Antibacterial and Antifungal Activity of Novel Synthesized Neodymium-Substituted Cobalt Ferrite Nanoparticles for Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CoNdxFe2−xO4 (0.0 ≤ x ≤ 0.2) NPs
2.2. Instrumentation
2.3. Determination of Antibacterial and Anti-Yeast Activity
Preparation of Inoculum
2.4. Evaluation of Growth Pattern in the Presence of Nanomaterial
2.5. Determination of Colony Forming Units
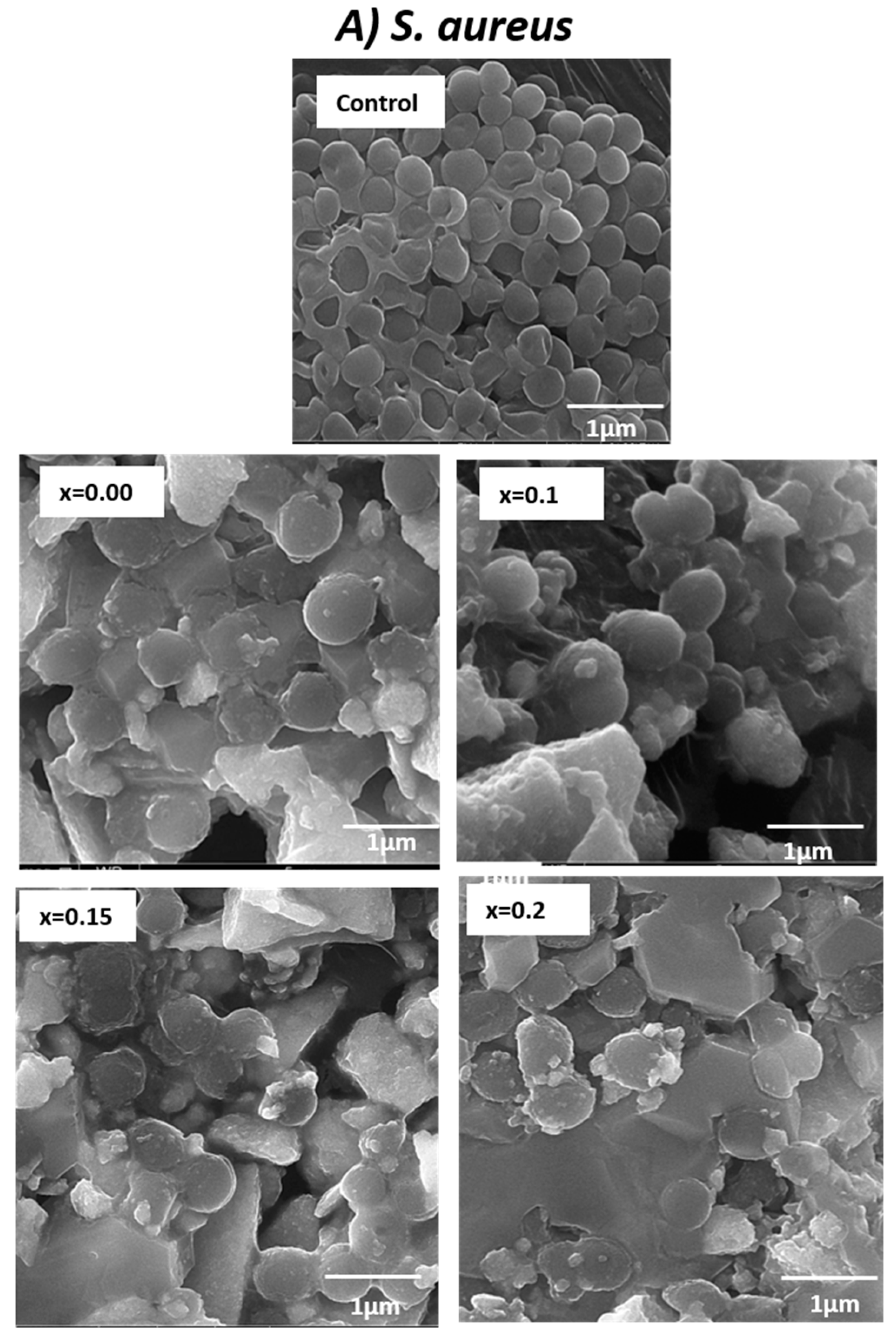
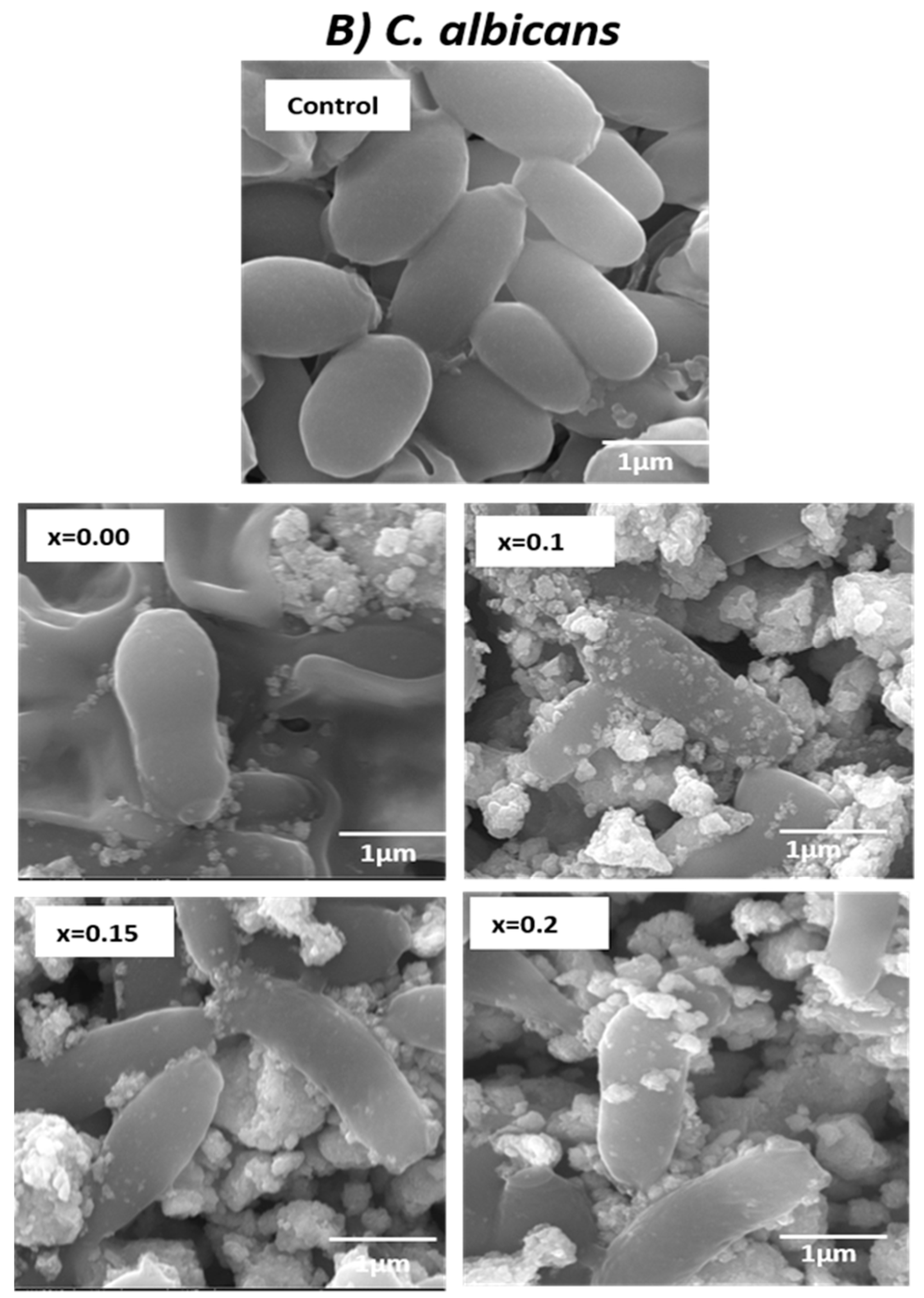
2.6. Study of Morphogenesis of the Treated Microbes
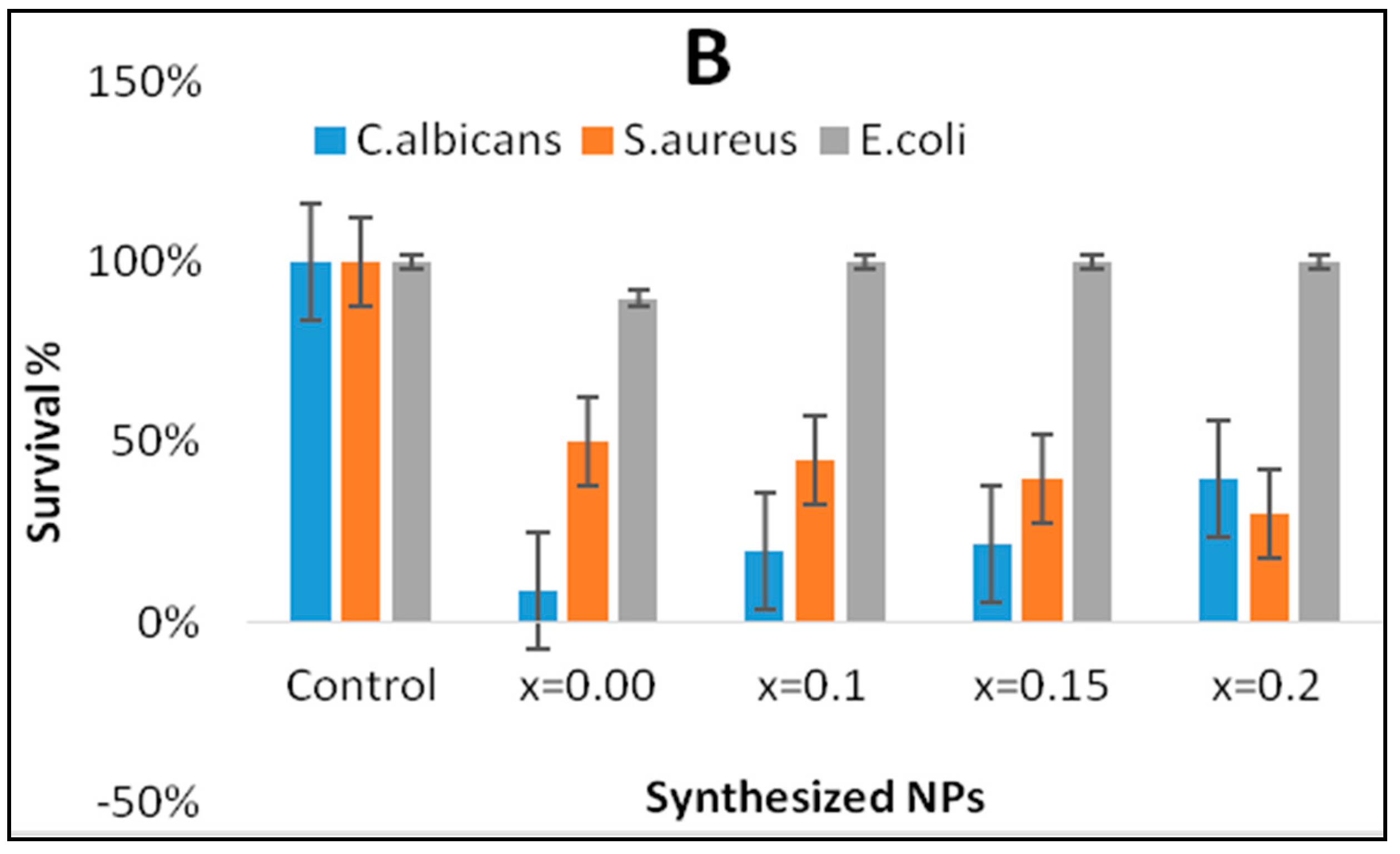
3. Results and Discussion
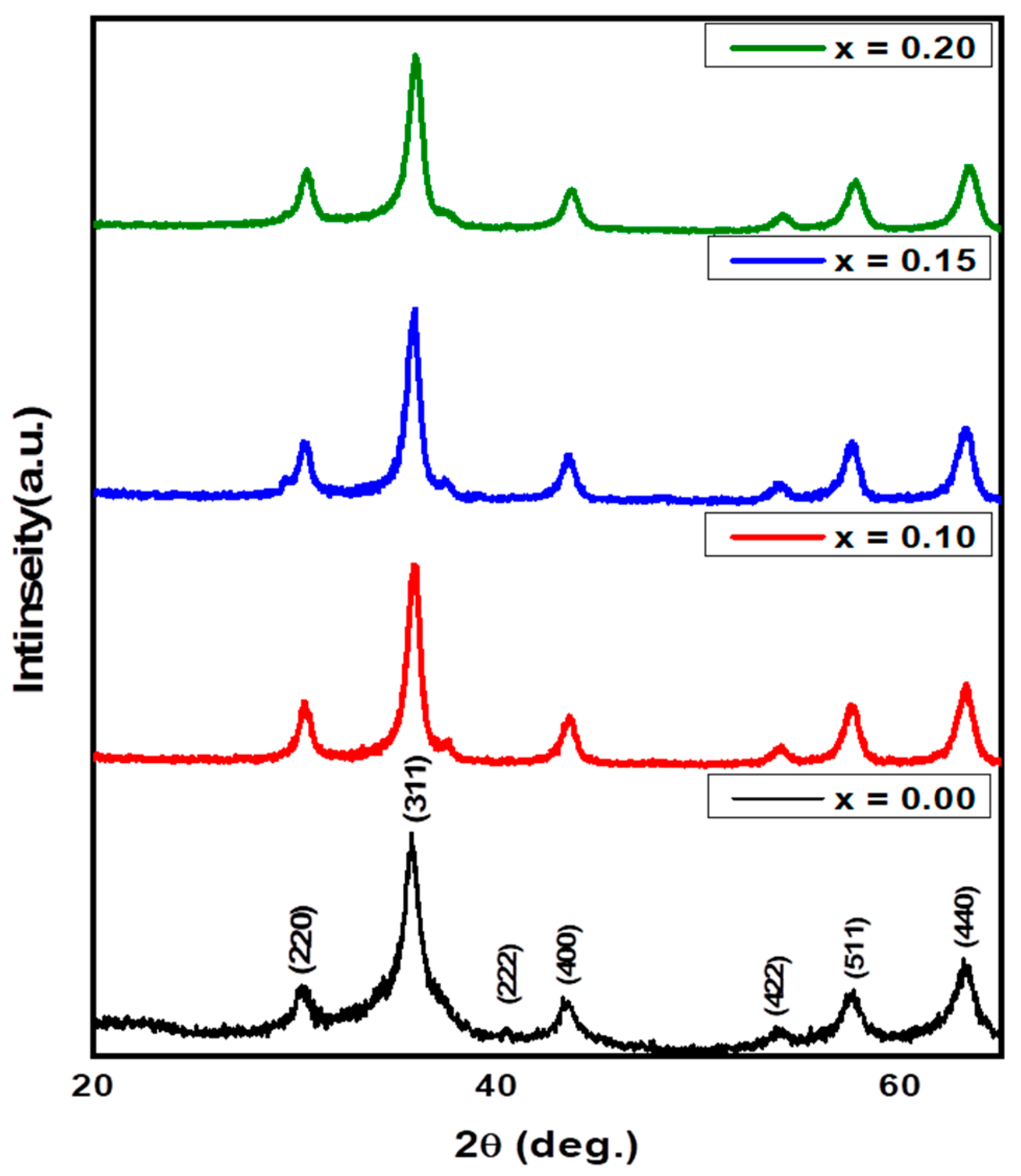
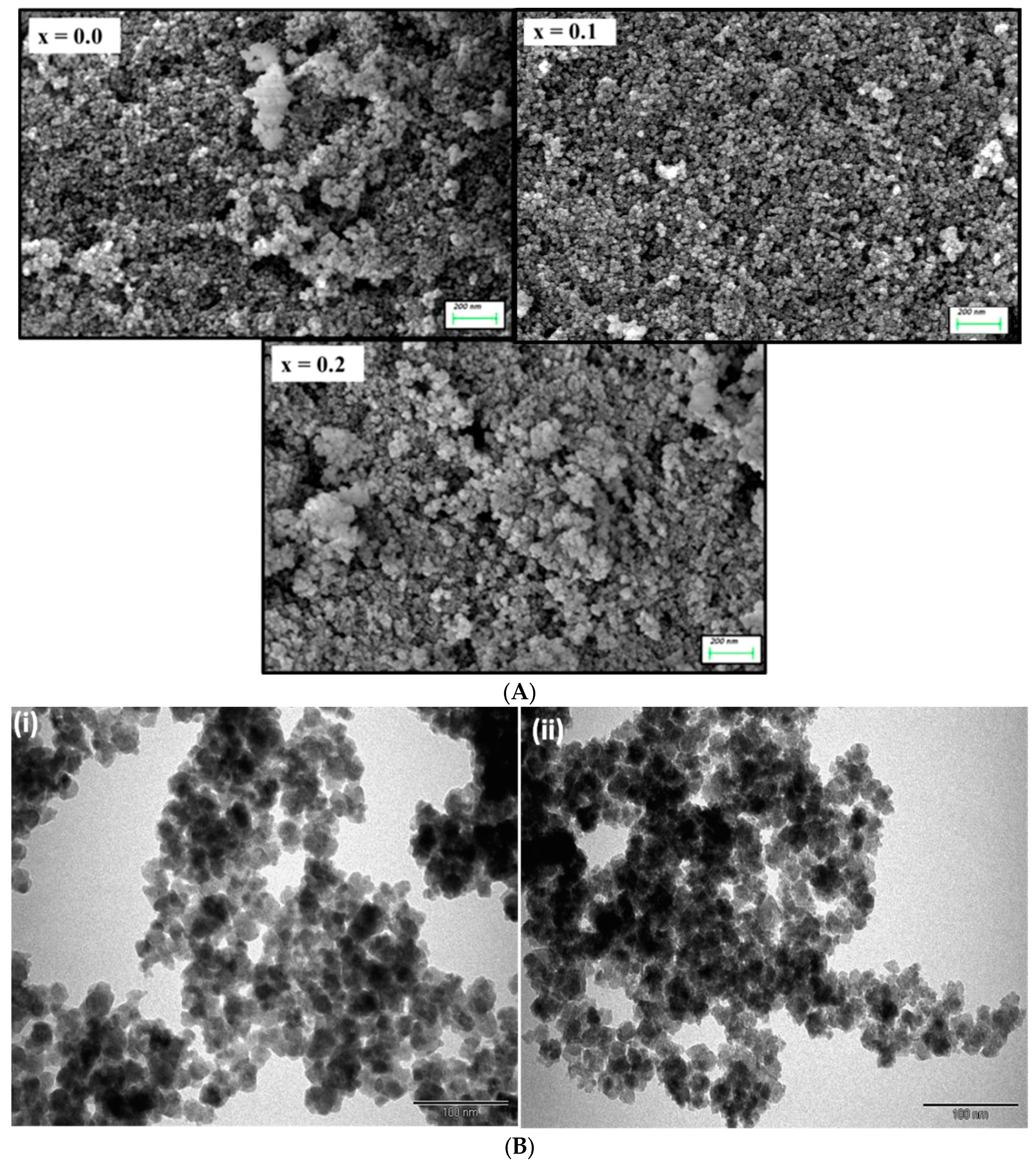
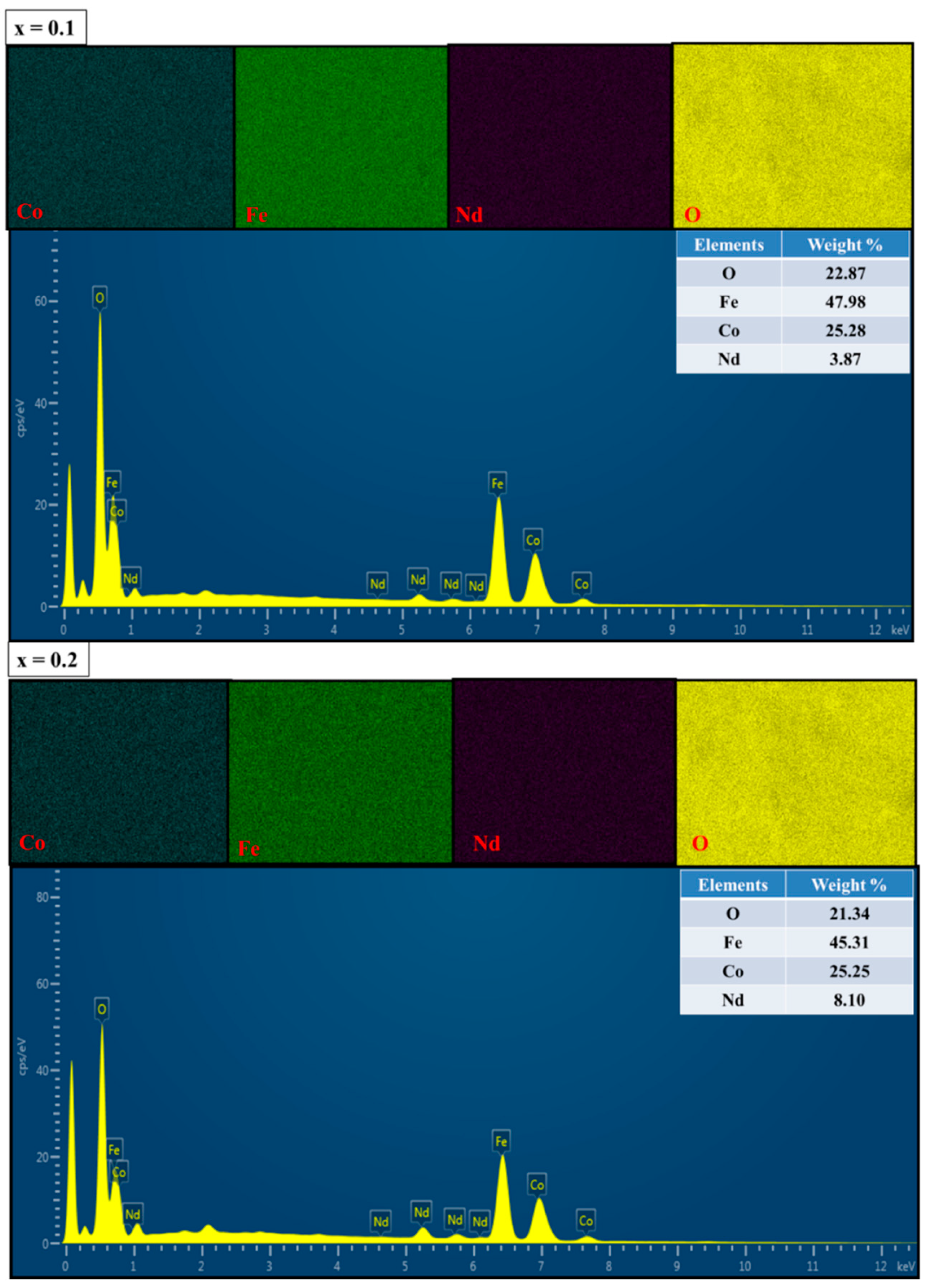
3.1. Structural and Morphological Analysis along with EDX and Elemental Mapping
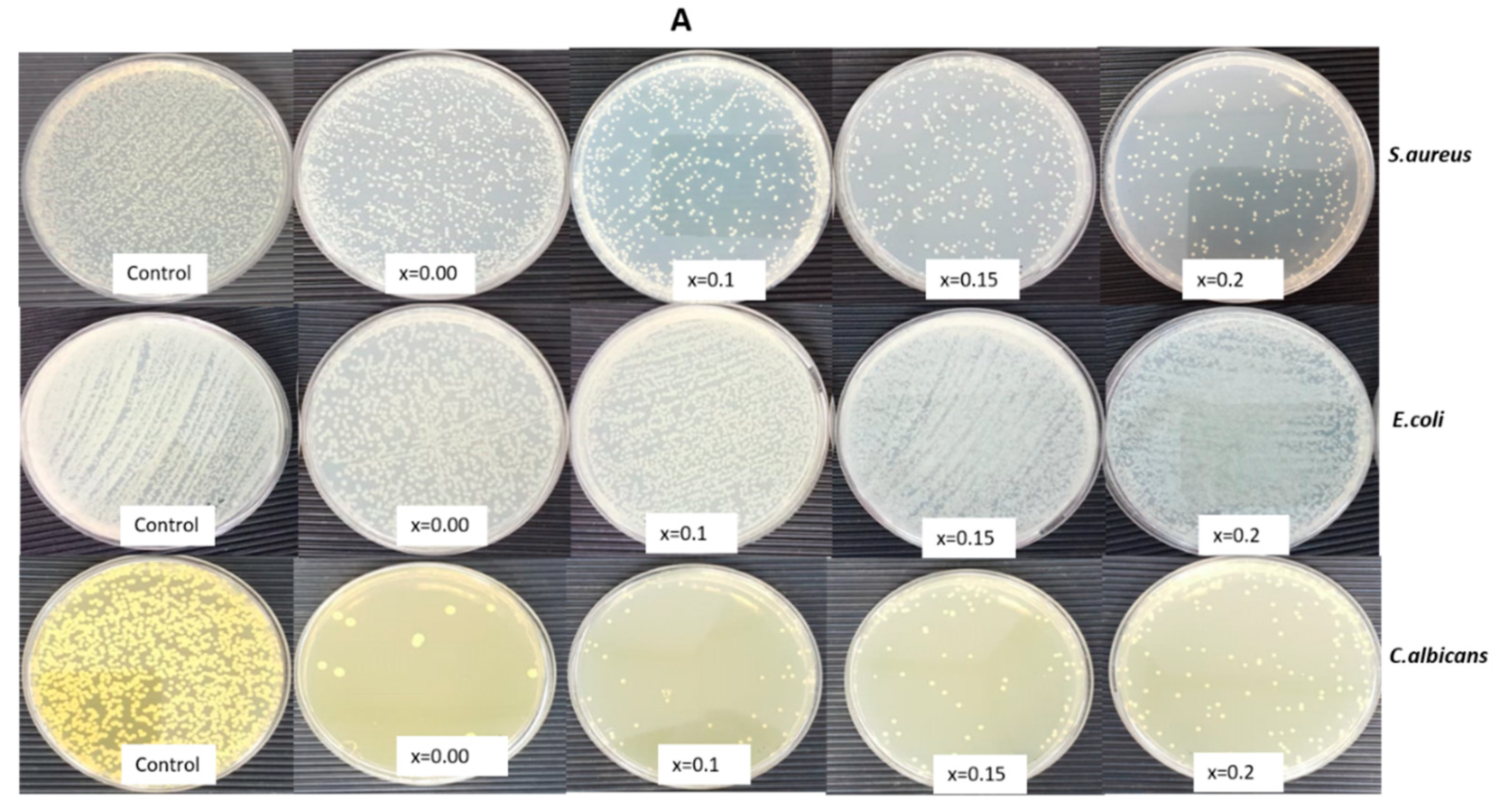
3.2. Determination of Morphogenesis of Treated Cells
4. Conclusions
Data Availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Organization WH. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Falagas, M.E.; Karageorgopoulos, D.E. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: Need for international harmonization in terminology. Clin. Infect. Dis. 2008, 46, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. Balancing the Drug-Resistance Equation; Elsevier Current Trends: Amsterdam, The Netherlands, 1994. [Google Scholar]
- El-Batal, A.I.; El-Sayyad, G.S.; El-Ghamery, A.; Gobara, M. Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J. Clust. Sci. 2017, 28, 1083–1112. [Google Scholar] [CrossRef]
- Žalnėravičius, R.; Paškevičius, A.; Kurtinaitiene, M.; Jagminas, A. Size-dependent antimicrobial properties of the cobalt ferrite nanoparticles. J. Nanopart. Res. 2016, 18, 300. [Google Scholar] [CrossRef]
- Goldman, A. Modern Ferrite Technology; Springer Science & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
- Sanpo, N.; Berndt, C.C.; Wen, C.; Wang, J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013, 9, 5830–5837. [Google Scholar] [CrossRef]
- Hatamie, S.; Nouri, M.; Karandikar, S.; Kulkarni, A.; Dhole, S.; Phase, D.; Kale, S.N. Complexes of cobalt nanoparticles and polyfunctional curcumin as antimicrobial agents. Mater. Sci. Eng. C 2012, 32, 92–97. [Google Scholar] [CrossRef]
- Cunningham, C.H.; Arai, T.; Yang, P.C.; McConnell, M.V.; Pauly, J.M.; Conolly, S.M. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn. Reson. Med Off. J. Int. Soc. Magn. Reson. Med. 2005, 53, 999–1005. [Google Scholar] [CrossRef]
- Lu, C.-W.; Hung, Y.; Hsiao, J.-K.; Yao, M.; Chung, T.-H.; Lin, Y.-S.; Wu, S.H.; Hsu, S.C.; Liu, H.M.; Mou, C.Y.; et al. Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett. 2007, 7, 149–154. [Google Scholar] [CrossRef]
- Sharma, R.P.; Raut, S.D.; Jadhav, V.V.; Kadam, A.S.; Mane, R.S. Anti-candida and anti-adhesion efficiencies of zinc ferrite nanoparticles. Mater. Lett. 2019, 237, 165–167. [Google Scholar] [CrossRef]
- Ghafoor, A.; Khan, M.A.; Islam, M.; Gilani, Z.A.; Manzoor, A.; Khan, H.M.; Ali, I.; Warsi, M.F. Structural and electromagnetic studies of Ni0.7Zn0.3HO2xFe2−2xO4 ferrites. Ceram. Int. 2016, 42, 14252–14256. [Google Scholar] [CrossRef]
- Routray, K.L.; Saha, S.; Sanyal, D.; Behera, D. Role of rare-earth (Nd3+) ions on structural, dielectric, magnetic and Mossbauer properties of nano-sized CoFe2O4: Useful for high frequency application. Mater. Res. Express 2018, 6, 026107. [Google Scholar] [CrossRef]
- Avazpour, L.; Shokrollahi, H.; Toroghinejad, M.R.; Khajeh, M.Z. Effect of rare earth substitution on magnetic and structural properties of Co1−xREx Fe2O4 (RE: Nd, Eu) nanoparticles prepared via EDTA/EG assisted sol–gel synthesis. J. Alloy. Compd. 2016, 662, 441–447. [Google Scholar] [CrossRef]
- Nikumbh, A.K.; Pawar, R.A.; Nighot, D.V.; Gugale, G.S.; Sangale, M.D.; Khanvilkar, M.B.; Nagawade, A.V. Structural, electrical, magnetic and dielectric properties of rare-earth substituted cobalt ferrites nanoparticles synthesized by the co-precipitation method. J. Magn. Magn. Mater. 2014, 355, 201–209. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Paliychuk, N.; Yaremiy, I.; Moklyak, V. Structural characterization and antistructure modeling of cobalt-substituted zinc ferrites. J. Alloy. Compd. 2017, 694, 777–791. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Pankhurst, Q.; Thanh, N.; Jones, S.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Amiri, S.; Shokrollahi, H. The role of cobalt ferrite magnetic nanoparticles in medical science. Mater. Sci. Eng. C 2013, 33, 1–8. [Google Scholar] [CrossRef]
- Hathout, A.S.; Aljawish, A.; Sabry, B.A.; El-Nekeety, A.A.; Roby, M.H.; Deraz, N.M.; Aly, S.E.; Abdel-Wahhab, M.A. Synthesis and characterization of cobalt ferrites nanoparticles with cytotoxic and antimicrobial properties. J. Appl. Pharm. Sci. 2017, 7, 086–092. [Google Scholar] [CrossRef]
- Samavati, A.; Ismail, A. Antibacterial properties of copper-substituted cobalt ferrite nanoparticles synthesized by co-precipitation method. Particuology 2017, 30, 158–163. [Google Scholar] [CrossRef]
- Elayakumar, K.; Dinesh, A.; Manikandan, A.; Palanivelu, M.; Kavitha, G.; Prakash, S.; Kumar, R.T.; Jaganathan, S.K.; Baykal, A. Structural, morphological, enhanced magnetic properties and antibacterial bio-medical activity of rare earth element (REE) Cerium (Ce3+) doped CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2018, 476, 157–165. [Google Scholar] [CrossRef]
- Maksoud, M.A.; El-Sayyad, G.S.; Ashour, A.H.; El-Batal, A.I.; Elsayed, M.A.; Gobara, M.; El-Khawaga, A.M.; Abdel-Khalek, E.K.; El-Okr, M.M. Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb. Pathog. 2019, 127, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.H.; El-Batal, A.I.; Maksoud, M.A.; El-Sayyad, G.S.; Labib, S.; Abdeltwab, E.; El-Okr, M.M. Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology 2018, 40, 141–151. [Google Scholar] [CrossRef]
- Deyno, S.; Toma, A.; Worku, M.; Bekele, M. Antimicrobial resistance profile of staphylococcus aureus isolates isolated from ear discharges of patients at University of Hawassa comprehensive specialized hospital. BMC Pharmacol. Toxicol. 2017, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Morrill, H.J.; Morton, J.B.; Caffrey, A.R.; Jiang, L.; Dosa, D.; Mermel, L.A.; LaPlante, K.L. Antimicrobial resistance of Escherichia coli urinary isolates in the Veterans Affairs Healthcare System. Antimicrob. Agents Chemother. 2017, 61, e02236-16. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Kalina, L.; Urbánek, P.; Machovsky, M.; Skoda, D.; Masař, M.; Holek, M. Sonochemical synthesis of Gd3+ doped CoFe2O4 spinel ferrite nanoparticles and its physical properties. Ultrason. Sonochem. 2018, 40, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Cobalt-doped nanohydroxyapatite: Synthesis, characterization, antimicrobial and hemolytic studies. J. Nanopart. Res. 2013, 15, 1644. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Ali, S.G.; Khan, H.M.; Rehman, S. Anticandidal activity of bioinspired ZnO NPs: Effect on growth, cell morphology and key virulence attributes of Candida species. Artif. Cells Nanomed. Biotechnol. 2018, 46, 912–925. [Google Scholar] [CrossRef]
- Rehman, S.; Jermy, B.R.; Akhtar, S.; Borgio, J.F.; Abdul Azeez, S.; Ravinayagam, V.; Al Jindan, R.; Alsalem, Z.H.; Buhameid, A.; Gani, A. Isolation and characterization of a novel thermophile; Bacillus haynesii, applied for the green synthesis of ZnO nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2072–2082. [Google Scholar] [CrossRef]
- Ishaq, K.; Saka, A.A.; Kamardeen, A.O.; Ahmed, A.; Alhassan MIh Abdullahi, H. Characterization and antibacterial activity of nickel ferrite doped α-alumina nanoparticle. Eng. Sci. Technol. Int. J. 2017, 20, 563–569. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumar, J.; Thakur, S.; Sharma, S.; Shrivastava, V. Antibacterial study of silver doped zinc oxide nanoparticles against Staphylococcus aureus and Bacillus subtilis. Drug Invent. Today 2013, 5, 50–54. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Patron, L.; Calderon-Moreno, J.M.; Mocioiu, O.C.; Preda, S.; Stanica, N.; Nita, S.; Dobre, N.; Popa, M.; et al. Green synthesis methods of CoFe2O4 and Ag-CoFe2O4 nanoparticles using hibiscus extracts and their antimicrobial potential. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Ansari, M.A.; Baykal, A.; Asiri, S.; Rehman, S. Synthesis and characterization of antibacterial activity of spinel chromium-substituted copper ferrite nanoparticles for biomedical application. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2316–2327. [Google Scholar] [CrossRef]
- Ansari, M.A.; Alzohairy, M.A. One-pot facile green synthesis of silver nanoparticles using seed extract of phoenix dactylifera and their bactericidal potential against MRSA. Evid. Based Complement. Altern. Med. 2018. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carriere, M. Size-, composition-and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef]
- Scheres, N.; Krom, B.P. Staphylococcus–Candida Interaction Models: Antibiotic Resistance Testing and Host Interactions. In Candida Species: Methods and Protocols; Calderone, R., Cihlar, R., Eds.; Springer: New York, NY, USA, 2016; pp. 153–161. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Jermy, B.R.; Shahzad, R.; Tashkandi, N.; Alsalem, Z.H. Antibacterial and Antifungal Activity of Novel Synthesized Neodymium-Substituted Cobalt Ferrite Nanoparticles for Biomedical Application. Processes 2019, 7, 714. https://doi.org/10.3390/pr7100714
Rehman S, Ansari MA, Alzohairy MA, Alomary MN, Jermy BR, Shahzad R, Tashkandi N, Alsalem ZH. Antibacterial and Antifungal Activity of Novel Synthesized Neodymium-Substituted Cobalt Ferrite Nanoparticles for Biomedical Application. Processes. 2019; 7(10):714. https://doi.org/10.3390/pr7100714
Chicago/Turabian StyleRehman, Suriya, Mohammad Azam Ansari, Mohammad A. Alzohairy, Mohammad N. Alomary, B. Rabindran Jermy, Raheem Shahzad, Neda Tashkandi, and Zainab Hassan Alsalem. 2019. "Antibacterial and Antifungal Activity of Novel Synthesized Neodymium-Substituted Cobalt Ferrite Nanoparticles for Biomedical Application" Processes 7, no. 10: 714. https://doi.org/10.3390/pr7100714
APA StyleRehman, S., Ansari, M. A., Alzohairy, M. A., Alomary, M. N., Jermy, B. R., Shahzad, R., Tashkandi, N., & Alsalem, Z. H. (2019). Antibacterial and Antifungal Activity of Novel Synthesized Neodymium-Substituted Cobalt Ferrite Nanoparticles for Biomedical Application. Processes, 7(10), 714. https://doi.org/10.3390/pr7100714





