Effects of Conventional Flotation Frothers on the Population of Mesophilic Microorganisms in Different Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Flotation Reagents
2.3. Experimental Procedure
2.4. Feature Selection
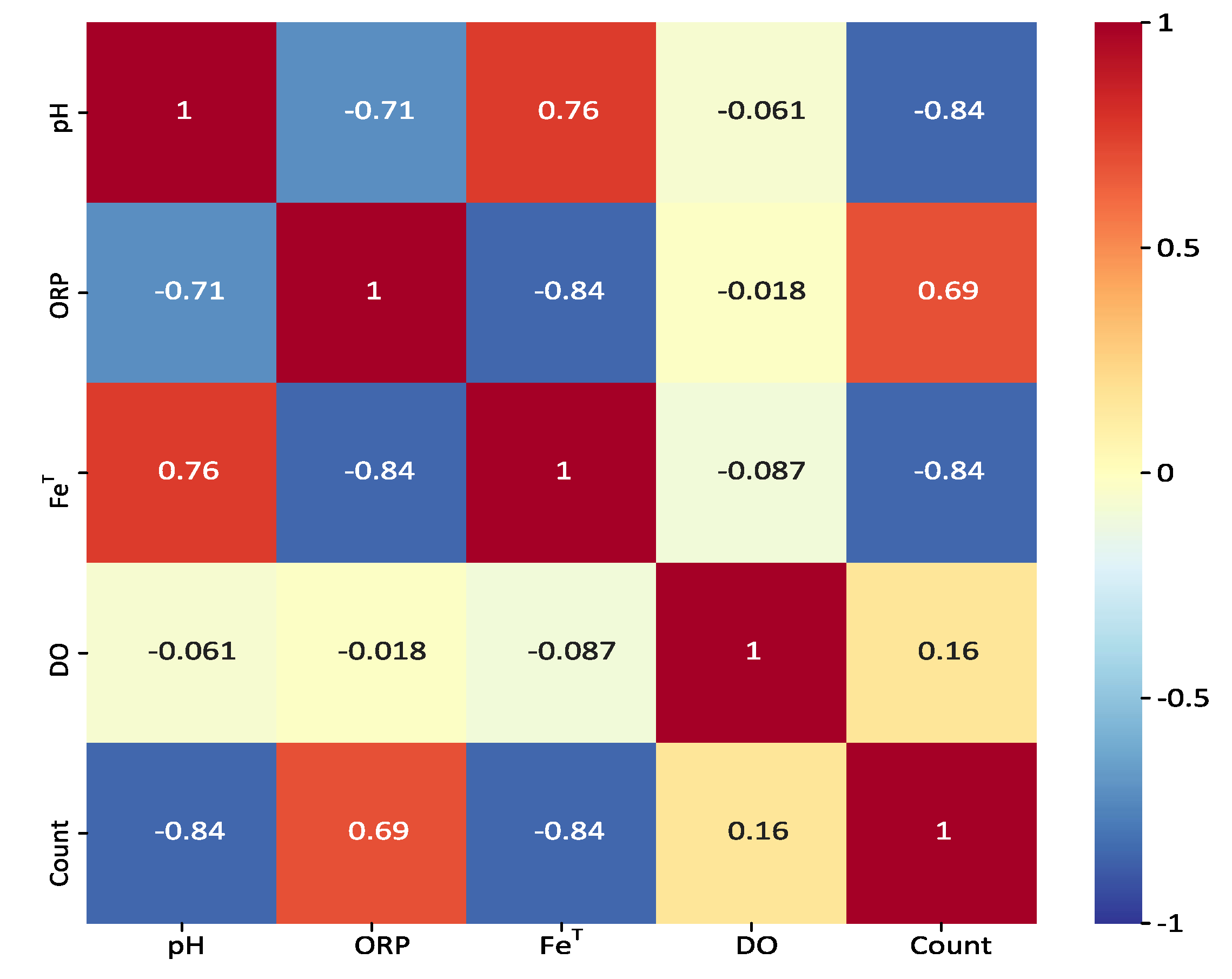
2.4.1. Pearson Correlation
2.4.2. Mutual Information
3. Results
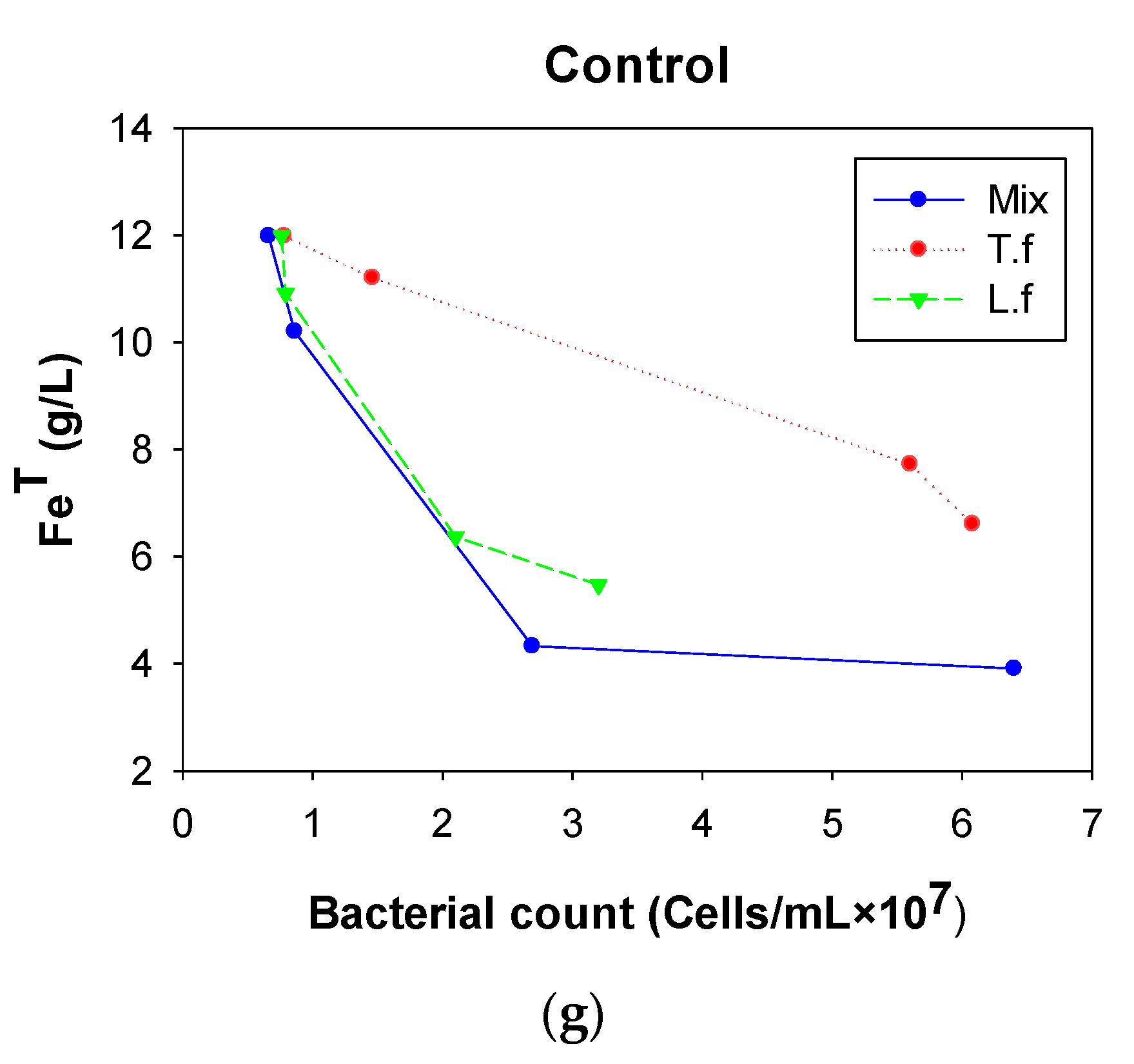
3.1. Control Test
Feature Selection
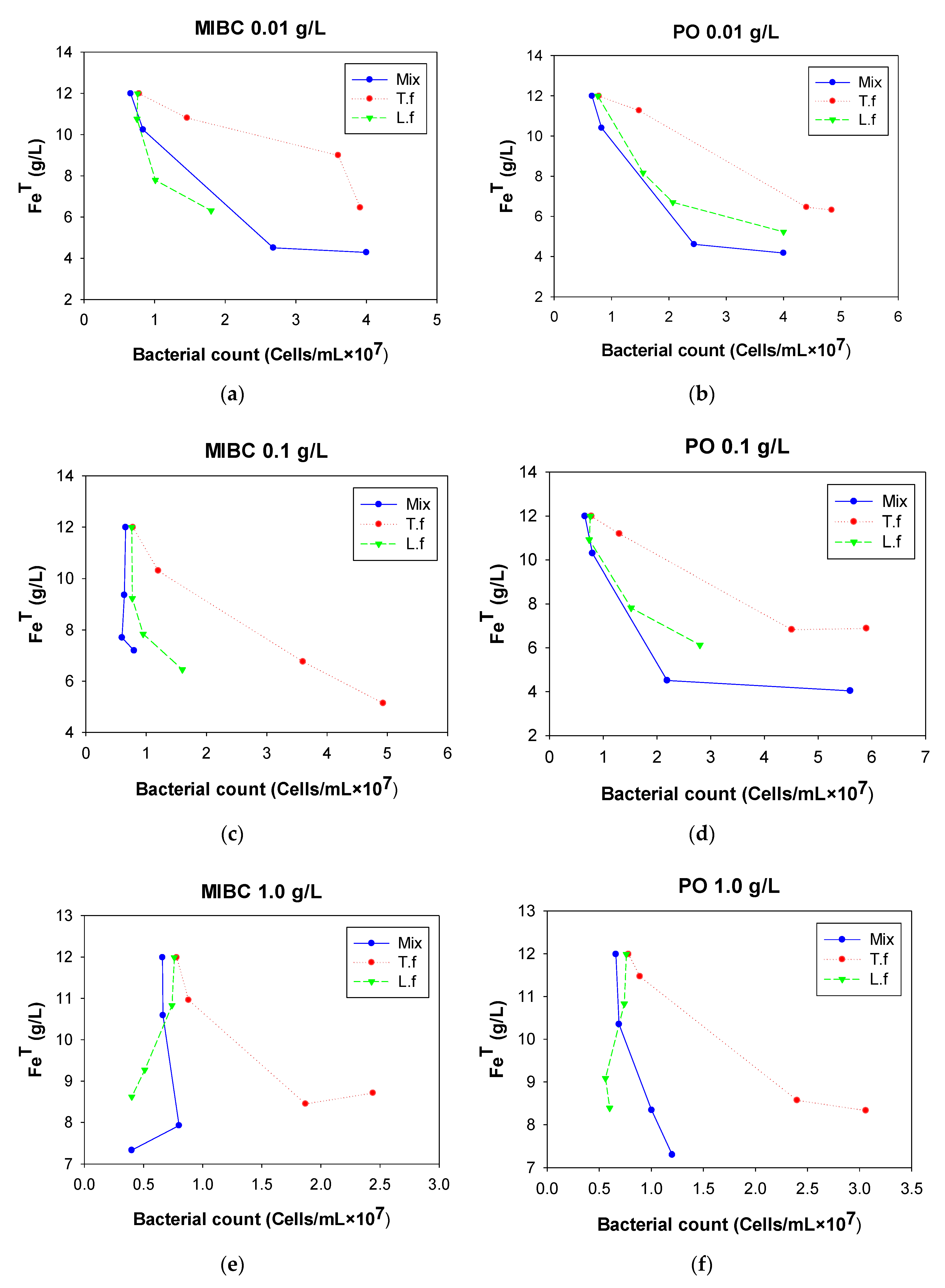
3.2. Frothers
3.2.1. Population of Microorganisms
3.2.2. Fe Total
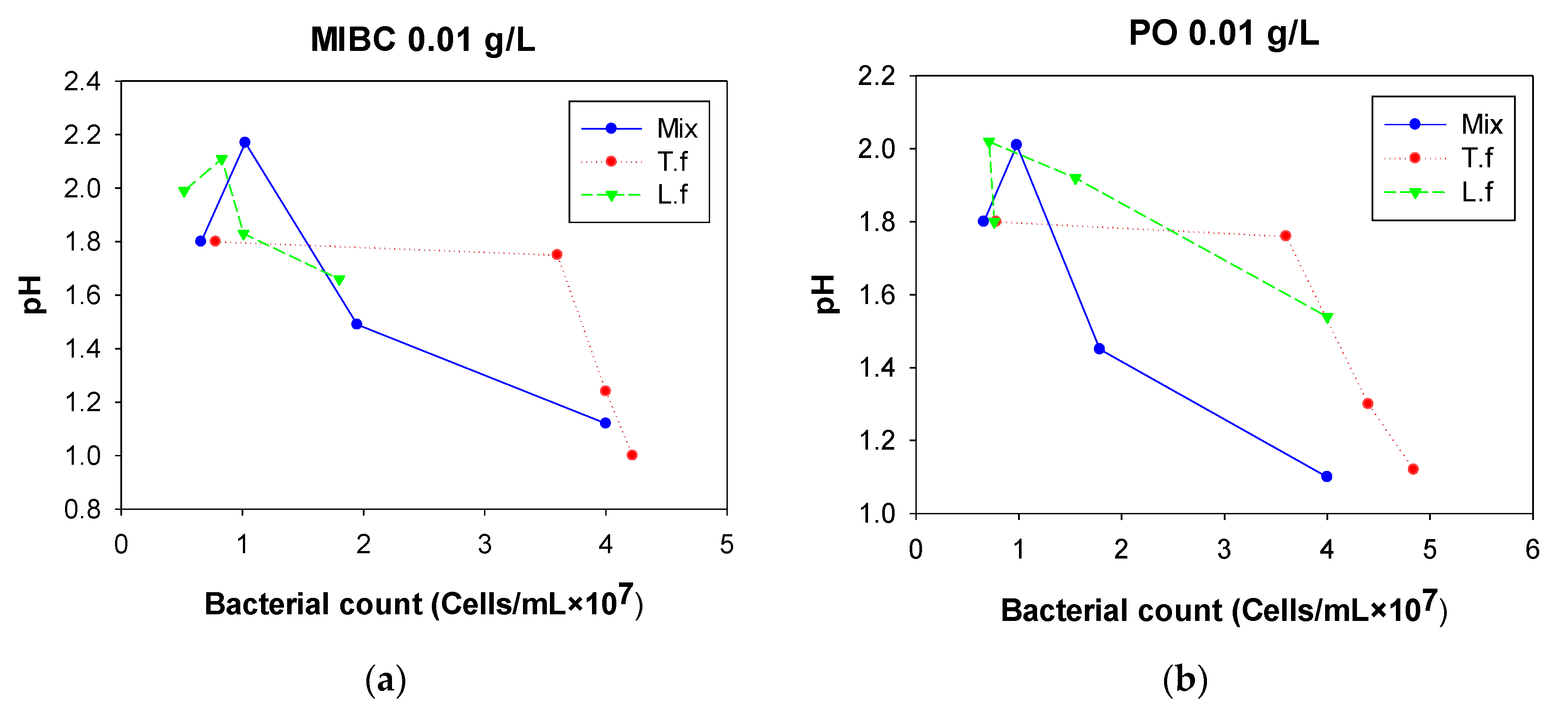
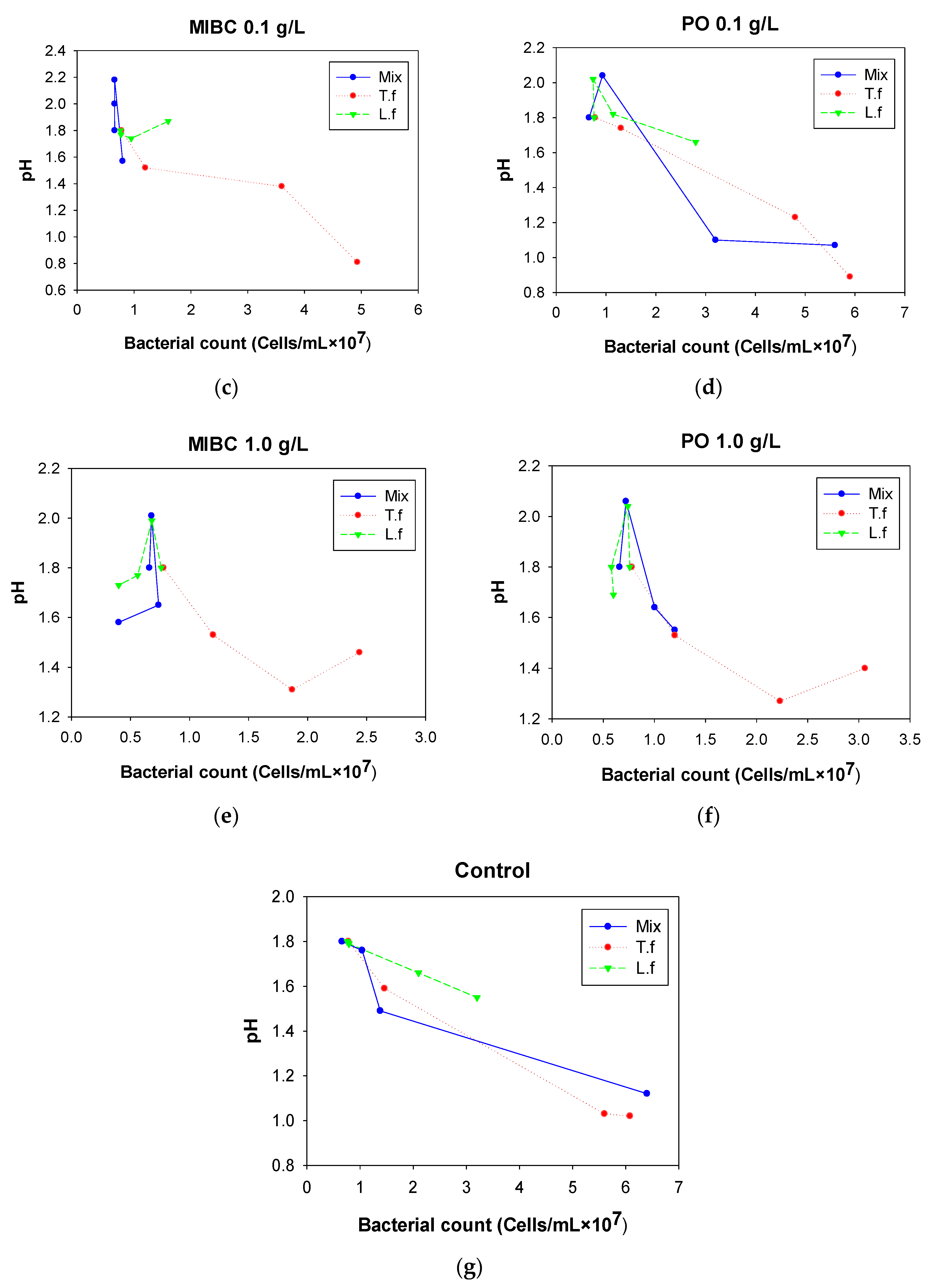
3.2.3. pH
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fugleberg, S.; Järvinen, A. Method for leaching zinc concentrate in atmospheric conditions. Google Patents: U.S. Patent No. 6,340,450, 22 January 2002. [Google Scholar]
- Baláž, P.; Achimovičová, M.; Bastl, Z.; Ohtani, T.; Sanchez, M. Influence of mechanical activation on the alkaline leaching of enargite concentrate. Hydrometallurgy 2000, 54, 205–216. [Google Scholar] [CrossRef]
- Babu, M.N.; Sahu, K.; Pandey, B. Zinc recovery from sphalerite concentrate by direct oxidative leaching with ammonium, sodium and potassium persulphates. Hydrometallurgy 2002, 64, 119–129. [Google Scholar] [CrossRef]
- Rawlings, D.E. Microbially-assisted dissolution of minerals and its use in the mining industry. Pure Appl. Chem. 2004, 76, 847–859. [Google Scholar] [CrossRef]
- Pradhan, N.; Das, B.; Gahan, C.S.; Kar, R.N.; Sukla, L.B. Beneficiation of iron ore slime using Aspergillus niger and Bacillus circulans. Bioresour. Technol. 2006, 97, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Brierley, C. Biohydrometallurgical prospects. Hydrometallurgy 2010, 104, 324–328. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, S.; Rao, D.S.; Pradhan, N.; Mohapatra, U.; Angadi, S.; Mishra, B.K. Extraction of copper from copper slag: Mineralogical insights, physical beneficiation and bioleaching studies. Korean J. Chem. Eng. 2015, 32, 667–676. [Google Scholar] [CrossRef]
- Akcil, A. Potential bioleaching developments towards commercial reality: Turkish metal mining’s future. Miner. Eng. 2004, 17, 477–480. [Google Scholar] [CrossRef]
- Anjum, F.; Bhatti, H.N.; Asgher, M.; Shahid, M. Leaching of metal ions from black shale by organic acids produced by Aspergillus niger. Appl. Clay Sci. 2010, 47, 356–361. [Google Scholar] [CrossRef]
- D’Hugues, P.; Spolaore, P.; Consortium, B. Biohydrometallurgy applied to exploitation of black shale resources: Overview of Bioshale FP6 European project. Trans. Nonferrous Met. Soc. China 2008, 18, 1485–1490. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Borja, D.; Nguyen, K.; Silva, R.; Park, J.; Gupta, V.; Han, Y.; Lee, Y.; Kim, H. Experiences and future challenges of bioleaching research in South Korea. Minerals 2016, 6, 128. [Google Scholar] [CrossRef]
- Borja, D.; Nguyen, K.A.; Silva, R.A.; Ngoma, E.; Petersen, J.; Harrison, S.T.; Park, J.H.; Kim, H. Continuous bioleaching of arsenopyrite from mine tailings using an adapted mesophilic microbial culture. Hydrometallurgy 2019, 187, 187–194. [Google Scholar] [CrossRef]
- Ngoma, E.; Borja, D.; Smart, M.; Shaik, K.; Kim, H.; Petersen, J.; Harrison, S.T. Bioleaching of arsenopyrite from Janggun mine tailings (South Korea) using an adapted mixed mesophilic culture. Hydrometallurgy 2018, 181, 21–28. [Google Scholar] [CrossRef]
- Hong, J.; Silva, R.A.; Park, J.; Lee, E.; Park, J.; Kim, H. Adaptation of a mixed culture of acidophiles for a tank biooxidation of refractory gold concentrates containing a high concentration of arsenic. J. Biosci. Bioeng. 2016, 121, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, Y.; Lee, E.; Choi, U.; Yoo, K.; Song, Y.; Kim, H. Bioleaching of highly concentrated arsenic mine tailings by Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2014, 133, 291–296. [Google Scholar] [CrossRef]
- Brierley, C. How will biomining be applied in future? Trans. Nonferrous Met. Soc. China 2008, 18, 1302–1310. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: a review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef]
- Torma, A.E.; Walden, C.; Branion, R. Microbiological leaching of a zinc sulfide concentrate. Biotechnol. Bioeng. 1970, 12, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Pourhossein, F.; Mousavi, S.M. A novel step-wise indirect bioleaching using biogenic ferric agent for enhancement recovery of valuable metals from waste light emitting diode (WLED). J. Hazard. Mater. 2019, 378, 120648. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Yan, W.; Chen, L.; Mahadevan, G.D.; Zhao, F. Enhanced bioleaching efficiency of metals from E-wastes driven by biochar. J. Hazard. Mater. 2016, 320, 393–400. [Google Scholar] [CrossRef]
- Gahan, C.S.; Sundkvist, J.E.; Sandström, Å. A study on the toxic effects of chloride on the biooxidation efficiency of pyrite. J. Hazard. Mater. 2009, 172, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Q.; Zheng, P.; Cai, J.; Wu, D.; Hu, B.; Li, J. Anoxic sulfide biooxidation using nitrite as electron acceptor. J. Hazard. Mater. 2007, 147, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Abdollahi, H.; Shafaei, S.Z.; Gharabaghi, M.; Jafari, H.; Akcil, A.; Panda, S. Acidophilic bioleaching: a review on the process and effect of organic–inorganic reagents and materials on its efficiency. Miner. Process. Extr. Met. Rev. 2018, 40, 87–107. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaei, S.; Abdollahi, H.; Gharabaghi, M.; Chehreh Chelgani, S. Effect of flotation reagents on the activity of L. ferrooxidans. Miner. Process. Extr. Met. Rev. 2018, 39, 34–43. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaei, S.Z.; Abdollahi, H.; Gharabaghi, M.; Chehreh Chelgani, S.; Ghassa, S. Examining the effects of typical reagents for sulfide flotation on bio-oxidation activity of ferrous iron oxidizing microorganisms. In Proceedings of Solid State Phenomena; Trans Tech Publications: freiberg, Germany, 2017; Volume 262, pp. 84–87. [Google Scholar]
- Dehghan, R.; Dianati, M. The effects of Pb-Zn flotation reagents on the bioleaching process by mesophilic bacteria. Int. J. Mineral. Process. 2015, 143, 80–86. [Google Scholar] [CrossRef]
- Loon, H.Y.; Madgwick, J. The effect of xanthate floatation reagents on bacterial leaching of chalcopyrite by Thiobacillus ferrooxidans. Biotechnol. Lett. 1995, 17, 997–1000. [Google Scholar] [CrossRef]
- Puhakka, J.; Tuovinen, O.H. Effect of organic compounds on the microbiological leaching of a complex sulphide ore material. MIRCEN J. Appl. Microbiol. Biotechnol. 1987, 3, 429–436. [Google Scholar] [CrossRef]
- Zhang, C.G.; Xia, J.L.; Zhang, R.Y.; Peng, A.A.; Nie, Z.Y.; Qiu, G.Z. Comparative study on effects of Tween-80 and sodium isobutyl-xanthate on growth and sulfur-oxidizing activities of Acidithiobacillus albertensis BY-05. Trans. Nonferrous Met. Soc. China 2008, 18, 1003–1007. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaie, S.; Abdollahi, H.; Gharabaghi, M.; Chehreh Chelgani, S. Study of the effects of conventional reagents for sulfide flotation on bio-oxidation activity of Acidithiobacillus ferrooxidans. Chem. Eng. Commun. 2019, 206, 365–377. [Google Scholar] [CrossRef]
- Rawlings, D.E. Biomining: Theory, Microbes And Industrial Processes; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Battaglia-Brunet, F.; d’Hugues, P.; Cabral, T.; Cezac, P.; Garcia, J.-L.; Morin, D. The mutual effect of mixed thiobacilli and leptospirilli populations on pyrite bioleaching. Miner. Eng. 1998, 11, 195–205. [Google Scholar] [CrossRef]
- Norris, P. Iron and mineral oxidation with Leptospirillum-like bacteria. In Proceedings of International symposium on biohydrometallurgy; Recent Progress in Biohydrometalurgy: Cagliari, Canada, 1983; pp. 83–96. [Google Scholar]
- Goebel, B.M.; Stackebrandt, E. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl. Environ. Microbiol. 1994, 60, 1614–1621. [Google Scholar] [PubMed]
- Gomez, E.; Ballester, A.; Blázquez, M.; Gonzalez, F. Silver-catalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms. Hydrometallurgy 1999, 51, 37–46. [Google Scholar] [CrossRef]
- Tuovinen, O.; Kelley, B.; Groudev, S. Mixed cultures in biological leaching processes and mineral biotechnology. In Mixed Cultures in Biotechnology; McGraw-Hill Book Co.: New York, NY, USA, 1991; pp. 373–427. [Google Scholar]
- Akcil, A.; Ciftci, H.; Deveci, H. Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner. Eng. 2007, 20, 310–318. [Google Scholar] [CrossRef]
- Akcil, A.; Ciftci, H. Bacterial leaching of Kure copper ore. J. Chamb. Min. Eng. Turk. 2003, 42, 15–25. [Google Scholar]
- Dong, Y.; Lin, H. Influences of flotation reagents on bioleaching of chalcopyrite by Acidthiobacillus ferrooxidans. Miner. Eng. 2012, 32, 27–29. [Google Scholar] [CrossRef]
- Huerta, G.; Escobar, B.; Rubio, J.; Badilla-Ohlbaum, R. Adverse effect of surface-active reagents on the bioleaching of pyrite and chalcopyrite by Thiobacillus ferrooxidans. World J. Microbiol. Biotechnol. 1995, 11, 599–600. [Google Scholar] [CrossRef]
- Dopson, M.; Sundkvist, J.-E.; Lindström, E.B. Toxicity of metal extraction and flotation chemicals to Sulfolobus metallicus and chalcopyrite bioleaching. Hydrometallurgy 2006, 81, 205–213. [Google Scholar] [CrossRef]
- Zeng, G.-M.; Shi, J.-G.; Yuan, X.-Z.; Liu, J.; Zhang, Z.-B.; Huang, G.-H.; Li, J.-B.; Xi, B.-D.; Liu, H.-L. Effects of Tween 80 and rhamnolipid on the extracellular enzymes of Penicillium simplicissimum isolated from compost. Enzym. Microb. Technol. 2006, 39, 1451–1456. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D.B. Toxicity of flotation reagents to moderately thermophilic bioleaching microorganisms. Biotechnol. Lett. 2002, 24, 2011–2016. [Google Scholar] [CrossRef]
- Foucher, S.; Battaglia-Brunet, F.; d’Hugues, P.; Clarens, M.; Godon, J.; Morin, D. Evolution of the bacterial population during the batch bioleaching of a cobaltiferous pyrite in a suspended-solids bubble column and comparison with a mechanically agitated reactor. Hydrometallurgy 2003, 71, 5–12. [Google Scholar] [CrossRef]
- Nazari, S.; Chelgani, S.C.; Shafaei, S.; Shahbazi, B.; Matin, S.; Gharabaghi, M. Flotation of coarse particles by hydrodynamic cavitation generated in the presence of conventional reagents. Sep. Purif. Technol. 2019, 220, 61–68. [Google Scholar] [CrossRef]
- Matin, S.; Farahzadi, L.; Makaremi, S.; Chelgani, S.C.; Sattari, G. Variable selection and prediction of uniaxial compressive strength and modulus of elasticity by random forest. Appl. Soft Comput. 2018, 70, 980–987. [Google Scholar] [CrossRef]
- Hadavandi, E.; Chelgani, S.C. Estimation of coking indexes based on parental coal properties by variable importance measurement and boosted-support vector regression method. Measurement 2019, 135, 306–311. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.; Shahbazi, B.; Chelgani, S.C. Study relationships between flotation variables and recovery of coarse particles in the absence and presence of nanobubble. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 284–288. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Matin, S.S. Study the relationship between coal properties with Gieseler plasticity parameters by random forest. Int. J. OilGas. Coal Technol. 2018, 17, 113–127. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Shahbazi, B.; Hadavandi, E. Support vector regression modeling of coal flotation based on variable importance measurements by mutual information method. Measurement 2018, 114, 102–108. [Google Scholar] [CrossRef]
- Schippers, A.; Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar]
- Bosecker, K. Bioleaching: metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Stott, M.; Watling, H.; Franzmann, P.; Sutton, D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Miner. Eng. 2000, 13, 1117–1127. [Google Scholar] [CrossRef]
- Rohwerder, T.; Sand, W. The sulfane sulfur of persulfides is the actual substrate of the sulfur-oxidizing enzymes from Acidithiobacillus and Acidiphilium spp. Microbiology 2003, 149, 1699–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeling, S.; Palmer, M.; Caracatsanis, F.; Johnson, J.; Watling, H. Leaching of chalcopyrite and sphalerite using bacteria enriched from a spent chalcocite heap. Miner. Eng. 2005, 18, 1289–1296. [Google Scholar] [CrossRef]
- Brandl, H. Microbial leaching of metals. In Biotechnology Set, 2nd ed.; WILEY: Zürich, Switzerland, 2008; pp. 191–224. [Google Scholar]
- Silva, R.A.; Park, J.; Lee, E.; Park, J.; Choi, S.Q.; Kim, H. Influence of bacterial adhesion on copper extraction from printed circuit boards. Sep. Purif. Technol. 2015, 143, 169–176. [Google Scholar] [CrossRef]
- Dutrizac, J. Factors affecting alkali jarosite precipitation. Metall. Trans. B 1983, 14, 531–539. [Google Scholar] [CrossRef]
- Chowdhury, F.; Ojumu, T. Investigation of ferrous-iron biooxidation kinetics by Leptospirillum ferriphilum in a novel packed-column bioreactor: Effects of temperature and jarosite accumulation. Hydrometallurgy 2014, 141, 36–42. [Google Scholar] [CrossRef]
- Silva, R.A.; Borja, D.; Hwang, G.; Hong, G.; Gupta, V.; Bradford, S.A.; Zhang, Y.; Kim, H. Analysis of the effects of natural organic matter in zinc beneficiation. J. Clean. Prod. 2017, 168, 814–822. [Google Scholar] [CrossRef]
- Deo, N.; Natarajan, K. Biological removal of some flotation collector reagents from aqueous solutions and mineral surfaces. Miner. Eng. 1998, 11, 717–738. [Google Scholar] [CrossRef]
- Pomianowski, A.; Leja, J. Spectrophotometric study of xanthate and dixanthogen solutions. Can. J. Chem. 1963, 41, 2219–2230. [Google Scholar] [CrossRef]
- Jones, M.; Woodcock, J. Decomposition of alkyl dixanthogens in aqueous solutions. Int. J. Mineral. Process. 1983, 10, 1–24. [Google Scholar] [CrossRef]
- Khoshdast, H.; Sam, A. Flotation frothers: review of their classifications, properties and preparation. Open Mineral. Process. J. 2011, 4, 25–44. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Sun, Z.; Forsling, W. The degradation kinetics of ethyl-xanthate as a function of pH in aqueous solution. Miner. Eng. 1997, 10, 389–400. [Google Scholar] [CrossRef]
- Boxall, N.J.; Cheng, K.Y.; Bruckard, W.; Kaksonen, A.H. Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries. J. Hazard. Mater. 2018, 360, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Akinci, G.; Guven, D.E. Bioleaching of heavy metals contaminated sediment by pure and mixed cultures of Acidithiobacillus spp. Desalination 2011, 268, 221–226. [Google Scholar] [CrossRef]
- Liu, G.; Yin, J.; Cong, W. Effect of fluid shear and particles collision on the oxidation of ferrous iron by Acidithiobacillus ferrooxidans. Miner. Eng. 2007, 20, 1227–1231. [Google Scholar] [CrossRef]
- Smith, R. Liquid and solid wastes from mineral processing plants. Mineral. Process. Extr. Metullargy Rev. 1996, 16, 1–22. [Google Scholar] [CrossRef]
- Pacholewska, M.; Cwalina, B.; Steindor, K. The influence of flotation reagents on sulfur-oxidizing bacteria Acidithiobacillus thiooxidans. Physicochem. Probl. Mineral. Process. 2008, 42, 37–46. [Google Scholar]
- Tuovinen, O.H. Inhibition of Thiobacillus ferrooxidans by mineral flotation reagents. Appl. Microbiol. Biotechnol. 1978, 5, 301–304. [Google Scholar] [CrossRef]
- Torma, A.E.; Gabra, G.; Guay, R.; Silver, M. Effects of surface active agents on the oxidation of chalcopyrite by Thiobacillus ferrooxidans. Hydrometallurgy 1976, 1, 301–309. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaei, S.; Abdollahi, H.; Gharabaghi, M.; Chehreh Chelgani, S. A comparative study on the effect of flotation reagents on growth and iron oxidation activities of Leptospirillum ferrooxidans and Acidithiobacillus ferrooxidans. Minerals 2016, 7, 2. [Google Scholar] [CrossRef]
- Iwasaki, I.; Cooke, S. Dissociation constant of xanthic acid as determined by spectrophotometric method. J. Phys. Chem. 1959, 63, 1321–1322. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Qin, W.-Q.; Jun, W.; Zhen, S.-J.; Yang, C.-R.; Zhang, J.-W.; Nai, S.-S.; Qiu, G.-Z. Bioleaching of chalcopyrite by pure and mixed culture. Trans. Nonferrous Met. Soc. China 2008, 18, 1491–1496. [Google Scholar] [CrossRef]

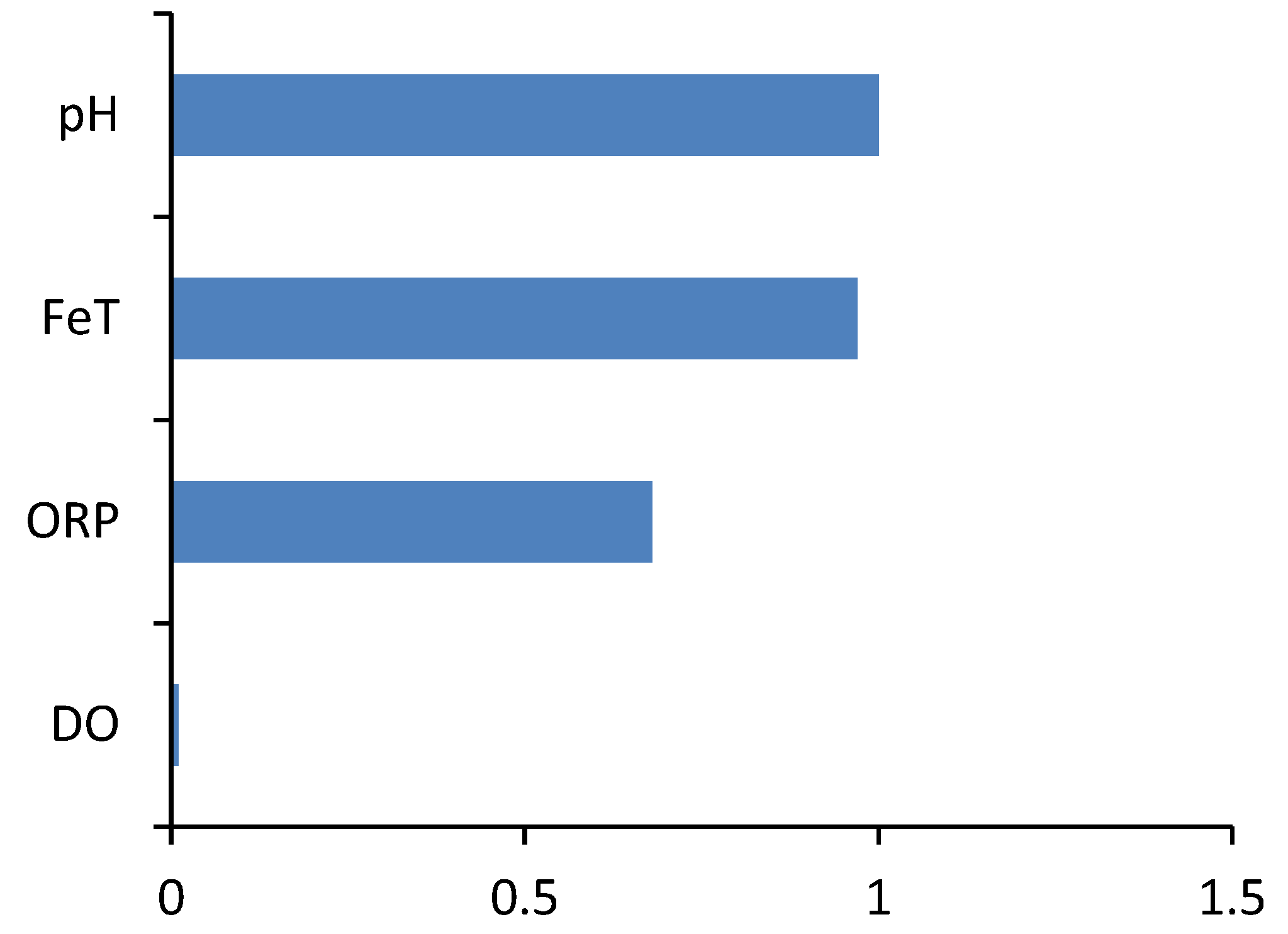
| No. | Microorganism | Goal Metal | Process | Reagent | Description | Ref |
|---|---|---|---|---|---|---|
| 1 | Acidithiobacillus ferrooxidans | Copper | Bioleaching | Butyl amine | −45.0% (Cu-Recovery) | [41] |
| Ethyl-xanthate | −36.7% (Cu-Recovery) | |||||
| Isoamyl-xanthate | −20.0% (Cu-Recovery) | |||||
| Butyl-xanthate | −11.7% (Cu-Recovery) | |||||
| 2 | Acidithiobacillus ferrooxidans | Copper | Bioleaching | Isopropyl-xanthate | −30.0% (Cu-Recovery) | [42] |
| Iron (Pyrite) | Biooxidation | −50.0% (Fe-Oxidation) | ||||
| 3 | Sulfolobus metallicus | Copper | Bioleaching | Hostaflot X23 | −14.0% (Cu-Recovery) | [43] |
| Aero 3477 | −34.0% (Cu-Recovery) | |||||
| Flotanol C-7 | −27.0% (Cu-Recovery) | |||||
| Montanol 800 | −30.0% (Cu-Recovery) | |||||
| 4 | Leptospirillum ferrooxidans | Iron (ferrous) | Biooxidation | Potassium amyl-xanthate | The inhibition effect of reagents (collector): NaEX > KAX > KIBX > KIPX > Aero3477 For frothers: MIBC > PO | [26] |
| Potassium isobutyl-xanthate | ||||||
| Sodium ethyl-xanthate | ||||||
| Potassium isopropyl-xanthate | ||||||
| Dithiophosphate (Aero 3477) | ||||||
| Methyl isobutyl carbinol (MIBC) | ||||||
| Pine oil | ||||||
| 5 | Acidithiobacillus ferrooxidans | Copper | Bioleaching | Isobutyl-xanthate | −53.0% (Cu-Recovery) | [29] |
| Amyl-xanthate | −77.0% (Cu-Recovery) | |||||
| 6 | Acidithiobacillus albertensis | Sulfur | Biooxidation | Sodium isobutyl-xanthate | + on the growth and S0 oxidation | [31] |
| Tween-80 | + on the growth and S0 oxidation | |||||
| 7 | Penicillium simplicissimum | Cellulose | Decomposition | Tween-80 | +11.60% | [44] |
| Hemicellulose | + 8.00% | |||||
| 8 | Acidithiobacillus ferrooxidans | Iron (ferrous) | Biooxidation | Potassium amyl-xanthate | The inhibition effect of reagents (collector): KAX > KIPX > KIBX > Aero3477 > NaEX For frothers: MIBC > PO | [32] |
| Potassium isobutyl-xanthate | ||||||
| Sodium ethyl-xanthate | ||||||
| Potassium isopropyl-xanthate | ||||||
| Dithiophosphate (Aero 3477) | ||||||
| MIBC | ||||||
| Pine oil | ||||||
| 9 | Ferroplasma | Iron (ferrous) | Biooxidation | Sodium ethyl xanthate | All reagents have a negative effect on the biooxidation of iron. | [45] |
| 10 | Acidithiobacillus ferrooxidans | Sodium (alkyl) dithiocarbamate | ||||
| 11 | Leptospirillum | Xanthate (Mix) | ||||
| Sodium ethyl-xanthate | ||||||
| Sodium n-propyl xanthate | ||||||
| Sodium isobutyl xanthate | ||||||
| Potassium amyl xanthate | ||||||
| Potassium n-butyl xanthate | ||||||
| Sodium (alkyl) dithiocarbamate | ||||||
| Sodium (alkyl) dithiocarbamate and sodium di-(alkyl) dithiophosphate | ||||||
| Isopropylthionocarbamate | ||||||
| Dithiophosphate(mixture) | ||||||
| Sodium 2-mercaptobenzthiazole |
| Microorganism | ||
|---|---|---|
| Acidithiobacillus ferrooxidans (T.f) | √ | √ |
| Leptospirillum ferrooxidans (L.f) | - | √ |
| Acidithiobacillus thiooxidans (T.f) | √ | - |
| 9K Culture | pH 1 | FeSO4·7H2O | S0 | Incubation Temperature | Rotation Speed | ||||
|---|---|---|---|---|---|---|---|---|---|
| (NH4)2SO4 | MgSO4·7H2O | K2HPO4 | KCL | Ca (NO3)2·H2O | |||||
| 3.00 g/L | 0.50 g/L | 0.50 g/L | 1.00 g/L | 0.01 g/L | 1.80 | 44.22 g/L | 10.00 g/L | 34.00 °C | 140 rpm |
| Parameter | Definition |
|---|---|
| pH | The pH and ORP value of tests were measured by pH-ORP analyzer (Mettler Toledo). |
| ORP | |
| DO | An oxygen-meter (Model JENWEY) was used to measure the amount of dissolved oxygen in the media. |
| FeT | The amounts of FeT were determined by atomic adsorption Spectro-photometer (AAS). |
| Count | The bacterial number (growth) was determined by using a Neubauer lam and 100 × magnification under a Zeiss biological microscope (Bacterial count per mL = N × 400 × 104), it could be indirect evidence of cell activity and cannot capture the non-culturable cells. |
| MIBC (g/L) | ||||
| Culture | Control | 0.01 | 0.1 | 1 |
| T.f | 7.8 | 5.4 | 6.3 | 3.1 |
| L.f | 4.2 | 2.4 | 2.1 | 0.5 |
| Mixed | 9.7 | 5.9 | 1.2 | 0.6 |
| PO (g/L) | ||||
| Culture | Control | 0.01 | 0.1 | 1 |
| T.f | 7.8 | 6.2 | 7.6 | 3.9 |
| L.f | 4.2 | 5.3 | 3.7 | 0.8 |
| Mixed | 9.7 | 6 | 8.5 | 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, M.; Golzadeh, M.; Shafaei, S.Z.; Abdollahi, H.; Gharabaghi, M.; Chehreh Chelgani, S. Effects of Conventional Flotation Frothers on the Population of Mesophilic Microorganisms in Different Cultures. Processes 2019, 7, 653. https://doi.org/10.3390/pr7100653
Jafari M, Golzadeh M, Shafaei SZ, Abdollahi H, Gharabaghi M, Chehreh Chelgani S. Effects of Conventional Flotation Frothers on the Population of Mesophilic Microorganisms in Different Cultures. Processes. 2019; 7(10):653. https://doi.org/10.3390/pr7100653
Chicago/Turabian StyleJafari, Mohammad, Mehdi Golzadeh, Sied Ziaedin Shafaei, Hadi Abdollahi, Mahdi Gharabaghi, and Saeed Chehreh Chelgani. 2019. "Effects of Conventional Flotation Frothers on the Population of Mesophilic Microorganisms in Different Cultures" Processes 7, no. 10: 653. https://doi.org/10.3390/pr7100653
APA StyleJafari, M., Golzadeh, M., Shafaei, S. Z., Abdollahi, H., Gharabaghi, M., & Chehreh Chelgani, S. (2019). Effects of Conventional Flotation Frothers on the Population of Mesophilic Microorganisms in Different Cultures. Processes, 7(10), 653. https://doi.org/10.3390/pr7100653








