Radical Copolymerization Kinetics of Bio-Renewable Butyrolactone Monomer in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
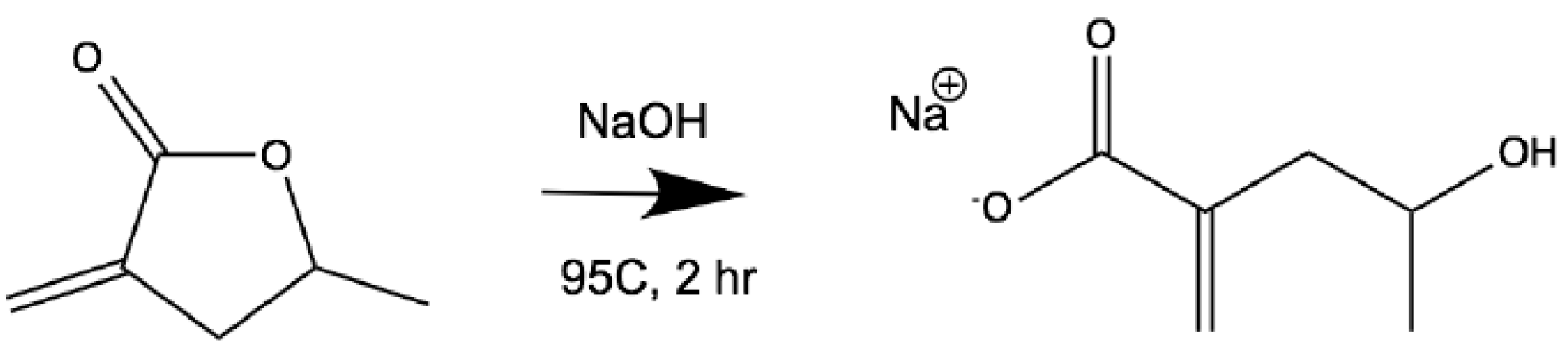
2.2. Ring-Opening Saponfication of MeMBL
2.3. Preparation for In Situ NMR
2.4. Kinetic Parameters for PREDICI Parameter Estimation
3. Results and Discussion
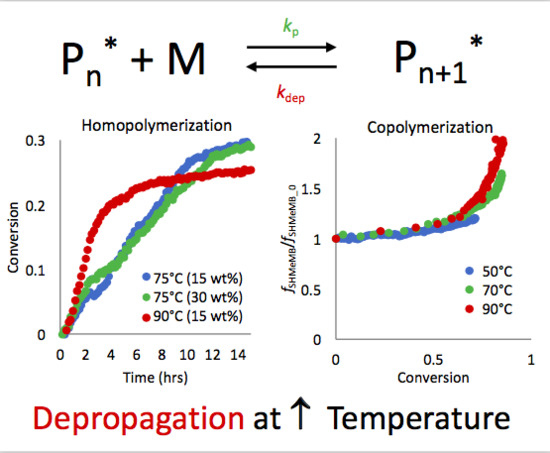
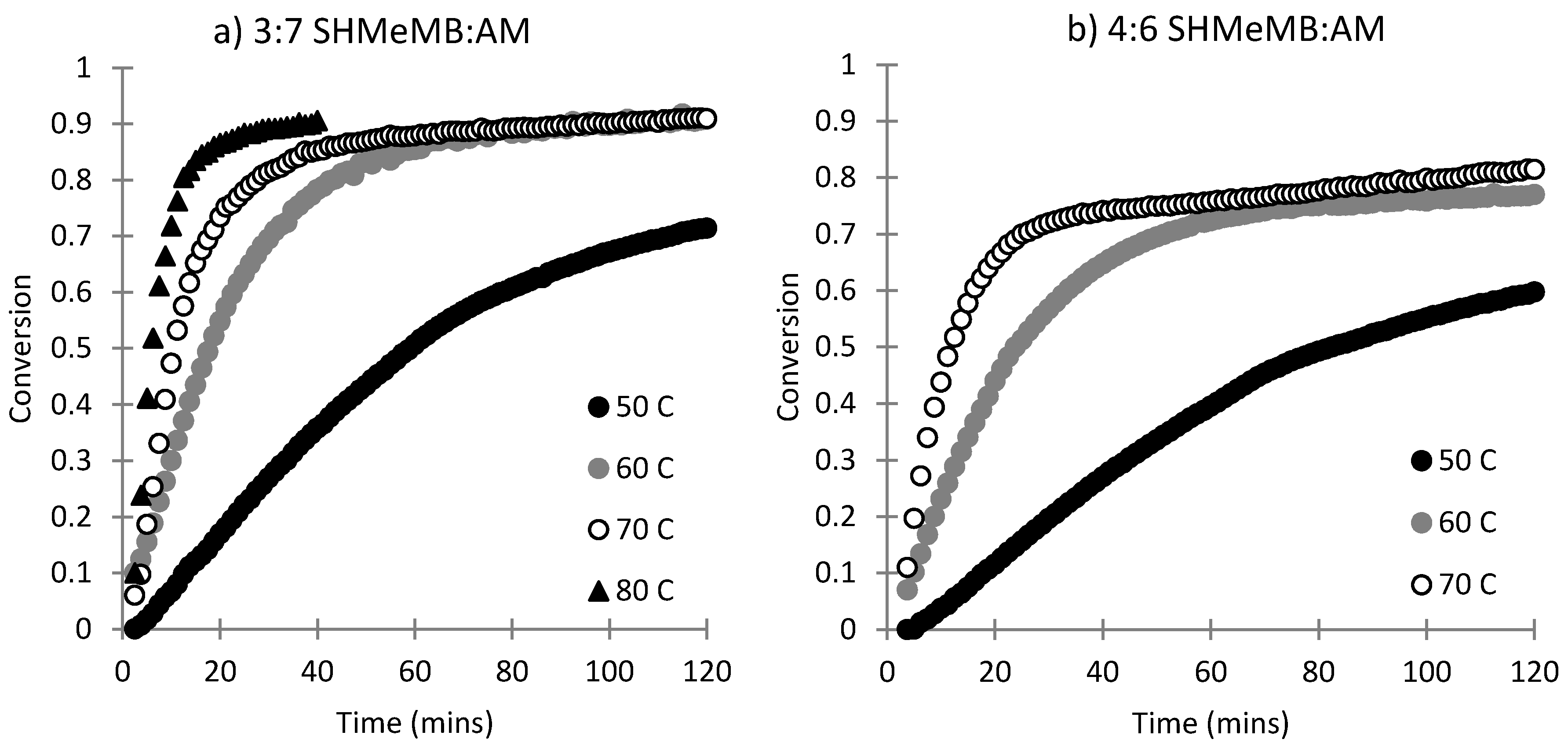
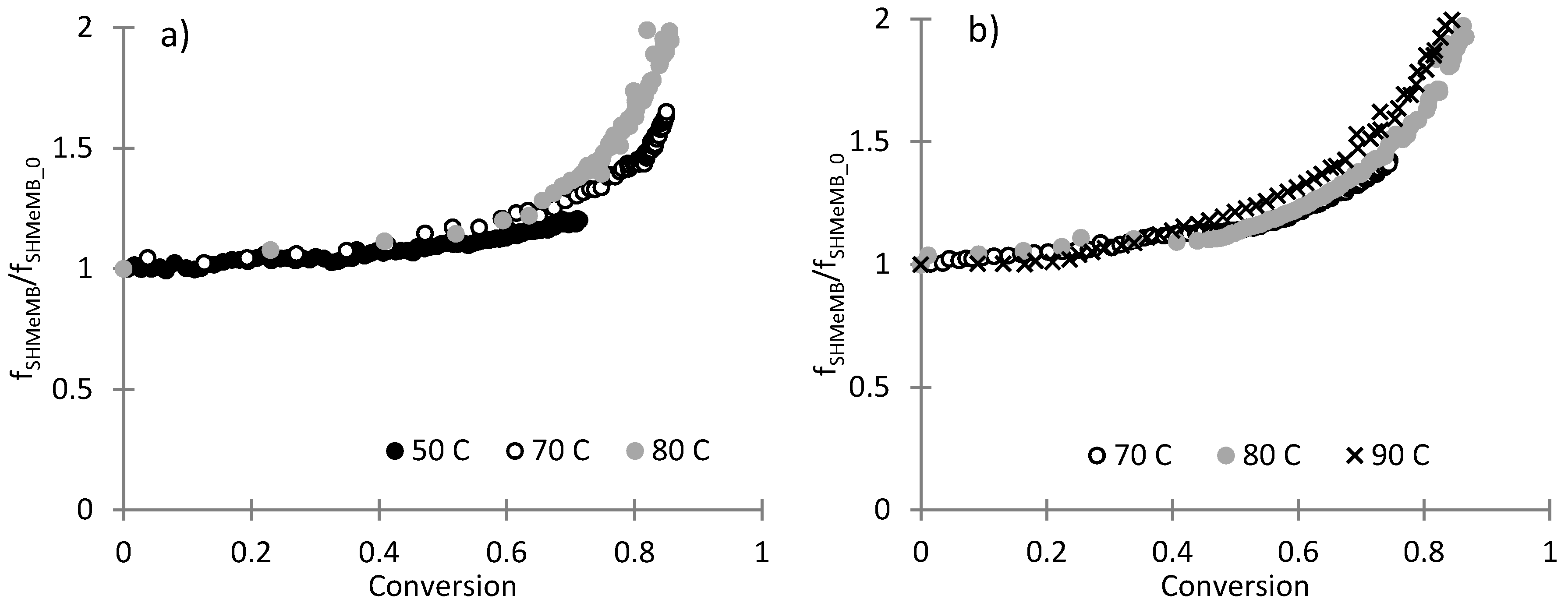
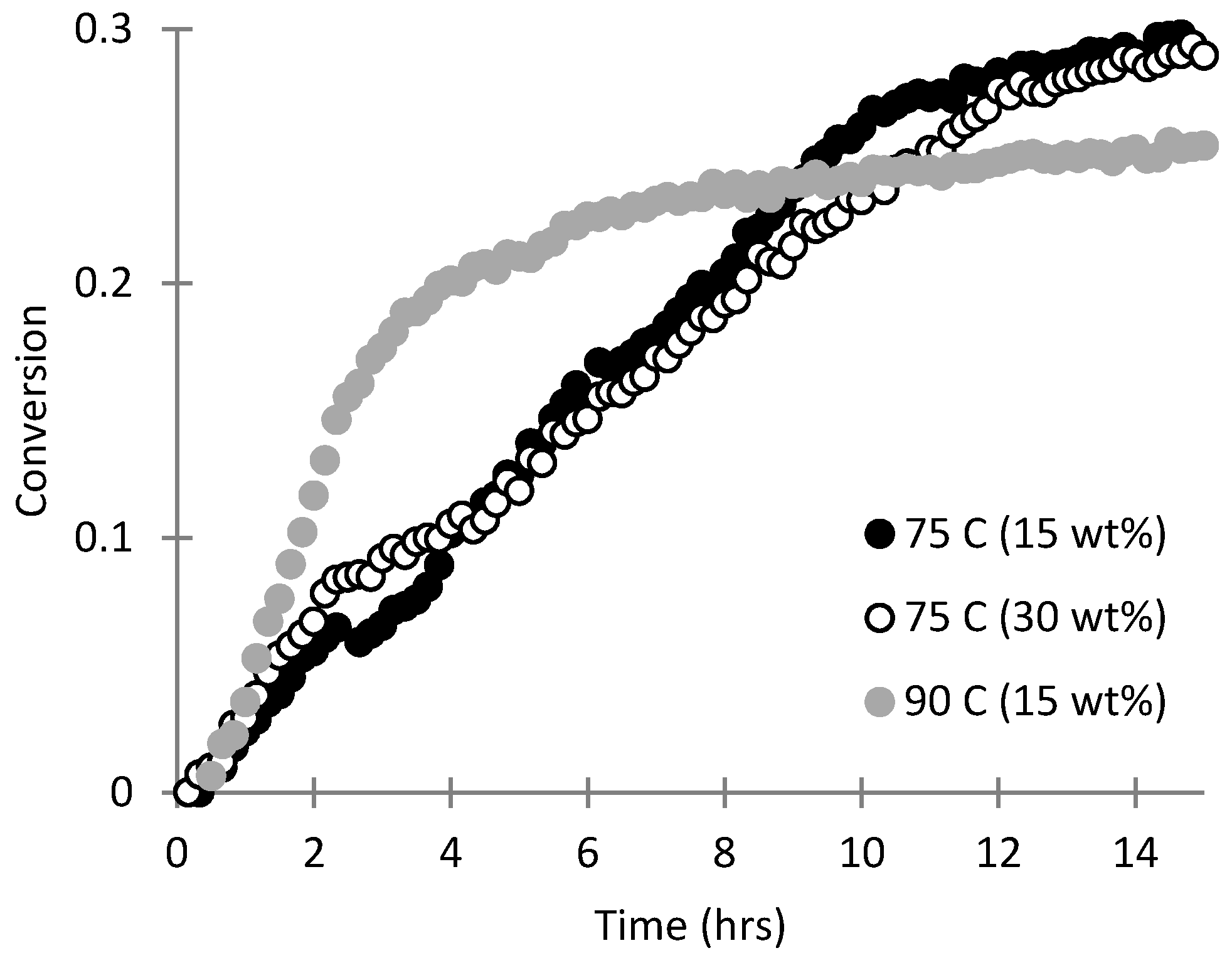
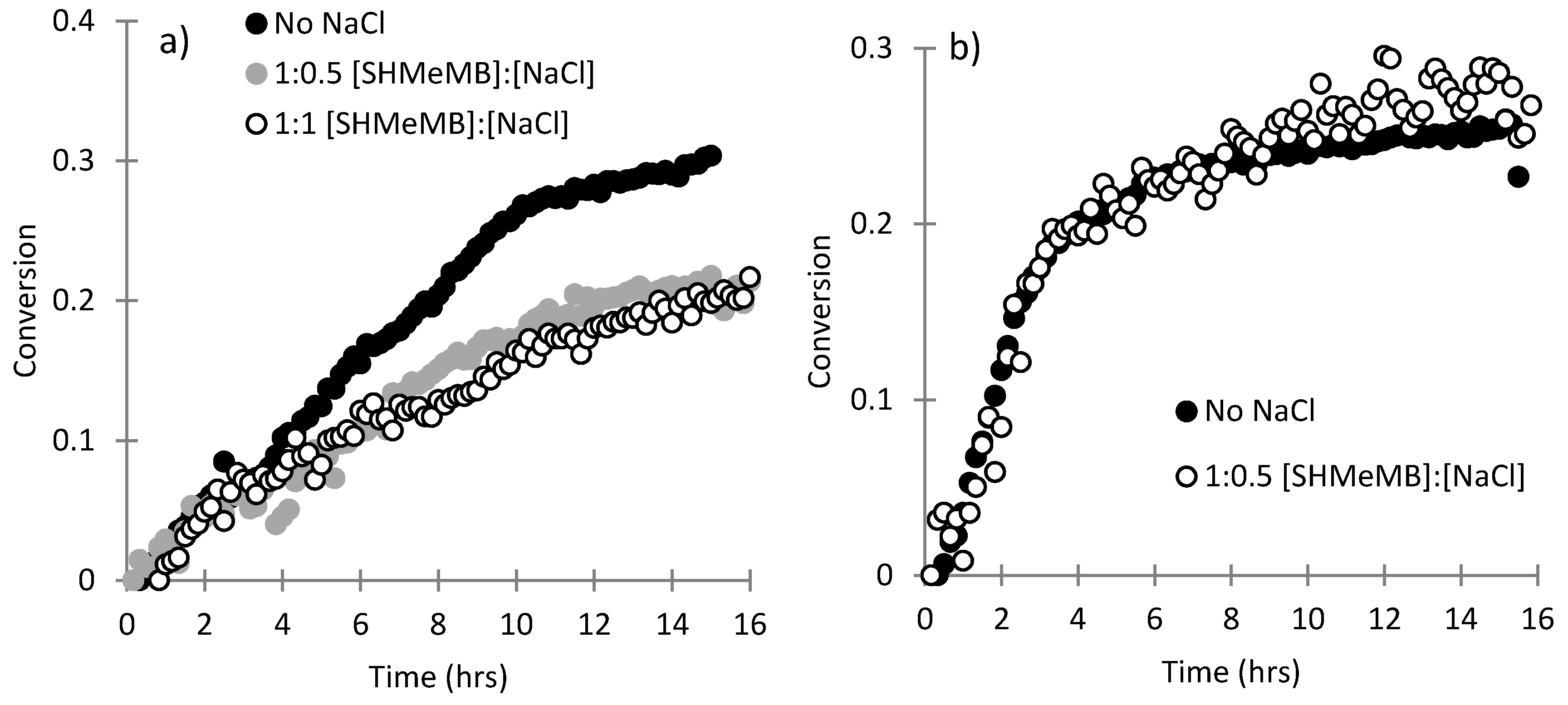
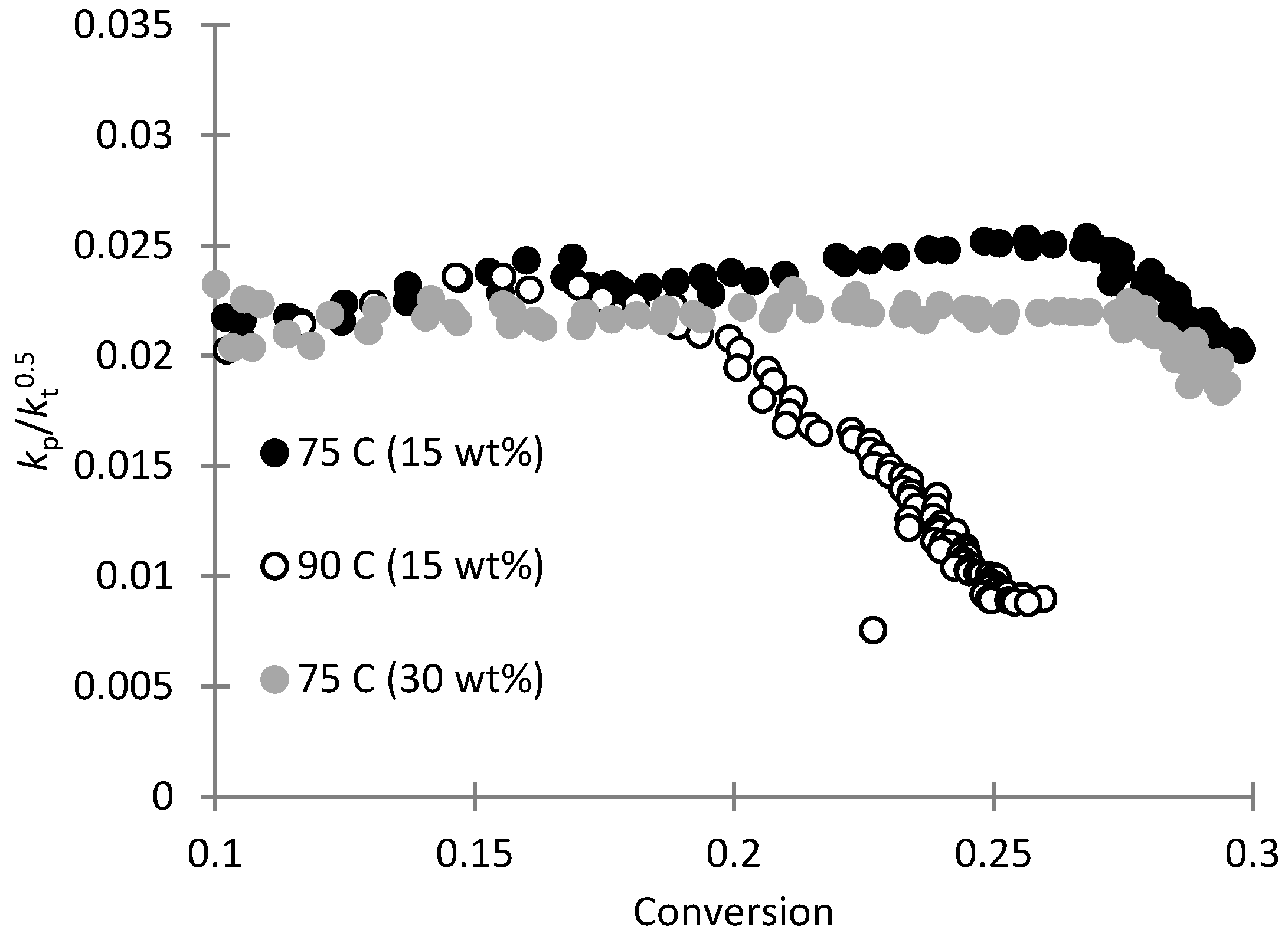
3.1. Copolymerization of SHMeMB:AM at Different Temperatures
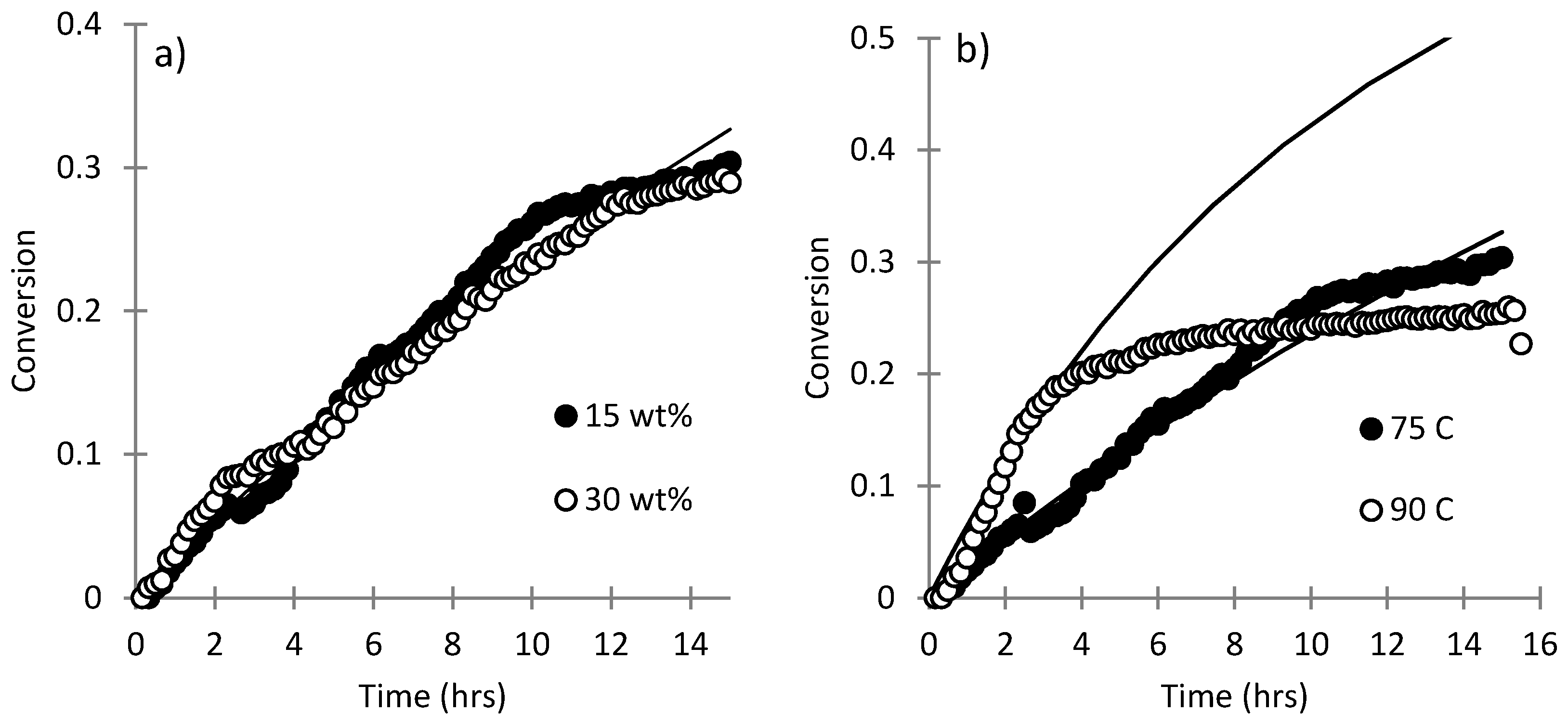
3.2. Homopolymerization Kinetics of SHMeMB
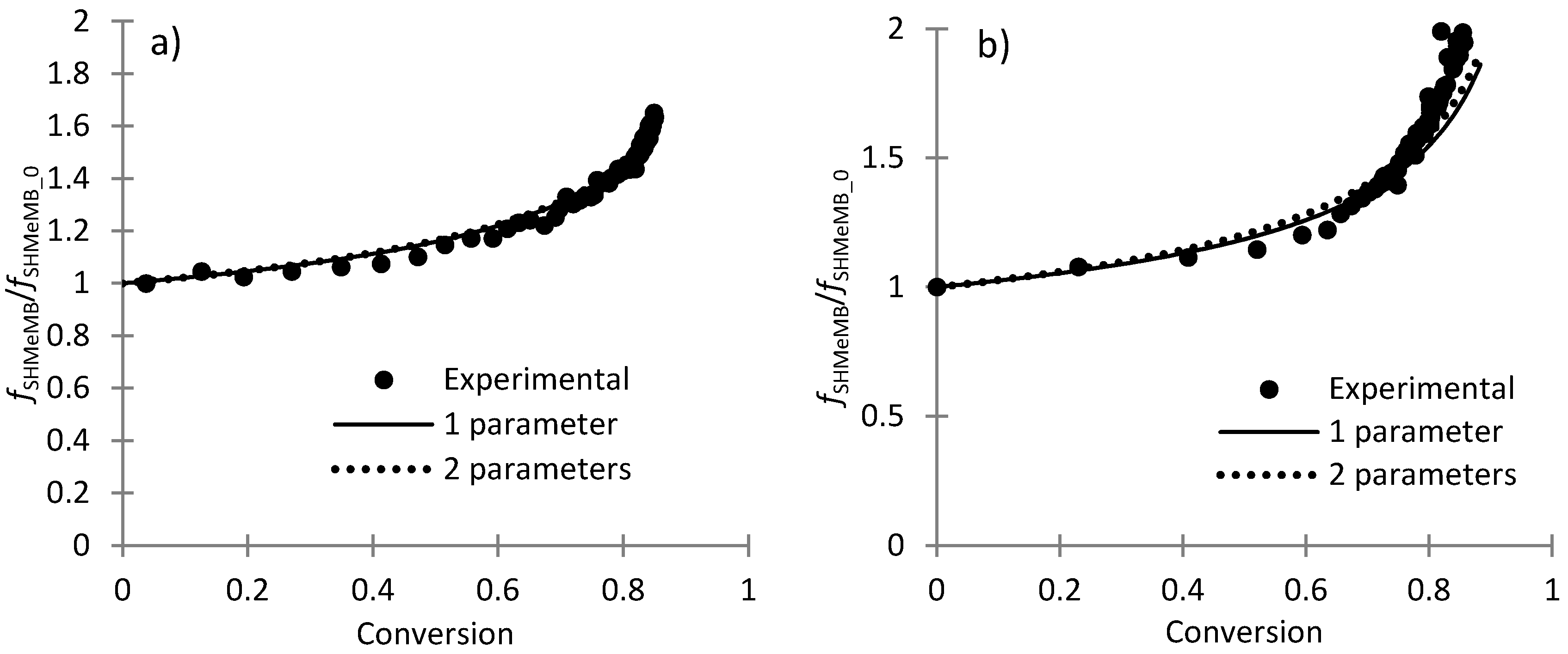
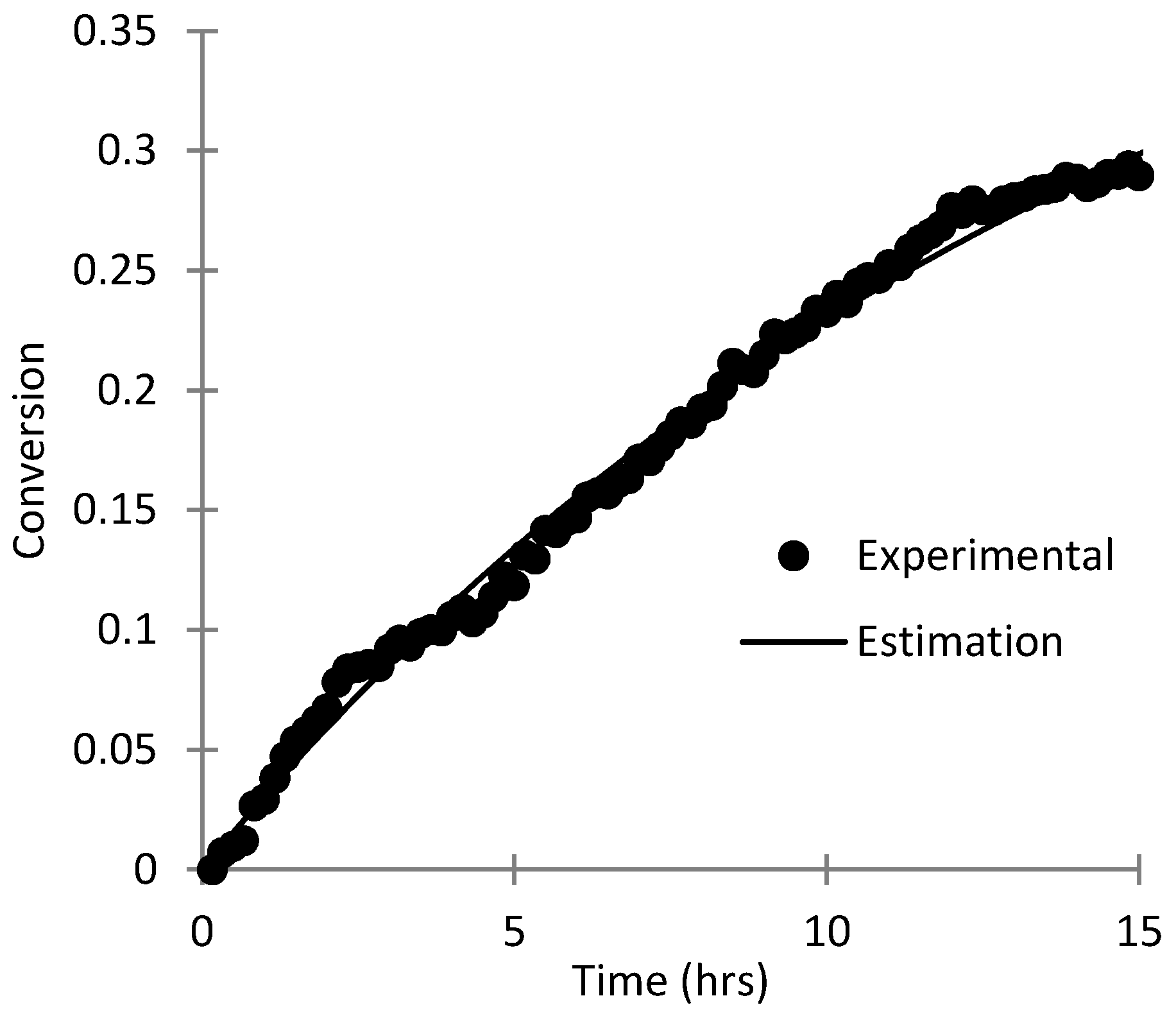
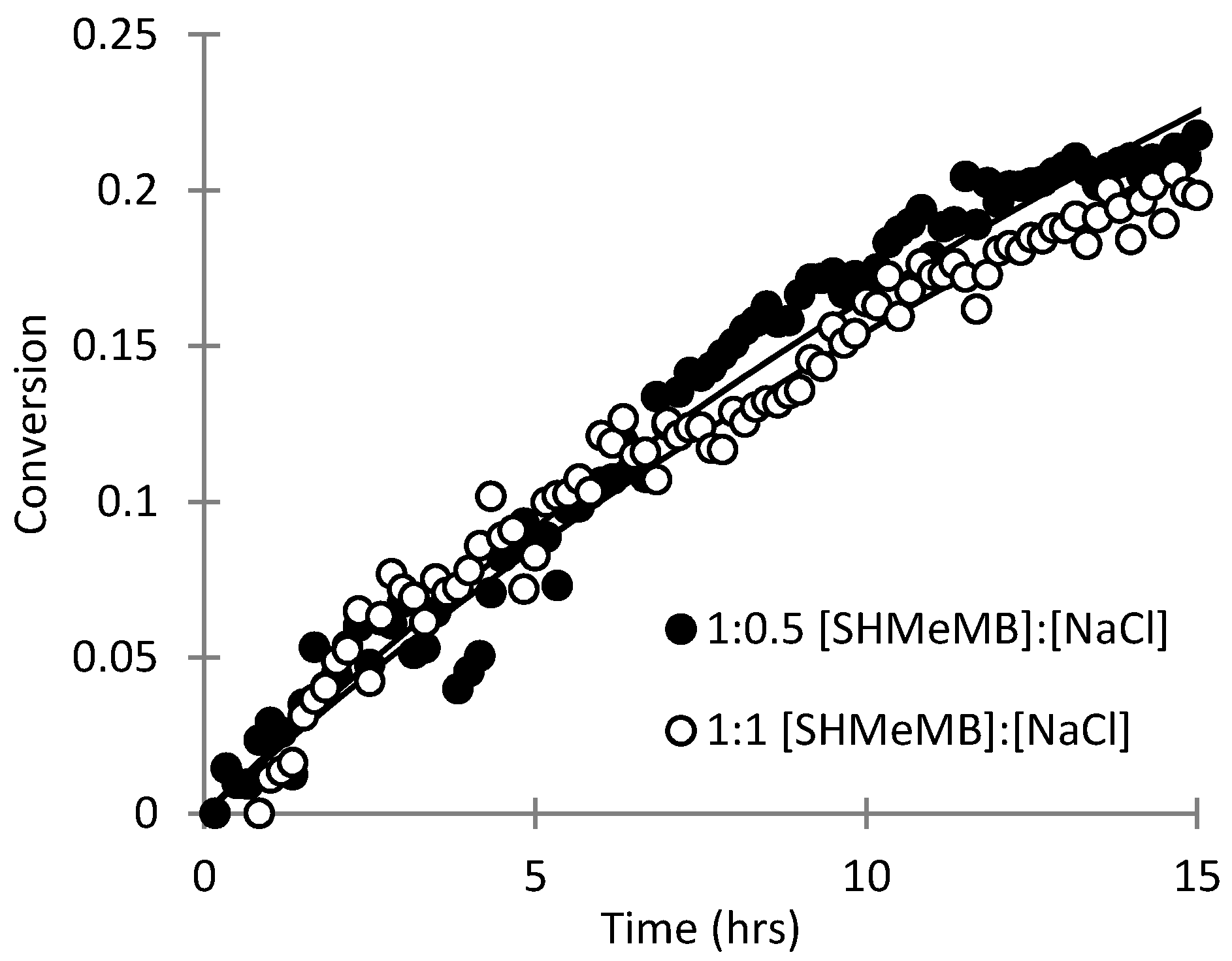
3.3. Parameter Estimation for SHMeMB Homopolymerizations
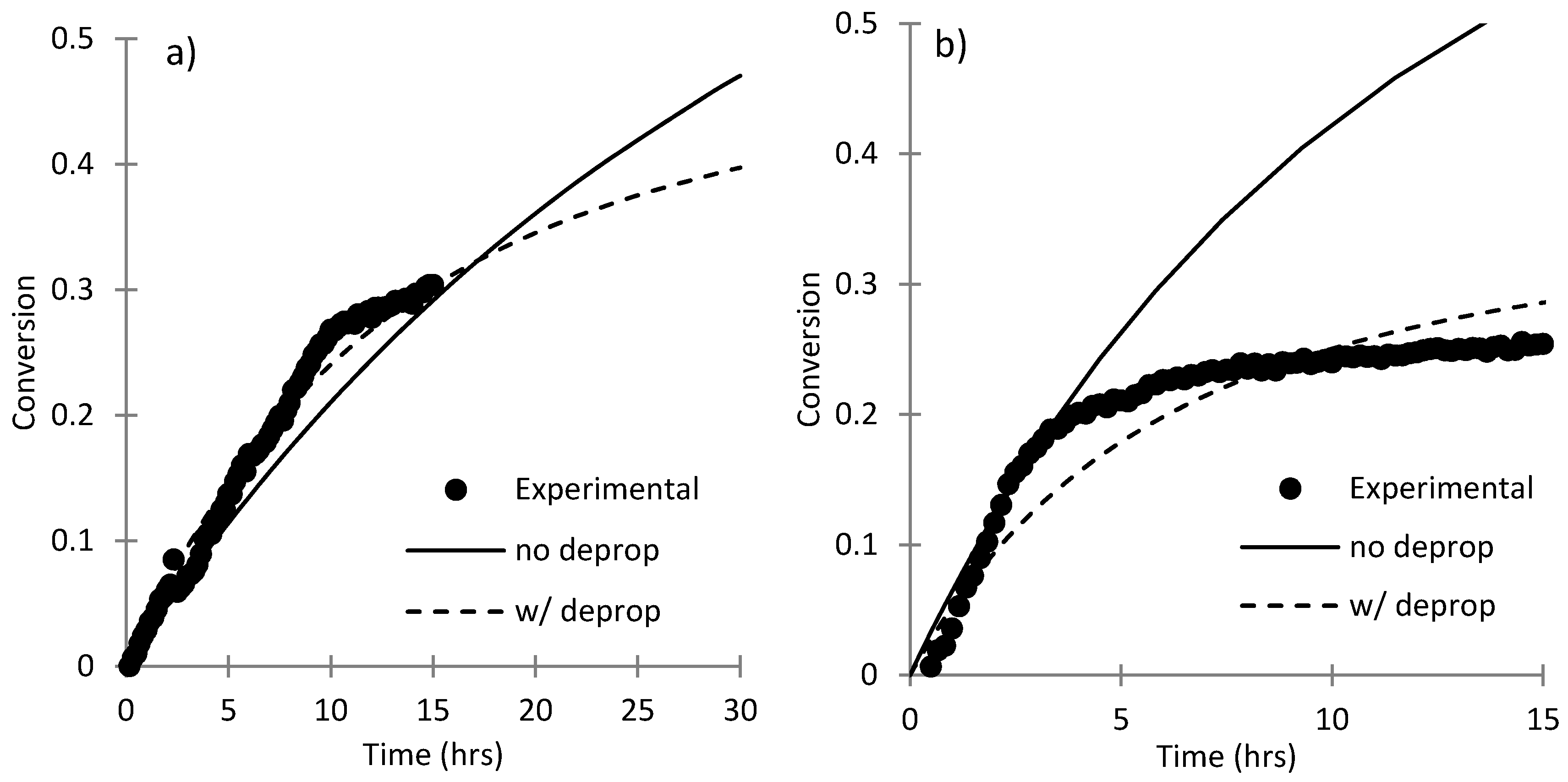
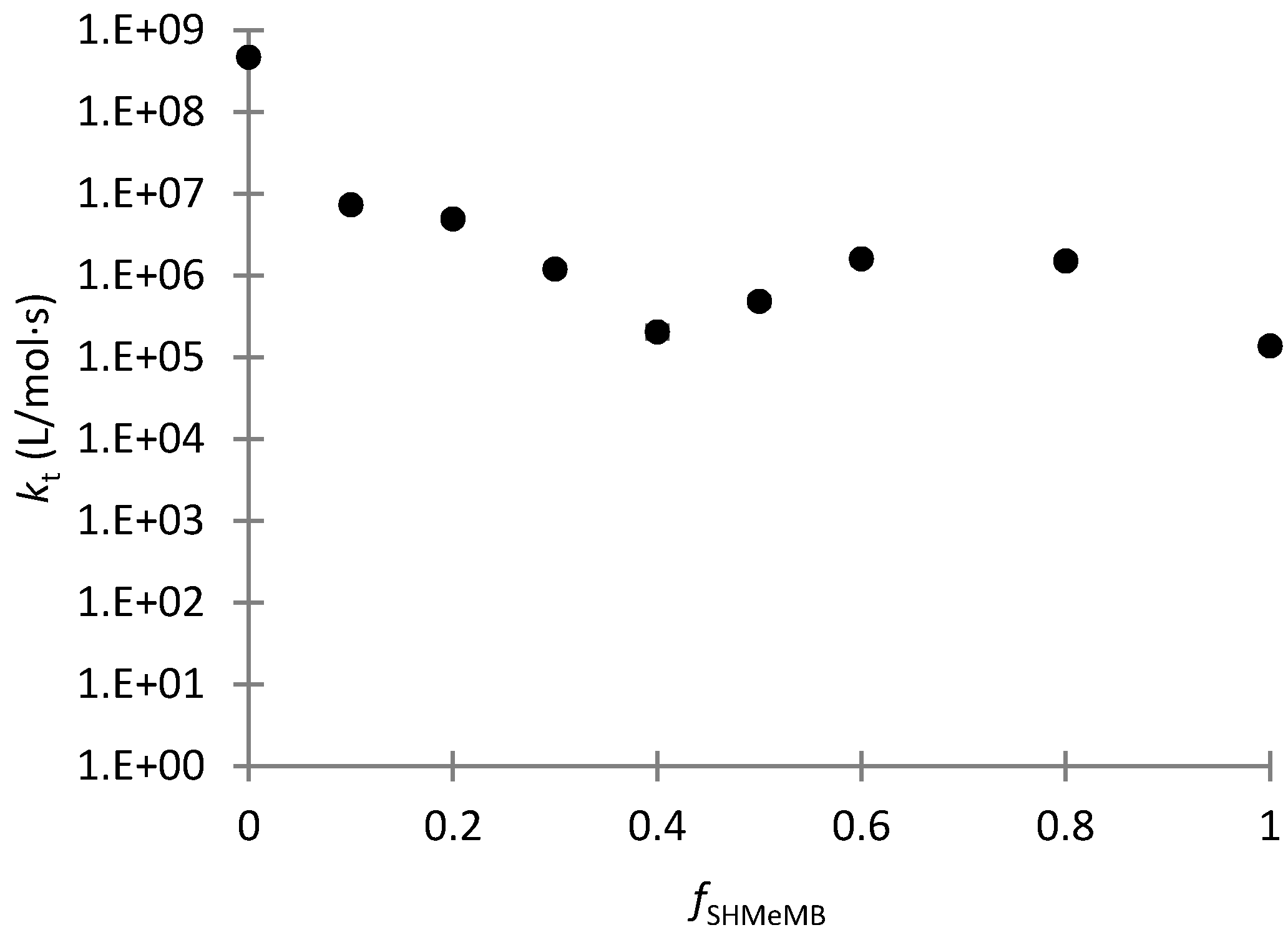
3.4. Parameter Estimation for SHMeMB:AM Copolymerizations with Depropagation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Syed, N.; Habib, W.W.; Kuhajda, A.M. Water-Soluble Polymers in Hair Care. In Water Soluble Polymers; Springer: Boston, MA, USA, 2002; pp. 231–244. ISBN 978-0-306-46915-2. [Google Scholar]
- Hayashi, Y.; Lu, D.; Kobayashi, N. Application of Ultra-High Molecular Weight Amphoteric Acrylamide Copolymers to Detergents. In Water Soluble Polymers; Springer: Boston, MA, USA, 2002; pp. 245–250. ISBN 978-0-306-46915-2. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Kadajji, V.; Betageri, G. Water Soluble Polymers for Pharmaceutical Applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Vedoy, D.; Soares, J. Water-soluble polymers for oil sands tailing treatment: A Review. Can. J. Chem. Eng. 2015, 93, 888–904. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pereira, E.; Palencia, M.; Sánchez, J. Water-soluble functional polymers in conjunction with membranes to remove pollutant ions from aqueous solutions. Prog. Polm. Sci. 2011, 36, 294–322. [Google Scholar] [CrossRef]
- Akkapeddi, M.K. Poly(α-methylene-γ-butyrolactone) Synthesis Configurational Structure, and Properties. Macromolecules 1979, 12, 546–551. [Google Scholar] [CrossRef]
- Suenaga, J.; Sutherlin, D.M.; Stille, J. Polymerization of (RS)-and (R)-a-Methylene-y-methyl-y-butyrolactone. Macromolecules 1984, 17, 2913–2916. [Google Scholar] [CrossRef]
- Ueda, M.; Takahashi, M. Radical-initiated homo- and copolymerization of α-methylene-γ-butyrolactone. J. Polym. Sci. 1982, 20, 2819–2828. [Google Scholar] [CrossRef]
- Cockburn, R.A.; McKenna, T.F.; Hutchinson, R.A. An Investigation of Free Radical Copolymerization Kinetics of the Bio-renewable Monomer γ-Methyl-α-methylene-γ-butyrolactone with Methyl methacrylate and Styrene. Macromol. Chem. Phys. 2010, 211, 501–509. [Google Scholar] [CrossRef]
- Kollár, J.; Mrlík, M.; Moravčíková, D.; Kroneková, Z.; Liptaj, T.; Lacík, I.; Mosnáček, J. Tulips: A Renewable Source of Monomer for Superabsorbent Hydrogels. Macromolecules 2016, 49, 4047–4056. [Google Scholar] [CrossRef]
- Luk, S.B.; Kollár, J.; Chovancová, A.; Mrlík, M.; Lacík, I.; Mosnáček, J.; Hutchinson, R.A. Superabsorbent hydrogels made from bio-derived butyrolactone monomers in aqueous solution. Polym. Chem. 2017. [CrossRef]
- Buback, M.; Gilbert, R.G.; Russell, G.T.; Hill, D.J.T.; Moad, G.; O’Driscoll, K.F.; Shen, J.; Winnik, M.A. Consistent values of rate parameters in free radical polymerization systems. II. Outstanding dilemmas and recommendations. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 851–863. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M.; Hesse, P.; Lacík, I. Free-Radical Propagation Rate Coefficient of Nonionized Methacrylic Acid in Aqueous Solution from Low Monomer Concentrations to Bulk Polymerization. Macromolecules 2006, 39, 184–193. [Google Scholar] [CrossRef]
- Lacík, I.; Beuermann, S.; Buback, M. PLP-SEC Study into Free-Radical Propagation Rate of Nonionized Acrylic Acid in Aqueous Solution. Macromolecules 2003, 36, 9355–9363. [Google Scholar] [CrossRef]
- Lacík, I.; Beuermann, S.; Buback, M. PLP-SEC Study into the Free-Radical Propagation Rate Coefficients of Partially and Fully Ionized Acrylic Acid in Aqueous Solution. Macromol. Chem. Phys. 2004, 205, 1080–1087. [Google Scholar] [CrossRef]
- Lacík, I.; Učňová, L.; Kukučková, S.; Buback, M.; Hesse, P.; Beuermann, S. Propagation Rate Coefficient of Free-Radical Polymerization of Partially and Fully Ionized Methacrylic Acid in Aqueous Solution. Macromolecules 2009, 42, 7753–7761. [Google Scholar] [CrossRef]
- Lacík, I.; Chovancová, A.; Uhelska, L.; Preusser, C.; Hutchinson, R.A.; Buback, M. PLP-SEC Studies into the Propagation Rate Coefficient of Acrylamide Radical Polymerization in Aqueous Solution. Macromolecules 2016, 49, 3244–3253. [Google Scholar] [CrossRef]
- Kuchta, F.D.; van Herk, A.M.; German, A.L. Propagation Kinetics of Acrylic and Methacrylic Acid in Water and Organic Solvents Studied by Pulsed-Laser Polymerization. Macromolecules 2000, 33, 3641–3649. [Google Scholar] [CrossRef]
- Preusser, C.; Chovancová, A.; Lacík, I.; Hutchinson, R.A. Modeling the Radical Batch Homopolymerization of Acrylamide and Aqueous Solution. Macromol. React. Eng. 2016, 10, 490–501. [Google Scholar] [CrossRef]
- Preusser, C.; Hutchinson, R.A. An In Situ NMR Study of Radical Copolymerization Kinetics of Acrylamide and Non-Ionized Acrylic Acid in Aqueous Solution. Macromol. Symp. 2013, 333, 122–137. [Google Scholar] [CrossRef]
- Preusser, C.; Ezenwajiaku, I.H.; Hutchinson, R.A. The Combined Influence of Monomer Concentration and Ionization on Acrylamide/Acrylic acid Composition in Aqueous Solution Radical Batch Copolymerization. Macromolecules 2016, 49, 4746–4756. [Google Scholar] [CrossRef]
- Riahinezhad, M.; McManus, N.; Penlidis, A. Effect of Monomer Concentration and pH on Reaction Kinetics and Copolymer Microsctructure of Acrylamide/Acrylic Acid Copolymer. Macromol. React. Eng. 2015, 9, 100–113. [Google Scholar] [CrossRef]
- Schier, J.E.; Hutchinson, R.A. The influence of hydrogen bonding on radical chain-growth parameters for butyl methacrylate/2-hydroxyethyl acrylate solution copolymerization. Polym. Chem. 2016, 7, 4567–4574. [Google Scholar] [CrossRef]
- Li, D.; Li, N.; Hutchinson, R.A. High-Temperature Free Radical Copolymerization of Styrene and Butyl Methacrylate with Depropagation and Penultimate Kinetics Effects. Macromolecules 2006, 39, 4366–4373. [Google Scholar] [CrossRef]
- Szablan, Z.; Stenzel, M.H.; Davis, T.P.; Barner, L.; Barner-Kowollik, C. Depropagation Kinetics of Sterically Demanding Monomers: A Pulsed Laser Size Exclusion Chromatography Study. Macromolecules 2005, 38, 5944–5954. [Google Scholar] [CrossRef]
- Penelle, J.; Collot, J.; Rufflard, G. Kinetic and thermodynamic analysis of methyl ethacrylate radical polymerization. J. Polym. Sci. 1993, 31, 2407–2412. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.; Grulke, E. Polymer Handbook, 4th ed.; John Wiley & Sons: New York, NY, USA, 1999; ISBN 978-0-471-47936-9. [Google Scholar]
- Morris, L.; Davis, T.; Chaplin, R. Radical copolymerization propagation kinetics of methyl ethacrylate and styrene. Polymer 2001, 42, 941–952. [Google Scholar] [CrossRef]
- Ueda, M.; Takahashi, M.; Imai, Y.; Pittman, C.U. Synthesis and homopolymerization kinetics of α-methylene-δ-valerolactone, an exo-methylene cyclic monomer with a nonplanar ring system spanning the radical center. Macromolecules 1983, 16, 1300–1305. [Google Scholar] [CrossRef]
- Drawe, P.; Buback, M.; Lacík, I. Radical Polymerization of Alkali Acrylates in Aqueous Solution. Macromol. Chem. Phys. 2015, 216, 1333–1340. [Google Scholar] [CrossRef]
- Drawe, P. Kinetic of the Radical Polymerization of Ionic Monomers in Aqueous Solution: Spectroscopic Analysis and Modelling. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2016. [Google Scholar]
- Barth, J.; Buback, M. SP-PLP-EPR Study into the Termination Kinetics of Methacrylic Acid Radical Polymerization in Aqueous Solution. Macromolecules 2011, 44, 1292–1297. [Google Scholar] [CrossRef]
- Wulkow, M. Computer Aided Modeling of Polymer Reaction Engineering—The Status of Predici, 1—Simulation. Macromol. React. Eng. 2008, 2, 461–494. [Google Scholar] [CrossRef]
- Wittenburg, N. Kinetics and Modeling of the Radical Polymerization of Acrylic Acid and of Methacrylic Acid in Aqueous Solution. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2013. [Google Scholar]
- Wako Pure Chemical Industries Ltd. “V-50”. Available online: http://www.wako-chem.co.jp/kaseihin_en/waterazo/V-50.htm (accessed on 31 March 2017).
- Wako Pure Chemical Industries Ltd. “VA-086”. Available online: http://www.wako-chem.co.jp/kaseihin_en/waterazo/VA-086.htm (accessed on 31 March 2017).
- Barth, J.; Buback, M. Termination and Transfer Kinetics of Sodium Acrylate Polymerization. Macromolecules 2012, 45, 4152–4157. [Google Scholar] [CrossRef]
- Luk, S.B. Radical Polymerization Kinetics of Bio-Renewable Monomers in Aqueous Solution. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2017. [Google Scholar]
- Kattner, H.; Drawe, P.; Buback, M. Chain-Length-Dependent Termination of Sodium Methacrylate Polymerization in Aqueous Solution Studied by SP-PLP-EPR. Macromolecules 2017, 50, 1386–1393. [Google Scholar] [CrossRef]
- Kattner, H.; Buback, M. Termination and Transfer Kinetics of Acrylamide Homopolymerization in Aqueous Solution. Macromolecules 2015, 48, 7410–7419. [Google Scholar] [CrossRef]
| Reaction Mechanisms | |
|---|---|
| Initiator decomposition | |
| Initiation | |
| Propagation | |
| Termination (by disproportionation) | |
| Depropagation |
| Reaction | Rate Expression | References |
|---|---|---|
| Decomposition of V-50 | kd = 9.385 × 1014 exp (–14,890/T(K)) | [36] |
| f = 0.8 | ||
| Decomposition of V-86 | kd = 1.24 × 1013 exp (–14,800/T(K)) | [35,37] |
| f = 0.38 | ||
| Propagation of AM | [18] | |
| Propagation of SHMeMB | kp = 25 L·mol−1·s−1 | [12] |
| Reactivity ratios at 50 °C | rAM = kp2,2/ kp2,1 = 0.95 ± 0.01 rSHMeMB = kp1,1/ kp1,2 = 0.17 ± 0.01 | [12] |
| 70 °C | 80 °C | |||
|---|---|---|---|---|
| 1 Parameter | 2 Parameters | 1 Parameter | 2 Parameters | |
| rSHMeMB | 0.005 ± 0.008 | 0.12 ± 0.22 | 7 × 10−6 ± 7 × 10−3 | 0.05 ± 0.17 |
| rAM | - | 1.04 ± 0.17 | - | 1.06 ± 0.21 |
| 1:0.5 [SHMeMB]:[NaCl] | 1:1 [SHMeMB]:[NaCl] | |||
|---|---|---|---|---|
| 95% Confidence | 95% Confidence | |||
| kp (L·mol−1·s−1) | 30.3 | ±50.9 | 29.2 | ±37.0 |
| kt (L·mol−1·s−1) | 9.98 × 105 | ±7.96 × 106 | 1.01 × 106 | ±6.32 × 106 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luk, S.B.; Hutchinson, R.A. Radical Copolymerization Kinetics of Bio-Renewable Butyrolactone Monomer in Aqueous Solution. Processes 2017, 5, 55. https://doi.org/10.3390/pr5040055
Luk SB, Hutchinson RA. Radical Copolymerization Kinetics of Bio-Renewable Butyrolactone Monomer in Aqueous Solution. Processes. 2017; 5(4):55. https://doi.org/10.3390/pr5040055
Chicago/Turabian StyleLuk, Sharmaine B., and Robin A. Hutchinson. 2017. "Radical Copolymerization Kinetics of Bio-Renewable Butyrolactone Monomer in Aqueous Solution" Processes 5, no. 4: 55. https://doi.org/10.3390/pr5040055
APA StyleLuk, S. B., & Hutchinson, R. A. (2017). Radical Copolymerization Kinetics of Bio-Renewable Butyrolactone Monomer in Aqueous Solution. Processes, 5(4), 55. https://doi.org/10.3390/pr5040055