The Influence of Cation Treatments on the Pervaporation Dehydration of NaA Zeolite Membranes Prepared on Hollow Fibers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of NaA Zeolite Membranes and Ion-Exchange Processes
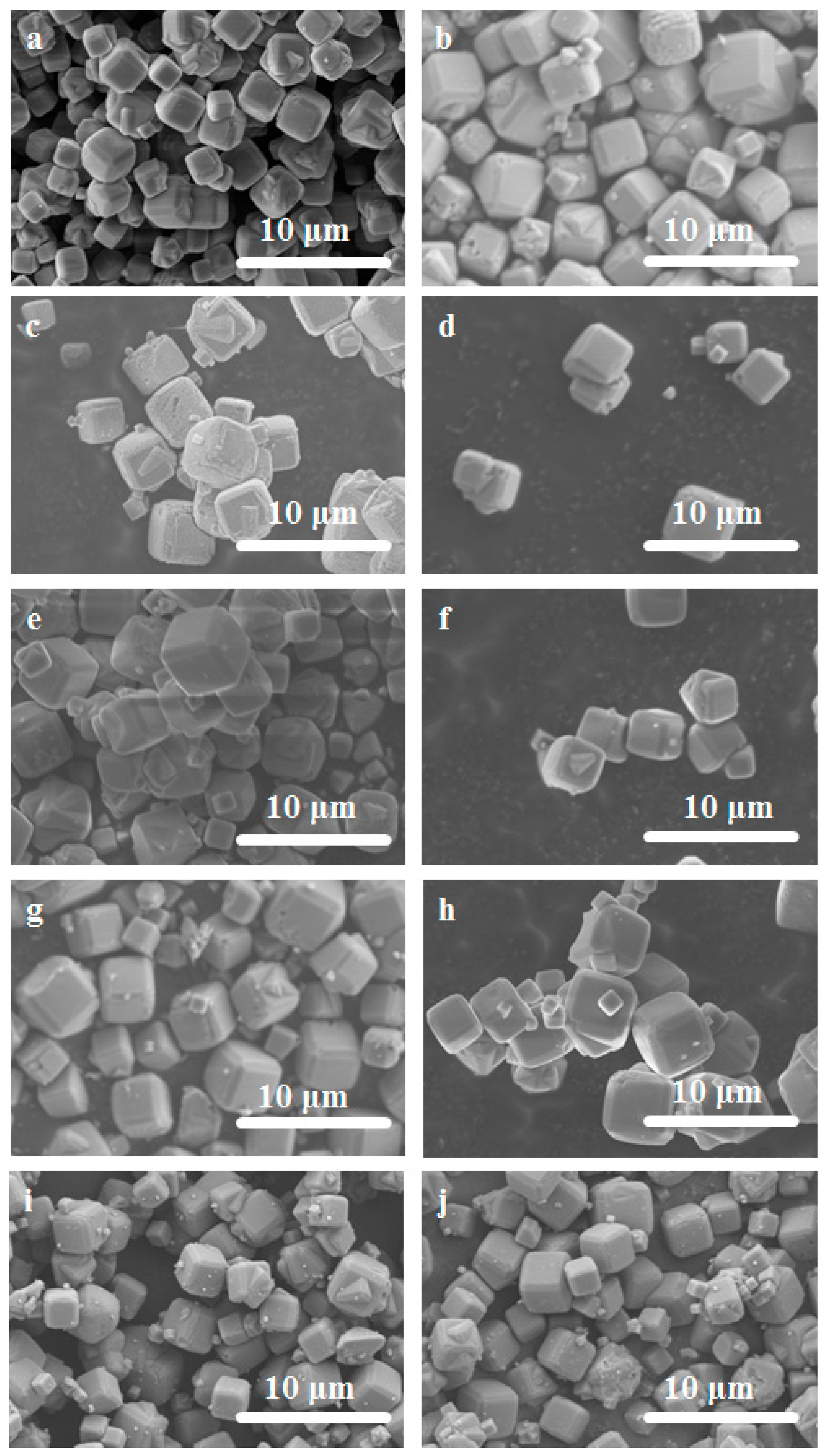
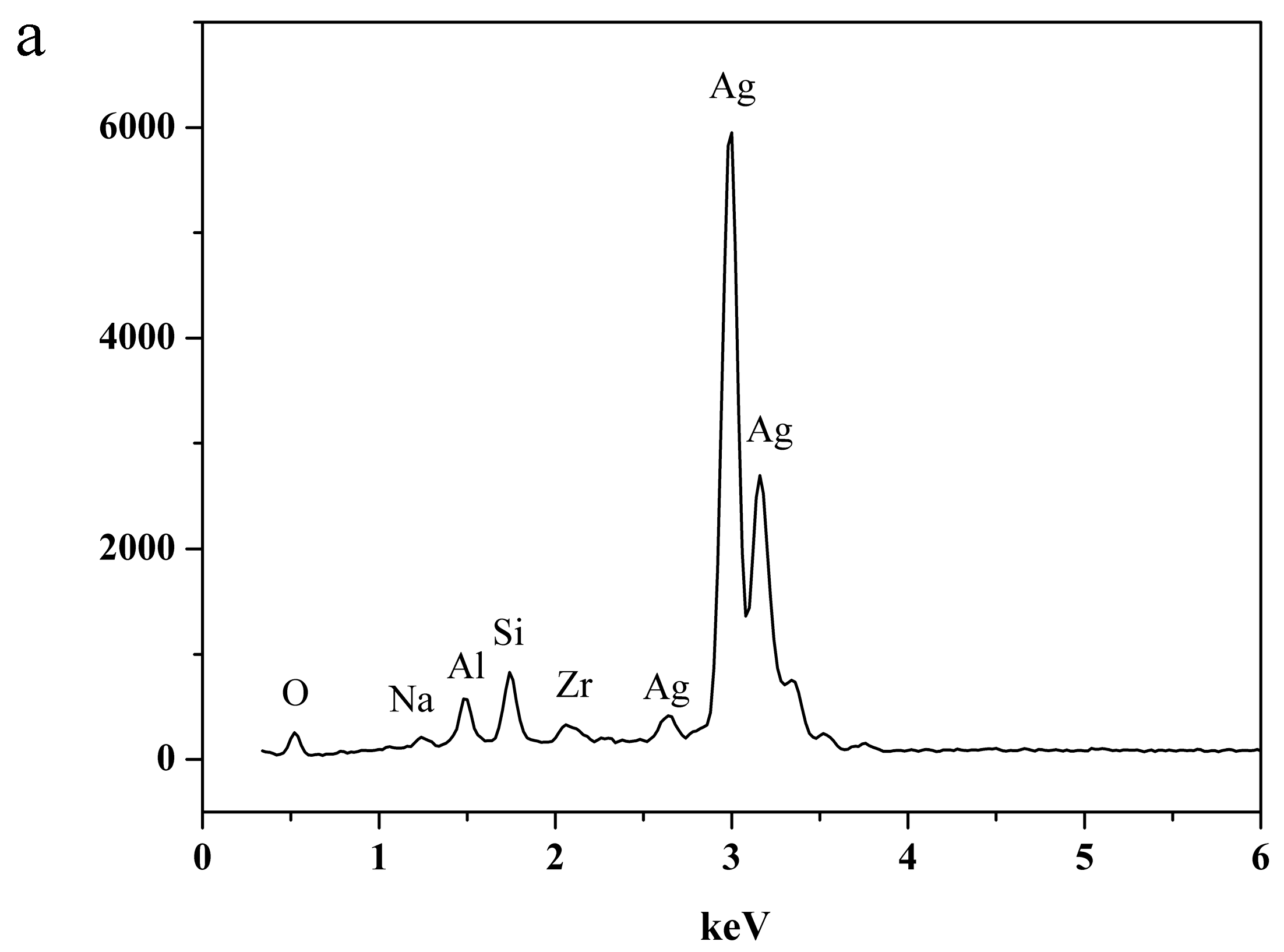
2.3. Characterization of NaA Zeolite Particles and Membranes
2.4. PV Test and Single Gas Permeation
3. Results and Discussion
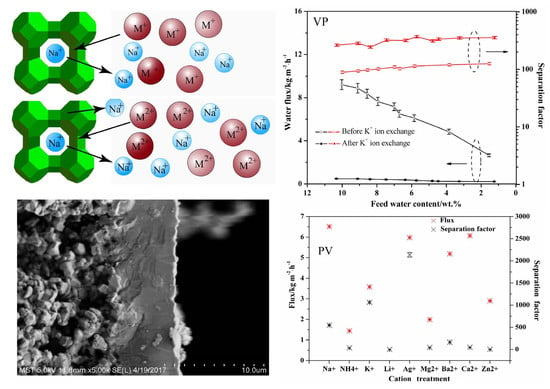
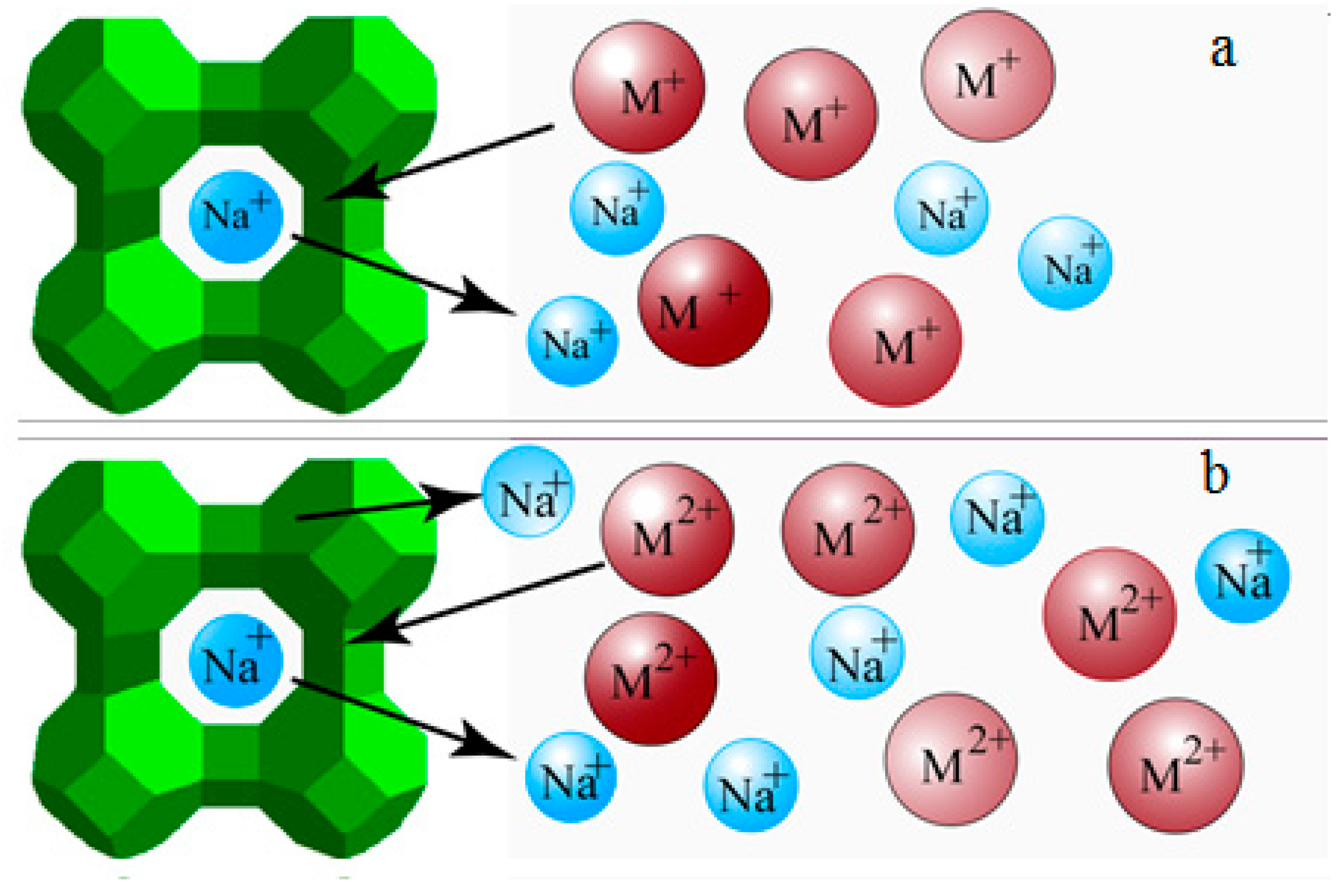
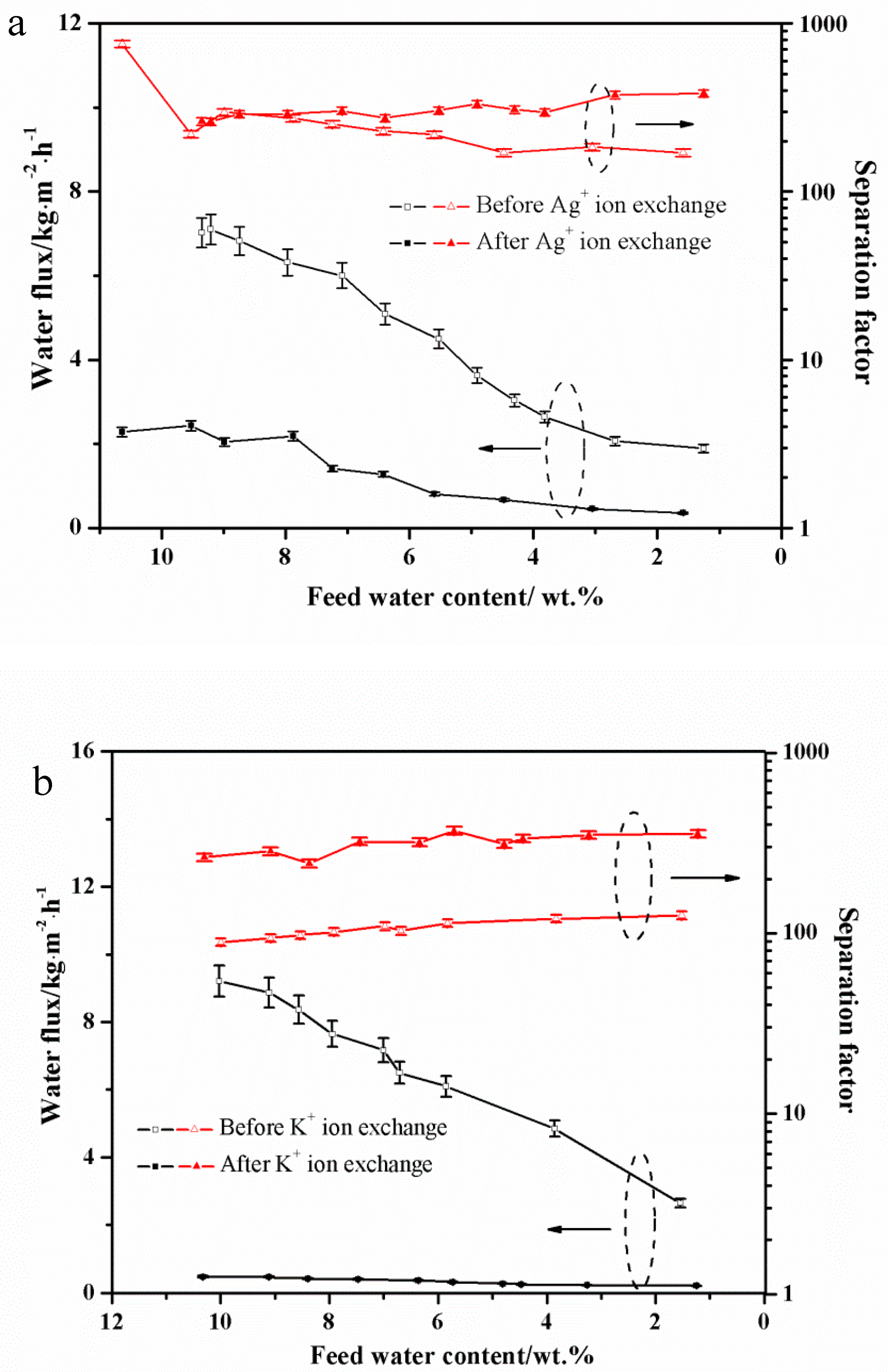
3.1. PV Performances of the Exchanged NaA Zeolite Membrane
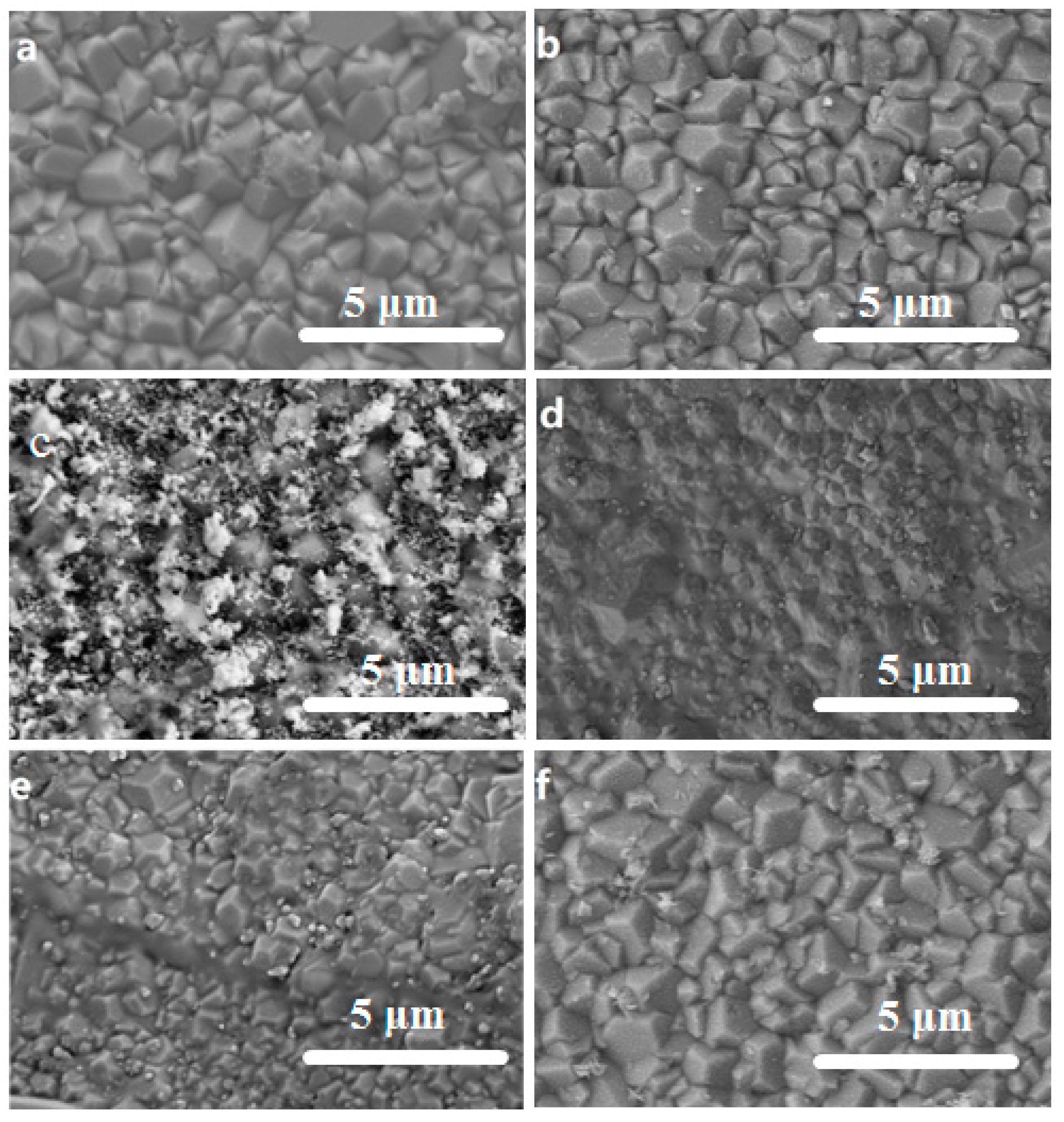
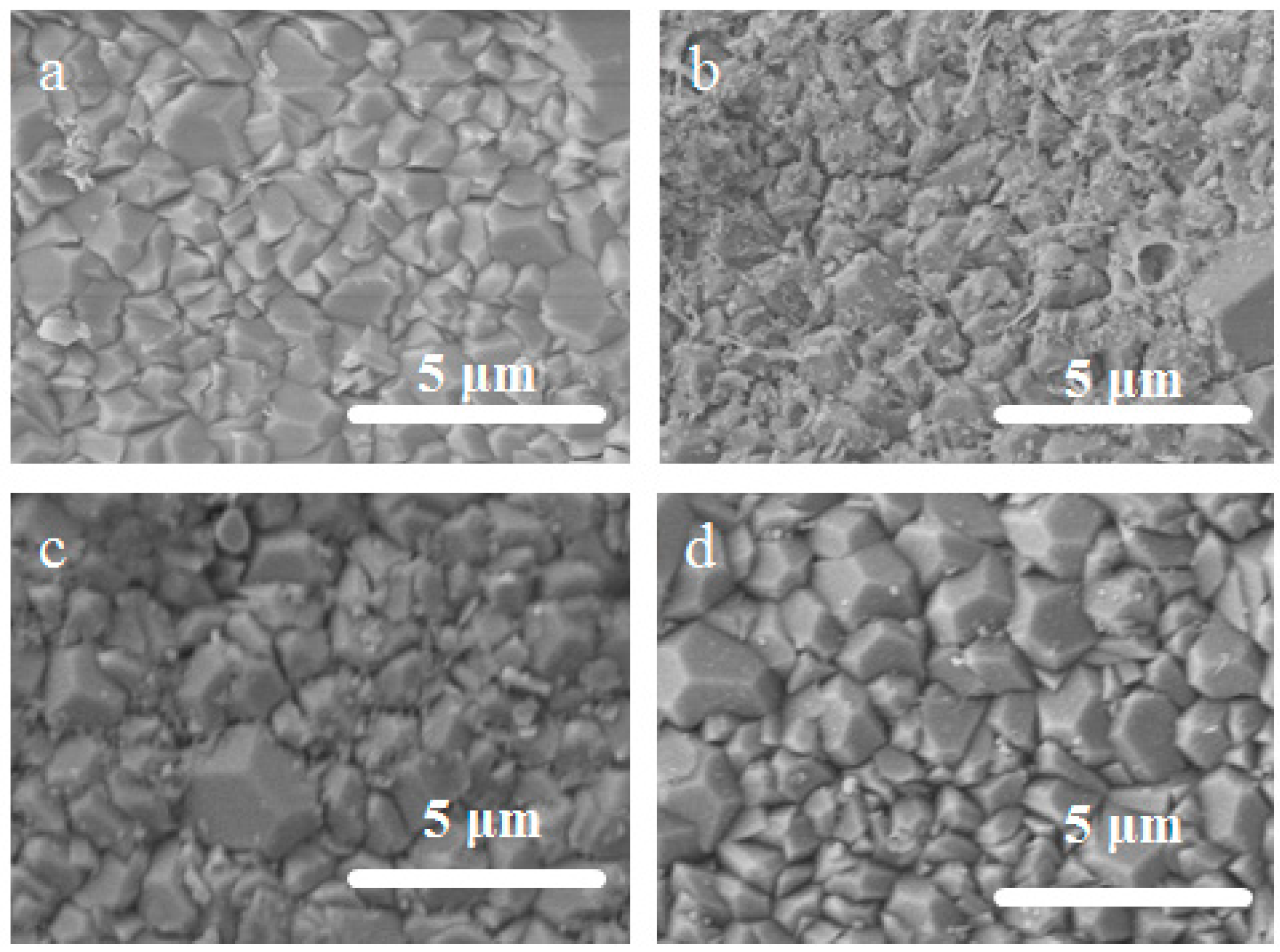
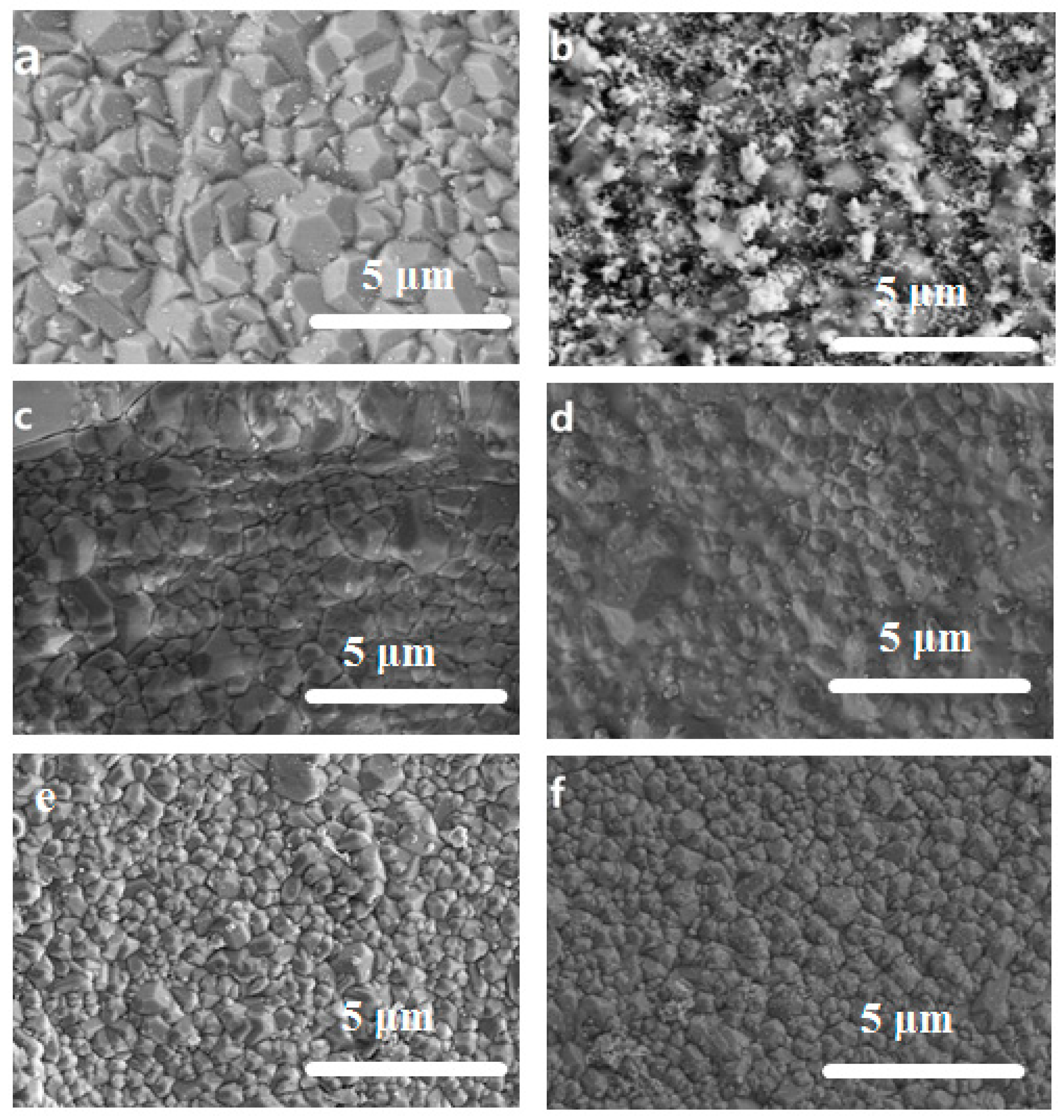
3.2. Surface Morphology of the Exchanged NaA Zeolite Membrane
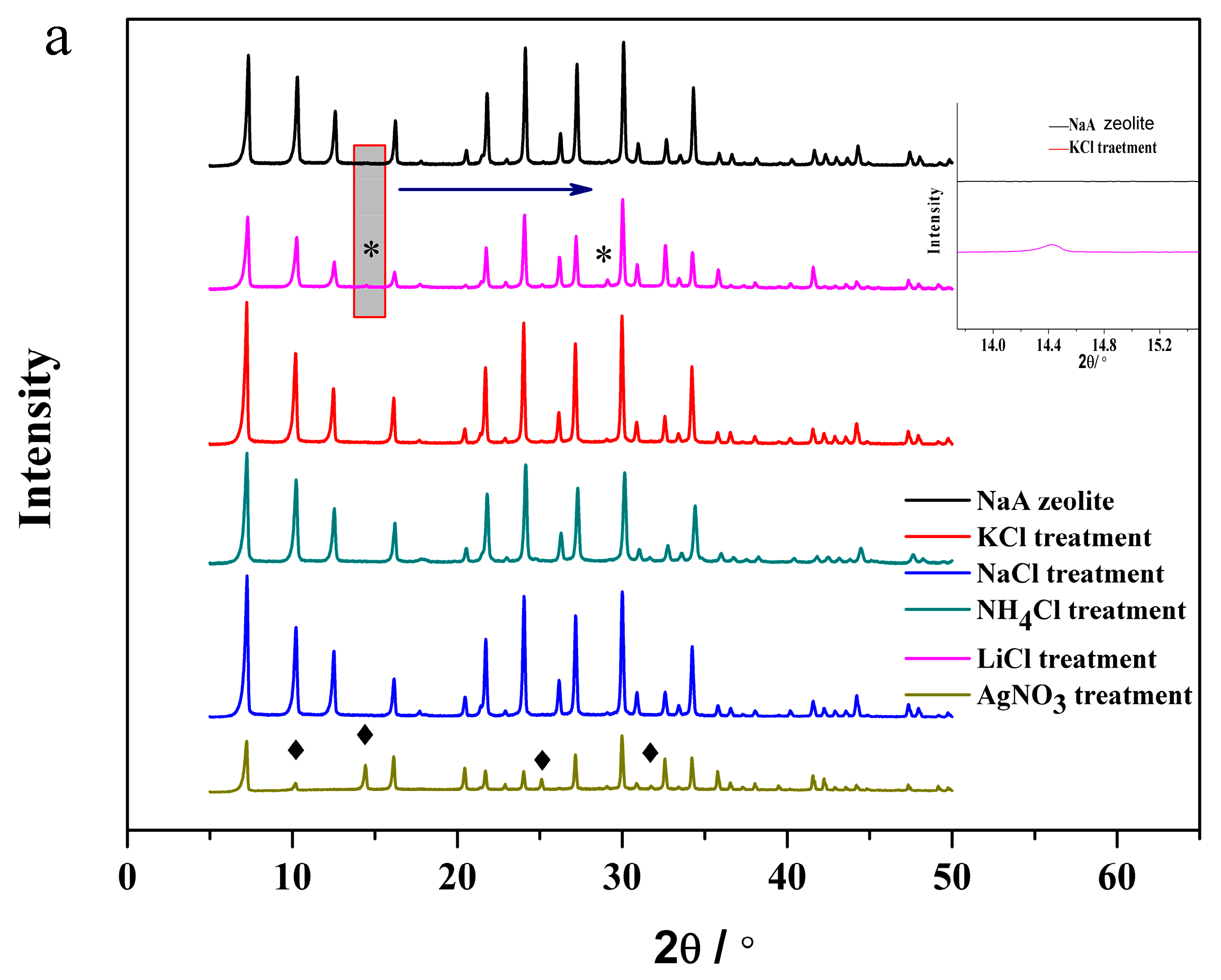
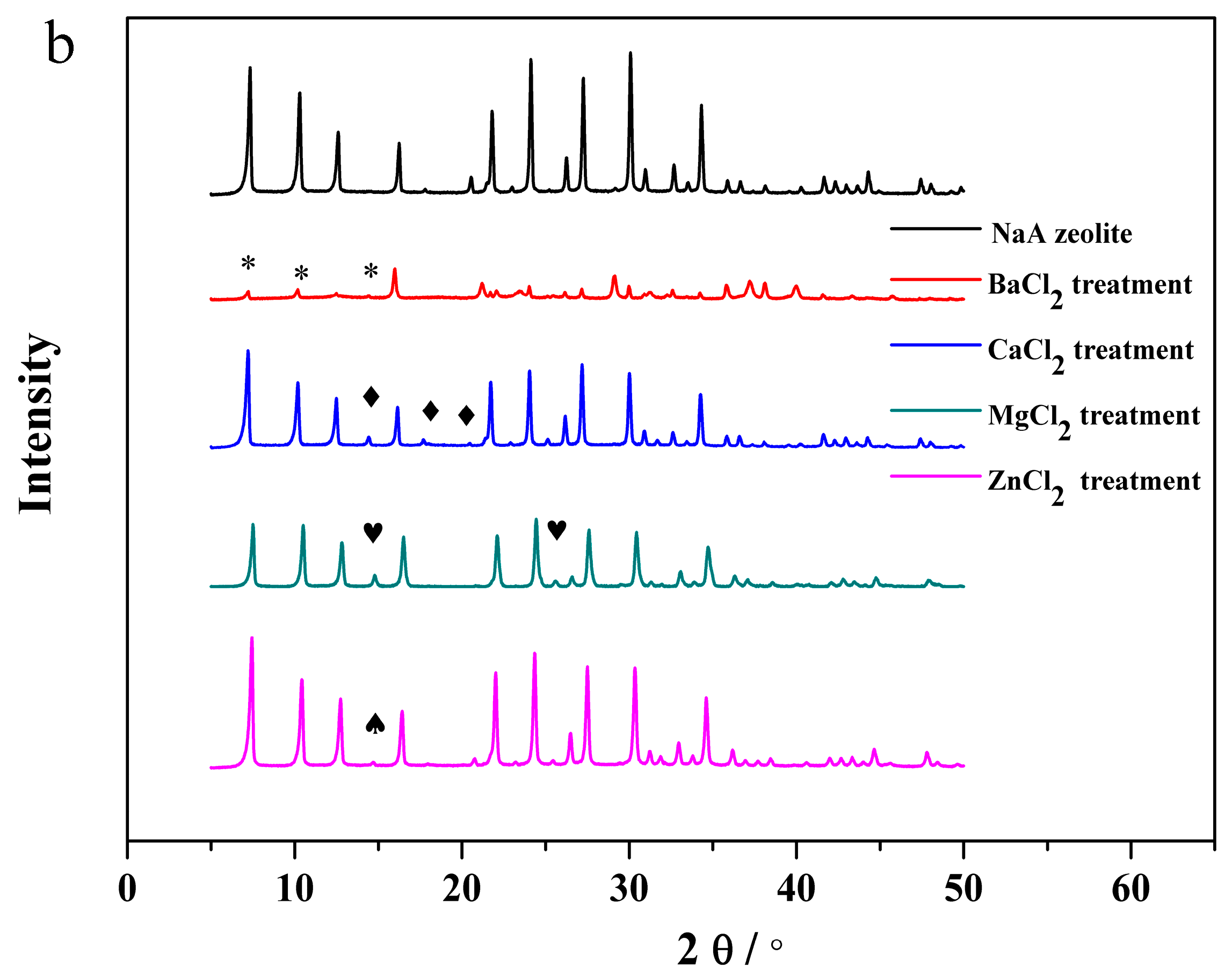
3.3. The Structural Analysis of the Exchanged NaA Zeolite Powders
3.4. The Concentration Effect on the Exchanged Membrane
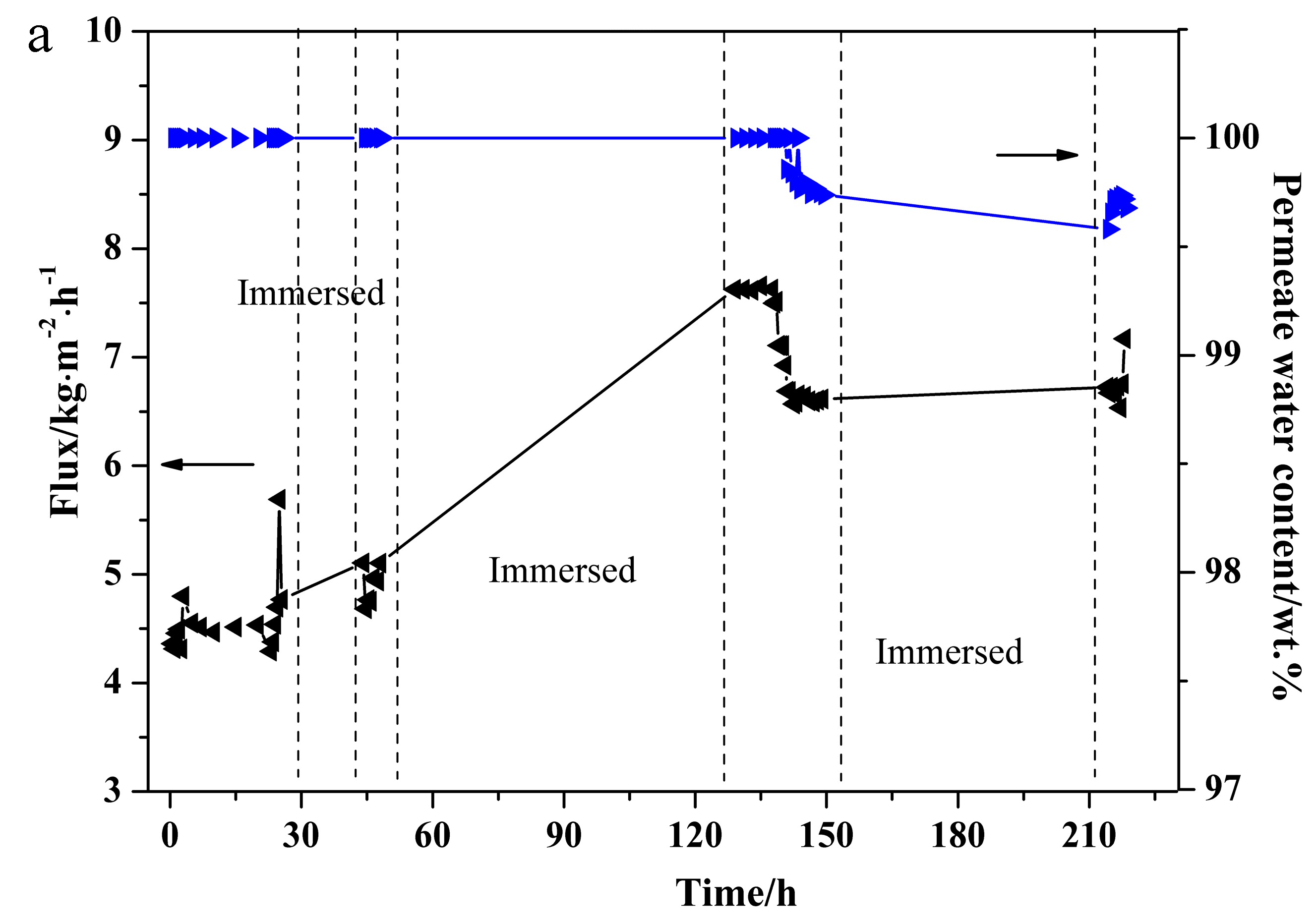
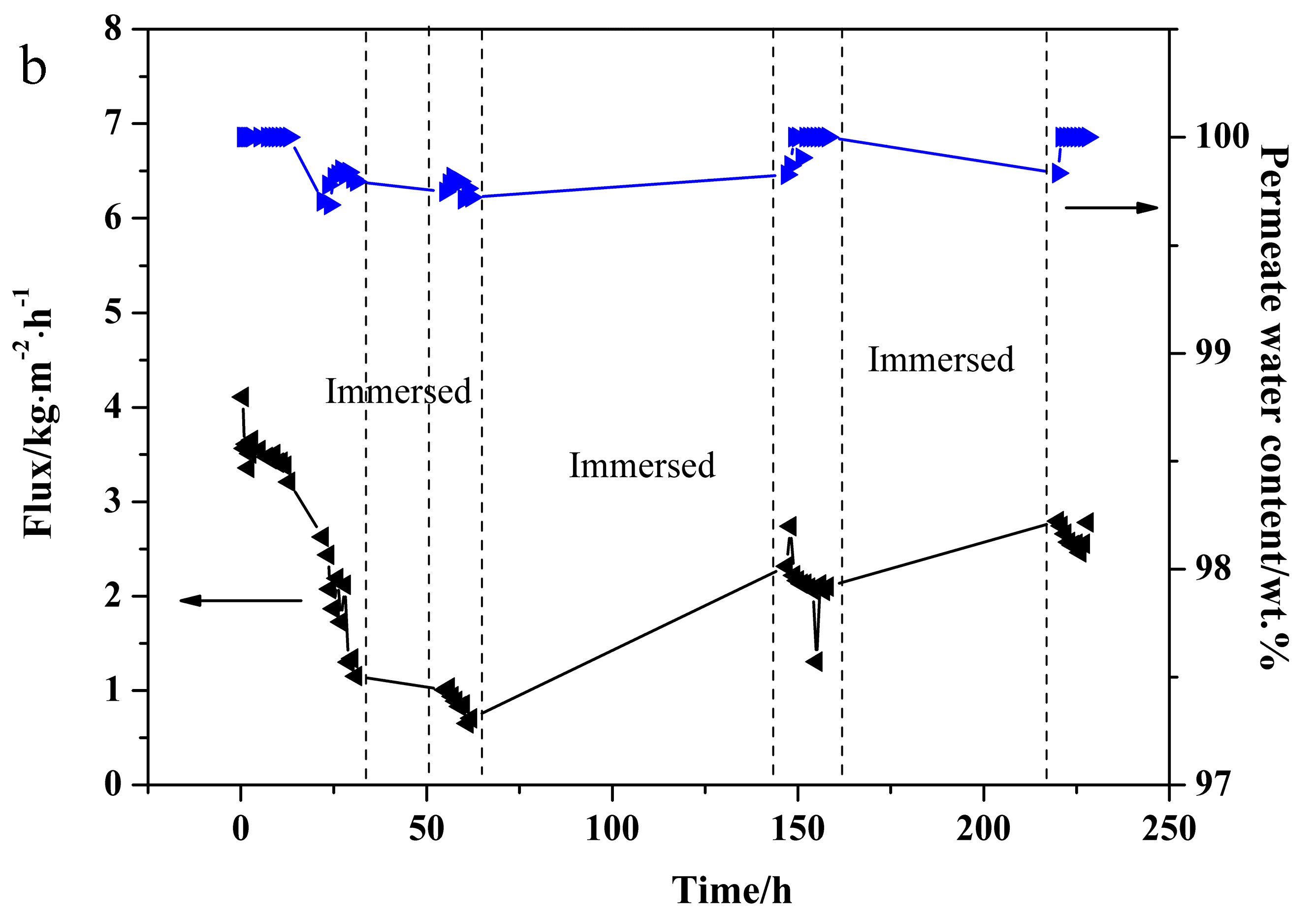
3.5. PV Stability Test of NaA Zeolite Membrane
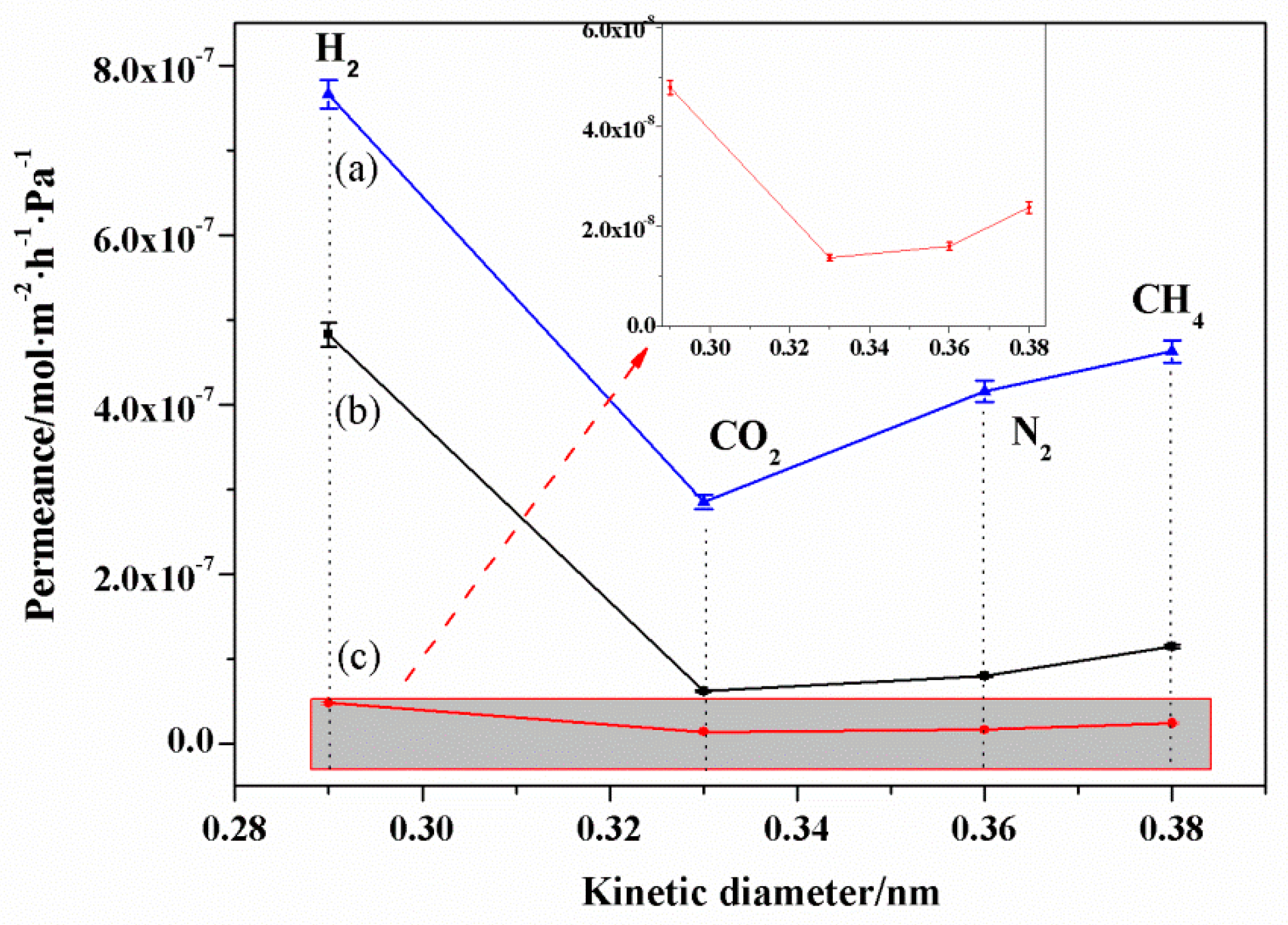
3.6. Gas Permeation of NaA Zeolite Membranes
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zeng, L.; Li, Z. A new process for fuel ethanol dehydration based on modeling the phase equilibria of the anhydrous MgCl2 + ethanol + water system. AIChE J. 2015, 61, 664–676. [Google Scholar] [CrossRef]
- Sanchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.L.; Koziel, J.A.; Jane, J.; Pometto, A.L., III. Reducing bacterial contamination in fuel ethanol fermentations by ozone treatment of uncooked corn mash. J. Agric. Food Chem. 2015, 63, 5239–5248. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Takenaka, A.; Fujita, H.; Mochidzuki, K.; Sakoda, A. Practical considerations for a simple ethanol concentration from a fermentation broth via a single adsorptive process using molecular-sieving carbon. Renew. Energ. 2018, 118, 257–264. [Google Scholar] [CrossRef]
- Zah, J.; Krieg, H.M.; Breytenbach, J.C. Single gas permeation through compositionally different zeolite NaA membranes: Observations on the intercrystalline porosity in an unconventional semicrystalline layer. J. Membr. Sci. 2007, 287, 300–310. [Google Scholar] [CrossRef]
- Aoki, K.; Kusakabe, K.; Morooka, S. Preparation of oriented A-type zeolite membranes. AIChE. J. 2000, 46, 221–224. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes-Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Shao, J.; Zhan, Z.; Li, J.; Wang, Z.; Li, K.; Yan, Y. Zeolite NaA membranes supported on alumina hollow fibers: Effect of support resistances on pervaporation performance. J. Membr. Sci. 2014, 451, 10–17. [Google Scholar] [CrossRef]
- Wenten, I.G.; Dharmawijaya, P.T.; Aryanti, P.T.P.; Mukti, R.R.; Khoiruddin, K. LTA zeolite membranes: Current progress and challenges in pervaporation. RSC Adv. 2017, 7, 29520–29539. [Google Scholar] [CrossRef]
- Kita, H.; Horii, K.; Ohtoshi, Y.; Tanaka, K.; Okamoto, K.I. Synthesis of a zeolite NaA membrane for pervaporation of water-organic liquid mixtures. J. Mater. Sci. Lett. 1995, 14, 206–208. [Google Scholar] [CrossRef]
- Shakarova, D.; Ojuva, A.; Bergstrom, L.; Akhtar, F. Methylcellulose-drected synthesis of nanocrystalline zeolite NaA with high CO2 uptake. Materials 2014, 7, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, V.; Xomeritakis, G.; Abibi, A.; Dickson, M.; Tsapatsis, M.; Vlachos, D.G. Growth of a faujasite-type zeolite membrane and its application in the separation of saturated/unsaturated hydrocarbon mixtures. J. Membr. Sci. 2001, 184, 209–219. [Google Scholar] [CrossRef]
- Cui, Y.; Kita, H.; Okamoto, K.I. Zeolite T membrane: Preparation, characterization, pervaporation of water/organic liquid mixtures and acid stability. J. Membr. Sci. 2004, 236, 17–27. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Zhu, M.; Hu, Z.; Duan, L.; Chen, X. Synthesis and pervaporation performance of mordenite membranes on mullite tubes. Chin. J. Inorg. Chem. 2010, 26, 469–475. [Google Scholar]
- Yang, S.; Arvanitis, A.; Cao, Z.; Sun, X.; Dong, J. Synthesis of silicalite membrane with an aluminum-containing surface for controlled modification of zeolitic pore entries for enhanced gas separation. Processes 2018, 6, 13. [Google Scholar] [CrossRef]
- Dincer, E.; Culfaz, A.; Kalipcilar, H. Effect of seeding on the properties of MFI type zeolite membranes. Desalination 2006, 200, 66–67. [Google Scholar] [CrossRef]
- Korelskiy, D.; Ye, P.; Zhou, H.; Mouzon, J.; Hedlund, J. An experimental study of micropore defects in MFI membranes. Microporous Mesoporous Mater. 2014, 197, 358. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zhang, Y.; He, Y.; Gu, X. Scale-up of NaA zeolite membranes on alpha-Al2O3 hollow fibers by a secondary growth method with vacuum seeding. Chin. J. Chem. Eng. 2015, 23, 1114–1122. [Google Scholar] [CrossRef]
- Guan, G.; Kusakabe, K.; Morooka, S. Gas permeation properties of ion-exchanged LTA-type zeolite membranes. Sep. Purif. Technol. 2001, 36, 2233–2245. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, J.; Yang, W. Microwave synthesis of LTA zeolite membranes without seeding. J. Membr. Sci. 2006, 277, 230–239. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Zhu, G.; Liu, J.; Yang, W. Hydrothermal stability of LTA zeolite membranes in pervaporation. J. Membr. Sci. 2007, 297, 10–15. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nagase, T.; Kiyozumi, Y.; Hanaoka, T.; Mizukami, F. Influence of acid on the permeation properties of NaA-type zeolite membranes. J. Membr. Sci. 2010, 349, 189–194. [Google Scholar] [CrossRef]
- Caro, J.; Albrecht, D.; Noack, M. Why is it so extremely difficult to prepare shape-selective Al-rich zeolite membranes like LTA and FAU for gas separation? Sep. Purif. Technol. 2009, 66, 143–147. [Google Scholar] [CrossRef]
- Huang, A.; Caro, J. Cationic polymer used to capture zeolite precursor particles for the facile synthesis of oriented zeolite LTA molecular sieve membrane. Chem. Mater. 2010, 22, 4353–4355. [Google Scholar] [CrossRef]
- Breck, D.W.; Eversole, W.G.; Milton, R.M.; Reed, T.B.; Thomas, T.L. Crystalline zeolites. I. The properties of a new synthetic zeolite, type A. J. Am. Chem. Soc. 1956, 78, 5963–5972. [Google Scholar] [CrossRef]
- Shirazian, S.; Ashrafizadeh, S.N. LTA and ion-exchanged LTA zeolite membranes for dehydration of natural gas. J. Ind. Eng. Chem. 2015, 22, 132–137. [Google Scholar] [CrossRef]
- Varela-Gandia, F.J.; Berenguer-Murcia, A.; Lozano-Castello, D.; Cazorla-Amoros, D. Hydrogen purification for PEM fuel cells using membranes prepared by ion-exchange of Na-LTA/carbon membranes. J. Membr. Sci. 2010, 351, 123–130. [Google Scholar] [CrossRef]
- Wei, X.; Liang, S.; Xu, Y.; Sun, Y.; An, J.; Chao, Z. Methylcellulose-assisted synthesis of a compact and thin NaA zeolite membrane. RSC Adv. 2016, 6, 71863–71866. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, Z.; Feng, B.; Huang, A. A graphene oxide layer as an acid-resisting barrier deposited on a zeolite LTA membrane for dehydration of acetic acid. RSC Adv. 2016, 6, 23354–23359. [Google Scholar] [CrossRef]
- Zhang, Y.; Avila, A.M.; Tokay, B.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Blocking defects in SAPO-34 membranes with cyclodextrin. J. Membr. Sci. 2010, 358, 7–12. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Xu, J.; Wang, J.; Meng, X.; Bai, K.; Lu, J.; Zhang, Y.; Yin, D. Influences of inorganic salts on the pervaporation properties of zeolite NaA membranes on macroporous supports. Microporous Mesoporous Mater. 2014, 192, 60–68. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Jiang, J.; Wang, X.; Zhang, C.; Gu, X. High-performance NaA zeolite membranes supported on four-channel ceramic hollow fibers for ethanol dehydration. RSC Adv. 2015, 5, 95866–95871. [Google Scholar] [CrossRef]
- Nightingaljer, E.R. Phenomenological the ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
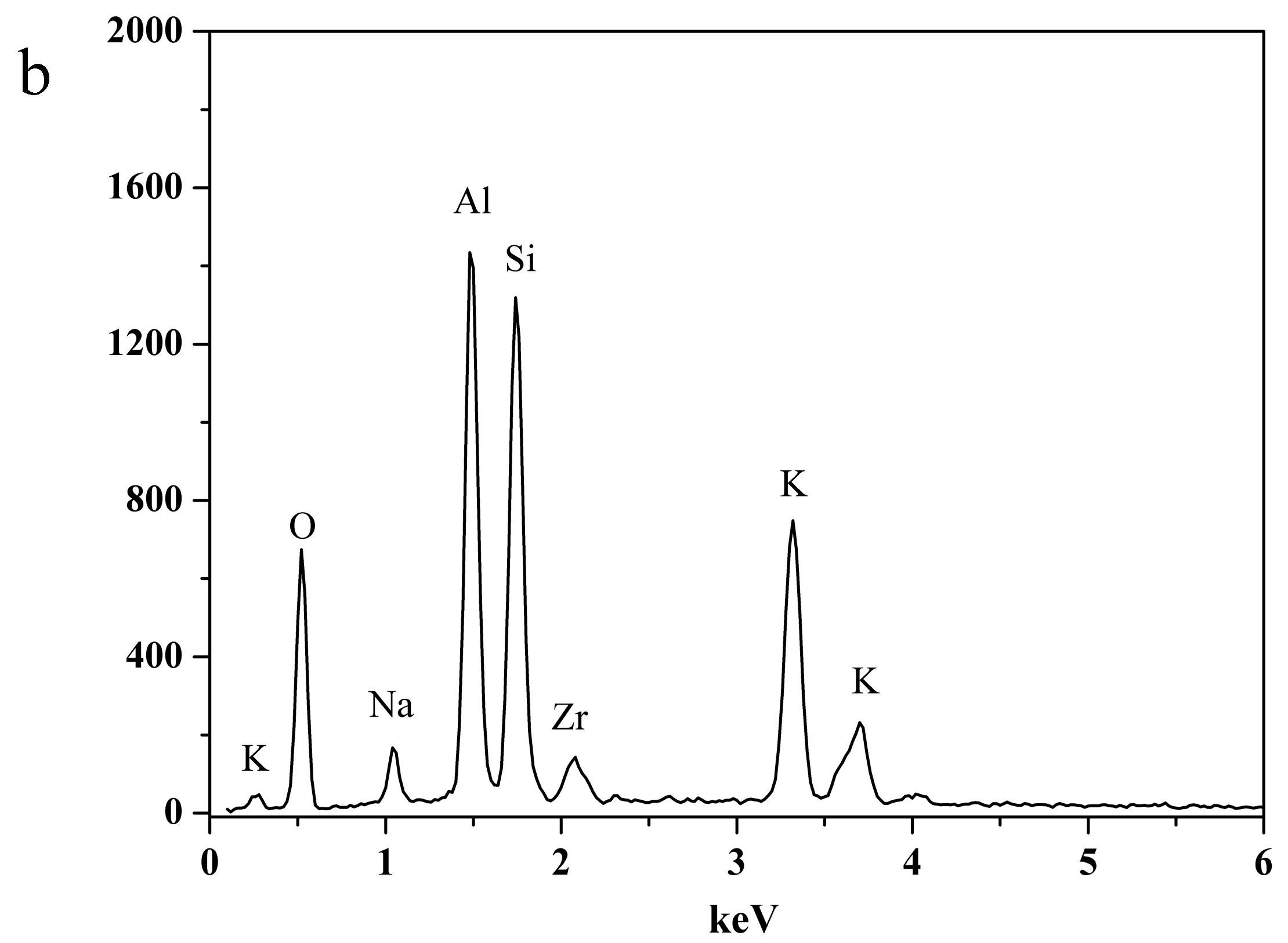
| Ion Valence State | Material | Processing Conditions | Fluxes/kg·m−2·h−1 | Separation Factor |
|---|---|---|---|---|
| 1 | KCl | Un-exchange | 4.05 ± 0.019 | 449 ± 2.4 |
| Exchange | 3.58 ± 0.023 | 1062 ± 12.8 | ||
| NH4Cl | Un-exchange | 7.42 ± 0.023 | 306 ± 1.1 | |
| Exchange | 1.44 ± 0.005 | 38 ± 0.1 | ||
| LiCl | Un-exchange | 4.58 ± 0.019 | 208 ± 0.5 | |
| Exchange | leaking | 1 ± 0.01 | ||
| NaCl | Un-exchange | 4.75 ± 0.023 | 449 ± 2.4 | |
| Exchange | 4.83 ± 0.018 | 519 ± 3.1 | ||
| AgNO3 | Un-exchange | 6.51 ± 0.027 | 548 ± 3.6 | |
| Exchange | 5.98 ± 0.025 | 2136 ± 50.7 | ||
| 2 | MgCl2 | Un-exchange | 5.05 ± 0.025 | 2167 ± 52.5 |
| Exchange | 1.99 ± 0.014 | 47 ± 0.1 | ||
| BaCl2 | Un-exchange | 4.80 ± 0.024 | 208 ± 0.6 | |
| Exchange | 5.19 ± 0.025 | 162 ± 0.4 | ||
| CaCl2 | Un-exchange | 6.45 ± 0.025 | 306 ± 1.2 | |
| Exchange | 6.07 ± 0.024 | 52 ± 0.1 | ||
| ZnCl2 | Un-exchange | 5.06 ± 0.019 | 2156 ± 53.4 | |
| Exchange | 2.90 ± 0.015 | 5 ± 0.04 |
| Cations | Hydrated Radius, Å [33] | Bare Radius, Å [34] |
|---|---|---|
| Li+ | 3.82 | 0.94 |
| Na+ | 3.58 | 1.17 |
| K+ | 3.31 | 1.49 |
| Ag+ | 3.41 | 1.26 |
| NH4+ | 3.31 | 1.48 |
| Mg2+ | 4.28 | 0.72 |
| Ca2+ | 4.12 | 1.00 |
| Ba2+ | 4.04 | 1.35 |
| Zn2+ | 4.30 | 0.74 |
| Type | Relative Content of Na | Relative Content of Positive Charge a |
|---|---|---|
| NaA zeolite | 1.00 | 0.96 |
| NaCl treatment | 1.12 | 1.03 |
| LiCl treatment | 0.84 | 0.93 |
| KCl treatment | 0.41 | 0.97 |
| AgNO3 treatment | 0.10 | 0.99 |
| ZnCl2 treatment | 0.22 | 0.97 |
| MgCl2 treatment | 0.51 | 1.06 |
| CaCl2 treatment | 0.34 | 1.02 |
| BaCl2 treatment | 0.19 | 0.99 |
| No. | Cations | Concentration/M | Flux/kg∙m−2∙h−1 | Separation Factor |
|---|---|---|---|---|
| M1 | - | - | 4.96 ± 0.03 | 449 ± 2.4 |
| M1′ | K+ | 0.05 | 4.45 ± 0.02 | >10,000 ± 198.2 |
| M1″ | K+ | 0.1 | 3.58 ± 0.02 | 1062 ± 12.8 |
| M2 | - | - | 6.51 ± 0.03 | 548 ± 3.6 |
| M2′ | Ag+ | 0.05 | 5.87 ± 0.03 | >10,000 ± 198.2 |
| M2″ | Ag+ | 0.1 | 5.98 ± 0.03 | 2136 ± 50.9 |
| M3 | - | - | 7.42 ± 0.03 | 306 ± 1.2 |
| M3′ | NH4+ | 0.05 | 4.95 ± 0.02 | 107 ± 0.2 |
| M3″ | NH4+ | 0.1 | 1.44 ± 0.01 | 38 ± 0.1 |
| Cation Type | Exchanged NaA Zeolite Membranes | Contact Angle/θ |
|---|---|---|
| Original | NaA zeolite membrane | 6.6° |
| Monovalent cation | Li+ exchanged NaA zeolite membrane | 46.5° |
| K+ exchanged NaA zeolite membrane | 43.0° | |
| Ag+ exchanged NaA zeolite membrane | 99.6° | |
| Divalent cation | Zn2+ exchanged NaA zeolite membrane | 72.6° |
| Mg2+ exchanged NaA zeolite membrane | 18.3° | |
| Ca2+ exchanged NaA zeolite membrane | 46.2° | |
| Ba2+ exchanged NaA zeolite membrane | 33.5° |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Gao, B.; Wang, X.; Shi, R.; Ur Rehman, R.; Gu, X. The Influence of Cation Treatments on the Pervaporation Dehydration of NaA Zeolite Membranes Prepared on Hollow Fibers. Processes 2018, 6, 70. https://doi.org/10.3390/pr6060070
Gao X, Gao B, Wang X, Shi R, Ur Rehman R, Gu X. The Influence of Cation Treatments on the Pervaporation Dehydration of NaA Zeolite Membranes Prepared on Hollow Fibers. Processes. 2018; 6(6):70. https://doi.org/10.3390/pr6060070
Chicago/Turabian StyleGao, Xuechao, Bing Gao, Xingchen Wang, Rui Shi, Rashid Ur Rehman, and Xuehong Gu. 2018. "The Influence of Cation Treatments on the Pervaporation Dehydration of NaA Zeolite Membranes Prepared on Hollow Fibers" Processes 6, no. 6: 70. https://doi.org/10.3390/pr6060070
APA StyleGao, X., Gao, B., Wang, X., Shi, R., Ur Rehman, R., & Gu, X. (2018). The Influence of Cation Treatments on the Pervaporation Dehydration of NaA Zeolite Membranes Prepared on Hollow Fibers. Processes, 6(6), 70. https://doi.org/10.3390/pr6060070