A Facile Synthesis of Hexagonal Spinel λ-MnO2 Ion-Sieves for Highly Selective Li+ Adsorption
Abstract
:1. Introduction
2. Experimental
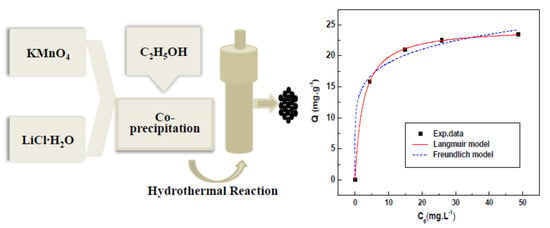
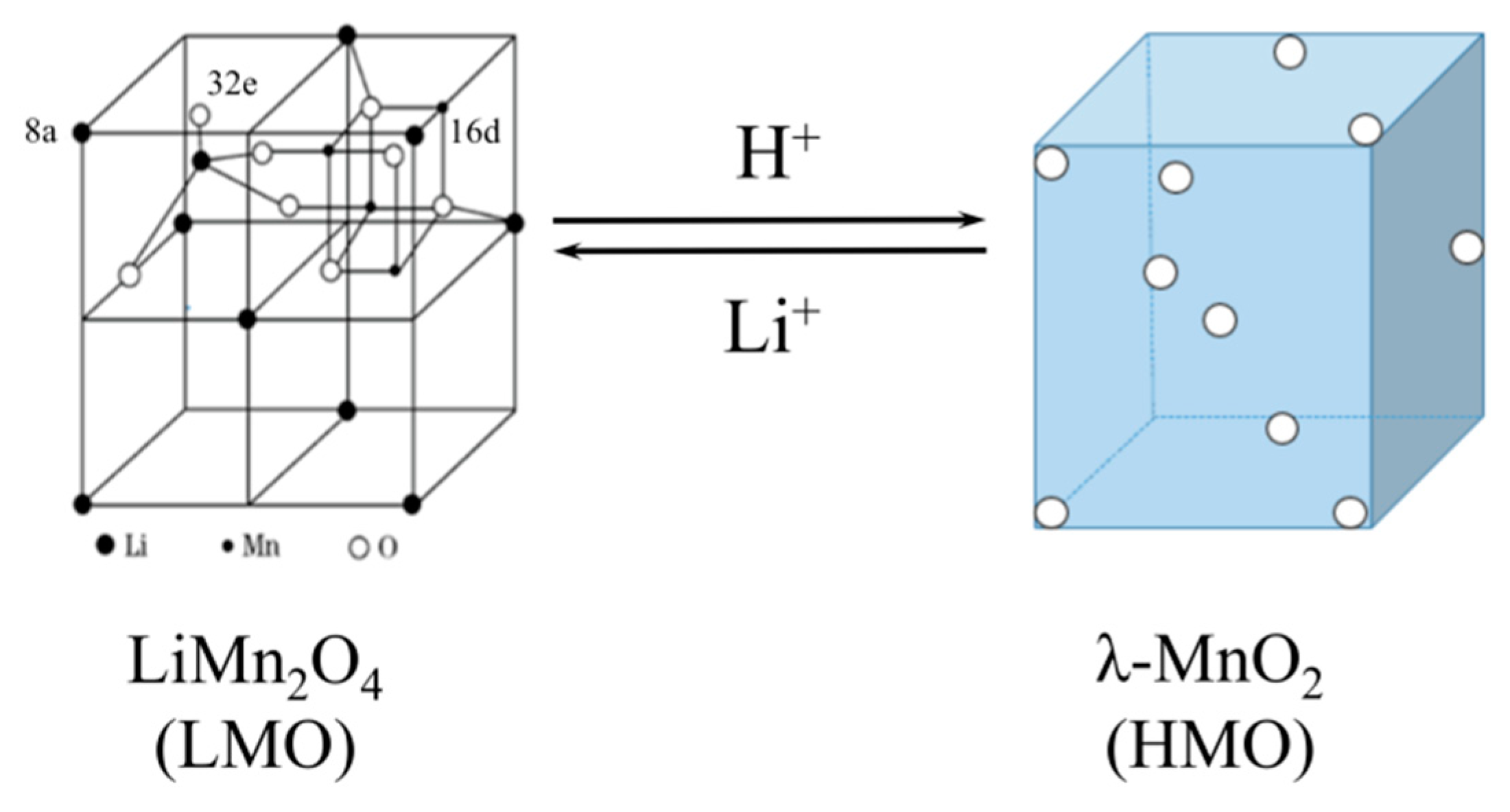
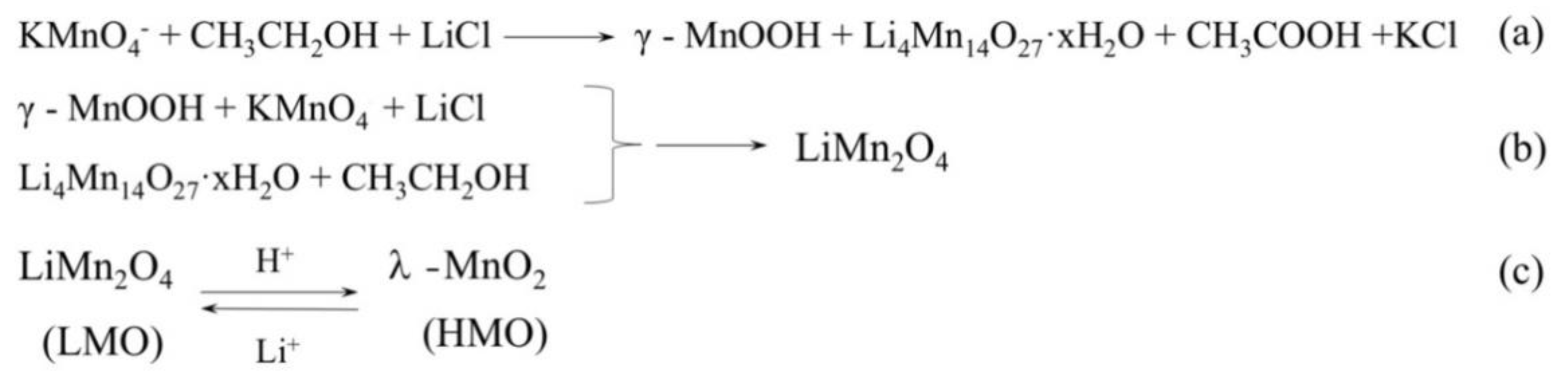
2.1. Preparation of LMO and HMO Ion Sieve
2.2. Characterization
2.3. Adsorption Behavior
2.3.1. Adsorption Capacity Test at Different pH Value
2.3.2. Static Kinetic Test
2.3.3. Adsorption Isotherm Test
2.4. Selective Adsorption Behavior
2.5. Desorption Behavior
3. Results and Discussion
3.1. Optimization of Synthesis Parameters
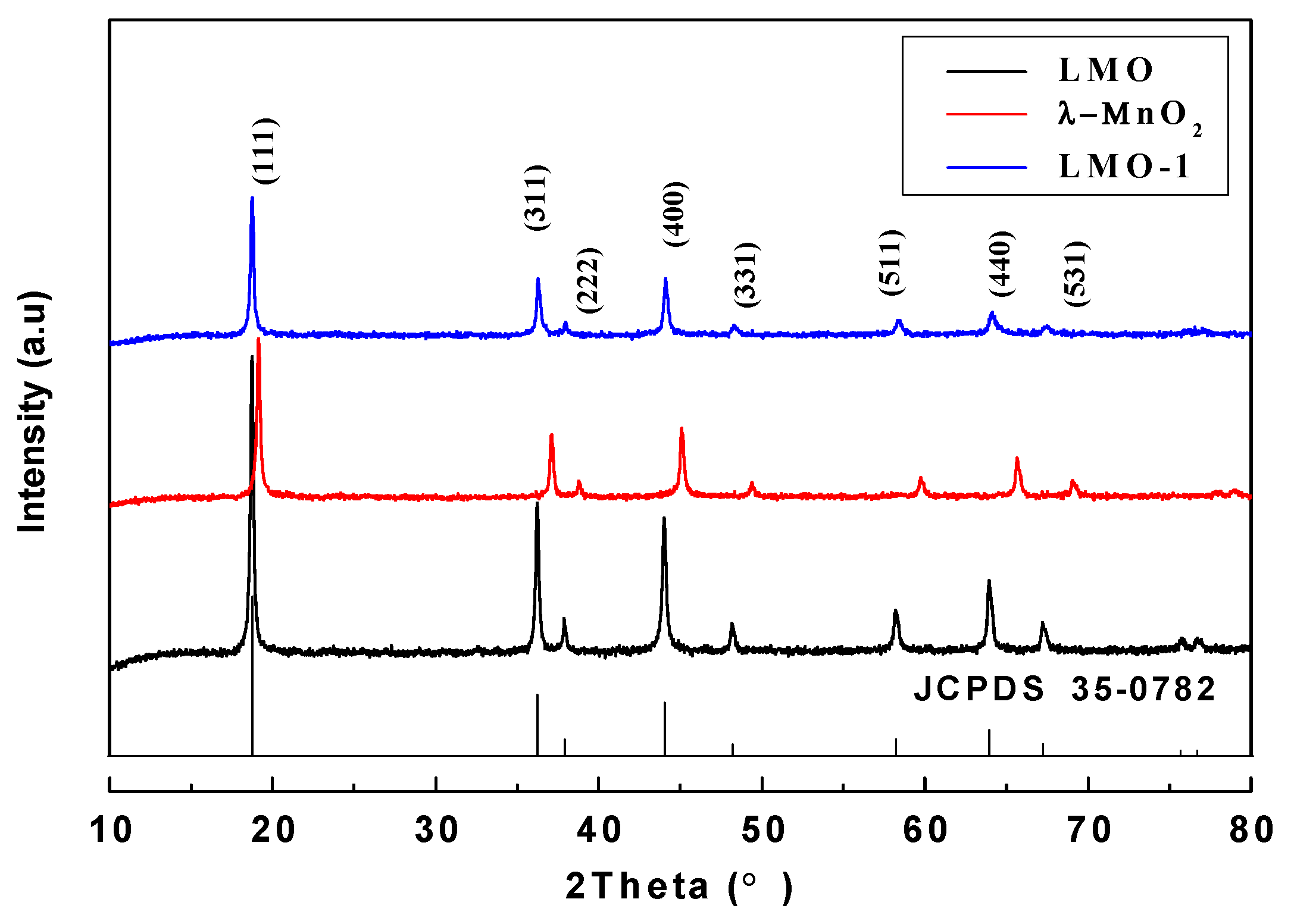
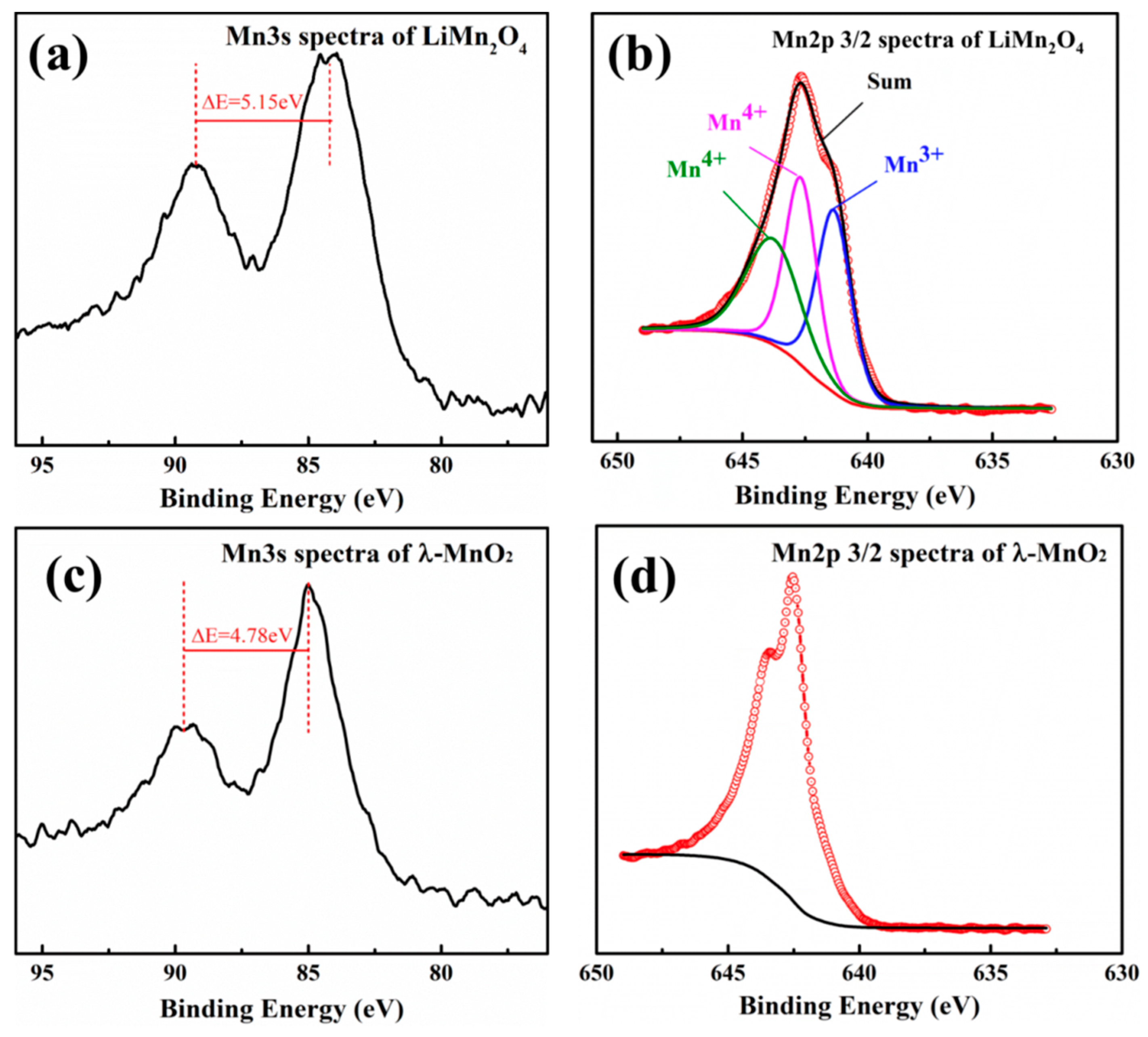
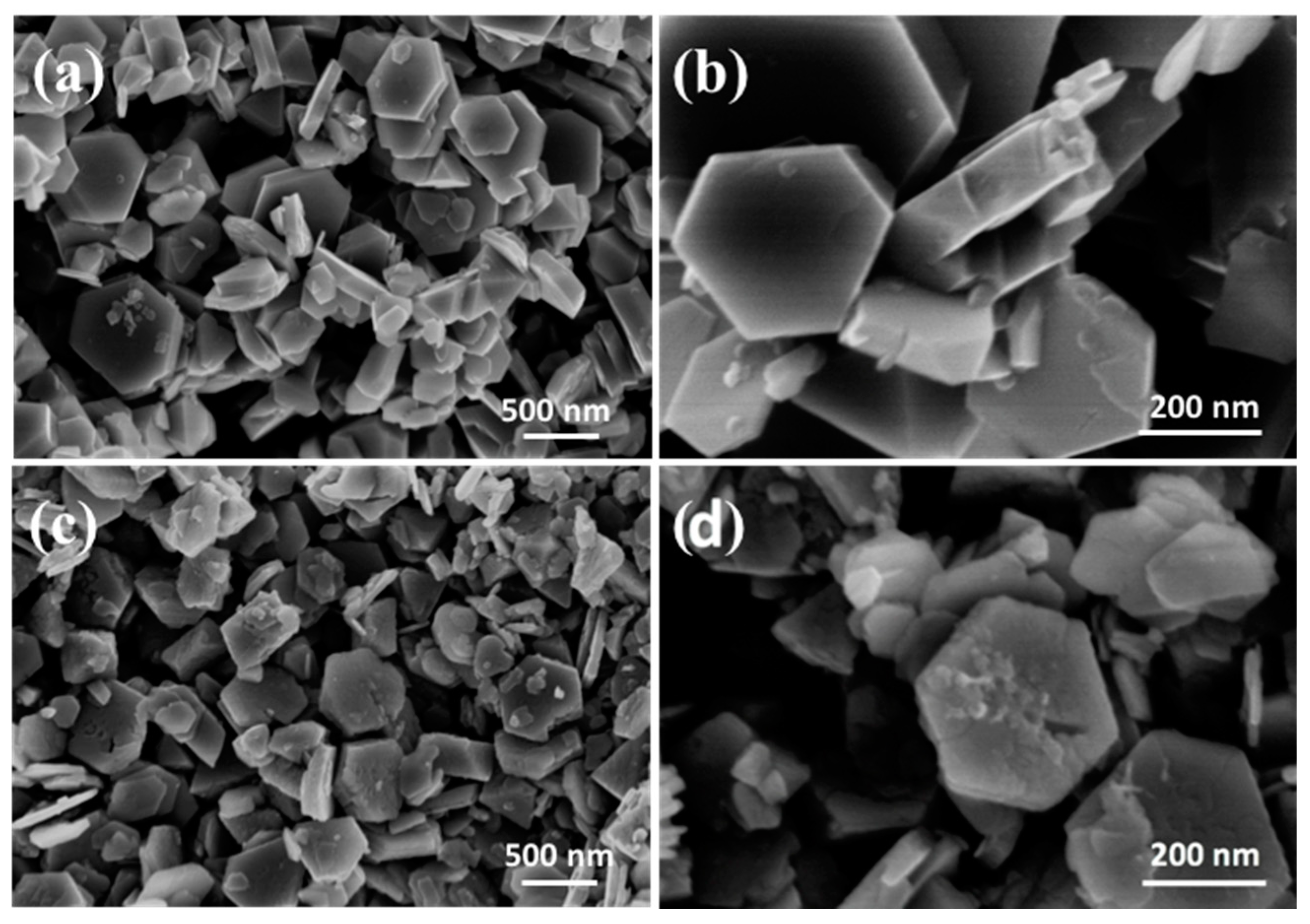
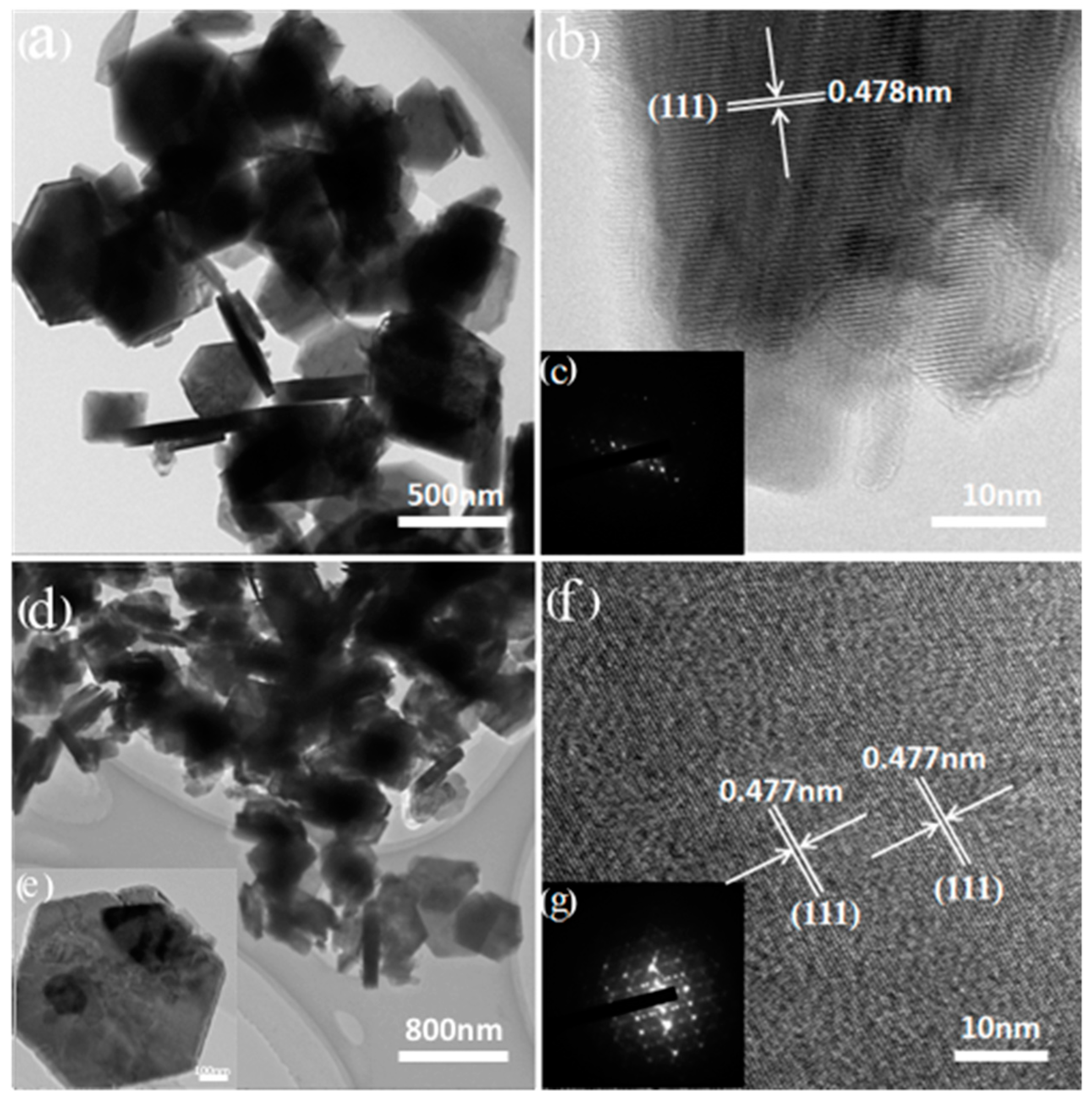
3.2. Ion-Sieves Characterization
3.3. Adsorption Behavior of the HMO
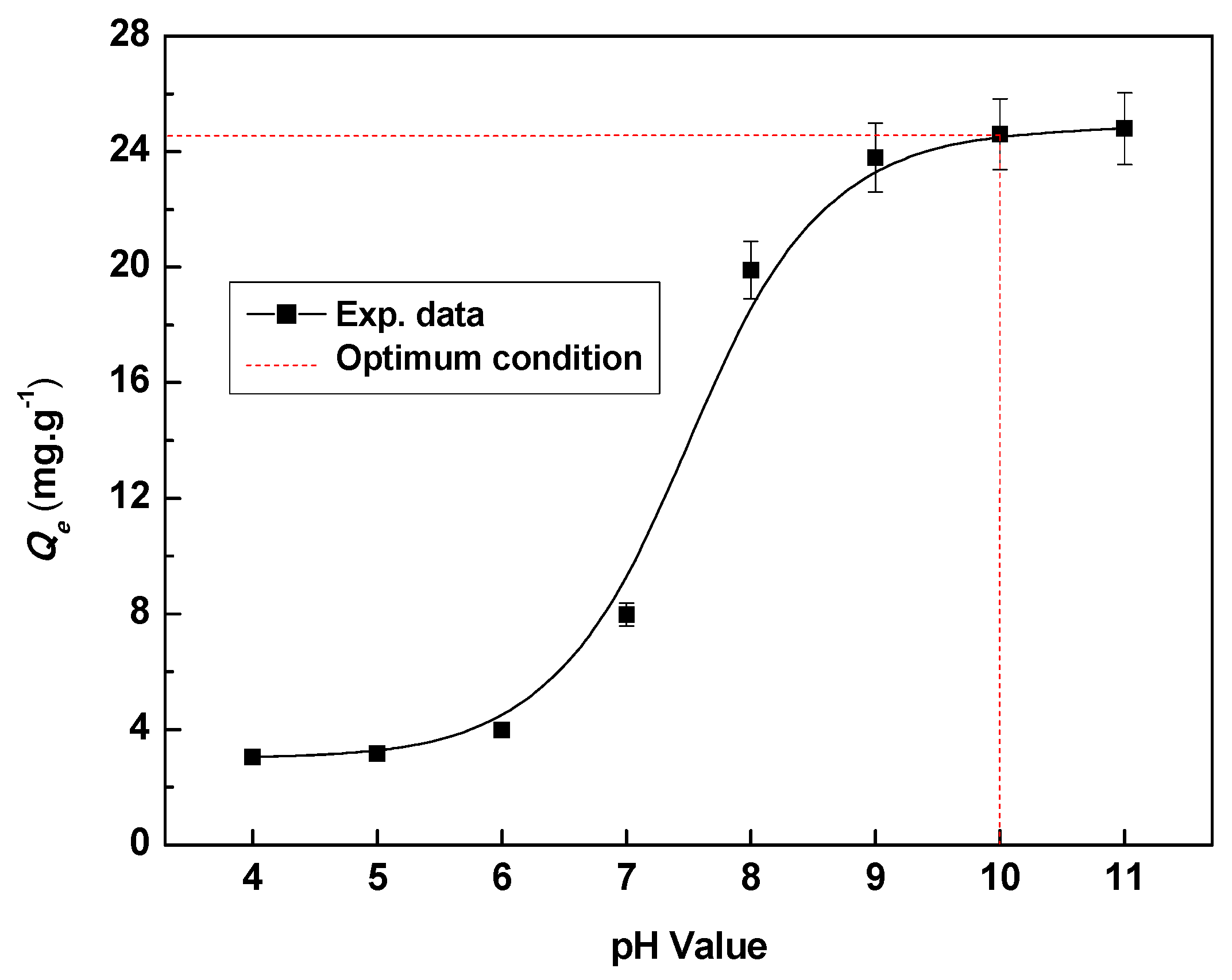
3.3.1. Effect of pH Value on Adsorption Capacity
3.3.2. Static Adsorption Test
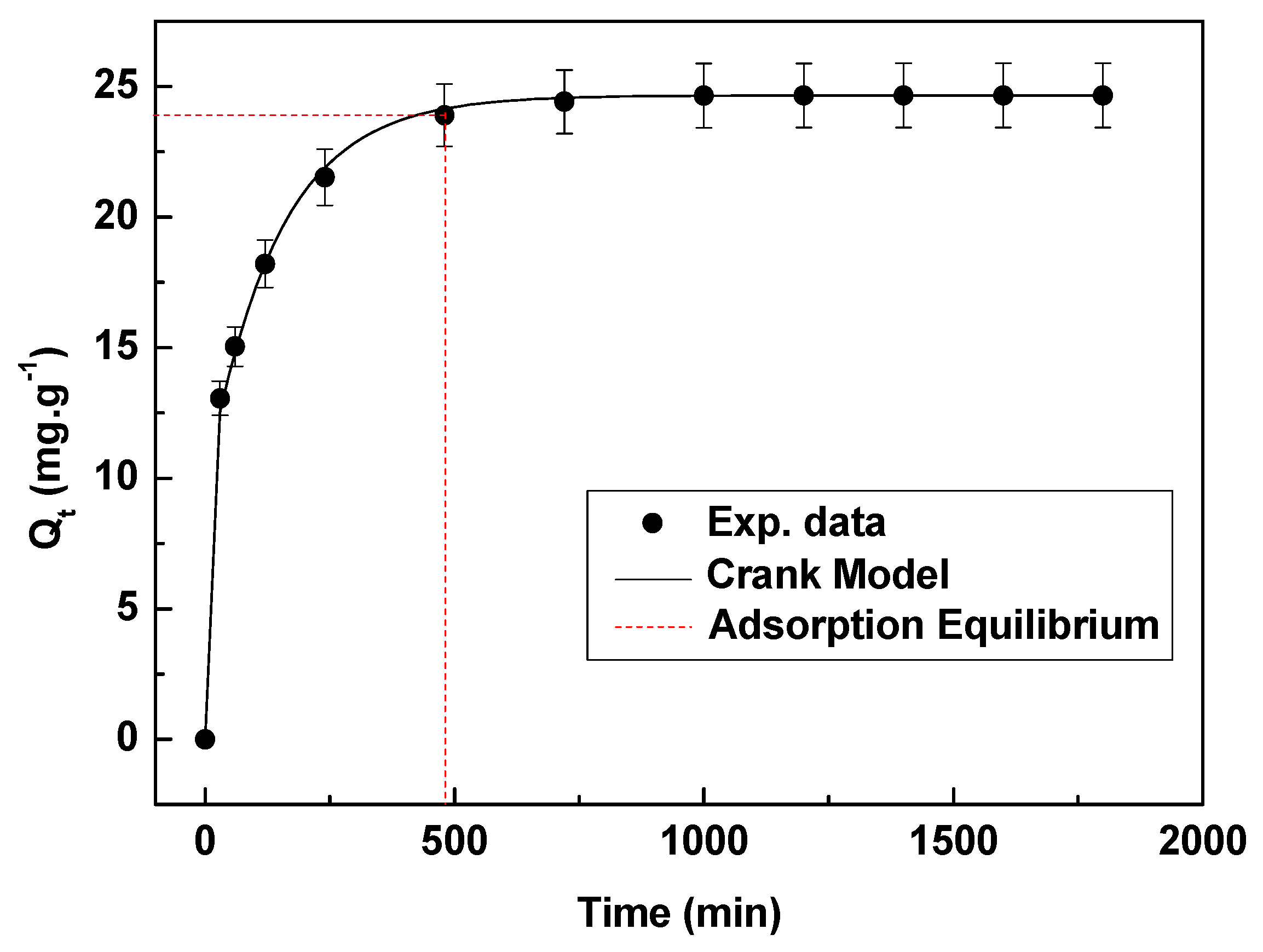
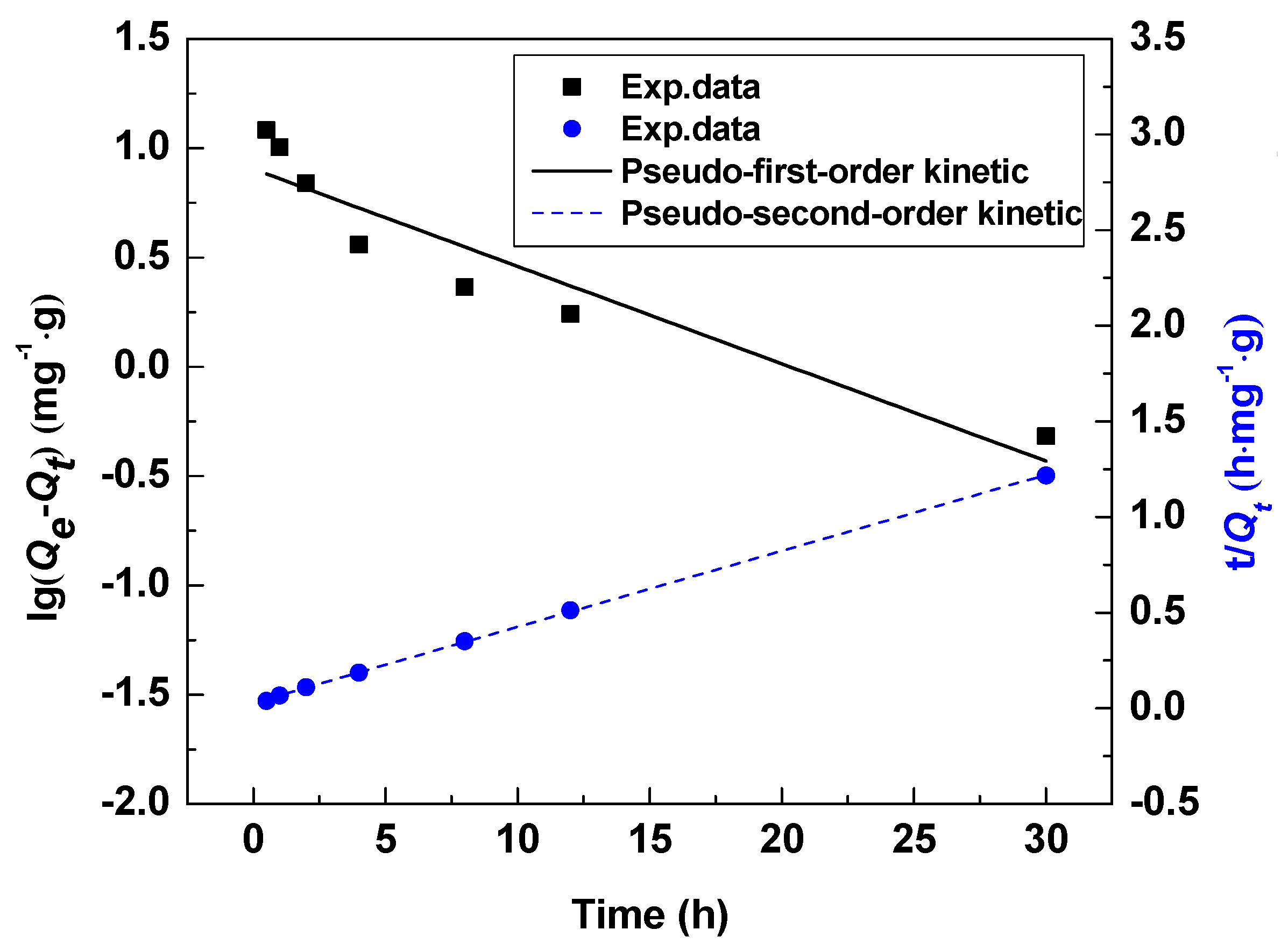
3.3.3. Adsorption Kinetic Test
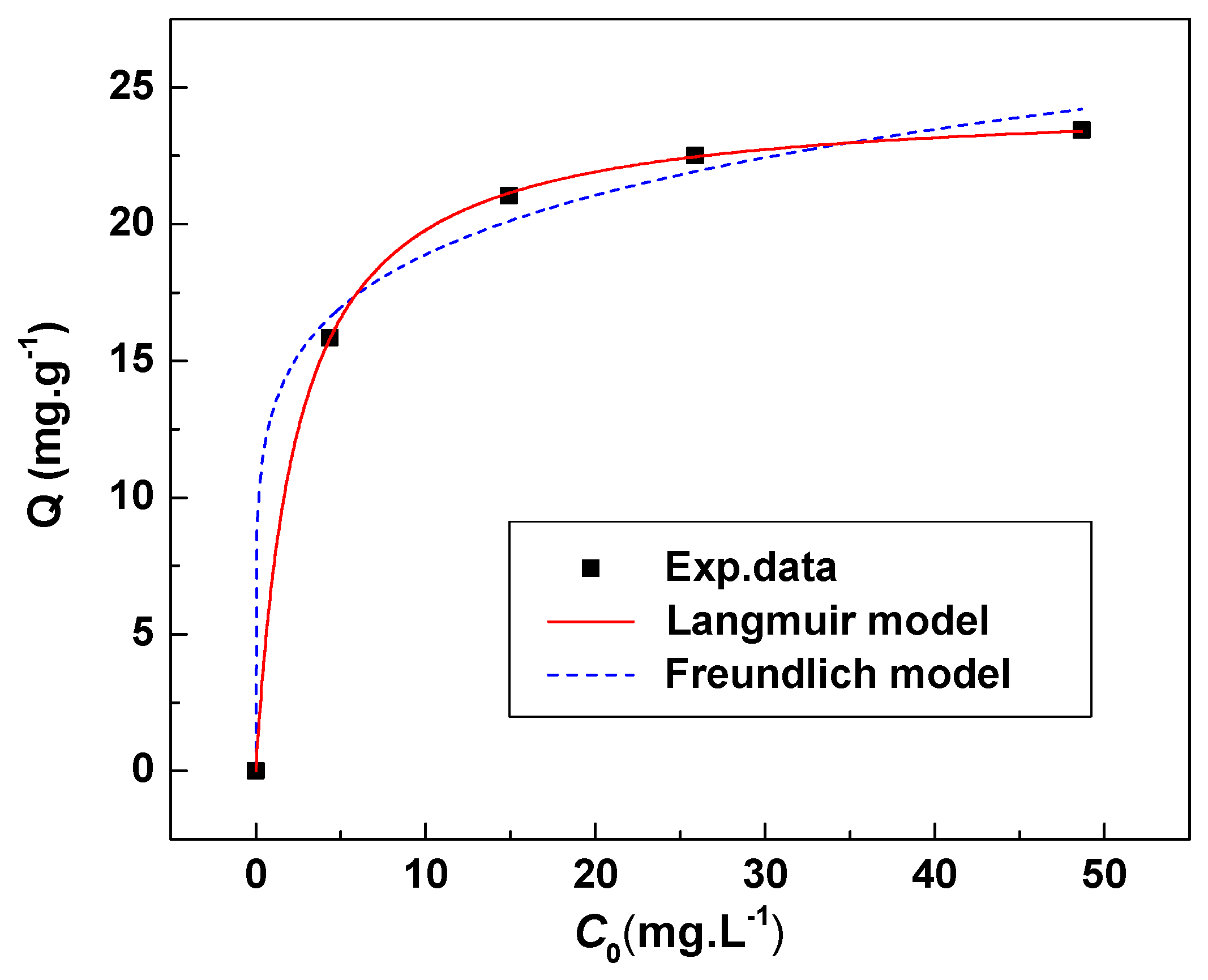
3.3.4. Adsorption Isotherm of Li+ on HMO
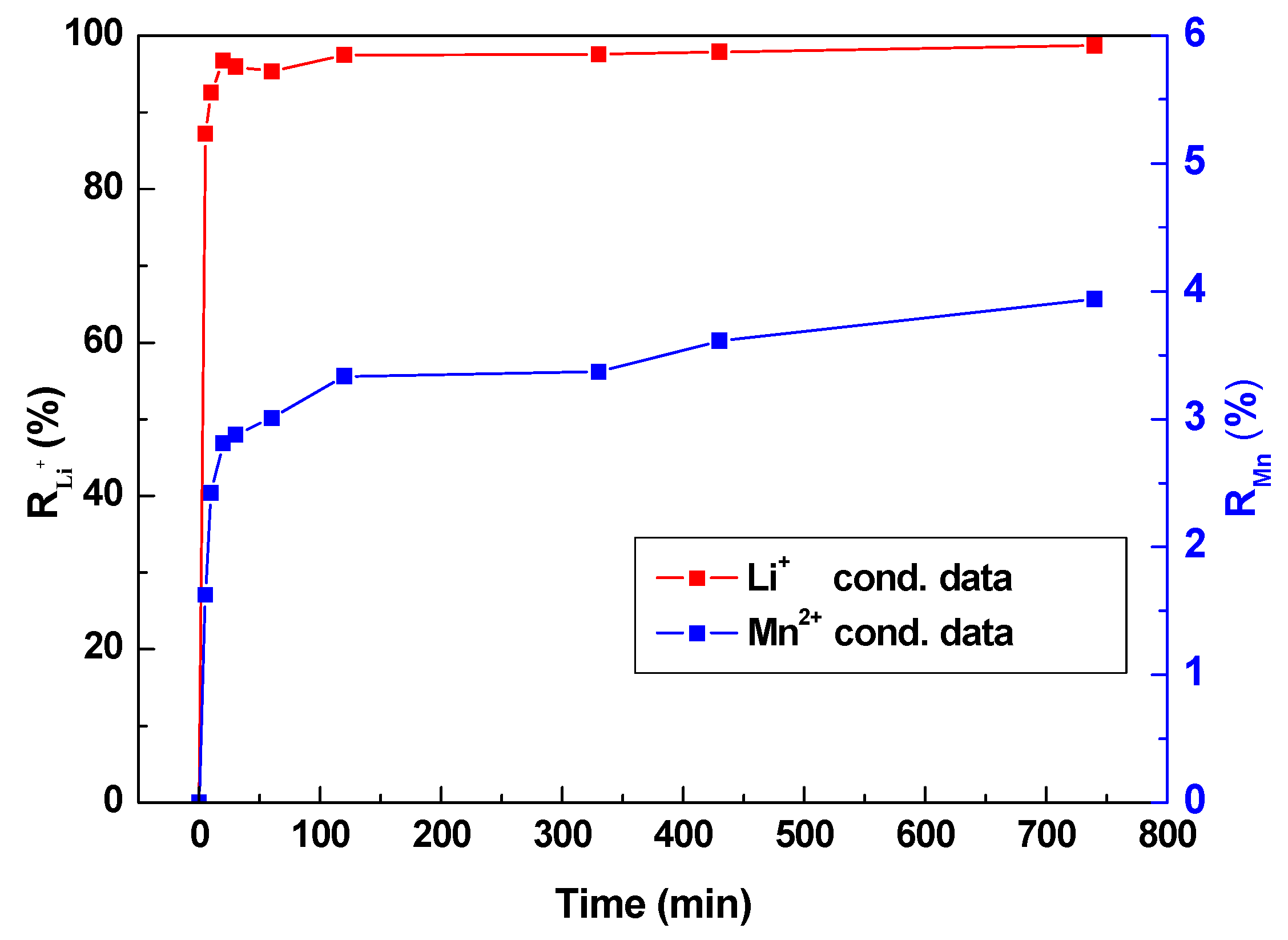
3.4. Absorption Selectivity of HMO
3.5. Desorption Behavior of LMO-1
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, S.; Huimin, R.; Shen, J.; Gao, C. Preparation methods and analyses of structural performance of spinel-type lithium manganese oxide ion sieves. Chem. Ind. Eng. Prog. 2015, 6, 1690–1699. [Google Scholar]
- Özgür, C. Preparation and characterization of LiMn2O4 ion-sieve with high Li+ adsorption rate by ultrasonic spray pyrolysis. Solid State Ion. 2010, 181, 1425–1428. [Google Scholar] [CrossRef]
- Xiao, J.L.; Sun, S.Y.; Song, X.; Li, P.; Yu, J.G. Lithium ion recovery from brine using granulated polyacry λ–MnO2 ion-sieve. Chem. Eng. J. 2015, 279, 659–666. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Feng, G.; Ping, Z.M.; Zhen, N.; Hua, L.J.; Sheng, S.P. Brine Lithium Resource in the Salt Lake and Advances in Its Exploitation. Acta Geosci. Sin. 2011, 32, 483–492. [Google Scholar]
- Xiao, J.L.; Sun, S.Y.; Wang, J.; Li, P.; Yu, J.G. Synthesis and Adsorption Properties of Li1.6Mn1.6O4 Spinel. Ind. Eng. Chem. Res. 2013, 52, 11967–11973. [Google Scholar] [CrossRef]
- Zandevakili, S.; Ranjbar, M.; Ehteshamzadeh, M. Improvement of lithium adsorption capacity by optimising the parameters affecting synthesised ion sieves. Micro Nano Lett. 2015, 10, 58–63. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Liu, F.; Zhao, T.; Zhang, X.; Chen, R.; Wu, F. Surface modification of spinel λ-MnO2 and its lithium adsorption properties from spent lithium ion batteries. Appl. Surf. Sci. 2014, 315, 59–65. [Google Scholar] [CrossRef]
- Xiao, G.; Tong, K.; Zhou, L.; Xiao, J.; Sun, S.; Li, P.; Yu, J. Adsorption and Desorption Behavior of Lithium Ion in Spherical PVC–MnO2 Ion Sieve. Ind. Eng. Chem. Res. 2012, 51, 10921–10929. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, P.; Qi, P.; Gao, C. Adsorption and desorption properties of Li+ on PVC-H1.6 Mn1.6O4 lithium ion-sieve membrane. Chem. Eng. J. 2014, 235, 340–348. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, Y.; Wang, X.; Zeng, M. Preparation and characterization of lithium λ-MnO2 ion-sieves. Front. Chem. Sci. Eng. 2014, 8, 471–477. [Google Scholar] [CrossRef]
- Xiao, J.; Nie, X.; Sun, S.; Song, X.; Ping, L.; Yu, J. Lithium ion adsorption–desorption properties on spinel Li4Mn5O12 and pH-dependent ion-exchange model. Adv. Powder Technol. 2015, 26, 589–594. [Google Scholar] [CrossRef]
- Singh, I.B.; Singh, A. A facile low-temperature synthesis of Li4Mn5O12 nanorods. Colloid Polym. Sci. 2017, 295, 689–693. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Zhang, Y.; Cao, D.; Zhao, X. Lithium extraction from seawater by manganese oxide ion sieve MnO2·0.5H2O. Colloids Surf. A. 2015, 468, 280–284. [Google Scholar] [CrossRef]
- Park, M.J.; Nisola, G.M.; Beltran, A.B.; Torrejos, R.E.C.; Seo, J.G.; Lee, S.; Kim, H.; Chung, W. Recyclable composite nanofiber adsorbent for Li+ recovery from seawater desalination retentate. Chem. Eng. J. 2014, 254, 73–81. [Google Scholar] [CrossRef]
- Sorour, M.H.; El-Rafei, A.M.; Hani, H.A. Synthesis and characterization of electrospun aluminum doped Li1.6Mn1.6O4 spinel. Ceram. Int. 2016, 42, 4911–4917. [Google Scholar] [CrossRef]
- Sun, D.; Meng, M.; Yin, Y.; Zhu, Y.; Li, H.; Yan, Y. Highly selective, regenerated ion-sieve microfiltration porous membrane for targeted separation of Li+. J. Porous Mater. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Yu, Q.; Sasaki, K. In situ X-ray diffraction investigation of the evolution of a nanocrystalline lithium-ion sieve from biogenic manganese oxide. Hydrometall 2014, 150, 253–258. [Google Scholar] [CrossRef]
- Yuan, J.S.; Yin, H.B.; Ji, Z.Y.; Deng, H.N. Effective Recycling Performance of Li+ Extraction from Spinel-Type LiMn2O4 with Persulfate. Ind. Eng. Chem. Res. 2014, 53, 9889–9896. [Google Scholar] [CrossRef]
- Park, H.K.; Rah, H.; Dong, J.K.; Chun, U.; Kim, S.G. Confined growth of lithium manganese oxide nanoparticles. J. Sol-Gel Sci. Technol. 2013, 67, 464–472. [Google Scholar] [CrossRef]
- Tang, W.; Tian, S.; Liu, L.L.; Li, L.; Zhang, H.P.; Yue, Y.B.; Bai, Y.; Wu, Y.P.; Zhu, K. Nanochain LiMn2O4 as ultra-fast cathode material for aqueous rechargeable lithium batteries. Electrochem. Commun. 2011, 13, 205–208. [Google Scholar] [CrossRef]
- Xiao, G.P. Granulation to LiMn2O4 Ion-Sieve and Its Lithium Adsorption Property. Chin. J. Inorg. Chem. 2010, 26, 435–439. [Google Scholar]
- Zhang, Q.H.; Li, S.P.; Sun, S.Y.; Yin, X.S.; Yu, J.G. LiMn2O4 spinel direct synthesis and lithium ion selective adsorption. Chem. Eng. Sci. 2010, 65, 169–173. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1977; Volume 7, pp. 1–10. [Google Scholar]
- Xue, F.; Xu, Y.; Lu, S.; Ju, S.; Xing, W. Adsorption of Cefocelis Hydrochloride on Macroporous Resin: Kinetics, Equilibrium, and Thermodynamic Studies. J. Chem. Eng. Data 2016, 61, 2179–2185. [Google Scholar] [CrossRef]
- Tian, L.; Ma, W.; Han, M. Adsorption behavior of Li+ onto nano-lithium ion sieve from hybrid magnesium/lithium manganese oxide. Chem. Eng. J. 2010, 156, 134–140. [Google Scholar] [CrossRef]
- Sun, S.Y.; Song, X.; Zhang, Q.H.; Wang, J.; Yu, J.G. Lithium extraction/insertion process on cubic Li-Mn-O precursors with different Li/Mn ratio and morphology. Adsorption 2011, 17, 881–887. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Sakakihara, J. Mechanism of lithium (Li+) insertion in spinel-type manganese oxide. Redox and ion-exchange reactions. Langmuir 1991, 7, 1167–1171. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, S.; Li, S.; Jiang, H.; Yu, J. Adsorption of lithium ions on novel nanocrystal MnO2. Chem. Eng. Sci. 2007, 62, 4869–4874. [Google Scholar] [CrossRef]
- Chen, L.; Lin, J.W.; Yang, C.L. Absorption of NO2 in a packed tower with Na2SO3 aqueous solution. Environ. Prog. Sustain. Energy 2002, 21, 225–230. [Google Scholar] [CrossRef]
- Tian, M.J.; Liao, F.; Ke, Q.F.; Guo, Y.J.; Guo, Y.P. Synergetic effect of titanium dioxide ultralong nanofibers and activated carbon fibers on adsorption and photodegradation of toluene. Chem. Eng. J. 2017, 328, 962–976. [Google Scholar] [CrossRef]
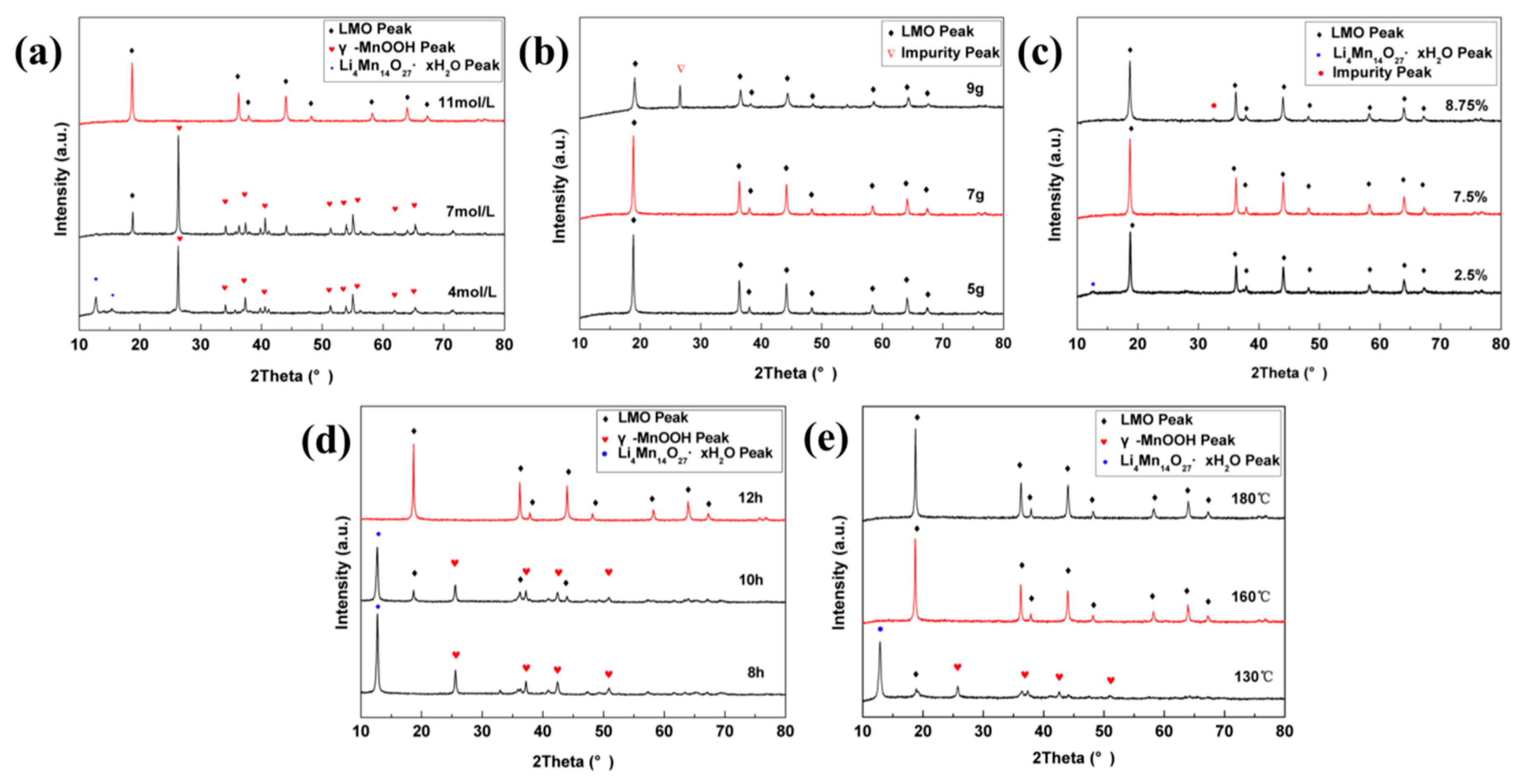
| Experiment Group | LiCl·H2O (mol·L−1) | KMnO4 (g) | Ethanol (V, %) | React. Time (h) | React. Temp. (°C) |
|---|---|---|---|---|---|
| 1 | a | 3 | 7.5 | 12 | 160 |
| 2 | 11 | b | 7.5 | 12 | 160 |
| 3 | 11 | 3 | c | 12 | 160 |
| 4 | 11 | 3 | 7.5 | d | 160 |
| 5 | 11 | 3 | 7.5 | 12 | e |
| Sample | Binding Energy (eV) | Chemical State | Peak Area | Average Valences |
|---|---|---|---|---|
| LMO | 643.76 | Mn2p3/2 Mn4+ | 38,795.51 | +3.65 |
| 642.66 | Mn2p3/2 Mn4+ | 40,557.36 | ||
| 641.33 | Mn2p3/2 Mn3+ | 42,725.95 | ||
| HMO | — | Mn2p3/2 Mn4+ | — | +4 |
| Ion Sieve | Raw Materials | Method | Temp. (°C) | t (h) | Crystal Morphology | Q (mg·g−1) | (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| λ-MnO2 | Mn(NO3)2, LiOH, H2O2 | hydrothermal | 110 | 10 | Nanowire | 23.7 | 61.9 | [29] |
| λ-MnO2 | MnSO4, (NH4)2S2O8 | hydrothermal | 150 650 | 12 6 | Nanowire | 16.9 | 49.2 | [23] |
| λ-MnO2 | LiNO3, Mn(NO3)2 | solid-phase | 700 | 1 | Sphere | - | [20] | |
| λ-MnO2 | Li2CO3, MnCO3 | solid-phase | 800 | 5 | - | - | [19] | |
| λ-MnO2 | LiCl KMnO4 ethanol | hydrothermal | 160 | 12 | Hexagonal | 24.7 | 64.4 | This work |
| Temperature | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|
| 18 °C | 0.115 | 8.41 | 0.7678 | 0.0687 | 25.3 | 0.9998 |
| Temperature | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| 18 °C | 0.415 | 24.6 | 0.9999 | 13.2 | 6.38 | 0.9918 |
| Metal Ion | (mg·L−1) | (mg·L−1)) | (L·g−1 × 10−3) | (mg·g−1) | (mL·g−1) | |
|---|---|---|---|---|---|---|
| Li+ | 319.3 | 288.0 | 19.6 | 6.26 | 19.6 | 1.00 |
| Na+ | 1810.0 | 1804.6 | 0.591 | 1.07 | 0.592 | 36.7 |
| K+ | 815.8 | 812.6 | 0.793 | 0.647 | 0.796 | 27.3 |
| Ca2+ | 121.8 | 120.2 | 2.63 | 0.320 | 2.63 | 8.16 |
| Mg2+ | 119,600.0 | 119,590.4 | 0.0161 | 1.93 | 0.0161 | 1.35 × 103 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Chen, S.; Shi, C.; Xue, F.; Zhang, X.; Ju, S.; Xing, W. A Facile Synthesis of Hexagonal Spinel λ-MnO2 Ion-Sieves for Highly Selective Li+ Adsorption. Processes 2018, 6, 59. https://doi.org/10.3390/pr6050059
Yang F, Chen S, Shi C, Xue F, Zhang X, Ju S, Xing W. A Facile Synthesis of Hexagonal Spinel λ-MnO2 Ion-Sieves for Highly Selective Li+ Adsorption. Processes. 2018; 6(5):59. https://doi.org/10.3390/pr6050059
Chicago/Turabian StyleYang, Fan, Sichong Chen, Chentao Shi, Feng Xue, Xiaoxian Zhang, Shengui Ju, and Weihong Xing. 2018. "A Facile Synthesis of Hexagonal Spinel λ-MnO2 Ion-Sieves for Highly Selective Li+ Adsorption" Processes 6, no. 5: 59. https://doi.org/10.3390/pr6050059
APA StyleYang, F., Chen, S., Shi, C., Xue, F., Zhang, X., Ju, S., & Xing, W. (2018). A Facile Synthesis of Hexagonal Spinel λ-MnO2 Ion-Sieves for Highly Selective Li+ Adsorption. Processes, 6(5), 59. https://doi.org/10.3390/pr6050059