Lower partial pressure of oxygen is required to ensure an effective arsenic removal rate when arsenic-bearing ore is roasted at lower temperatures. Arsenic is removed in the form of As2O3(g) by the roasting method, and residual arsenic reacts with oxides in the ore to generate arsenates.
- (3)
- The arsenic recovery from dust ash by roasting in the atmosphere of an air or anaerobic atmosphere is difficult, and arsenic easily reacts with oxides to form arsenate and remains in the dust ash. The method of roasting in a reducing atmosphere is feasible for arsenic recovery from dust ash. When the arsenic-bearing dust ash is roasted with a carbon mass ratio below 1.63%, the arsenic removal products are the majority of AlAsO4(s) and a small amount of As2O3(g). When the carbon mass ratio is 1.83%, the arsenic removal product is almost volatilized and recovered in the form of As2O3(g).
Author Contributions
Funding
Conflicts of Interest
References
- Riveros, P.A.; Dutrizac, J.E.; Spencer, P. Arsenic disposal practices in the metallurgical industry. Can. Metall. Q. 2001, 40, 395–420. [Google Scholar] [CrossRef]
- Díaz, J.A.; Serrano, J.; Leiva, E. Bioleaching of Arsenic-Bearing Copper Ores. Minerals 2018, 8, 215. [Google Scholar] [CrossRef]
- Xin, W.B.; Song, B.; Yang, Z.B.; Yang, Y.H.; Li, L.F. Effect of Arsenic and Copper+Arsenic on high temperature oxidation and hot shortness behavior of C–Mn steel. ISIJ Int. 2016, 56, 1232–1240. [Google Scholar] [CrossRef]
- Xin, W.B.; Song, B.; Huang, C.G.; Song, M.M.; Song, G.Y. Effect of Arsenic content and quenching temperature on solidification microstructure and Arsenic distribution in iron-arsenic alloys. Int. J. Miner. Metall. Mater. 2015, 22, 704–713. [Google Scholar] [CrossRef]
- Yin, L.; Seetharaman, S. Effects of residual elements Arsenic, Antimony, and Tin on surface hot shortness. Metall. Mater. Trans. B 2011, 42, 1031–1043. [Google Scholar] [CrossRef]
- Huang, C.G.; Song, B.; Xin, W.B.; Jia, S.J.; Yang, Y.H. Influence of rare earth La on hot ductility of low carbon steel containing As and Sn. Heat Treat. Met. 2015, 40, 1–6. [Google Scholar]
- Zhu, Y.Z.; Li, B.L.; Liu, P. Effect of annealing and hot rolling on grain boundary segregation of Arsenic in an Mn-steel microalloyed by Ti, Cr and Nb. J. Iron Steel Res. Int. 2013, 20, 67–72. [Google Scholar] [CrossRef]
- Xin, W.B.; Song, B.; Song, M.M.; Song, G.Y. Effect of cerium on characteristic of inclusions and grain boundary segregation of arsenic in iron melts. Steel Res. Int. 2015, 86, 1430–1438. [Google Scholar] [CrossRef]
- Geng, M.S.; Xiang, L.; Wang, X.H.; Zhang, J.M.; Xiao, J.G. Effect of residual elements on continuous cast slab and surface quality of hot rolled plate. J. Iron Steel Res. 2009, 21, 19–21. (In Chinese) [Google Scholar]
- Wang, J.J.; Luo, L.G.; Kong, H.; Zhou, L. The Arsenic removal from molten steel. High Temp. Mater. Proc. 2011, 30, 171–173. [Google Scholar] [CrossRef]
- Valenzuela, A. Arsenic Management in the Metallurgical Industry. Master’s Thesis, University of Laval, Quebec, QC, Canada, 2000. [Google Scholar]
- Mihajlovic, I.; Strbac, N.; Nikolic, D.; Živkovic, Z. Potential metallurgical treatment of Copper concentrates with high Arsenic contents. J. S. Afr. Inst. Min. Metall. 2011, 111, 409–416. [Google Scholar]
- Yin, Z.L.; Lu, W.H.; Xiao, H. Arsenic removal from copper-silver ore by roasting in vacuum. Vacuum 2014, 101, 350–353. [Google Scholar] [CrossRef]
- Lu, W.H.; Yin, Z.L. Study on thermal decomposition and Arsenic removal of a Silver bearing Copper ore. Int. J. Miner. Process. 2016, 153, 1–7. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, S.H.; Hu, X. Study on removal Arsenic from iron ore with Arsenic in sintering process. Adv. Mater. Res. 2011, 284, 238–241. [Google Scholar]
- Cheng, R.J.; Ni, H.W.; Zhang, H.; He, H.Y.; Yang, H.H.; Xiong, S. Experimental study on arsenic removal from low arsenic-bearing iron ore with sintering process. Sinter. Pelletizing 2016, 41, 13–16. (In Chinese) [Google Scholar]
- Chakraborti, N.; Lynch, D.C. Thermodynamic analysis of the As–S–O vapor system. Can. Metall. Q. 1985, 24, 39–45. [Google Scholar] [CrossRef]
- Contreras, M.L.; Arostegui, J.M.; Armesto, L. Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations. Fuel 2009, 88, 539–546. [Google Scholar] [CrossRef]
- Nakazawa, S.; Yazawa, A.; Jorgensen, F.R.A. Simulation of the removal of Arsenic during the roasting of Copper concentrate. Metall. Mater. Trans. B 1999, 30, 393–401. [Google Scholar] [CrossRef]
- Zhang, S.H.; Lü, Q.; Hu, X. Thermodynamics of arsenic removal from arsenic-bearing iron ores. Chin. J. Nonferrous Met. 2011, 21, 1705–1712. (In Chinese) [Google Scholar]
- Cheng, R.J.; Ni, H.W.; Zhang, H.; Jia, S.K.; Xiong, S. Thermodynamics of arsenic removal from arsenic-bearing iron ores with sintering process and dust ash by roasting. Iron Steel. 2017, 52, 26–33. (In Chinese) [Google Scholar]
- Jiang, T.; Huang, Y.F.; Zhang, Y.B.; Han, G.H.; Li, G.H.; Guo, Y.F. Behavior of arsenic in arsenic-bearing iron concentrate pellets by preoxidizing–weak reduction roasting process. J. Cent. South Univ. Sci. Technol. 2010, 41, 1–7. (In Chinese) [Google Scholar]
- Cheng, R.J.; Ni, H.W.; Zhang, H.; Zhang, X.K.; Bai, S.C. Mechanism research on arsenic removal from arsenopyrite ore during sintering process. Int. J. Miner. Metall. Mater. 2017, 24, 353–359. [Google Scholar] [CrossRef]
- Li, Y.Z.; Tong, H.L.; Zhuo, Y.Q.; Li, Y.; Xu, X.C. Simultaneous removal of SO2 and trace As2O3 from flue gas: Mechanism, kinetics study, and effect of main gases on arsenic capture. Environ. Sci. Technol. 2007, 41, 2894–2900. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Somoano, M.; López-Antón, M.A.; Huggins, F.E.; Martínez-Tarazona, M.R. The stability of arsenic and selenium compounds that were retained in limestone in a coal gasification atmosphere. J. Hazard. Mater. 2010, 173, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Nadia, M.V.; Roberto, B.G.; José, A.R.L.; Miguel, A.B.; Alan, D.C.; Elías, R.F.; Mario, V. Arsenic mobility controlled by solid calcium arsenates-A case study in Mexico showcasing a potentially widespread environmental problem. Environ. Pollut. 2013, 176, 114–122. [Google Scholar]
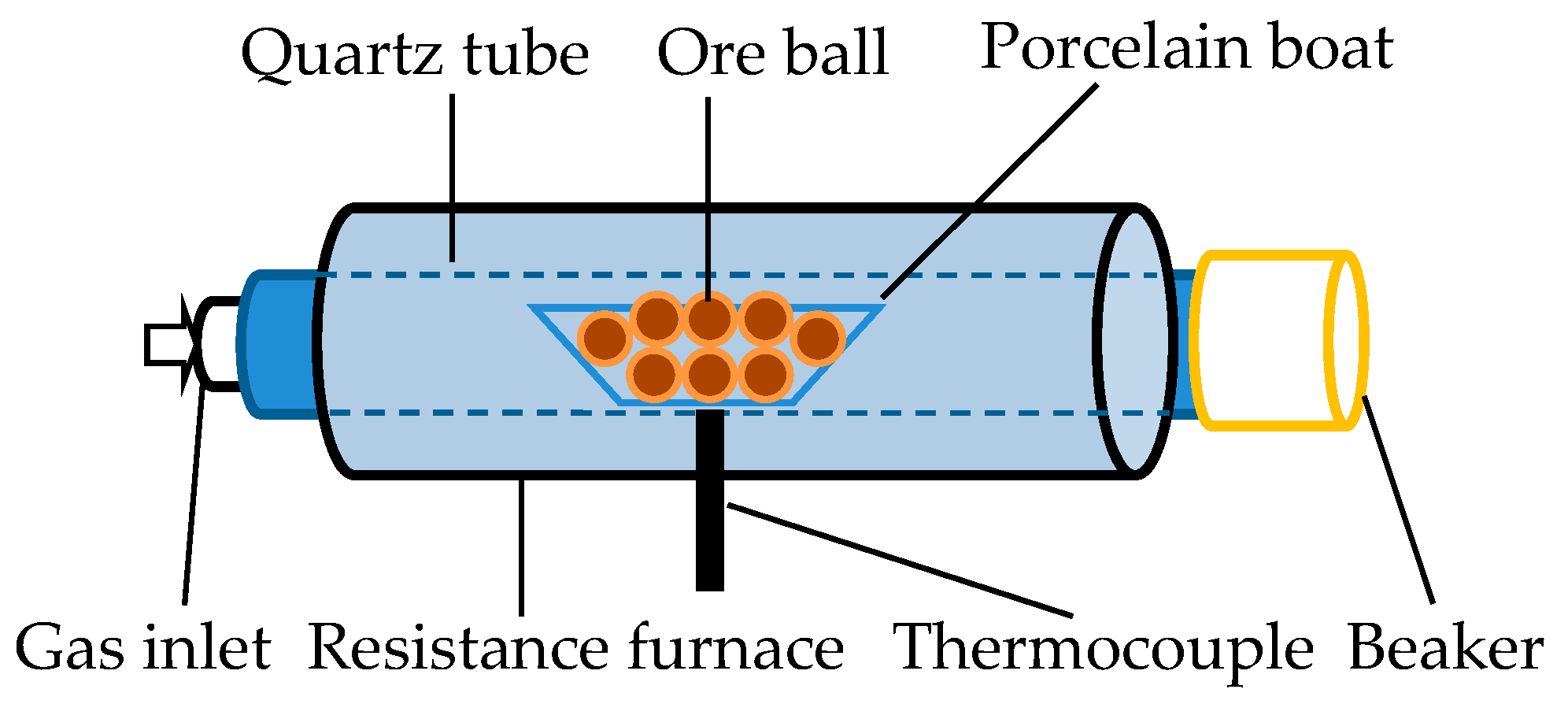
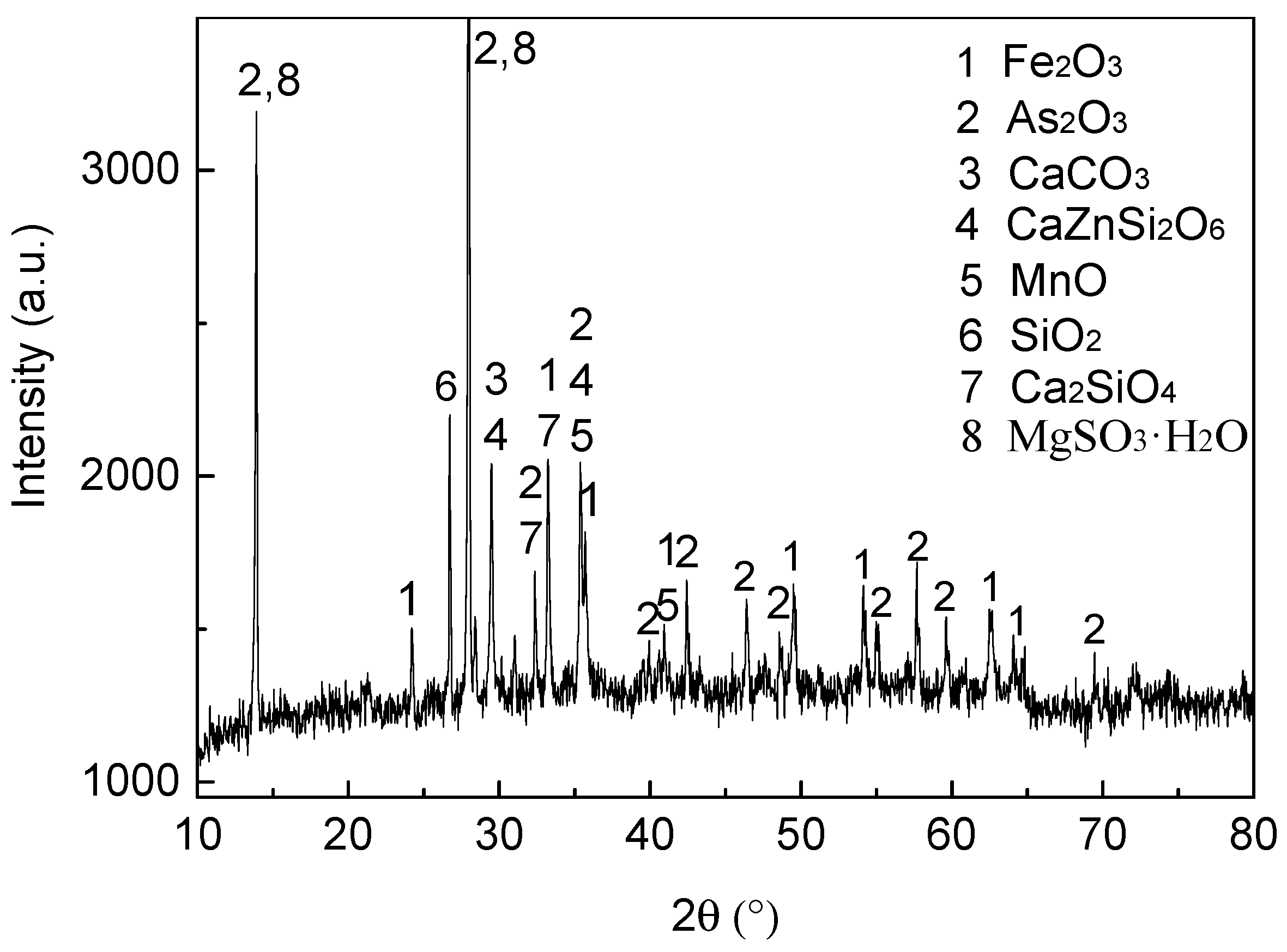

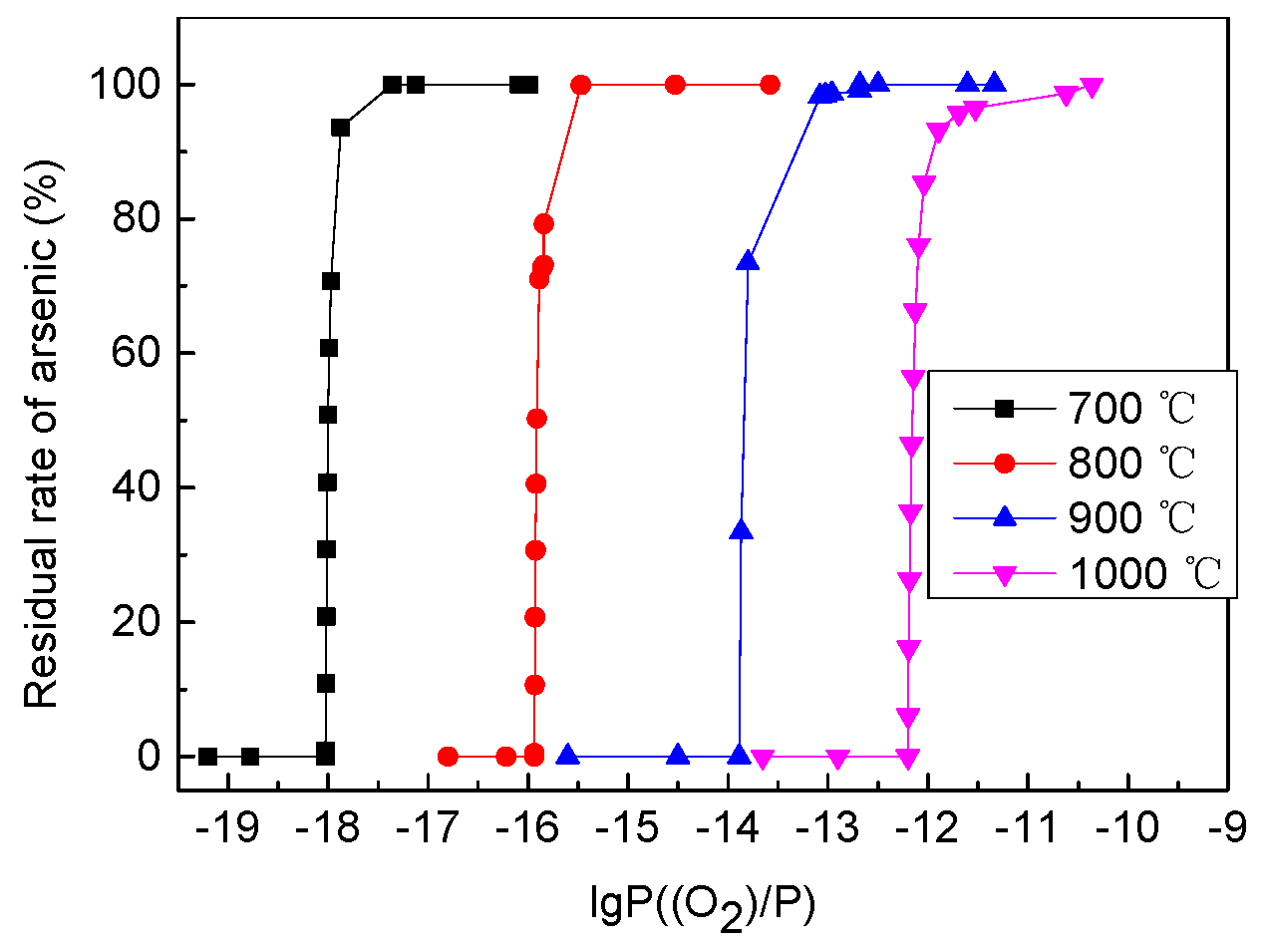
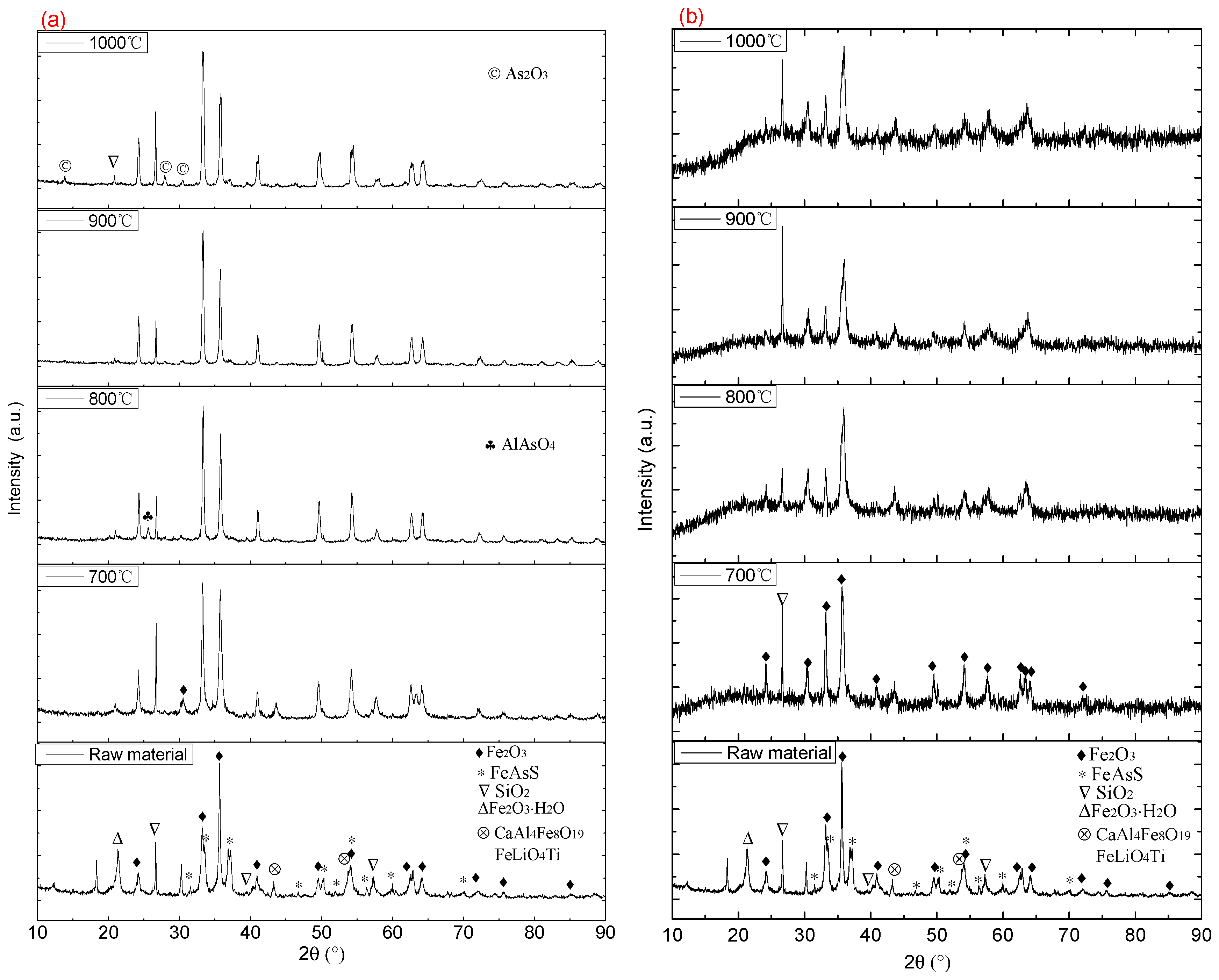

| Ore Name | Component/wt % | Proportion/% | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | CaO | Al2O3 | MgO | FeAsS | S | ||
| Iron ore | 71.36 | 10.64 | 0.40 | 7.39 | 0.31 | 0.05 | 0.06 | 90 |
| Arsenopyrite | / | / | / | / | / | 92.71 | / | 10 |
| Mixed ore | 64.22 | 9.58 | 0.36 | 6.65 | 0.28 | 9.37 | 0.05 | 100 |
| Fe | CaO | MgO | SiO2 | Al2O3 | TiO2 | S | K2O | Na2O | Cl− |
|---|---|---|---|---|---|---|---|---|---|
| 42.34 | 7.08 | 0.82 | 4.80 | 3.04 | 0.72 | 0.65 | 1.16 | 0.15 | 1.06 |
| Temperature/°C | Arsenic Removal Rate in Air Atmosphere/wt % | Arsenic Removal Rate in Nitrogen Atmosphere/wt % | ||||
|---|---|---|---|---|---|---|
| Before Roasting | After Roasting | Arsenic Removal Rate | Before Roasting | After Roasting | Arsenic Removal Rate | |
| 700 | 4.31 | 3.78 | 12.30 | 4.31 | 1.000 | 76.80 |
| 800 | 4.31 | 3.86 | 10.44 | 4.31 | 0.186 | 95.68 |
| 900 | 4.31 | 0.88 | 79.58 | 4.31 | 0.051 | 98.82 |
| 1000 | 4.31 | 0.57 | 86.77 | 4.31 | 0.027 | 99.37 |
| Figure No. | Point No. | Atomic Ratio of Elements/at % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Al | Ca | Cr | Si | S | Mn | Fe | Ni | As | Nb | O | ||
| 6a (raw ore) | A | - | - | - | - | 5.40 | - | - | 33.53 | - | - | - | 61.08 |
| B | 5.97 | - | - | - | 10.86 | - | - | 22.20 | - | - | - | 60.98 | |
| C | - | 9.18 | - | - | 6.73 | - | - | 22.75 | - | - | - | 61.35 | |
| D | - | - | - | - | 7.50 | 0.26 | 0.43 | 28.61 | - | 1.64 | - | 61.57 | |
| E | - | - | - | - | - | - | - | 40 | - | - | - | 60 | |
| F | - | - | - | - | - | 5.91 | - | 32.42 | - | 0.48 | - | 61.18 | |
| G | - | - | - | - | 7.23 | 2.14 | - | 22.82 | - | 5.08 | - | 62.73 | |
| 6b (700 °C) | A | - | - | - | - | 7.30 | - | - | 24.31 | - | 0.082 | 4.36 | 63.21 |
| B | - | - | - | - | 11.16 | - | 1.15 | 24.35 | 0.80 | 0.70 | - | 61.84 | |
| C | - | - | - | - | 3.12 | - | - | 35.84 | - | 0.42 | - | 60.62 | |
| D | - | - | 0.46 | - | 10.45 | - | - | 25.06 | 0.42 | 1.70 | - | 61.92 | |
| E | - | - | 0.64 | - | 8.38 | - | - | 28.03 | - | 0.81 | - | 61.65 | |
| F | - | - | - | 0.36 | 7.73 | - | - | 29.08 | - | 1.28 | - | 61.55 | |
| G | - | - | - | - | 2.10 | - | - | 37.48 | - | - | - | 60.42 | |
| 6c (800 °C) | A | - | - | - | 0.36 | 10.00 | - | 0.38 | 27.33 | - | - | - | 61.92 |
| B | - | - | - | 0.34 | 8.42 | - | - | 29.56 | - | - | - | 61.68 | |
| C | - | - | - | - | 22.59 | - | - | 12.55 | - | 0.34 | - | 64.52 | |
| D | - | - | - | - | 11.86 | - | - | 25.76 | - | - | - | 62.37 | |
| E | - | - | - | - | 8.02 | - | 3.49 | 27.58 | - | - | - | 60.91 | |
| F | 2.59 | - | - | - | 19.04 | - | - | 15.08 | - | - | - | 63.29 | |
| G | - | - | - | - | 24.37 | - | 0.40 | 9.85 | - | 0.58 | - | 64.79 | |
| H | - | - | - | - | 12.95 | - | - | 19.00 | - | 0.25 | 3.72 | 64.08 | |
| 6d (900 °C) | A | - | - | - | - | 9.79 | - | - | 28.25 | - | - | - | 61.96 |
| B | - | - | - | - | 7.12 | - | - | 23.55 | - | 2.00 | 4.22 | 63.11 | |
| C | - | - | - | - | 9.72 | - | - | 25.08 | - | 3.25 | - | 61.94 | |
| D | - | 7.01 | - | 0.39 | 3.87 | - | - | 27.95 | - | - | - | 60.77 | |
| E | - | - | - | 0.46 | 6.49 | - | - | 31.75 | - | - | - | 60.30 | |
| F | - | - | - | 0.34 | 5.44 | - | - | 32.65 | - | 0.49 | - | 61.09 | |
| 6e (1000 °C) | A | - | - | - | 0.96 | 7.34 | - | - | 30.23 | - | - | - | 61.47 |
| B | - | 1.16 | - | - | 1.75 | - | - | 36.74 | - | - | - | 60.35 | |
| C | - | 2.93 | - | - | 2.50 | - | - | 26.14 | - | - | 5.66 | 62.76 | |
| D | - | 1.46 | - | - | - | - | - | 38.54 | - | - | - | 60.00 | |
| E | - | - | - | - | 2.45 | - | - | 35.38 | - | 1.68 | - | 60.49 | |
| F | - | 0.89 | - | 0.49 | 16.73 | - | - | 18.54 | - | - | - | 63.35 | |
| G | - | - | - | 1.02 | 8.67 | - | - | 26.13 | - | 2.44 | - | 61.73 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.; Zhang, H.; Ni, H. Arsenic Removal from Arsenopyrite-Bearing Iron Ore and Arsenic Recovery from Dust Ash by Roasting Method. Processes 2019, 7, 754. https://doi.org/10.3390/pr7100754
Cheng R, Zhang H, Ni H. Arsenic Removal from Arsenopyrite-Bearing Iron Ore and Arsenic Recovery from Dust Ash by Roasting Method. Processes. 2019; 7(10):754. https://doi.org/10.3390/pr7100754
Chicago/Turabian StyleCheng, Rijin, Hua Zhang, and Hongwei Ni. 2019. "Arsenic Removal from Arsenopyrite-Bearing Iron Ore and Arsenic Recovery from Dust Ash by Roasting Method" Processes 7, no. 10: 754. https://doi.org/10.3390/pr7100754
APA StyleCheng, R., Zhang, H., & Ni, H. (2019). Arsenic Removal from Arsenopyrite-Bearing Iron Ore and Arsenic Recovery from Dust Ash by Roasting Method. Processes, 7(10), 754. https://doi.org/10.3390/pr7100754





