1. Introduction
The high worldwide demand for energy, the uncertainty about crude oil availability and cost as well as increasing concern about global climate change have led to a revival of interest in developing alternative energy sources that can displace fossil transportation fuels [
1]. This interest is further intensified by national and international policies, such as the European Union (EU) Renewable Energy Directive of 2009 that identifies future targets for the use of renewable energy and the US Renewable Fuel Standard that requires transportation fuel sold in the US to contain a minimum volume of renewable fuels. In addition, during the 2015 ‘United Nations Framework Convention on Climate Change’ in Paris, 195 countries recognized the ‘need for an effective and progressive response to the urgent threat of climate change on the basis of the best available scientific knowledge [
2]. Among the many candidate substitutes to fossil fuels (e.g., hydrogen, natural gas, syngas, etc.), biofuels have been identified as the most promising alternative, due to their renewability and potential for social, economic and environmental sustainability [
3].
Biofuels produced from crop biomass have attracted immense global attention in the past two decades. The most well-known first-generation biofuels are bioethanol produced from sugar or starch-based feedstocks (e.g., sugarcane, molasses and corn) and biodiesel produced from vegetable oils (e.g., soybean and palm oil) by transesterification processes or cracking. In particular, bioethanol is a well-established biofuel for blending with gasoline with the US currently producing about 60.5 million cubic meters per year, mainly from corn grain [
4]. However, there are several drawbacks associated with ethanol use. Specifically, ethanol has a lower energy content compared to gasoline, it is not amenable to pipeline distribution, and the amount that can be blended into gasoline for use in conventional vehicles is limited by environmental regulations and engine compatibility restrictions. The production of biodiesel, on the other hand, has lately received a lot of criticism due to the problems introduced by the occurring change in land use. In order to create space for the cultivation of biodiesel crops, existing forest and agricultural land is diverted to the production of biomass. This has a negative effect, not only on the levels of CO
2 released to the atmosphere but also on food availability and prices. As a response to the above concerns, the EU has recently resolved to phase out first-generation biofuels by 2030 [
5].
To overcome these issues and limitations, research and development initiatives have been focused on the production of higher molecular weight alcohols such as butanol. Butanol is an alcohol with four carbons and four isomers namely, n-butanol, sec-butanol, isobutanol and tert-butanol. Among these isomers, n-butanol and isobutanol are those mostly employed in the chemical industry and can be used as gasoline replacements or additives. A comparison of the different liquid fuels’ properties is presented in
Table 1. Butanol exhibits similar advantages as ethanol while possessing several superior characteristics [
6]. Compared with ethanol, butanol has a higher energy content, closer to that of gasoline. It has a low vapor pressure so it can be easily added to conventional gasoline. Butanol can also be used in higher blend concentrations than ethanol without requiring specially adapted vehicles that would impose compromises on performance in order to meet environmental regulations. Fuel specifications in the US for example, allow biobutanol to be blended to up to 16% by volume (equivalent to 10% by volume ethanol in terms of oxygen content) without compromising performance, durability, fuel economy or emissions. Because butanol-gasoline blends are less susceptible to separation in the presence of water and less corrosive than ethanol-gasoline blends, butanol can use the industry’s existing distribution infrastructure without requiring modifications to blending facilities, storage tanks or retail station pumps [
7]. Finally, butanol can be produced using existing ethanol production facilities with relatively minor modifications.
Compared to n-butanol, isobutanol has a similar energy content but a higher octane number which is an advantage for blending into gasoline. Furthermore, isobutanol is less toxic than n-butanol and requires significantly less energy for its downstream processing, mainly due to the need to purify the acetone and ethanol that are produced as by-products of n-butanol [
8]. Several global companies are pioneering the production of biobutanol including Gevo (USA), Butamax (USA) and Green Biologics (USA and UK).
n-Butanol is produced through acetone-butanol-ethanol (ABE) fermentation by solvent-producing bacteria of the
Clostridium species, with acetone and ethanol as major by-products with a butanol-acetone-ethanol mass ratio of 6:3:1 [
9]. On the other hand, isobutanol can be produced by engineered
E. Coli and
S. Cerevisiae strains [
10]. The technical challenges that must be overcome in order to render the production of biobutanol economically competitive have been extensively reviewed in the literature [
11,
12,
13] Research initiatives are focused on two main objectives: the reduction of the cost of raw materials by the utilization of alternative feedstocks and the improvement of the fermentation yield, titer and productivity by genetic engineering and application of new process technologies.
The production cost of biobutanol is considerably affected by the feedstock price which accounts for as much as 60–65% of the total cost [
14]. At present, biobutanol production mainly relies on sugar and starch-based feedstocks such as sugarcane, corn or wheat. While such feedstocks are globally available, they are also used as food supplies or main food ingredients for animals and humans. Therefore, alternative feedstocks with similar availability that are not food-related need to be utilized. A promising approach is the use of renewable lignocellulosic biomass from agricultural residues (corn stover, sugarcane, bagasse, wheat straw and rice bran), forest residues (sawdust, forest thinning waste) and energy crops (switchgrass and miscanthus). These raw materials are available in greater abundance and at a lower cost than the traditional edible feedstocks.
However, lignocellulosic biomass cannot be directly converted into fuel. Cellulose and hemicellulose chains must be converted into fermentable monosaccharides before they can be utilized by microorganisms. The breakdown of cellulose by water molecules needs to be catalyzed either by chemical (acidic or alkaline) or biochemical (enzymatic) agents. Among the two, enzymatic hydrolysis is considered a more environmentally-friendly option but requires large amounts of cellulases which substantially increase the overall production cost [
15]. The hydrolysis of cellulose generates glucose which can be used by a variety of microorganisms to produce biobutanol. On the other hand, the hydrolysis of hemicellulose results in different sugars, mainly pentoses, which can be used by certain microorganisms, although with potentially lower conversion percentages. To increase the efficiency of enzymatic hydrolysis, the lignocellulosic biomass must be first subjected to a pretreatment method. The pretreatment of biomass facilitates the separation and bioconversion of cellulose, hemicellulose and lignin by increasing the surface area so that enzymes can penetrate more effectively. Common pretreatment methods include wet oxidation, liquid hot water, steam explosion, ammonia fiber explosion and supercritical CO
2 explosion [
16].
The fermentative production of biobutanol is typically characterized by low yields, limited biomass growth and product inhibition. Butanol is toxic to microorganisms even at concentrations as low as 13 g/L for n-butanol and 8 g/L for isobutanol [
17,
18]. This dictates that the concentration of the substrate must be sufficiently low so that its utilization is maximized. Substrate concentrations in the fermentation broth are typically limited to less than 60 g/L [
11,
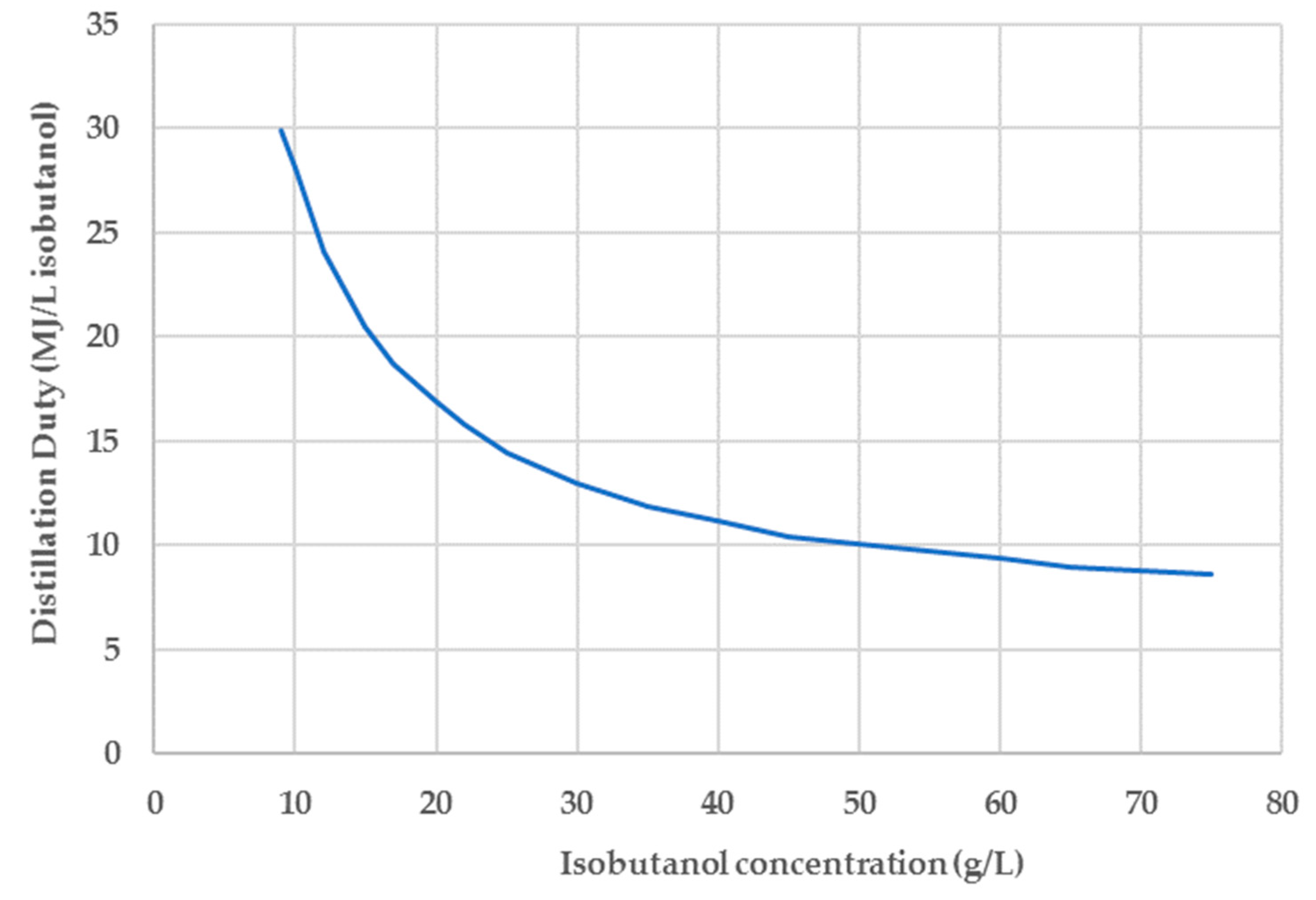
19], which results in increased water consumption and low butanol titers after fermentation. This has a negative effect both on the energy requirements and the amount of wastewater generated in downstream processing by distillation. In other words, the feasibility of the process depends, to a large extent, on the fermentation product titer [
20].
The development of robust strains with improved butanol yield and tolerance is a key point towards the economic competitiveness of biobutanol production. Atsumi et al. [
21], achieved an isobutanol titer of 22 g/L in 112 h of fermentation with a yield of 0.35 g/g, using an engineered
Escherichia coli strain. Smith and Liao achieved a final titer of 21.2 g/L in 99 h with a yield of 0.31 g/g by implementing an evolutionary strategy for isolating high-isobutanol-producing
Escherichia coli strains [
22]. Shen et al. [
23], were able to achieve a n-butanol titer of 15 g/L in 3 days with a yield of 0.36 g/g by constructing a modified clostridial 1-butanol pathway in
Escherichia coli. Jang et al. [
24], reported a n-butanol yield of 15.4 g/L in 60 h with a yield of 0.2 g/g by engineering a hyper-ABE-producing
Clostridium acetobutylicum strain to convert acetone into isopropanol. Some notable reports on biobutanol production enhancement by metabolic engineering are summarized in
Table 2.
In addition to the genetic engineering, improvement of the fermentation performance can be achieved by the implementation of integrated (in situ) product recovery during fermentation. The objective of this approach is to maintain butanol concentration in the fermentor below toxic levels, thereby enabling the use of denser substrate solutions and increased product titers, yields and productivities. Such integrated butanol recovery technologies include adsorption, liquid–liquid extraction, pervaporation, reverse osmosis, vacuum fermentation, vacuum stripping (flash fermentation) and gas stripping. The different alternatives have been extensively reviewed in several publications [
17,
27,
28,
29,
30,
31]. A summary of results obtained either from laboratory scale experiments or computational modeling is presented in
Table 3. By using a stoichiometric fermentation model and rigorous vapor liquid equilibrium (VLE) calculations, Mariano et al. concluded that continuous flash fermentation could reduce the energy requirements of the process by up to 39% compared to conventional fermentation without product removal [
32]. Qureshi et al. achieved a final n-butanol titer of 105.3 g/L with a yield of 0.23 g/g in a fed-batch fermentation system with butanol removal by pervaporation [
33]. Finally, Baez et al. reported an isobutanol titer of 50 g/L in 72 h with a yield of 0.29 g/g using gas stripping and an
Escherichia coli strain engineered for high isobutanol production [
34].
The development of sustainable biofuel processes is a key factor for the replacement of fossil fuels. The assessment of such processes at an economic and life cycle aspect requires information which is usually not available at the early stage of development. To this end, process simulation tools can offer a valuable alternative. Such tools have been used for many decades in the chemical industry to facilitate process development and optimization. Similar benefits can be obtained in the biotechnology arena where simulation tools can be used for the design, economic feasibility evaluation and environmental impact assessment of biorefinery plants. Despite a large number of publications on the improvement of fermentation characteristics by metabolic engineering and integrated product recovery methods, only a few publications exist on the economic assessment of industrial-scale biobutanol production plants (see
Table 4). Most of these publications refer to large-scale n-butanol production plants with capital investments of the order of
$300–400M. To the best of the authors’ knowledge, there is only one techno-economic study that focuses on isobutanol production [
8]. An evident issue from what is presented in
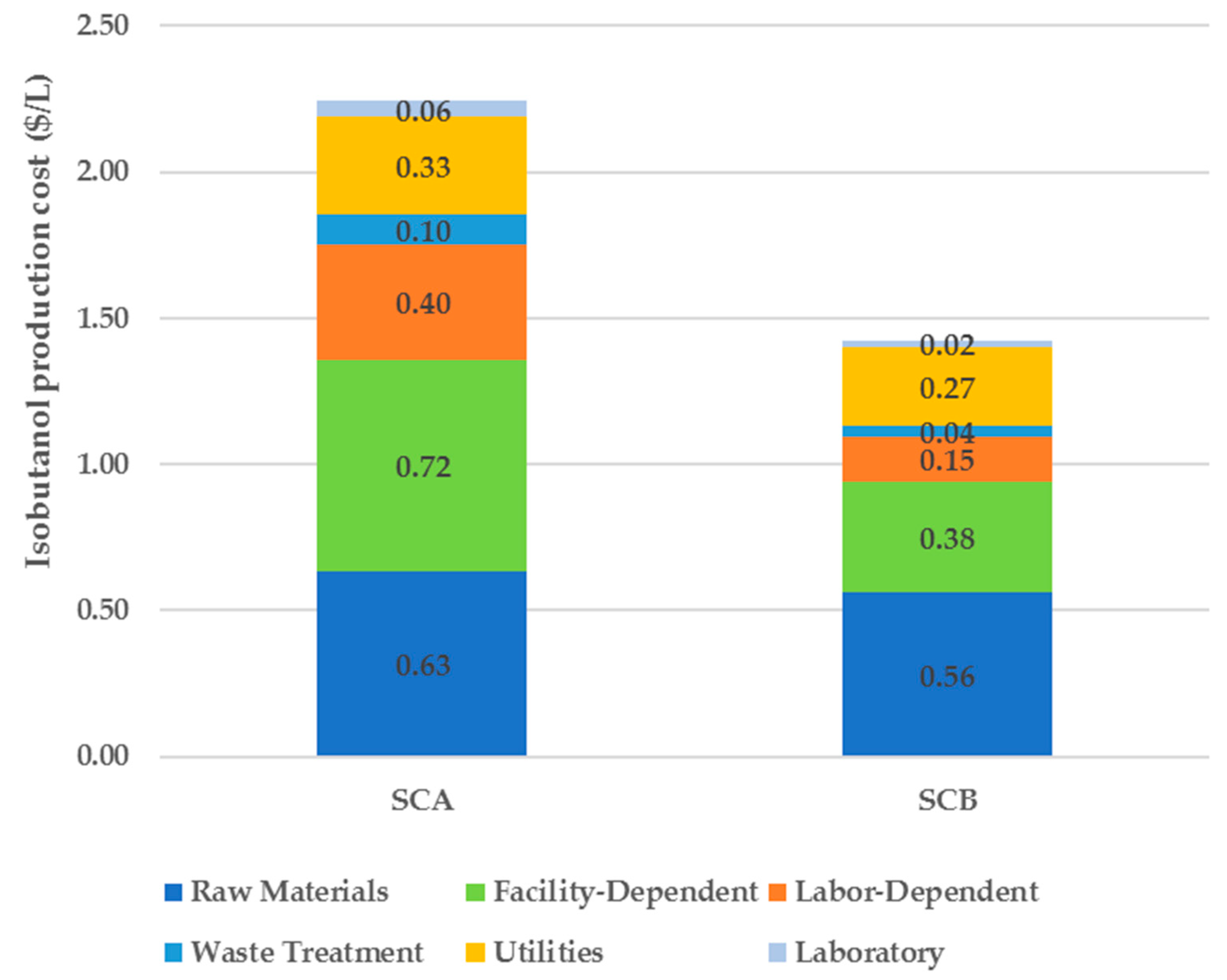
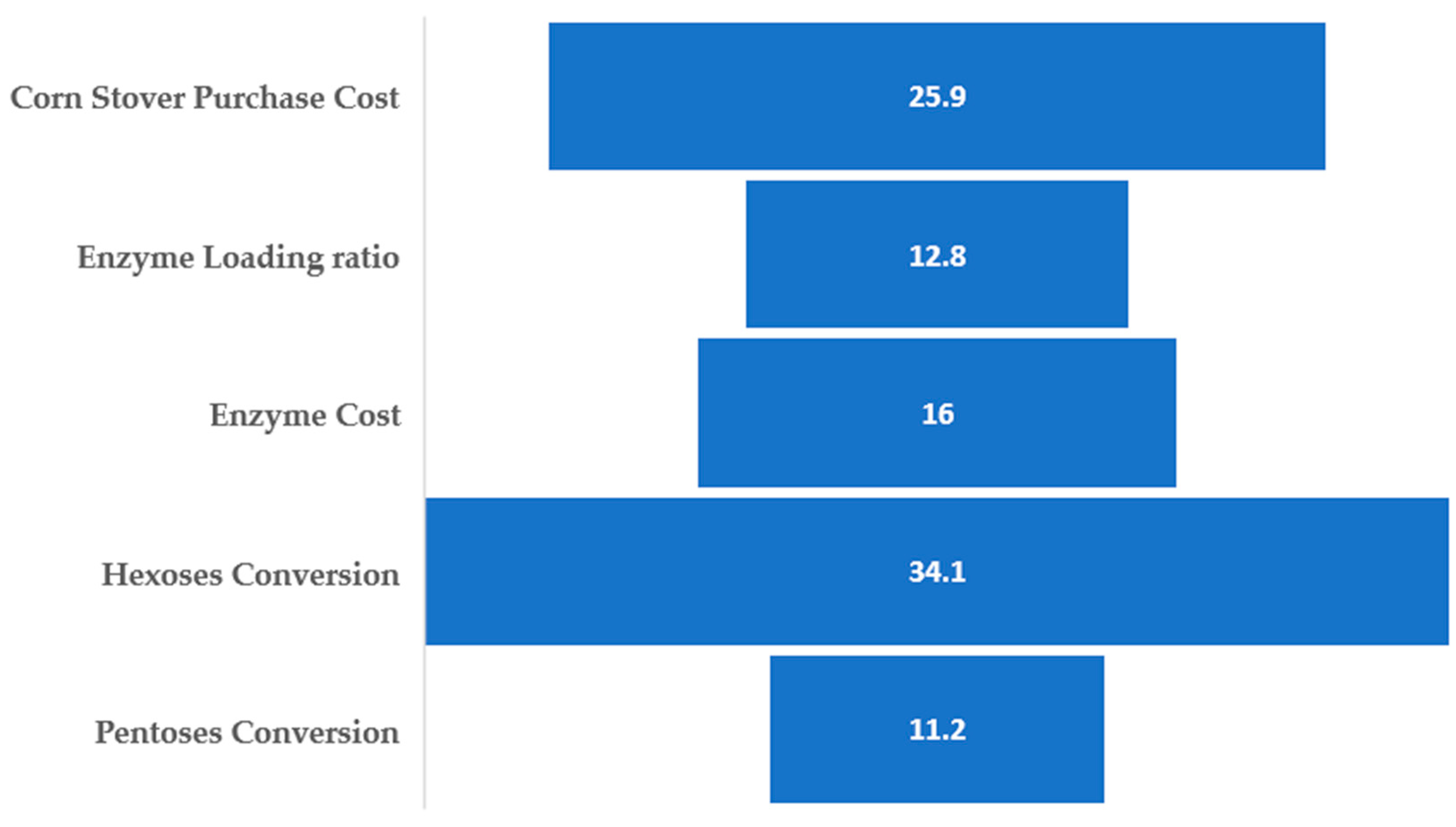
Table 4 is the ambiguity introduced by the economic and modeling assumptions. This is reflected by the large discrepancy in feedstock costs, specific TCI (i.e., the total capital investment per annual throughput), the overall reported yields per amount of dry feed, etc. As a result, the unit production cost and other related output parameters (e.g., minimum product selling price, net present value, return on investment) which provide an insight into the economic feasibility of a project may vary substantially. This kind of uncertainty is usually not considered at all or is, at best, partially addressed by single-point sensitivity analysis where specific key parameters are varied independently.
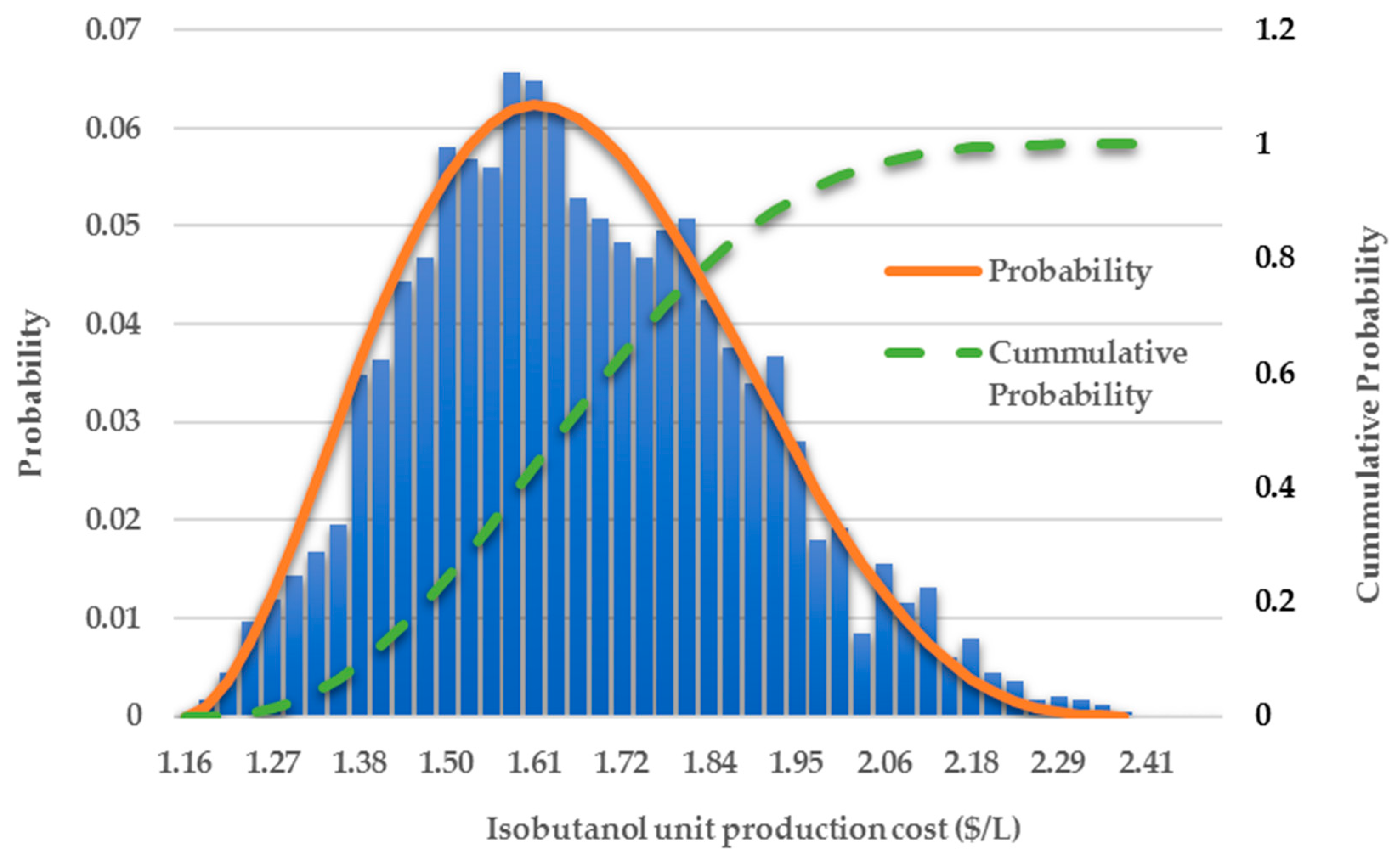
This work presents a techno-economic analysis of two alternative process scenarios for a small-scale biorefinery that produces isobutanol from corn stover. The uncertainty in the estimation of the unit production cost associated with the choice of certain key parameters (e.g., sugars conversion, corn stover cost, enzyme price and loading ratio) is addressed by a Monte Carlo simulation. This is a much better approach than the single-point sensitivity analysis because the parameters are varied simultaneously within specified ranges, so a probability distribution of the unit production cost is generated. It is then possible to estimate the probability that the unit production cost will be below a certain value that is considered to make the project economically viable.
2. Materials and Methods
The two conceptual biorefinery models for the production of isobutanol from corn stover were developed and evaluated with SuperPro Designer, a process simulator marketed by Intelligen, Inc. (Scotch Plains, NJ, USA). The first scenario (SCA) simulates a conventional batch fermentation plant without integrated product removal. In the second scenario (SCB), an integrated isobutanol removal system by vacuum stripping is considered, in order to maintain the isobutanol concentration in the fermentor below the toxic threshold. The two scenarios were compared based on equal fermentation volume and specifically, by considering eight fermentors with 500 m
3 of working volume capacity each. The main modeling and economic assumptions made in the analysis are presented in
Table 5. Those values were selected based on previous relevant publications [
8,
31,
36,
37,
38] as well as on the authors’ engineering judgement. In the case of SCA, the initial concentration of sugars in the fermentor is limited to 60 g/L and the conversion of sugars was assumed to be 85% (assuming equal conversion of pentoses and hexoses) with the remaining percentage converted to biomass cells. The integrated product removal in SCB allows for the utilization of a concentrated sugars feed with an effective concentration of 158 g/L and a slightly improved sugars conversion of 90%. Notice that experimental evidence has revealed that certain strains (e.g.,
C. beijerinckii BA101 and
C. acetobutylicum) can be fermented without substrate inhibitory effects at substrate concentrations of 180 g/L and even 200 g/L in the presence of integrated product removal [
29,
35,
39]. Alternatively, a fed-batch strategy can be adopted without changing the model results, where the substrate concentration in the fermentor at any given time does not exceed 90 g/L, as described in Ezeji et al. [
40]. Based on the above assumptions, the plant in the case of SCA has the capacity to process 6.8 MT/h of corn stover and produce 900 L/h of isobutanol while the plant in the case of SCB processes 18 MT/h of corn stover and produces 2514 L/h of isobutanol.
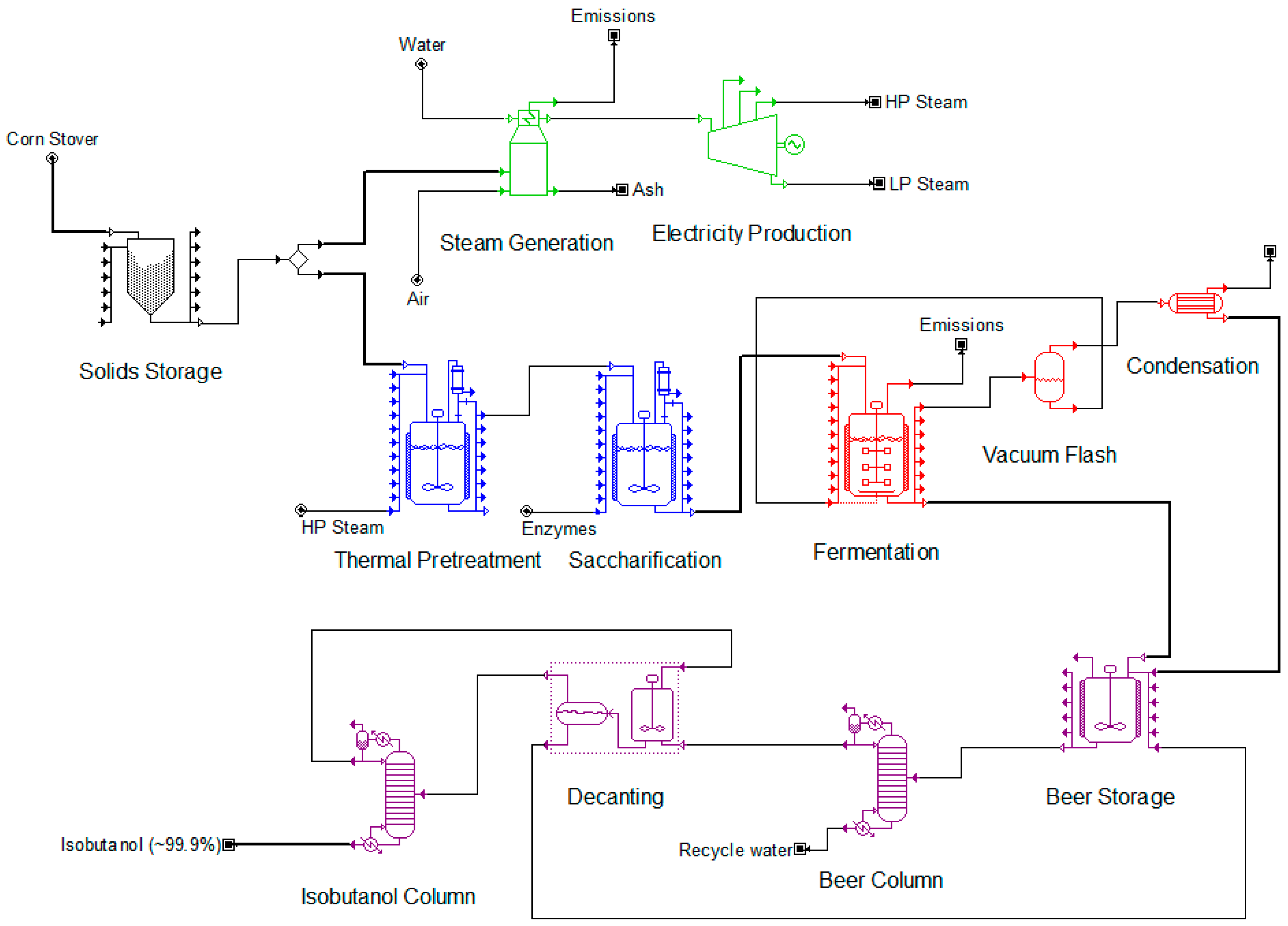
A simplified flowchart of the process illustrating its main sections, namely, the pretreatment and enzymatic hydrolysis section, the fermentation section, the purification section and the utilities section, is shown in
Figure 1. The vacuum flash chamber and condenser downstream the fermentation reactor, comprise the integrated product removal system implemented in SCB. The detailed SuperPro Designer models for the two scenarios analyzed in this study are available upon request.
2.1. Pretreatment and Enzymatic Hydrolysis Section
Corn stover is initially mixed with water and sulfuric acid (at a proportion of 0.3 wt%) and the slurry is preheated before entering the thermal pretreatment stage. During the thermal pretreatment by steam explosion, it is assumed that 10% of cellulose is converted to hexoses and 80% of hemicellulose is converted to pentoses. Subsequently, the slurry is neutralized with calcium hydroxide (at a proportion of 1 wt%) and is fed to the saccharification reactor where the enzymatic hydrolysis takes place. According to the literature, the enzyme amount required for saccharification varies from 5 to 35 mg/g cellulose [
41]. In this study, a baseline value of 20 mg/g cellulose was selected. The enzymatic hydrolysis is assumed to convert 90% of the remaining cellulose and hemicellulose to hexoses and pentoses, respectively. The hydrolyzed stream which contains 15.3 wt% hexoses, 8.9 wt% pentoses and 71.7 wt% water is filtered and directed to the fermentation section. The filtration residues are directed to the utilities section for the generation of steam and electricity.
2.2. Fermentation Section
In the case of SCA, it was assumed that the final isobutanol titer is 22 g/L with a yield of 0.37 g/g. In the case of SCB, the fermentors are equipped with a vacuum stripping system that processes ~47 m
3/h of fermentation broth, in order to maintain the isobutanol concentration in the fermentor below 22 g/L. The use of vacuum was originally proposed for the reduction of ethanol toxicity to yeast in ethanol production processes [
42,
43] and has been shown to be effective for butanol, despite the fact that the latter is less volatile than water [
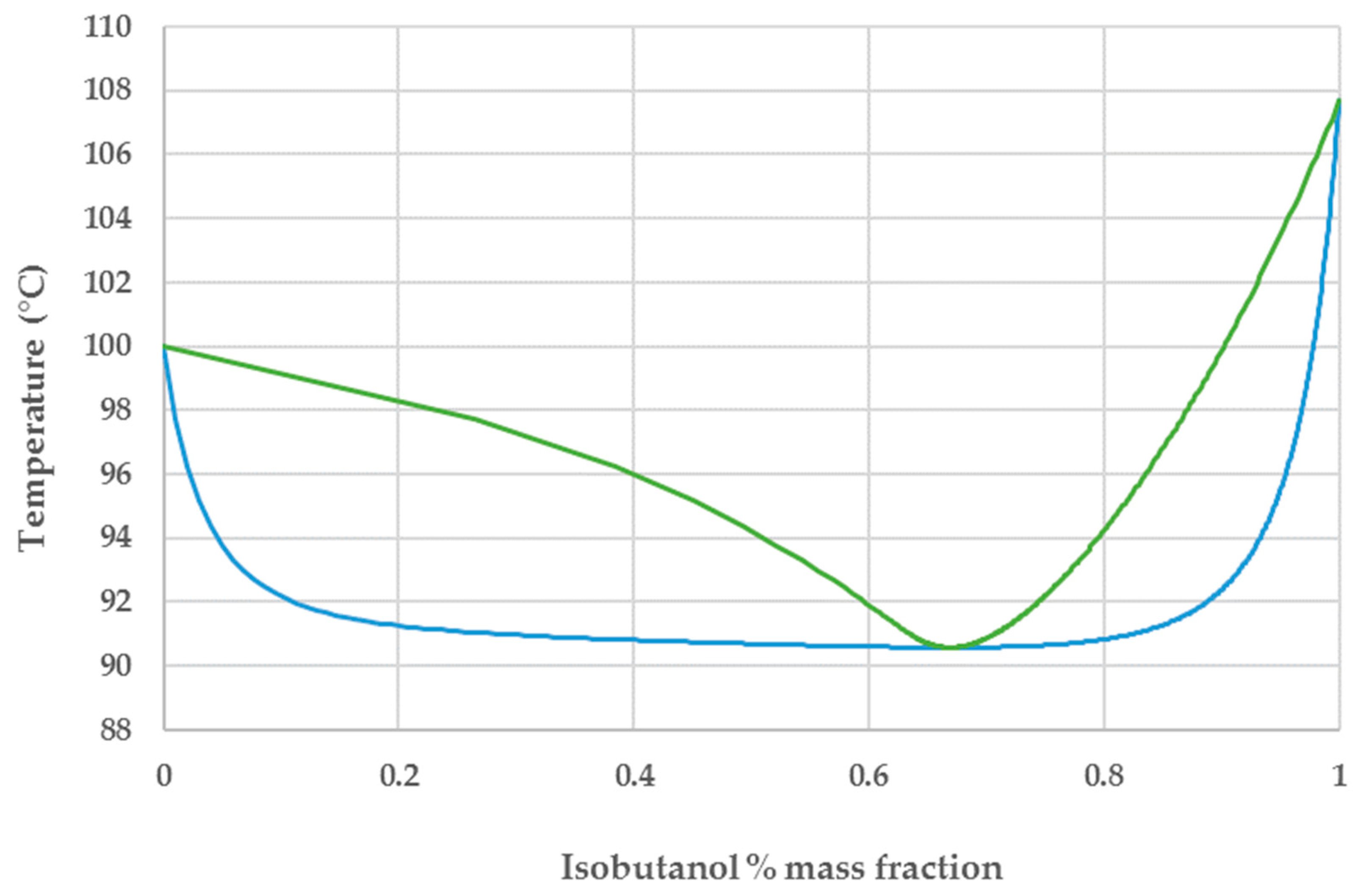
44]. The feasibility of vacuum stripping is evident from the water-isobutanol VLE chart depicted in
Figure 2. When a water–isobutanol mixture with sub-azeotropic composition is heated at a temperature above its bubble point, it forms a vapor phase where the isobutanol content is substantially increased. The fermentation broth is circulated through a vacuum flash tank operating at very low pressure (i.e., ≈7 kPa) where an isobutanol-rich vapor phase and an isobutanol-depleted liquid phase are generated. The vapor phase, after condensation, is sent to the purification section while the liquid phase is recycled back to the fermentor. The isobutanol titer in the combined stream from the final fermentation broth and condensed vapor phase is around 50 g/L, the same as the value reported by Baez et al. for gas-stripping integrated product removal [
34]. In this case, the fermentation yield is equal to 0.39 g/g.
2.3. Purification Section
As depicted in
Figure 2, the isobutanol-water mixture is characterized by an azeotrope at 66.9 wt% isobutanol [
46]. The azeotropic behavior was captured by using a modified Raoult’s law with activity coefficients calculated by the Wilson model. Isobutanol can be purified to a very high degree using a system of two distillation columns in combination with a decanter that exploits its low solubility in water to break the azeotrope [
30]. In the first column the product is concentrated by removing almost 90% of the water content. When the concentrated top stream (with sub-azeotropic composition) is allowed to settle in the decanter it forms two liquid phases, an aqueous phase with 8.7 wt% and an organic phase with 80 wt% isobutanol (i.e., above the azeotropic composition) [
8]. The aqueous phase is recycled back to the first distillation column and the organic phase is directed to the second distillation column where isobutanol can be separated to a purity of 99.9 wt%. Notice that, due to the higher amount of isobutanol in the feed of the downstream processing section in the case of SCB (i.e., three times more), the second distillation column processes almost twice the amount of material compared to SCA. Also notice that, to minimize the distillation energy requirements, the fermentation beer is preheated by the wastewater stream that comes out of the first distillation column.
2.4. Utilities Section
The utilities section uses the residual biomass from the pretreatment and enzymatic hydrolysis section to produce high-pressure and low-pressure steam as well as a modest amount of electricity. The quantity of high- and low-pressure steam produced is adequate to match the requirements of the entire process. In the case of SCA, 0.5 MT/h of corn stover needs to be diverted directly to the boiler, additionally to the residual biomass, to satisfy the above assumption. The electricity that is produced is used to cover a portion of the power requirements of the process and reduce the annual operating cost.