Supported Ionic Liquid Membranes for Separation of Lignin Aqueous Solutions
Abstract
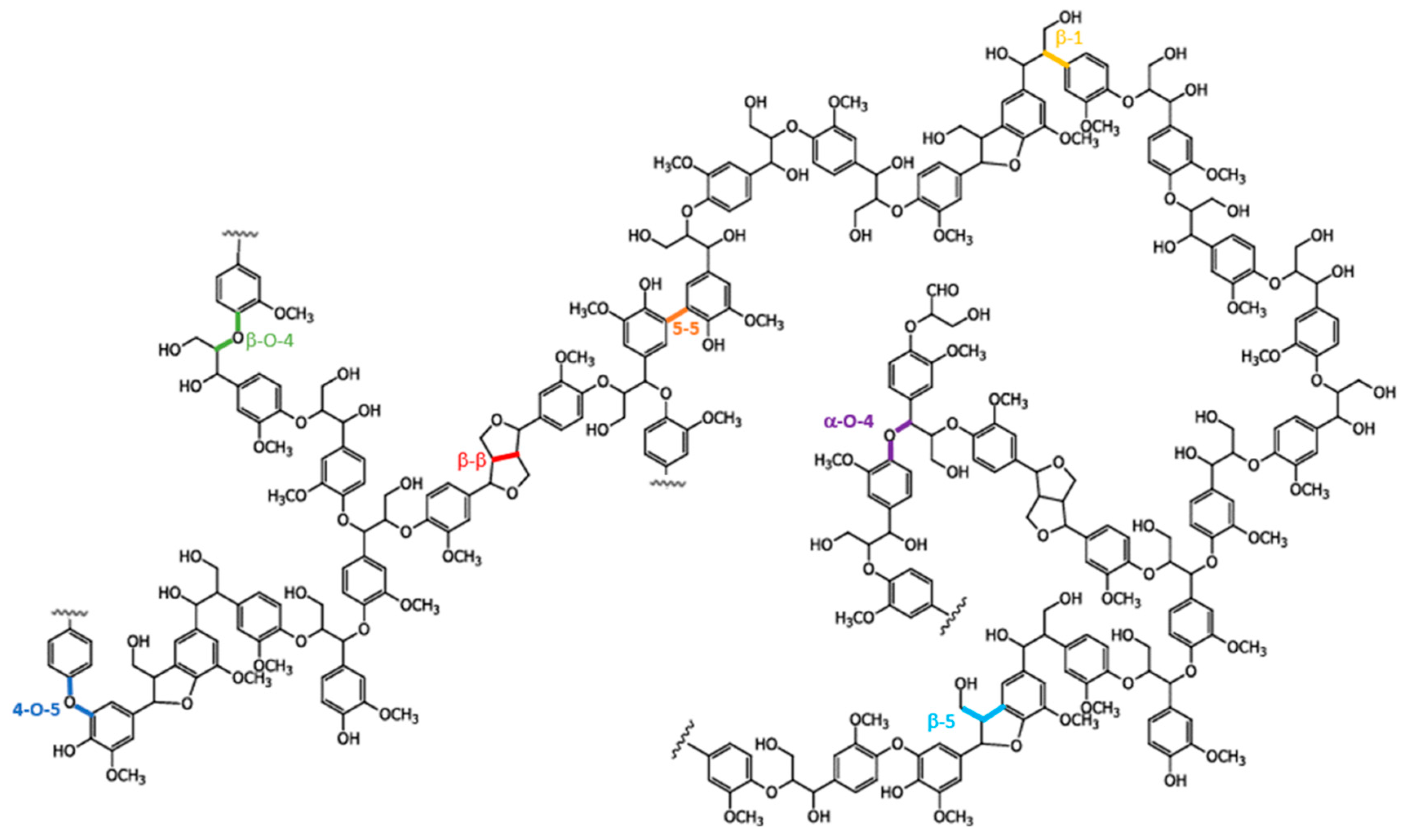
1. Introduction
2. Experimental
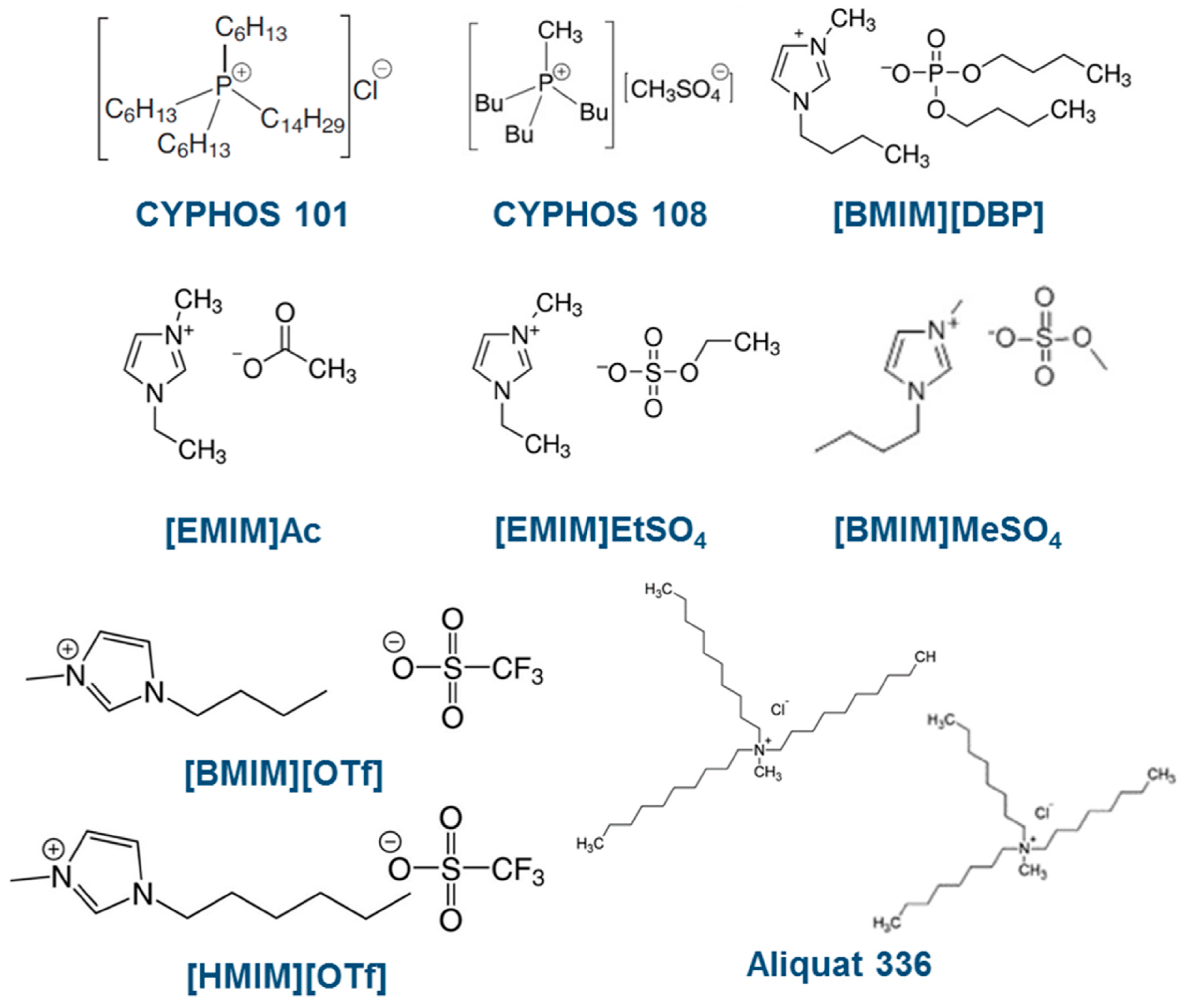
2.1. Chemicals and Materials
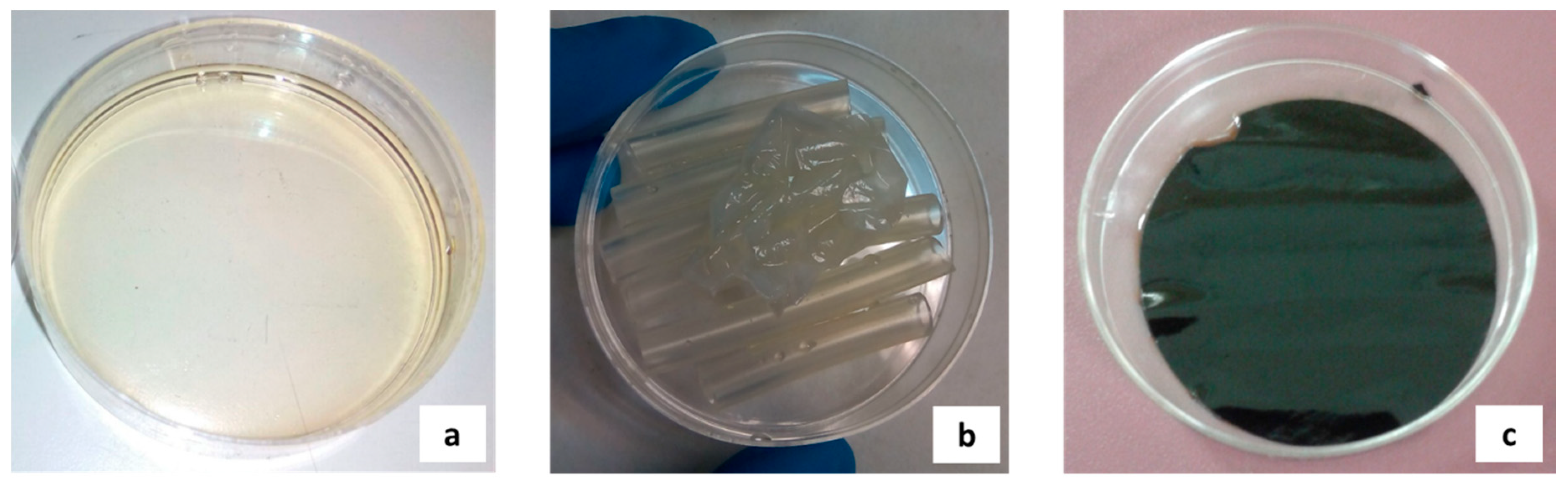
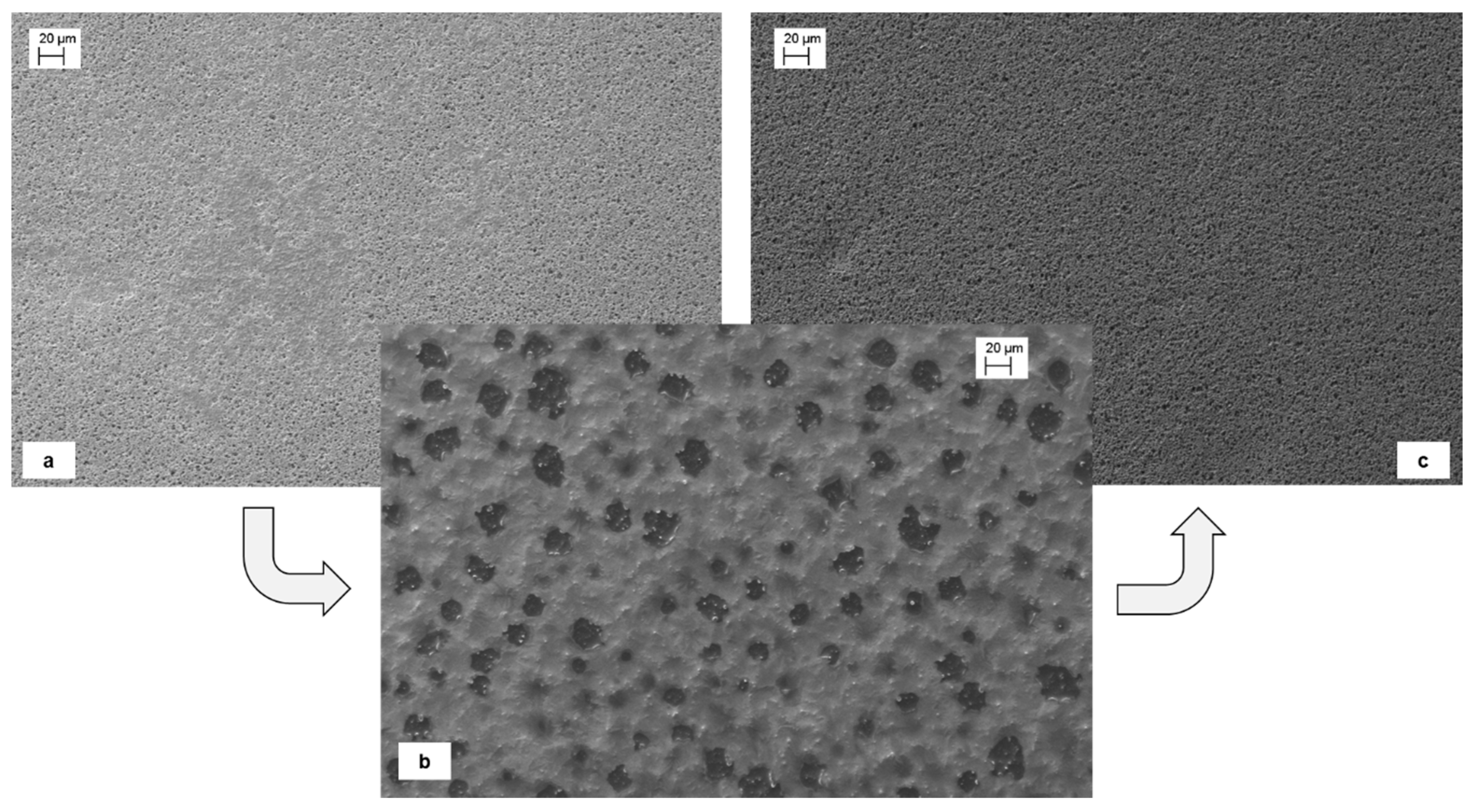
2.2. SILMs Preparation
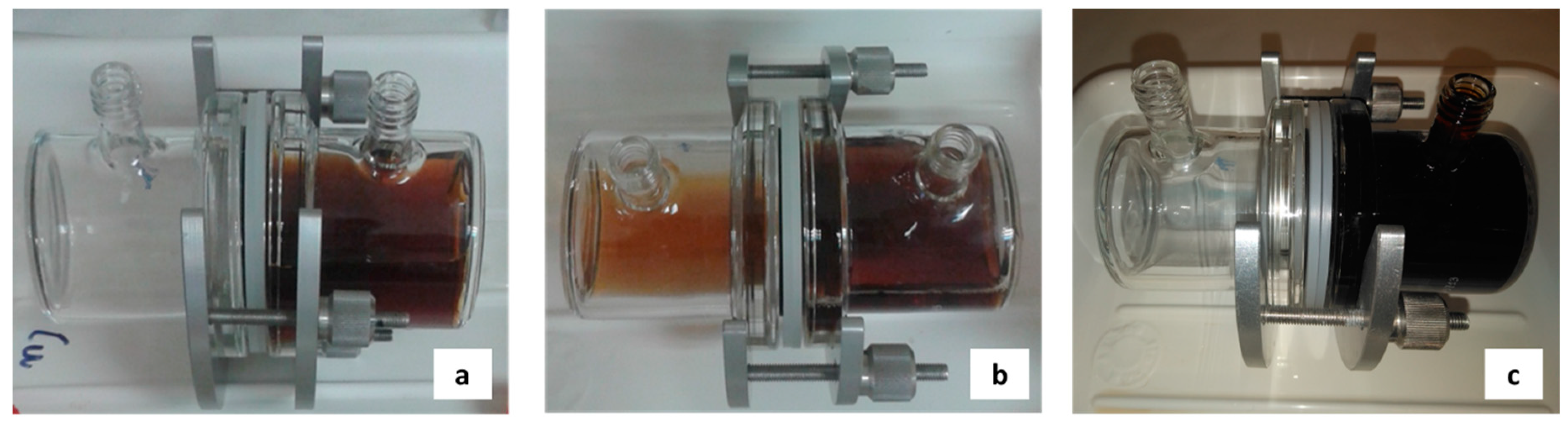
2.3. Installation and Analytical Procedures
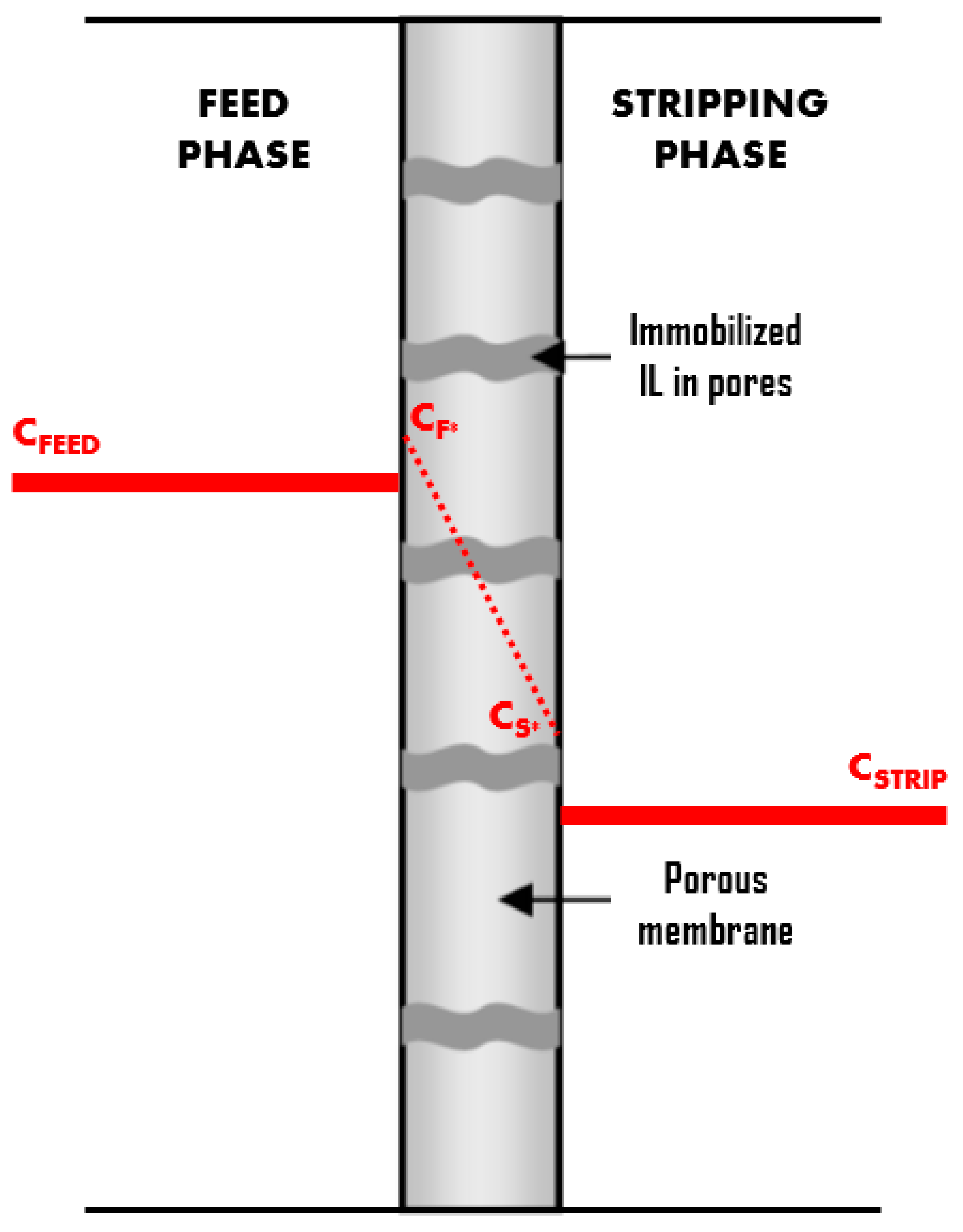
2.4. Membrane Transport Characterization
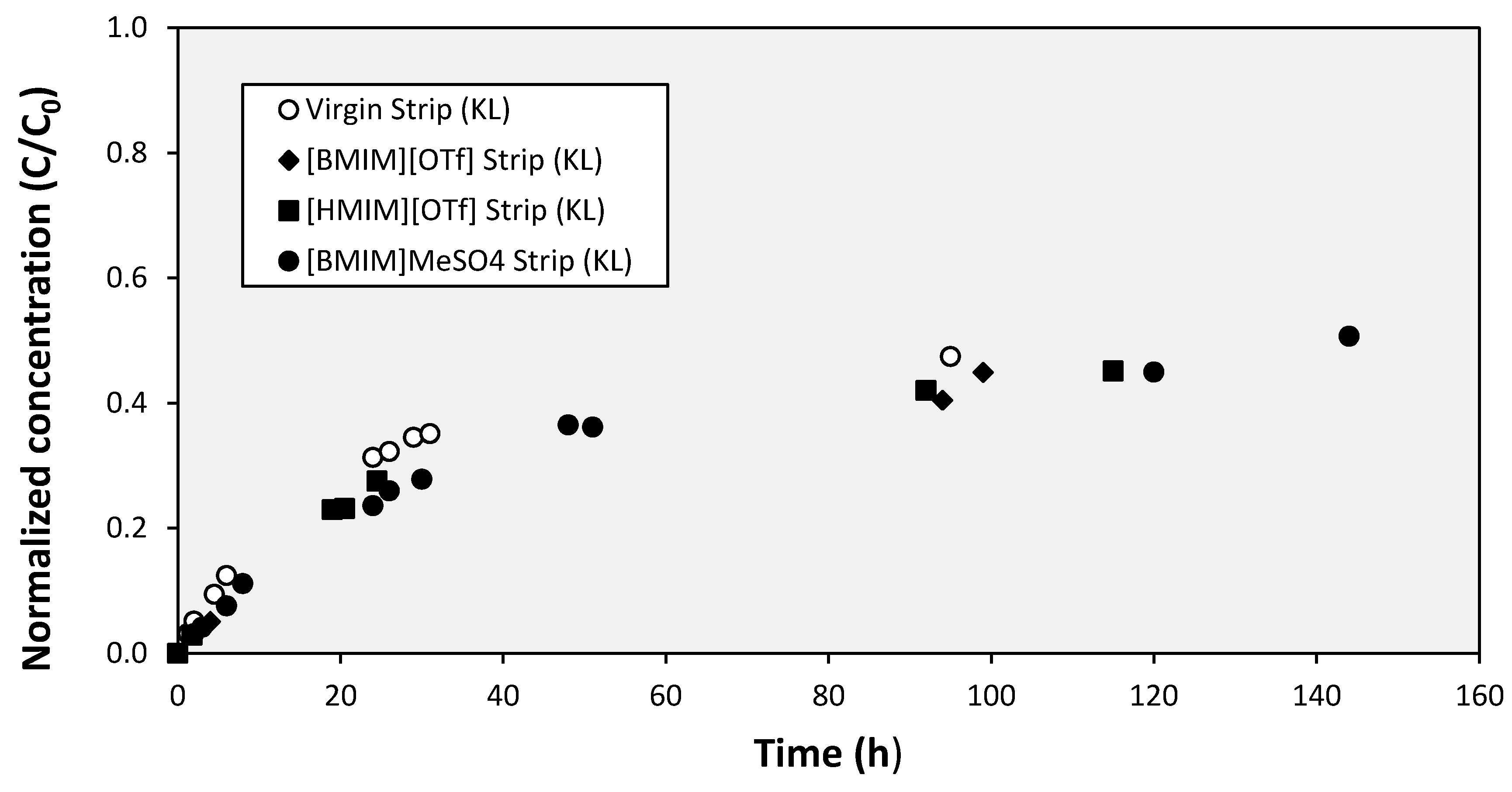
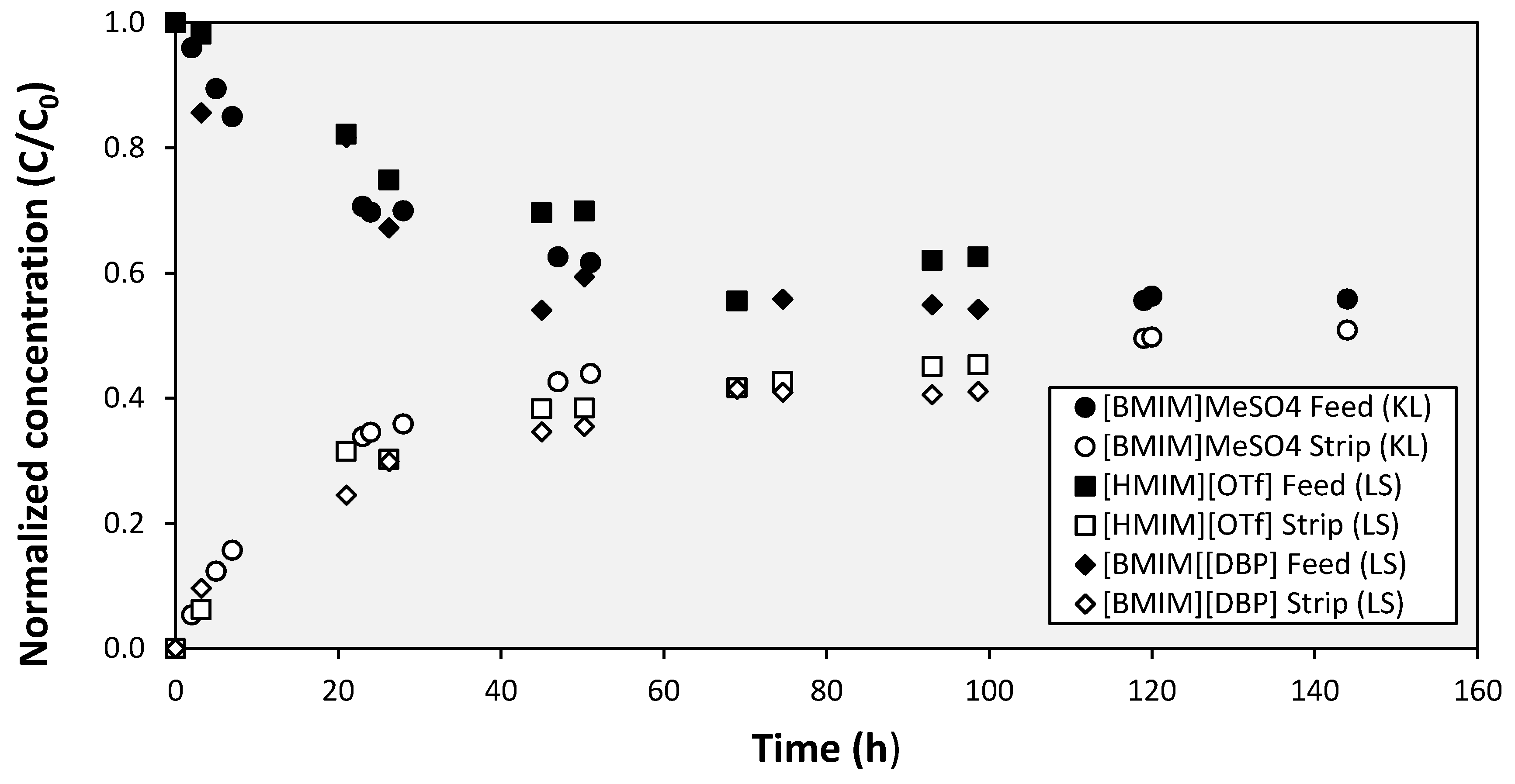
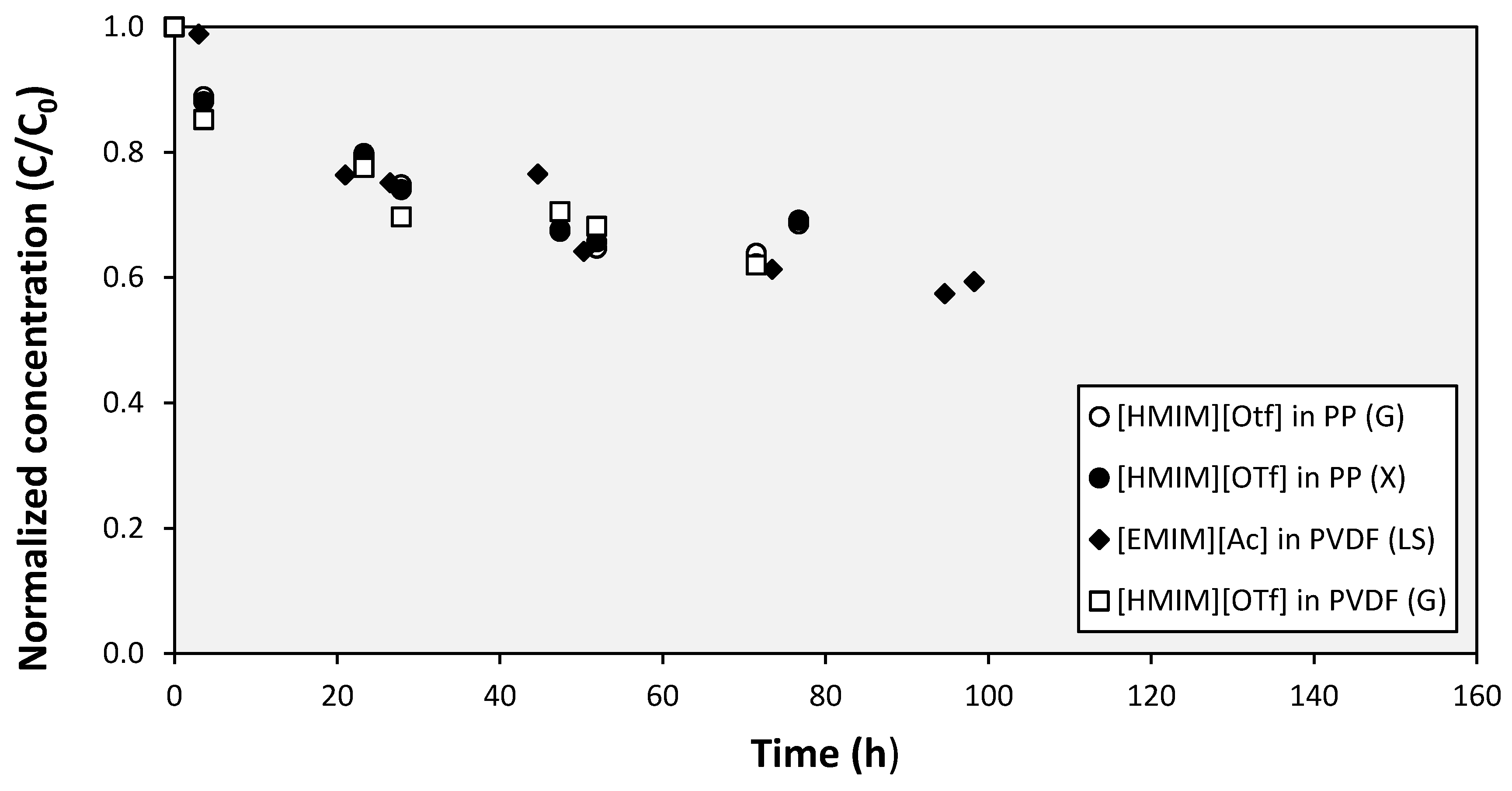
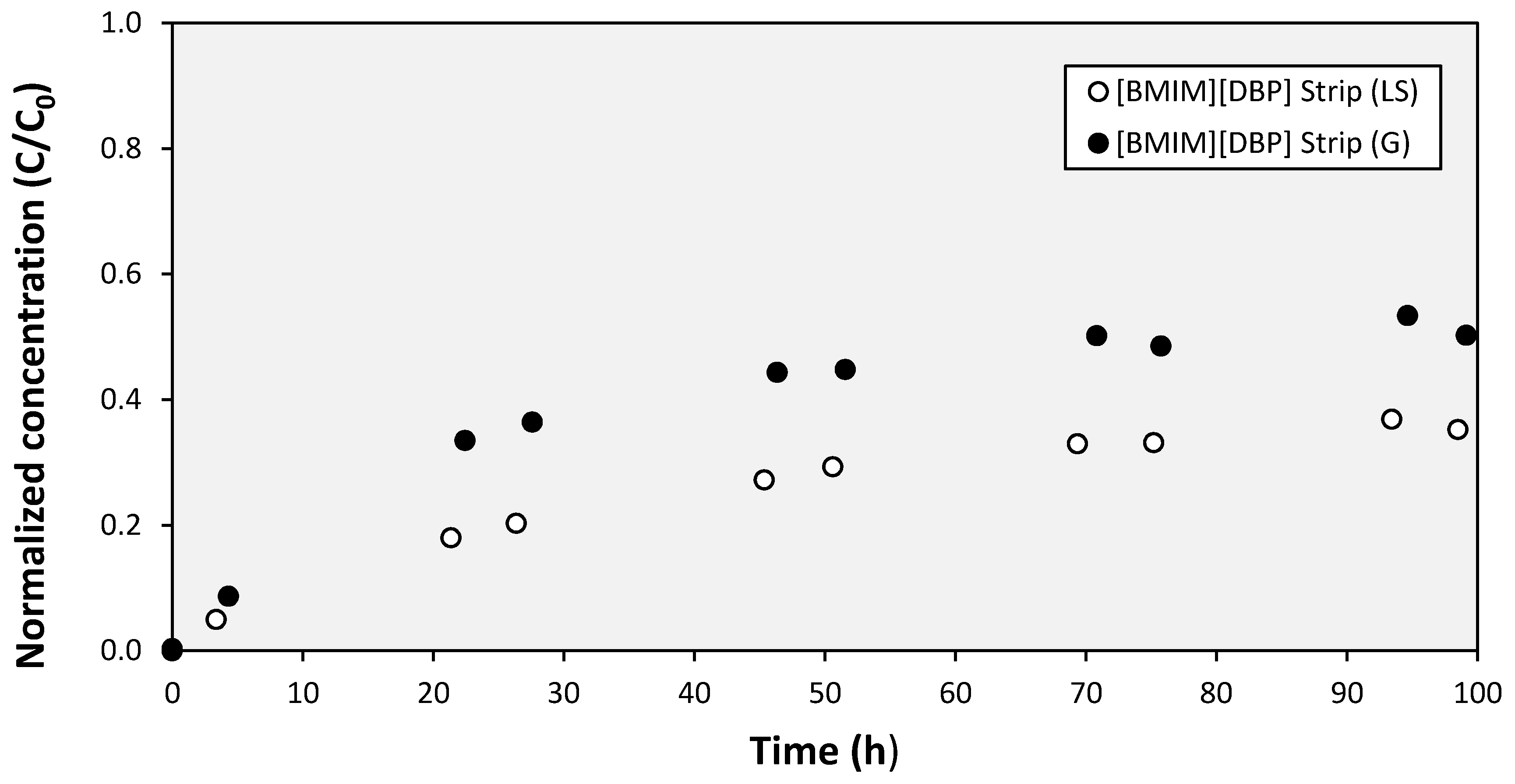
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bjärstig, T.; Sténs, A. Social Values of Forests and Production of New Goods and Services: The Views of Swedish Family Forest Owners. Small-Scale For. 2018, 17, 125–146. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kautto, J.; Vakkilainen, E. Effects of hemicellulose extraction on the kraft pulp mill operation and energy use: Review and case study with lignin removal. Chem. Eng. Res. Des. 2013, 91, 1284–1291. [Google Scholar] [CrossRef]
- Abejón, R. A Bibliometric Study of Scientific Publications regarding Hemicellulose Valorization during the 2000–2016 Period: Identification of Alternatives and Hot Topics. ChemEngineering 2018, 2, 7. [Google Scholar] [CrossRef]
- Abejón, R.; Pérez-Acebo, H.; Clavijo, L. Alternatives for Chemical and Biochemical Lignin Valorization: Hot Topics from a Bibliometric Analysis of the Research Published During the 2000–2016 Period. Processes 2018, 6, 98. [Google Scholar] [CrossRef]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefin. 2018, 1–32. [Google Scholar] [CrossRef]
- Lee, H.S.; Jae, J.; Ha, J.M.; Suh, D.J. Hydro- and solvothermolysis of kraft lignin for maximizing production of monomeric aromatic chemicals. Bioresour. Technol. 2016, 203, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ceapraz, I.L.; Kotbi, G.; Sauvee, L. The territorial biorefinery as a new business model. Bio-Based Appl. Econ. 2016, 5, 47–62. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, C.A.; Maeda, R.N.; Betancur, G.J.V.; Pereira, N. The essentialness of delignification on enzymatic hydrolysis of sugar cane bagasse cellulignin for second generation ethanol production. Waste Biomass Valorization 2013, 4, 341–346. [Google Scholar] [CrossRef]
- Pihlajaniemi, V.; Sipponen, M.H.; Pastinen, O.; Nyyssölä, A.; Laakso, S. The effect of direct and counter-current flow-through delignification on enzymatic hydrolysis of wheat straw, and flow limits due to compressibility. Biotechnol. Bioeng. 2016, 113, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Rogers, R.D. Review: Oxidation of lignin using ionic liquids-an innovative strategy to produce renewable chemicals. ACS Sustain. Chem. Eng. 2014, 2, 322–339. [Google Scholar] [CrossRef]
- Davis, K.M.; Rover, M.; Brown, R.C.; Bai, X.; Wen, Z.; Jarboe, L.R. Recovery and utilization of lignin monomers as part of the biorefinery approach. Energies 2016, 9, 808. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, C.; Sun, X.; Su, S.; Li, Q.; Linhardt, R.J. Efficient, environmentally-friendly and specific valorization of lignin: Promising role of non-radical lignolytic enzymes. World J. Microbiol. Biotechnol. 2017, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.H.; Deng, S.M.; Wang, T.J.; Li, Q.X. Production of BTX through catalytic depolymerization of lignin. Chin. J. Chem. Phys. 2014, 27, 221–226. [Google Scholar] [CrossRef]
- Elfadly, A.M.; Zeid, I.F.; Yehia, F.Z.; Abouelela, M.M.; Rabie, A.M. Production of aromatic hydrocarbons from catalytic pyrolysis of lignin over acid-activated bentonite clay. Fuel Process. Technol. 2017, 163, 1–7. [Google Scholar] [CrossRef]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; DOE Scientific and Technical Information; US Department of Energy: Oak Ridge, TN, USA, 2007; ISBN PNNL-16983.
- Abels, C.; Carstensen, F.; Wessling, M. Membrane processes in biorefinery applications. J. Memb. Sci. 2013, 444, 285–317. [Google Scholar] [CrossRef]
- Dafinov, A.; Font, J.; Garcia-Valls, R. Processing of black liquors by UF/NF ceramic membranes. Desalination 2005, 173, 83–90. [Google Scholar] [CrossRef]
- Bhattacharya, P.K.; Todi, R.K.; Tiwari, M.; Bhattacharjee, C.; Bhattacharjee, S.; Datta, S. Studies on ultrafiltration of spent sulfite liquor using various membranes for the recovery of lignosulphonates. Desalination 2005, 174, 287–297. [Google Scholar] [CrossRef]
- Moniz, P.; Serralheiro, C.; Matos, C.T.; Boeriu, C.G.; Frissen, A.E.; Duarte, L.C.; Roseiro, L.B.; Pereira, H.; Carvalheiro, F. Membrane separation and characterisation of lignin and its derived products obtained by a mild ethanol organosolv treatment of rice straw. Process Biochem. 2018, 65, 136–145. [Google Scholar] [CrossRef]
- Mota, I.F.; Pinto, P.R.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Downstream processing of an oxidized industrial kraft liquor by membrane fractionation for vanillin and syringaldehyde recovery. Sep. Purif. Technol. 2018, 197, 360–371. [Google Scholar] [CrossRef]
- Servaes, K.; Varhimo, A.; Dubreuil, M.; Bulut, M.; Vandezande, P.; Siika-aho, M.; Sirviö, J.; Kruus, K.; Porto-Carrero, W.; Bongers, B. Purification and concentration of lignin from the spent liquor of the alkaline oxidation of woody biomass through membrane separation technology. Ind. Crops Prod. 2017, 106, 86–96. [Google Scholar] [CrossRef]
- Weinwurm, F.; Drljo, A.; Waldmüller, W.; Fiala, B.; Niedermayer, J.; Friedl, A. Lignin concentration and fractionation from ethanol organosolv liquors by ultra- and nanofiltration. J. Clean. Prod. 2016, 136, 62–71. [Google Scholar] [CrossRef]
- Jönsson, A.S.; Nordin, A.K.; Wallberg, O. Concentration and purification of lignin in hardwood kraft pulping liquor by ultrafiltration and nanofiltration. Chem. Eng. Res. Des. 2008, 86, 1271–1280. [Google Scholar] [CrossRef]
- Toledano, A.; García, A.; Mondragon, I.; Labidi, J. Lignin separation and fractionation by ultrafiltration. Sep. Purif. Technol. 2010, 71, 38–43. [Google Scholar] [CrossRef]
- Sevastyanova, O.; Helander, M.; Chowdhury, S.; Lange, H.; Wedin, H.; Zhang, L.; Ek, M.; Kadla, J.F.; Crestini, C.; Lindström, M.E. Tailoring the molecular and thermo-mechanical properties of kraft lignin by ultrafiltration. J. Appl. Polym. Sci. 2014, 131, 9505–9515. [Google Scholar] [CrossRef]
- Žabková, M.; da Silva, E.A.B.; Rodrigues, A.E. Recovery of vanillin from lignin/vanillin mixture by using tubular ceramic ultrafiltration membranes. J. Memb. Sci. 2007, 301, 221–237. [Google Scholar] [CrossRef]
- Dubreuil, M.F.S.; Servaes, K.; Ormerod, D.; Van Houtven, D.; Porto-Carrero, W.; Vandezande, P.; Vanermen, G.; Buekenhoudt, A. Selective membrane separation technology for biomass valorization towards bio-aromatics. Sep. Purif. Technol. 2017, 178, 56–65. [Google Scholar] [CrossRef]
- Werhan, H.; Farshori, A.; von Rohr, P.R. Separation of lignin oxidation products by organic solvent nanofiltration. J. Memb. Sci. 2012, 423–424, 404–412. [Google Scholar] [CrossRef]
- Mai, N.L.; Koo, Y.M. Computer-Aided Design of Ionic Liquids for High Cellulose Dissolution. ACS Sustain. Chem. Eng. 2016, 4, 541–547. [Google Scholar] [CrossRef]
- Hossain, M.M.; Aldous, L. Ionic liquids for lignin processing: Dissolution, isolation, and conversion. Aust. J. Chem. 2012, 65, 1465–1477. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, H.; Zhang, J.; Fu, L.; Liu, M.; Fu, J.; Ren, P. Dissolution of cellulose in phosphate-based ionic liquids. Carbohydr. Polym. 2012, 87, 1490–1494. [Google Scholar] [CrossRef]
- Lan, W.; Liu, C.F.; Yue, F.X.; Sun, R.C.; Kennedy, J.F. Ultrasound-assisted dissolution of cellulose in ionic liquid. Carbohydr. Polym. 2011, 86, 672–677. [Google Scholar] [CrossRef]
- Stolarska, O.; Pawlowska-Zygarowicz, A.; Soto, A.; Rodríguez, H.; Smiglak, M. Mixtures of ionic liquids as more efficient media for cellulose dissolution. Carbohydr. Polym. 2017, 178, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Z.; Ren, H.; Duan, E. Synthesis and properties of non-aromatic ionic liquids and their role in cellulose dissolution. BioResources 2017, 12, 5407–5416. [Google Scholar] [CrossRef]
- Roselli, A.; Asikainen, S.; Stepan, A.; Monshizadeh, A.; Von Weymarn, N.; Kovasin, K.; Wang, Y.; Xiong, H.; Turunen, O.; Hummel, M.; et al. Comparison of pulp species in IONCELL-P: Selective hemicellulose extraction method with ionic liquids. Holzforschung 2016, 70, 291–296. [Google Scholar] [CrossRef]
- Heggset, E.B.; Syverud, K.; Øyaas, K. Novel pretreatment pathways for dissolution of lignocellulosic biomass based on ionic liquid and low temperature alkaline treatment. Biomass Bioenergy 2016, 93, 194–200. [Google Scholar] [CrossRef]
- Carneiro, A.P.; Rodriguez, O.; Macedo, E.A. Dissolution and fractionation of nut shells in ionic liquids. Bioresour. Technol. 2017, 227, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Arvela, P.; Anugwom, I.; Virtanen, P.; Sjöholm, R.; Mikkola, J.P. Dissolution of lignocellulosic materials and its constituents using ionic liquids-A review. Ind. Crops Prod. 2010, 32, 175–201. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.Y.; MacFarlane, D.R.; Upfal, J.; Edye, L.A.; Doherty, W.O.S.; Patti, A.F.; Pringle, J.M.; Scott, J.L. Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem. 2009, 11, 339. [Google Scholar] [CrossRef]
- Balaji, C.; Banerjee, T.; Goud, V.V. COSMO-RS based predictions for the extraction of lignin from lignocellulosic biomass using ionic liquids: Effect of cation and anion combination. J. Solut. Chem. 2012, 41, 1610–1630. [Google Scholar] [CrossRef]
- Hamada, Y.; Yoshida, K.; Asai, R.; Hayase, S.; Nokami, T.; Izumi, S.; Itoh, T. A possible means of realizing a sacrifice-free three component separation of lignocellulose from wood biomass using an amino acid ionic liquid. Green Chem. 2013, 15, 1863. [Google Scholar] [CrossRef]
- Prado, R.; Erdocia, X.; Labidi, J. Lignin extraction and purification with ionic liquids. J. Chem. Technol. Biotechnol. 2013, 88, 1248–1257. [Google Scholar] [CrossRef]
- Glas, D.; Van Doorslaer, C.; Depuydt, D.; Liebner, F.; Rosenau, T.; Binnemans, K.; De Vos, D.E. Lignin solubility in non-imidazolium ionic liquids. J. Chem. Technol. Biotechnol. 2015, 90, 1821–1826. [Google Scholar] [CrossRef]
- Konda, N.M.; Shi, J.; Singh, S.; Blanch, H.W.; Simmons, B.A.; Klein-Marcuschamer, D. Understanding cost drivers and economic potential of two variants of ionic liquid pretreatment for cellulosic biofuel production. Biotechnol. Biofuels 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parhi, P.K. Supported liquid membrane principle and its practices: A short review. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Kocherginsky, N.M.; Yang, Q.; Seelam, L. Recent advances in supported liquid membrane technology. Sep. Purif. Technol. 2007, 53, 171–177. [Google Scholar] [CrossRef]
- Abejón, R.; Pérez-Acebo, H.; Garea, A. A Bibliometric Analysis of Research on Supported Ionic Liquid Membranes during the 1995–2015 Period: Study of the Main Applications and Trending Topics. Membranes 2017, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Lozano, L.J.; Godínez, C.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Sánchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Memb. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef]
- Kilulya, K.F.; Msagati, T.A.M.; Mamba, B.B.; Ngila, J.C.; Bush, T. Ionic liquid-liquid extraction and supported liquid membrane analysis of lipophilic wood extractives from dissolving-grade pulp. Chromatographia 2012, 75, 513–520. [Google Scholar] [CrossRef]
- Venkateswaran, P. Di (2-ethylhexyl) phosphoric acid-coconut oil supported liquid membrane for the separation of copper ions from copper plating wastewater. J. Environ. Sci. 2007, 19, 1446–1453. [Google Scholar] [CrossRef]
- Abejón, R.; Abejón, A.; Garea, A.; Irabien, A. Transport of lignin and other lignocellulosic components through supported ionic liquid membranes. Chem. Eng. Trans. 2017, 57, 1153–1158. [Google Scholar]
- Alén, R.; Hartus, T. UV spectrophotometric determination of lignin from alkaline pulping liquors. Cell. Chem. Technol. 1987, 618, 613–618. [Google Scholar]
- Lee, R.A.; Bédard, C.; Berberi, V.; Beauchet, R.; Lavoie, J.M. UV-Vis as quantification tool for solubilized lignin following a single-shot steam process. Bioresour. Technol. 2013, 144, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Oveissi, F.; Fatehi, P. Isolating lignin from spent liquor of thermomechanical pulping process via adsorption. Environ. Technol. 2014, 35, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Iolitec Ionic liquids: Catalogue. Available online: https://iolitec.de/index.php/en/products/ionic_liquids/catalogue (accessed on 6 June 2018).
- Zhang, S.; Zhou, Q.; Lu, X.; Song, Y.; Wang, X. Physicochemical Properties of Ionic Liquid Mixtures; Springer: Dordrecht, The Netherlands, 2016; ISBN 9789401775717. [Google Scholar]
- Palgunadi, J.; Kang, J.E.; Cheong, M.; Kim, H.; Lee, H.; Kim, H.S. Fluorine-free imidazolium-based ionic liquids with a phosphorous-containing anion as potential CO2 absorbents. Bull. Korean Chem. Soc. 2009, 30, 1749–1754. [Google Scholar] [CrossRef]
- Ge, M.-L.; Zhao, R.-S.; Yi, Y.-F.; Zhang, Q.; Wang, L.-S. Densities and Viscosities of 1-Butyl-3-methylimidazolium Tetrafluoroborate + H2O Binary Mixtures at T = (303.15 to 343.15) K. J. Chem. Eng. Data 2008, 53, 2408–2411. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Fraser, K.J.; MacFarlane, D.R. Phosphonium-based ionic liquids: An overview. Aust. J. Chem. 2009, 62, 309–321. [Google Scholar] [CrossRef]


| Absorptivity Coefficients (L/g·cm) | ||
|---|---|---|
| Kraft lignin | 14.706 | - |
| Lignosulphonate | 7.604 | - |
| Xylose | - | 0.687 |
| Glucose | - | 0.608 |
| KP (h−1) | FP (m/h) | RMEMB (h/m) | RIL (h/m) | Viscosity 1 (mPa·s) | Density (g/cm3) | |
|---|---|---|---|---|---|---|
| Virgin HPVDF | 0.0334 | 1.37 × 10−3 | 729 | - | - | - |
| HPVDF + [EMIM]EtSO4 | 0.0263 | 1.08 × 10−3 | - | 197 | 122 | 1.20 (80 °C) |
| HPVDF +[BMIM]MeSO4 | 0.0216 | 0.89 × 10−3 | - | 398 | 214 | 1.17 (80 °C) |
| HPVDF + CYPHOS 108 | 0.0238 | 0.98 × 10−3 | - | 294 | 409 | 1.07 (25 °C) |
| HPVDF + [EMIM]Ac | 0.0248 | 1.02 × 10−3 | - | 253 | 93 | 1.07 (80 °C) |
| HPVDF + [BMIM][DBP] | 0.0141 | 0.58 × 10−3 | - | 997 | 1539 | 1.04 (40 °C) |
| HPVDF + [HMIM][OTf] | 0.0157 | 0.65 × 10−3 | - | 821 | 135 | 1.24 (29 °C) |
| HPVDF + [BMIM][OTf] | 0.0225 | 0.92 × 10−3 | - | 353 | 75 | 1.30 (25 °C) |
| Membrane Support | KP (h−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CYPHOS 101 | CYPHOS 108 | ALIQUAT | [BMIM]MeSO4 | [BMIM][DBP] | [BMIM][OTf] | [HMIM][OTf] | [EMIM]EtSO4 | [EMIM]Ac | |
| HPVDF | No tested | 0.0238 | No tested | 0.0216 | 0.0141 | 0.0225 | 0.0157 | 0.0263 | 0.0248 |
| PVDF | No tested | 0.0254 | No tested | 0.0238 | 0.0247 | 0.0221 | 0.0171 | 0.0237 | 0.0186 |
| PCTE | No tested | No tested | No tested | 0.0284 | 0.0225 | No tested | 0.0227 | No tested | No tested |
| PP | No tested | 0.0248 | No tested | No transport | 0.0139 | 0.0179 | 0.0182 | No transport | No transport |
| PTFE | No tested | No transport | No tested | No transport | Selective transport | No transport | No transport | No transport | No transport |
| K (h−1) | k (m/h) | |
|---|---|---|
| Kraft lignin (KL) | 0.0172 | 0.71 × 10−3 |
| Lignosulphonate (LS) | 0.0162 | 0.67 × 10−3 |
| Glucose (G) | 0.0453 | 1.86 × 10−3 |
| Xylose (X) | 0.0429 | 1.76 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abejón, R.; Rabadán, J.; Lanza, S.; Abejón, A.; Garea, A.; Irabien, A. Supported Ionic Liquid Membranes for Separation of Lignin Aqueous Solutions. Processes 2018, 6, 143. https://doi.org/10.3390/pr6090143
Abejón R, Rabadán J, Lanza S, Abejón A, Garea A, Irabien A. Supported Ionic Liquid Membranes for Separation of Lignin Aqueous Solutions. Processes. 2018; 6(9):143. https://doi.org/10.3390/pr6090143
Chicago/Turabian StyleAbejón, Ricardo, Javier Rabadán, Silvia Lanza, Azucena Abejón, Aurora Garea, and Angel Irabien. 2018. "Supported Ionic Liquid Membranes for Separation of Lignin Aqueous Solutions" Processes 6, no. 9: 143. https://doi.org/10.3390/pr6090143
APA StyleAbejón, R., Rabadán, J., Lanza, S., Abejón, A., Garea, A., & Irabien, A. (2018). Supported Ionic Liquid Membranes for Separation of Lignin Aqueous Solutions. Processes, 6(9), 143. https://doi.org/10.3390/pr6090143







