A Hybrid MPC-PID Control System Design for the Continuous Purification and Processing of Active Pharmaceutical Ingredients
Abstract
:1. Introduction and Objective
1.1. Objectives
- Present a system-wide hybrid MPC-PID control scheme.
- Quantify the importance of a cascade control scheme in case of pharmaceutical unit operations.
- Present a comparative study between PID-only control and MPC-PID control schemes based on set point tracking and disturbance rejection criteria.
2. Background
3. Flowsheet Model Description
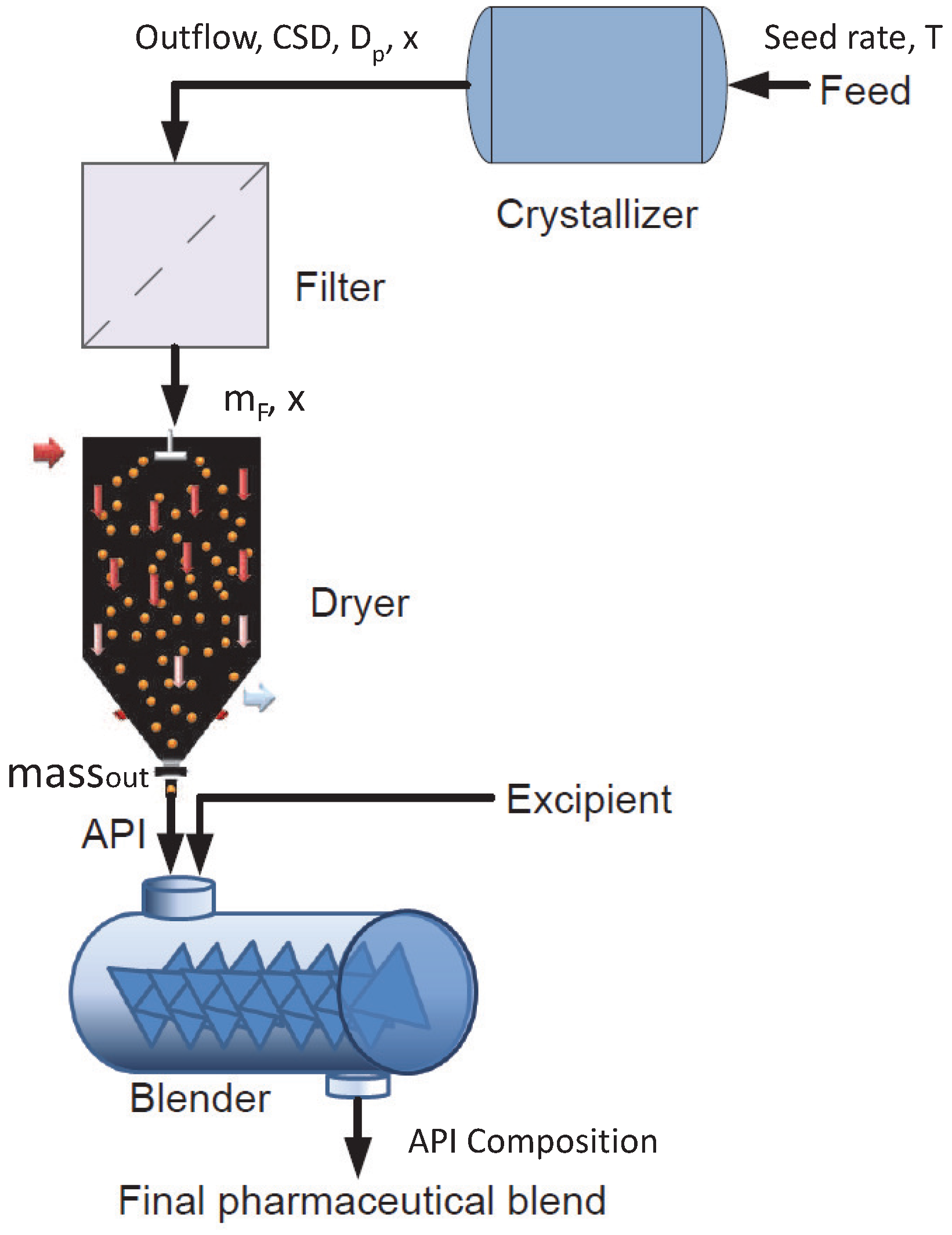
3.1. Numerical Technique
4. A Systematic Framework for Hybrid Control Design
- Identify the critical control variables.
- Identify suitable actuators or manipulating variables to be paired with the control variables.
- Obtain the response of the control variables by implementing a step change to the manipulating variables (from the actual flowsheet model, which has been developed in gPROMS™).
- Fit a transfer function model to relate the control and manipulating variables from the response obtained in the previous step. This has been done using the system identification tool box of MATLAB™.
- Group the control loops into two categories (i.e., MPC controlled and PID controlled)
- Design the hybrid MPC-PID control loops in Simulink™.
- Evaluate the performance of the designed control system in terms of set point tracking and disturbance rejection ability.
5. Design Strategy of the Control System
5.1. Selection of the Control Variables and Pairing with Suitable Actuators
| Process | Control variable | Actuators (Manipulating variable) |
|---|---|---|
| Crystallization | Temperature cooling schedule | Coolant temperature |
| Saturation concentration | ||
| Drying | Drying gas temperature | Super-heated steam temperature |
| Blending | API composition | Excipient flow rate |
| Holdup | Weir height |
5.2. Design of Controller
6. Results and Discussion
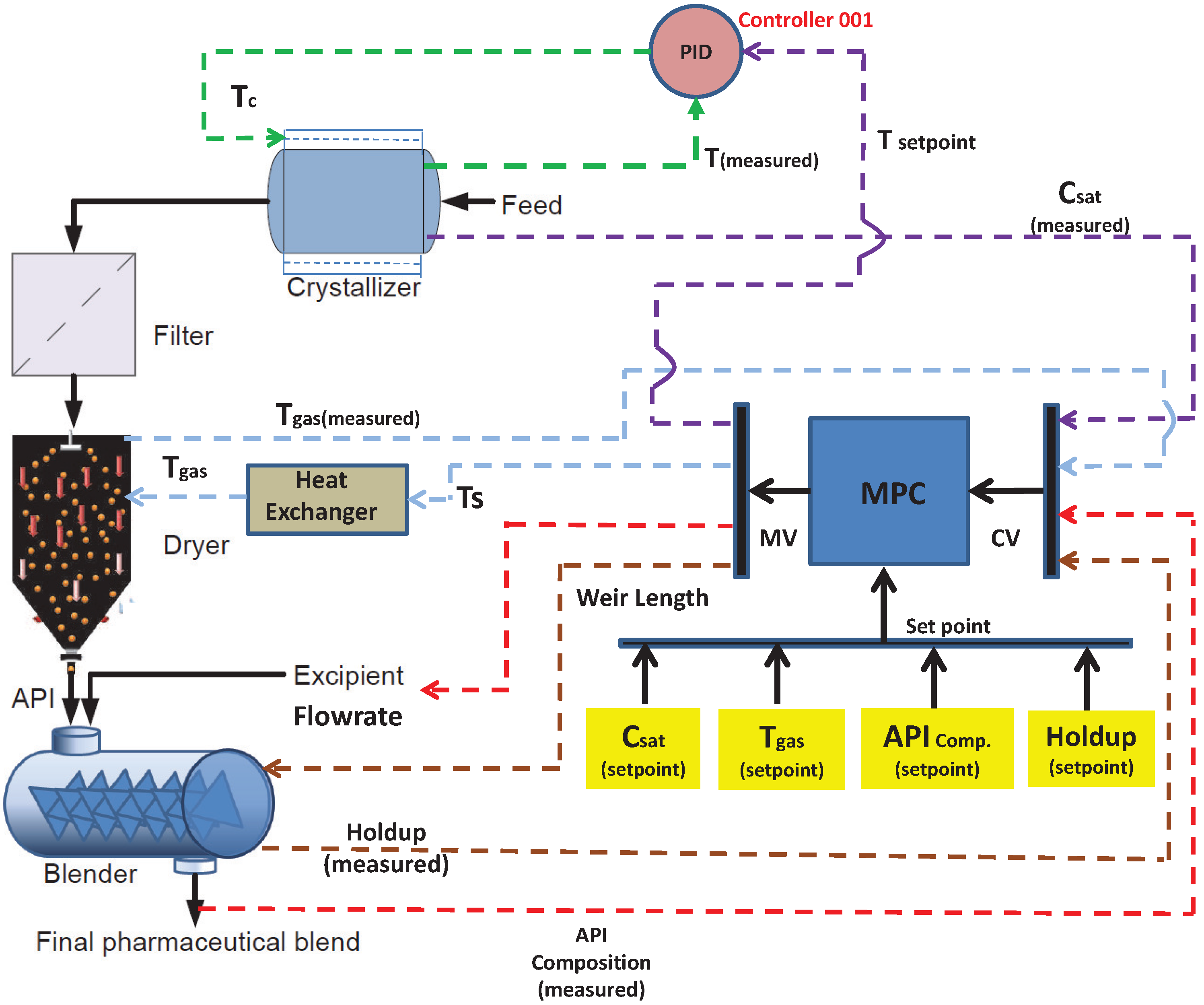
6.1. Control System Design
| PID parameters | |||
|---|---|---|---|
| Control loop | Gain | Reset Time | Rate |
| Crystallization (slave loop) | −0.166 | 2.95 × s | 1.09 × s |
| MPC parameters | |||
| Control loop | Input Weight | Rate Weight | Output Weight |
| Crystallization (master loop) | 0 | 0.1 | 1 |
| Drying | 0 | 0.1 | 1 |
| Mixing (API composition) | 0 | 0.1 | 1 |
| Mixing (holdup) | 0 | 0.1 | 1 |
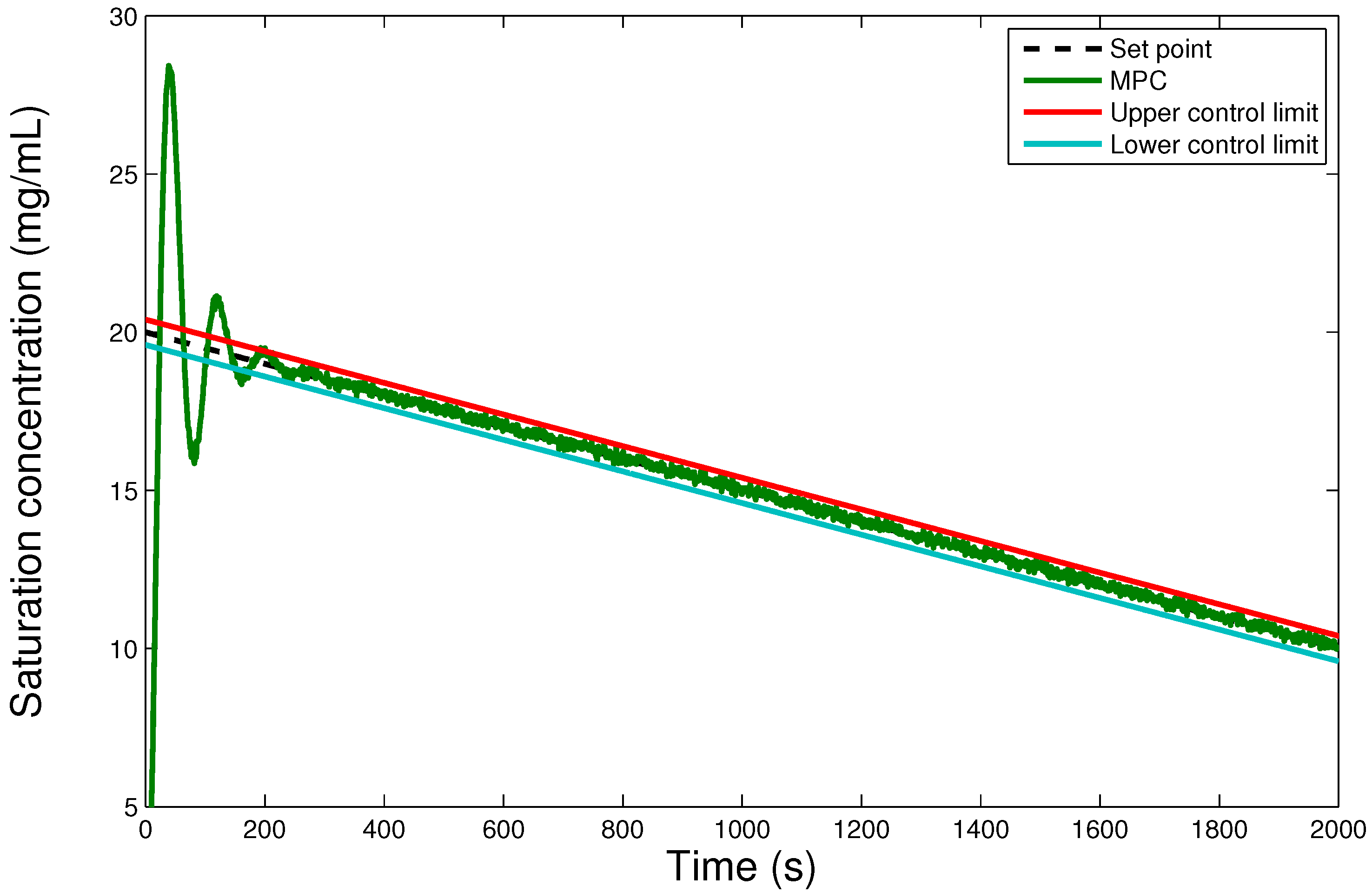
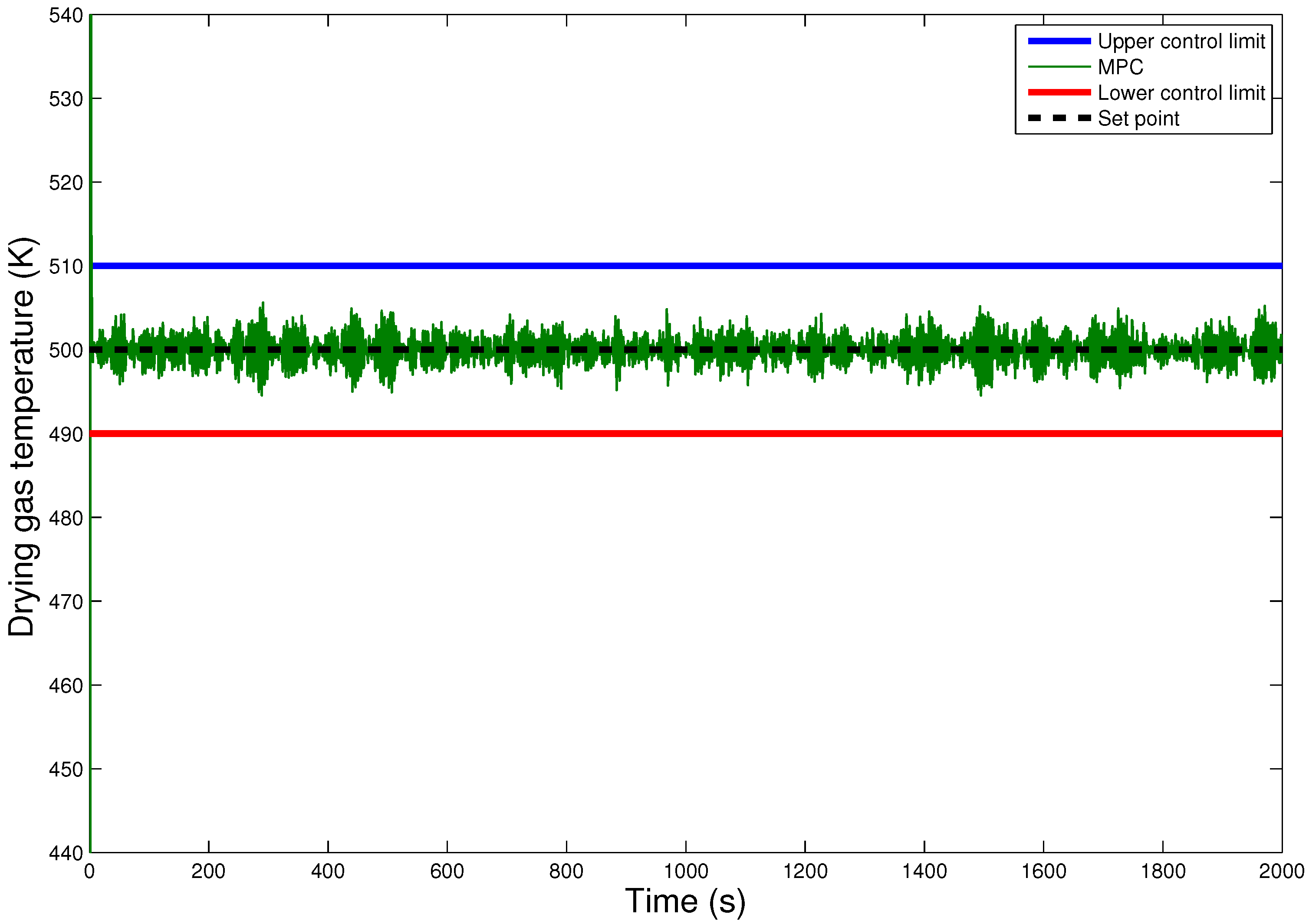
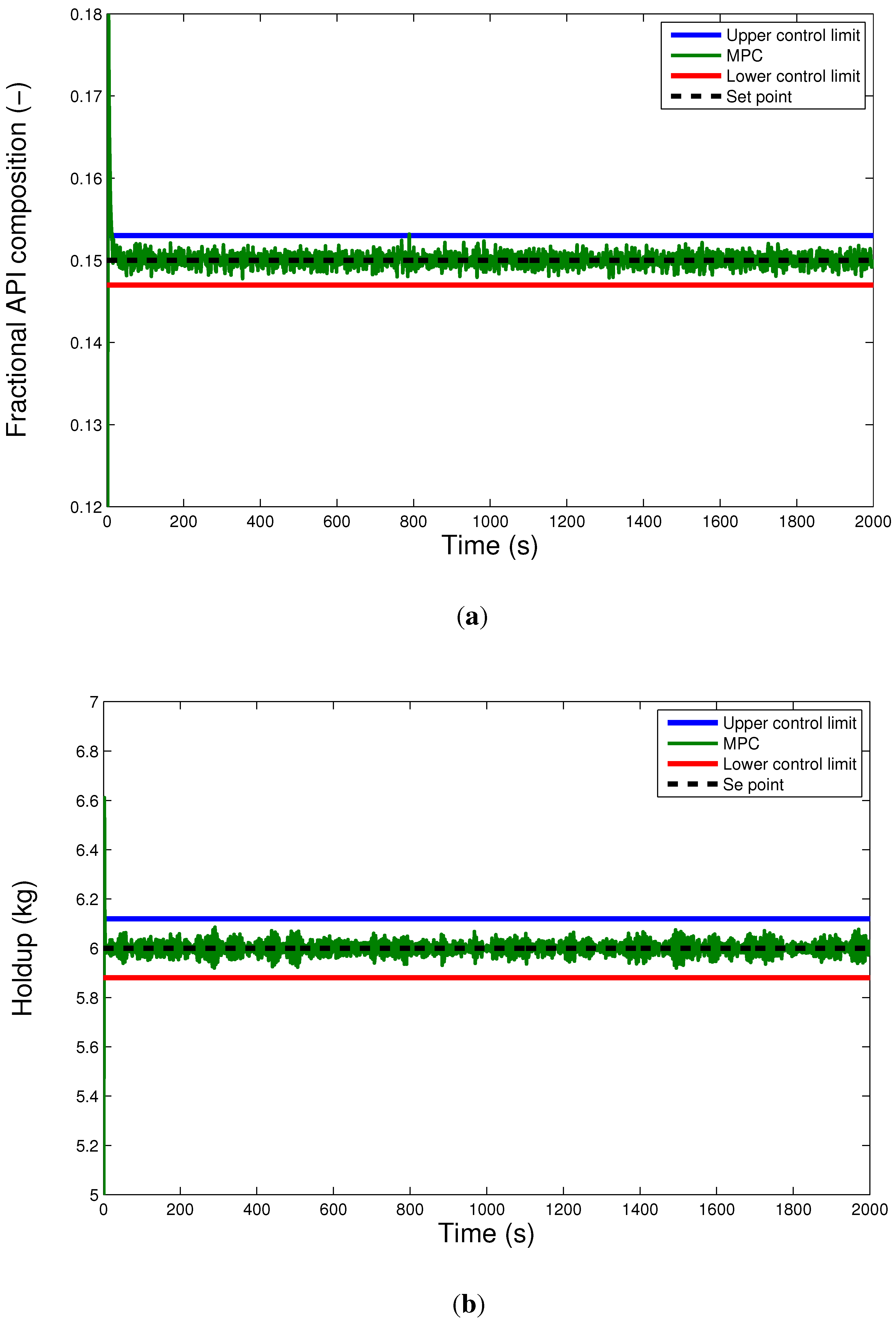
6.2. Comparison of the Hybrid MPC-PID Scheme with PID-only Scheme
| Set point tracking | ||
|---|---|---|
| Control loop | MPC-PID | PID-only |
| Saturation concentration | 6.322 × | 2.935 × |
| Drying gas temperature | 7.371 × | 2.068 × |
| API composition | 1.584 × | 1.892 × |
| Disturbance rejection | ||
| Control loop | MPC-PID | PID only |
| Saturation concentration | 3.317 × | 4.540 × |
| Drying gas temperature | 1.556 × | 6.193 × |


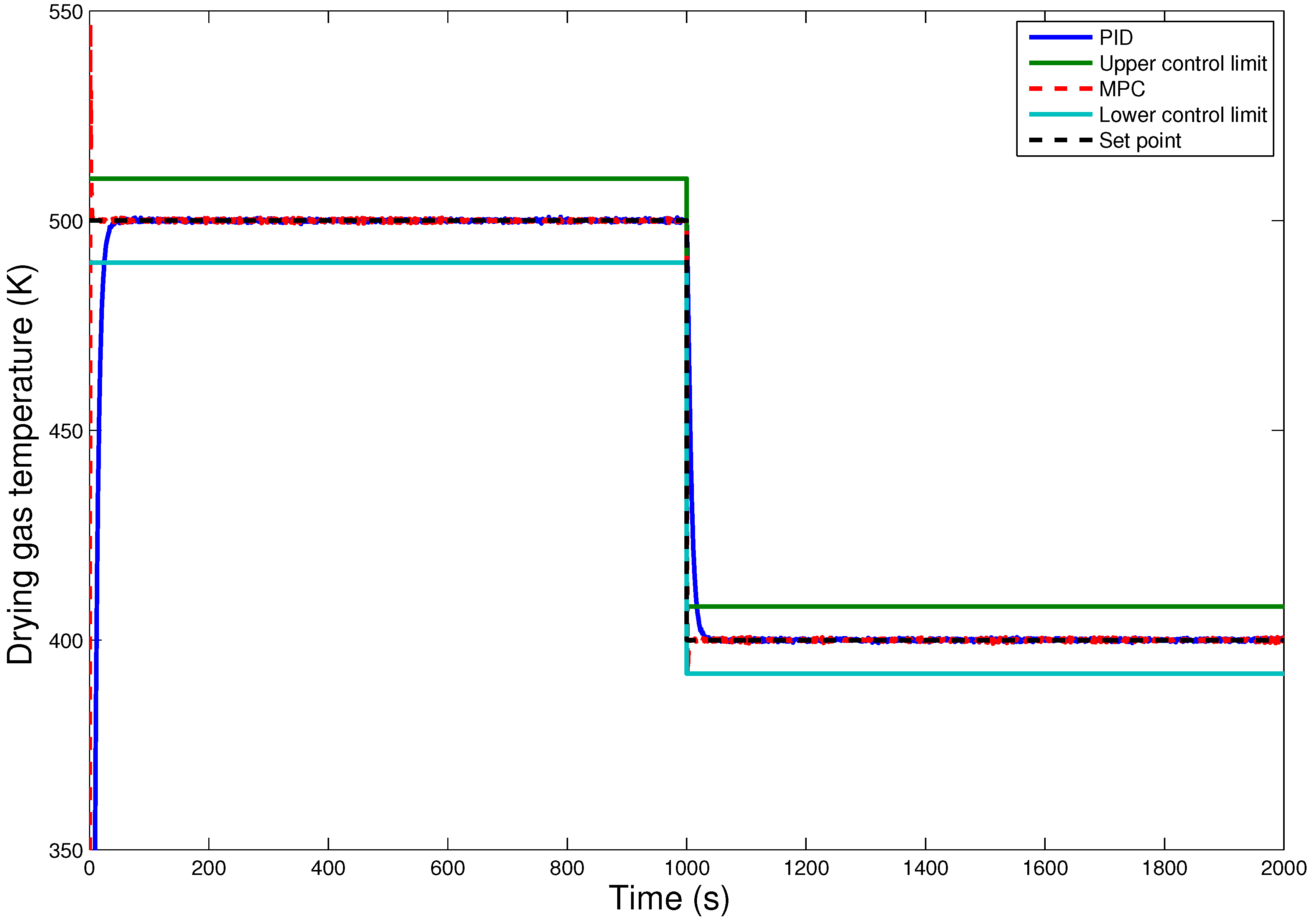
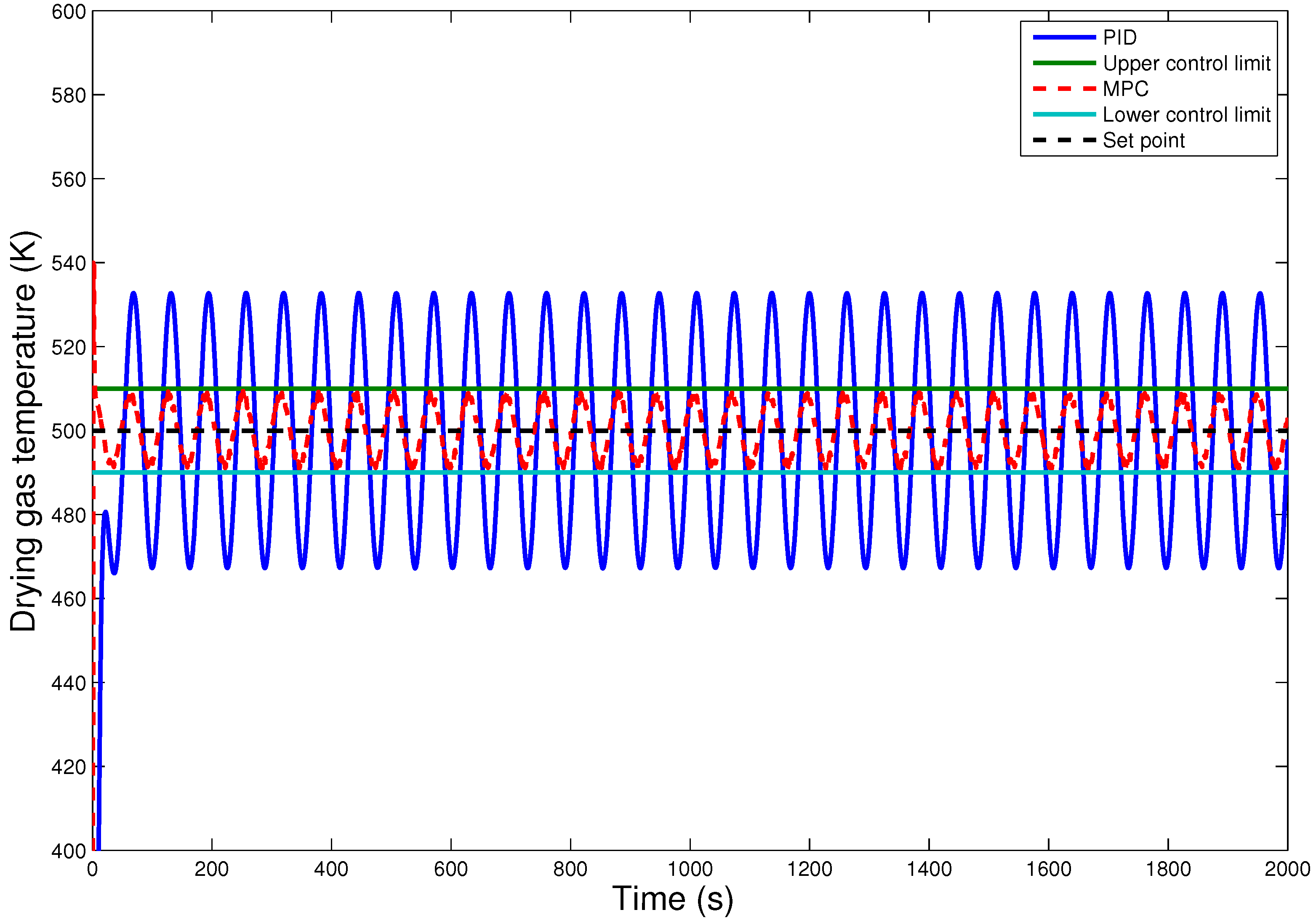
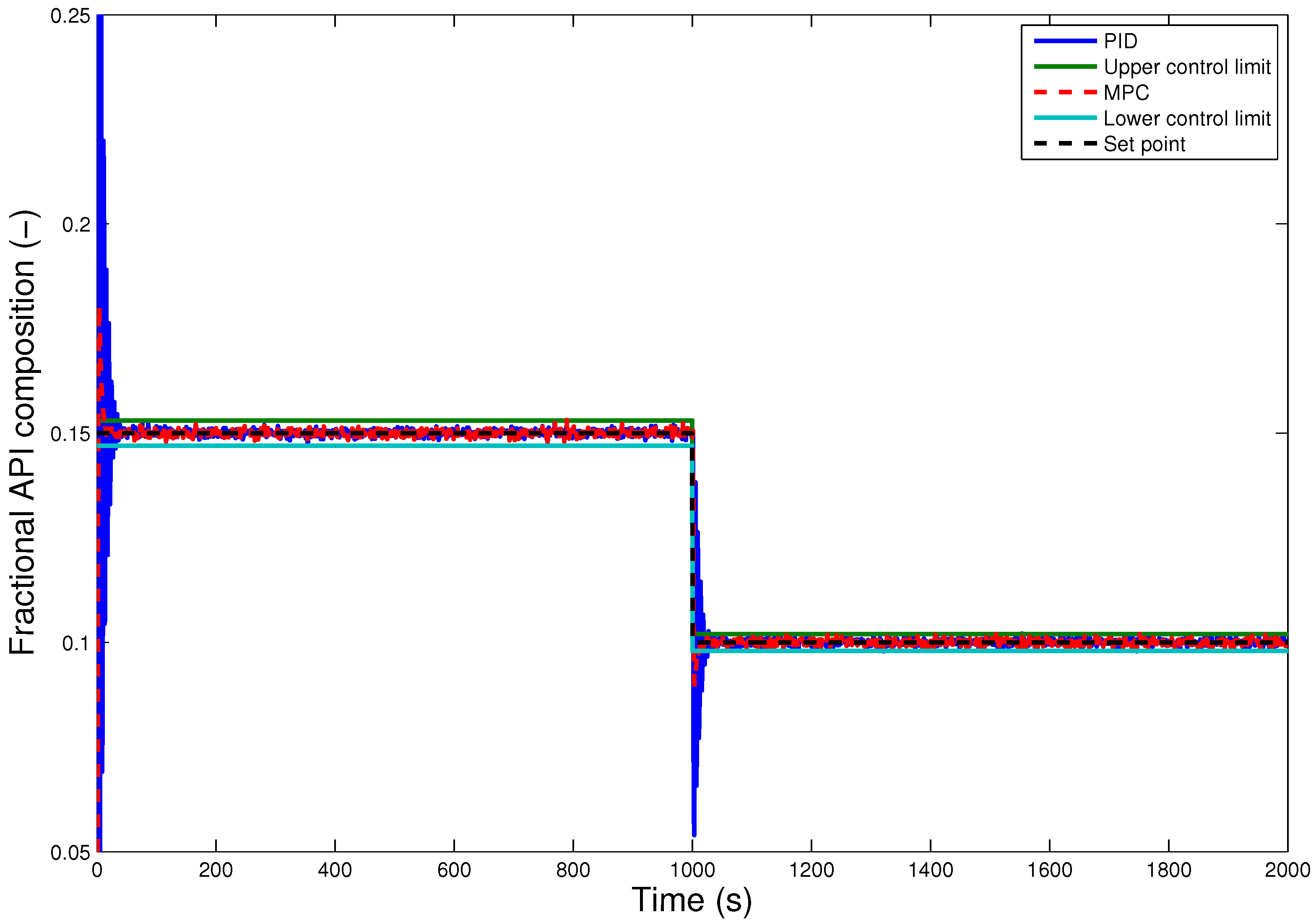
7. Conclusions
Acknowledgments
Nomenclature
| Symbol | Description | Units |
| Ac | Area of heat transfer | m2 |
| B0 | Primary nucleation term | particles/(m3/s) |
| b | Kinetic parameter for crystallization | Dimensionless |
| C | Solute concentration in crystallization | moles/m3 |
| Csat | Saturation concentration of solute | moles/m3 |
| Cpw | Specific heat constant for water | J/K |
| Dp | Crystal diameter | μm |
| F | Particle density | particles/m3 |
| G1 | Growth rate | m/s |
| G2 | Growth rate | m/s |
| g1 | Kinetic parameter for crystallization | Dimensionless |
| g2 | Kinetic parameter for crystallization | Dimensionless |
| kg1 | Kinetic parameter for crystallization | m/s |
| kg2 | Kinetic parameter for crystallization | m/s |
| kb | Kinetic parameter for crystallization | particles/(m3/s) |
| L1 | Internal coordinate for length of solid | m |
| L2 | Internal coordinate for length of solid | m |
| L3 | Internal coordinate for length of liquid | m |
| Mw | Cooling water flow rate | kg/s |
| T | Temperature (cooling schedule) | Kelvin |
| Tc | Temperature of cooling water | Kelvin |
| Tin | Inlet temperature of water | Kelvin |
| U | Overall heat transfer coefficient | W/(m2·K) |
| ϵ | Porosity of the cake | Dimensionless |
| ρavg | Average density of wet particles | kg/m3 |
| ρs | Density of solid | kg/m3 |
| ∆P | Filter pressure difference | kPa |
| μ | Fluid viscosity | kg/(m·s) |
| α | Specific cake resistance | m/kg |
| A | Filter surface area | m2 |
| CF | Concentration of solutes in slurry | moles/m3 |
| c | Mass of solute deposited on filter per unit volume of filtrate | kg/m3 |
| mF | Mass of wet cake | kg |
| mc | Mass of dry cake | kg |
| mv | Rate of evaporation during drying | kg/s |
| Na | Avogadro number | Dimensionless |
| Rm | Filter medium resistance | 1/m |
| V | Filtrate volume | m3 |
| Vp | Particle volume | m3 |
| As | Area of heat transfer | m2 |
| Cps | Specific heat capacity | J/(kg·K) |
| Cpsteam | Specific heat constant for steam | J/K |
| k | Mass transfer coefficient | m/s |
| hfg | Specific heat of evaporation | J/kg |
| h | Heat transfer coefficient | W/(M2·K) |
| Ts | Temperature of steam | Kelvin |
| Tp | Temperature of particle | Kelvin |
| Tg | Drying gas temperature | Kelvin |
| Tin-steam | Inlet temperature of steam | Kelvin |
| massout | Outlet flow rate of API crystals from dryer | kg/s |
| Ms | Steam flow rate | kg/s |
| Nu | Nusselt’s Number | Dimensionless |
| Pr | Prandtl Number | Dimensionless |
| Re | Reynolds Number | Dimensionless |
| Sh | Sherwood Number | Dimensionless |
| U | Overall heat transfer coefficient | W/(m2·K) |
| xp | Liquid content of solid particle | Dimensionless |
| xeql | Liquid content of solid particle at equilibrium | Dimensionless |
| n | Counter for number of components | Dimensionless |
| nmax | Maximum number of components | - |
| t | Time | s |
| Vf | Forward axial velocity | m/s |
| Vb | Backward axial velocity | m/s |
| Vr | Radial velocity | m/s |
| x | Spatial coordinate in axial direction | - |
| xmax | Maximum number of axial compartments | - |
| y | Spatial coordinate in axial direction | - |
| ymax | Maximum number of radial compartments | - |
| yAPI | Fractional API composition at mixer outlet | Dimensionless |
| yavg | Average spatial composition of component A | moles/m3 |
| yi | Composition of component A in i-th compartment | moles/m3 |
Appendix: Process Model Integrated with the Hybrid MPC-PID Scheme
Crystallizer
Filtration Process
Drying Process
Mixer
MPC-PID Controller Equations
Conflicts of Interest
References
- Food and Drug Administration. Guidance for Industry. Q8 Pharmaceutical Development; U.S. Food and Drug Administration: Rockville, MD, USA, 2006.
- Food and Drug Administration. Guidance for Industry. PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance; U.S. Food and Drug Administration: Rockville, MD, USA, 2004.
- Ogunnaike, B.A.; Ray, W.H. Process Dynamics, Modeling, and Control; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Richalet, J. Industrial applications of model based predictive control. Automatica 1993, 29, 1251–1274. [Google Scholar] [CrossRef]
- Garcia, C.E.; Prett, D.M.; Morari, M. Model predictive control: Theory and practice-A survey. Automatica 1989, 25, 335–348. [Google Scholar] [CrossRef]
- Singh, R.; Ierapetritou, M.; Ramachandran, R. System-wide hybrid MPC-PID control of a continuous pharmaceutical tablet manufacturing process via direct compaction. Eur. J. Pharm. Biopharm. 2013, 85, 1164–1182. [Google Scholar] [CrossRef] [PubMed]
- Pantelides, C.C.; Renfro, J.G. The online use of first-principles models in process operations: Review, current status and future needs. Comput. Chem. Eng. 2013, 51, 136–148. [Google Scholar] [CrossRef]
- Boukouvala, F.; Niotis, V.; Ramachandran, R.; Muzzio, F.; Ierapetritou, M.G. An integrated approach for dynamic flowsheet modeling and sensitivity analysis of a continuous tablet manufacturing process: An integrated approach. Comput. Chem. Eng. 2012, 42, 30–47. [Google Scholar] [CrossRef]
- Boukouvala, F.; Chaudhury, A.; Sen, M.; Zhou, R.; Mioduszewski, L.; Ierapetritou, M.G.; Ramachandran, R. Computer-aided flowsheet simulation of a pharmaceutical tablet manufacturing process incorporating wet granulation. J. Pharm. Innov. 2013, 8, 11–27. [Google Scholar] [CrossRef]
- Sen, M.; Chaudhury, A.; Singh, R.; John, J.; Ramachandran, R. Multi-scale flowsheet simulation of an integrated continuous purification-downstream pharmaceutical manufacturing process. Int. J. Pharm. 2013, 445, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Rogers, A.; Singh, R.; Chaudhury, A.; John, J.; Ierapetritou, M.G.; Ramachandran, R. Flowsheet optimization of an integrated continuous purification-processing pharmaceutical manufacturing operation. Chem. Eng. Sci. 2013, 102, 56–66. [Google Scholar] [CrossRef]
- Gnoth, S.; Jenzsch, M.; Simutis, R.; Luubert, A. Process analytical technology (PAT): Batch-to-batch reproducibility of fermentation processes by robust process operational design and control. J. Biotechnol. 2007, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Singh, R.; Ramachandran, R. Simulation-based design of an efficient control system for the continuous purification and processing of active pharmaceutical ingredients. J. Pharm. Innov. 2014. [Google Scholar] [CrossRef]
- Singh, R.; Ierapetritou, M.; Ramachandran, R. An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction. Int. J. Pharm. 2012, 438, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.; Cooper, D. A practical multiple model adaptive strategy for single-loop MPC. Control Eng. Pract. 2003, 11, 141–159. [Google Scholar] [CrossRef]
- Sanders, C.F.W.; Hounslow, M.J.; Doyle, F.J., III. Identification of models for control of wet granulation. Powder Technol. 2009, 188, 255–263. [Google Scholar] [CrossRef]
- Mesbah, A.; Nagy, Z.K.; Huesman, A.E.M.; Kramer, H.J.M.; van den Hof, P.M.J. Nonlinear model-based control of a semi-industrial batch crystallizer using population balance modeling framework. IEEE Trans. Control Syst. Technol. 2012, 20, 1188–1201. [Google Scholar] [CrossRef]
- Kwon, J.S.; Nayhouse, M.; Christofides, P.D.; Orkoulas, G. Protein crystal shape and size control in batch crystallization: Comparing model predictive control with conventional operating policies. Ind. Eng. Chem. Res. 2014, 53, 5002–5014. [Google Scholar] [CrossRef]
- Dufour, P. Control engineering in drying technology: Review and trends. Dry. Technol. 2006, 24, 889–904. [Google Scholar] [CrossRef]
- Daraoui, N.; Dufour, P.; Hammouri, H.; Hottot, A. Model predictive control during the primary drying stage of lyophilisation. Control Eng. Pract. 2010, 18, 483–494. [Google Scholar] [CrossRef]
- Pisano, R.; Fissore, D.; Barresi, A.A. Freeze drying cycle optimization using model predictive control techniques. Ind. Eng. Chem. Res. 2011, 50, 7363–7379. [Google Scholar] [CrossRef]
- Hsu, S.; Reklaitis, G.V.; Venkatasubramanian, V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part I: Process dynamics and control framework. J. Pharm. Innov. 2010, 5, 14–23. [Google Scholar] [CrossRef]
- Hsu, S.; Reklaitis, G.V.; Venkatasubramanian, V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part II: Control and system design. J. Pharm. Innov. 2010, 5, 24–36. [Google Scholar] [CrossRef]
- Bardin, M.; Knight, P.C.; Seville, J.P.K. On control of particle size distribution in granulation using high shear mixers. Powder Technol. 2004, 140, 169–175. [Google Scholar] [CrossRef]
- Burggraeve, A.; Monteyne, T.; Vervaet, C.; Remon, J.P.; Beer, T.D. Process analytical tools for monitoring, understanding, and control of pharmaceutical fluidized bed granulation: A review. Eur. J. Pharm. Biopharm. 2013, 83, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Chaudhury, A. Model-based design and control of continuous drum granulation processes. Chem. Eng. Res. Design 2011, 90, 1063–1073. [Google Scholar] [CrossRef]
- Gatzke, E.P.; Doyle, F.J. Model predictive control of a granulation system using soft output constraints and prioritized control objectives. Powder Technol. 2001, 121, 149–158. [Google Scholar] [CrossRef]
- Long, C.E.; Polisetty, P.K.; Gatzke, E.P. Deterministic global optimization for non-linear model predictive control of hybrid dynamic systems. Int. J. Rob. Nonlin. Control 2007, 17, 1232–1250. [Google Scholar]
- Pottmann, M.; Ogunnaike, B.A.; Adetayo, A.A.; Ennis, B.J. Model-based control of a granulation process. Powder Technol. 2000, 108, 192–201. [Google Scholar] [CrossRef]
- Ramachandran, R.; Arjunan, J.; Chaudhury, A.; Ierapetritou, M.G. Model-based control loop performance assessment of a continuous direct compaction pharmaceutical processes. J. Pharm. Innov. 2012, 6, 249–263. [Google Scholar] [CrossRef]
- Singh, R.; Ierapetritou, M.; Ramachandran, R. Hybrid advanced control of flexible multipurpose continuous tablet manufacturing process via direct compaction. Comput. Aid. Chem. Eng. 2013, 32, 757–762. [Google Scholar]
- Singh, R.; Barrasso, D.; Chaudhury, A.; Sen, M.; Ierapetritou, M.; Ramachandran, R. Closed-loop feedback control of a continuous pharmaceutical tablet manufacturing process via wet granulation. J. Pharm. Innov. 2014. [Google Scholar] [CrossRef]
- Singh, R.; Sahay, A.; Oka, S.; Liu, X.; Ramachandran, R.; Ierapetritou, M.; Muzzio, F. Online monitoring, advanced control and operation of robust continuous pharmaceutical tablet manufacturing process. BioPharma Mag. Asia 2013, 2, 18–23. [Google Scholar]
- Benyahia, B.; Lakerveld, R.; Barton, P.I. A plant-wide dynamic model of a continuous pharmaceutical process. Ind. Eng. Chem. Res. 2012, 51, 15393–15412. [Google Scholar] [CrossRef]
- Lakerveld, R.; Benyahia, B.; Braatz, R.D.; Barton, P.I. Model-based design of a plant-wide control strategy for a continuous pharmaceutical plant. AIChE J. 2013, 59, 3671–3685. [Google Scholar] [CrossRef]
- Lakerveld, R.; Benyahia, B.; Heider, P.L.; Zhang, H.; Braatz, R.D.; Barton, P.I. Averaging level control to reduce off-spec material in a continuous pharmaceutical pilot plant. Processes 2013, 1, 330–348. [Google Scholar] [CrossRef]
- Pregelj, B.; Gerksic, S. Hybrid explicit model predictive control of a nonlinear process approximated with a piecewise affine model. J. Process Control 2010, 20, 832–839. [Google Scholar] [CrossRef]
- Zhu, G.; Henson, M.A.; Ogunnaike, B.A. A hybrid model predictive control strategy for nonlinear plant-wide control. J. Process Control 2000, 10, 449–458. [Google Scholar] [CrossRef]
- Song, C.; Wu, B.; Li, P. A hybrid model-based optimal control method for nonlinear systems using simultaneous dynamic optimization strategies. J. Process Control 2012, 22, 852–860. [Google Scholar] [CrossRef]
- Mccabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Mezhericher, A.; Levy, A.; Borde, I. Modelling of particle breakage during drying. Chem. Eng. Process. 2008, 47, 1404–1411. [Google Scholar] [CrossRef]
- Sen, M.; Singh, R.; Vanarase, A.; John, J.; Ramachandran, R. Multi-dimensional population balance modeling and experimental validation of continuous powder mixing processes. Chem. Eng. Sci. 2012, 80, 349–360. [Google Scholar] [CrossRef]
- Robles, A.; Ruano, M.V.; Ribes, J.; Ferrer, J. Advanced control system for optimal filtration in submerged anaerobic MBRs (SAnMBRs). J. Membr. Sci. 2013, 430, 330–340. [Google Scholar] [CrossRef]
- Peiris, R.H.; Budman, H.; Moresoli, C.; Legge, R.L. Fouling control and optimization of a drinking water membrane filtration process with real-time model parameter adaptation using fluorescence and permeate flux measurements. J. Process Control 2013, 23, 70–77. [Google Scholar] [CrossRef]
- Hammond, A.; Smith, M. The role of PAT, condition monitoring and adaptive control in delivering QbD. In Proceedings of the AIChE Annual Meeting, Pittsburg, PA, USA, 28 October–2 November 2012.
- Sen, M.; Dubey, A.; Singh, R.; Ramachandran, R. Mathematical development and comparison of a hybrid PBM-DEM description of a continuous powder mixing process. J. Powder Technol. 2013. [Google Scholar] [CrossRef]
- Stephanopoulos, G. Chemical Process Control; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 2006. [Google Scholar]
- Schultz, W.C.; Rideout, V.C. Control system performance measures: Past, present and future. IRE Trans. Autom. Control 1961, AC-6, 22–35. [Google Scholar] [CrossRef]
- Singh, R.; Sahay, A.; Muzzio, F.; Ierapetritou, M.; Ramachandran, R. Systematic framework for onsite design and implementation of the control system in continuous tablet manufacturing process. Comput. Chem. Eng. 2014. [Google Scholar] [CrossRef]
- Miki, H.; Terashima, T.; Asakuma, Y.; Maeda, K.; Fukui, K. Inclusion of mother liquor inside KDP crystals in a continuous MSMPR crystallizer. Separ. Purif. Technol. 2005, 43, 71–76. [Google Scholar] [CrossRef]
- Gunawan, R.; Fusman, I.; Braatz, R.D. High resolution algorithms for multidimensional population balance equations. AIChE J. 2004, 50, 2738–2749. [Google Scholar] [CrossRef]
- Sen, M.; Ramachandran, R. A multi-dimensional population balance model approach to continuous powder mixing processes. Adv. Powder Technol. 2013, 24, 51–59. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sen, M.; Singh, R.; Ramachandran, R. A Hybrid MPC-PID Control System Design for the Continuous Purification and Processing of Active Pharmaceutical Ingredients. Processes 2014, 2, 392-418. https://doi.org/10.3390/pr2020392
Sen M, Singh R, Ramachandran R. A Hybrid MPC-PID Control System Design for the Continuous Purification and Processing of Active Pharmaceutical Ingredients. Processes. 2014; 2(2):392-418. https://doi.org/10.3390/pr2020392
Chicago/Turabian StyleSen, Maitraye, Ravendra Singh, and Rohit Ramachandran. 2014. "A Hybrid MPC-PID Control System Design for the Continuous Purification and Processing of Active Pharmaceutical Ingredients" Processes 2, no. 2: 392-418. https://doi.org/10.3390/pr2020392
APA StyleSen, M., Singh, R., & Ramachandran, R. (2014). A Hybrid MPC-PID Control System Design for the Continuous Purification and Processing of Active Pharmaceutical Ingredients. Processes, 2(2), 392-418. https://doi.org/10.3390/pr2020392





