A Combined Glutaraldehyde and Denitrifying Bacteria Strategy for Enhanced Control of SRB-Induced Corrosion in Shale Gas Infrastructure
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of SRB from Shale Gas Production Liquid
2.2. Enrichment and Identification of DNB Communities
2.3. The Design of the Experimental Group Division
2.4. Determination of Minimum Inhibitory Concentration (MIC)
2.5. Electrochemical Corrosion Testing
2.6. Static Weight Loss Testing
- CR—Average corrosion rate, mm/year;
- Δm—the reduced weight, g;
- S—exposed surface area of steel coupon, m2;
- t—immersion duration, hours;
- ρ—density of steel, 7.85 g/cm3.
2.7. Microbial Community Composition Analysis
3. Results and Discussion
3.1. Isolation of SRB
3.2. Enrichment of DNB
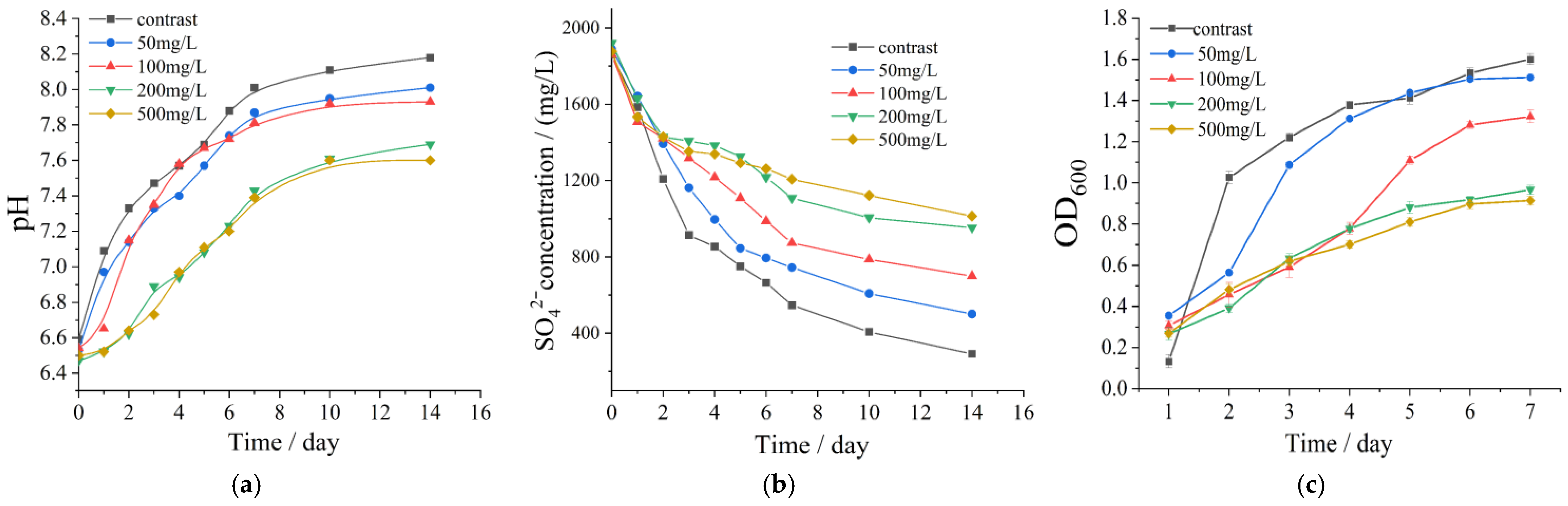
3.3. Influence of Nitrate Concentration and DNB on SRB Activity
3.4. Minimum Inhibitory Concentration of Glutaraldehyde
3.5. Electrochemical Corrosion Analysis
3.5.1. Open Circuit Potential and Linear Polarization Resistance
3.5.2. Electrochemical Impedance Spectroscopy Analysis
3.6. Weight Loss Measurements and Coupon Surface Characterization
3.7. Microbial Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SRB | sulfate-reducing bacteria |
| DNB | denitrifying bacteria |
| H2S | hydrogen sulfide |
| BCX | bio-competitive exclusion |
| MIC | determination of minimum inhibitory concentration |
| OCP | open circuit potential |
| EIS | electrochemical impedance spectroscopy |
| LPR | linear polarization resistance |
| OTU | operational taxonomic units |
| SEM | scanning electron microscopy |
| OD600 | the optical density at 600 nm |
References
- Jiang, X.; Zhang, Q.; Qu, D.; Xu, K.; Song, X. Corrosion behavior of L360 N and L415 N mild steel in a shale gas gathering environment—Laboratory and on-site studies. J. Nat. Gas Sci. Eng. 2020, 82, 103492. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Tang, Y.; Wen, S.; Hou, X. Influence of initial cell counts on the microbiologically influenced corrosion of L245N steel in shale gas environments. Bioelectrochemistry 2026, 168, 109157. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lewandowski, Z.; Nielsen, P.H.; Hamilton, W.A. Role of sulfate-reducing bacteria in corrosion of mild steel: A review. Biofouling 1995, 8, 165–194. [Google Scholar] [CrossRef]
- Liu, H.X.; Jin, Z.Y.; Liu, H.F.; Meng, G.Z.; Liu, H.W. Microbiological corrosion acceleration of N80 steel in shale gas field produced water containing Citrobacter amalonaticus at 60? Bioelectrochemistry 2022, 148, 108253. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, K.; Gu, T.; Raad, I.I. A green biocide enhancer for the treatment of sulfate-reducing bacteria (SRB) biofilms on carbon steel surfaces using glutaraldehyde. Int. Biodeterior. Biodegrad. 2009, 63, 1102–1106. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, J.; Wei, B.; Qin, Q.; Bai, Y.; Yu, C.; Sun, C. Biologically competitive effect of Desulfovibrio desulfurican and Pseudomonas stutzeri on corrosion of X80 pipeline steel in the Shenyang soil solution. Bioelectrochemistry 2022, 145, 108051. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wu, Y.; Chen, M.; Fu, Y.; Li, W.; Liu, S.; Wang, J.; Chen, Y. Efficient nitrogen removal by alternating axial distribution of nitrification and denitrification on conductive aeration membrane in membrane aerated biofilm reactor(MABR). Sep. Purif. Technol. 2026, 382, 136037. [Google Scholar] [CrossRef]
- Yamamoto-Ikemoto, R.; Matsui, S.; Komori, T.; Bosque-Hamilton, E.J. Symbiosis and competition among sulfate reduction, filamentous sulfur, denitrification, and poly-p accumulation bacteria in the anaerobic-oxic activated sludge of a municipal plant. Water Sci. Technol. 1996, 34, 119–128. [Google Scholar] [CrossRef]
- Yamamoto-Ikemoto, R.; Matsui, S.; Komori, T. Ecological interactions among denitrification, poly-P accumulation, sulfate reduction, and filamentous sulfur bacteria in activated sludge. Water Sci. Technol. 1994, 30, 201–210. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, J.; Wei, B.; Qin, Q.; Bai, Y.; Yu, C.; Sun, C. Mechanistic diversity between nitrate and nitrite on biocorrosion of X80 pipeline steel caused by Desulfovibrio desulfurican and Pseudomonas stutzeri. Corros. Sci. 2022, 207, 110573. [Google Scholar] [CrossRef]
- Myhr, S.; Lillebø, B.L.; Sunde, E.; Beeder, J.; Torsvik, T. Inhibition of microbial H2S production in an oil reservoir model column by nitrate injection. Appl. Microbiol. Biotechnol. 2002, 58, 400–408. [Google Scholar] [CrossRef]
- Zhao, M.L.; Sui, G.Z.; Xu, X.K.; Sun, W.G.; Sun, X.Y.; Kang, Y. Biological Inhibition of Sulfate-reducing Bacteria in Produced Water from Wunan Oilfield. Drill. Prod. Technol. 2021, 44, 106–110. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, B.; Liu, J.; Cai, Q.; Lin, W.; Chen, B. Towards sulfide removal and sulfate reducing bacteria inhibition: Function of biosurfactants produced by indigenous isolated nitrate reducing bacteria. Chemosphere 2020, 238, 124655. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Song, F.; Gu, T. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci. 2013, 77, 385–390. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Y.; Zhang, Y.; Yu, L.; Liu, L.; Wang, L.; Huang, S. First Report of Bacterial Panicle Blight of Rice Caused by Burkholderia glumae in Southern China. Plant Dis. 2019, 104, 1252. [Google Scholar] [CrossRef]
- Blasco, R.; Castillo, F.; MartinezLuque, M. The assimilatory nitrate reductase from the phototrophic bacterium, Rhodobacter capsulatus E1F1, is a flavoprotein. FEBS Lett. 1997, 414, 45–49. [Google Scholar] [CrossRef]
- Wysocka, J.; Cieslik, M.; Krakowiak, S.; Ryl, J. Carboxylic acids as efficient corrosion inhibitors of aluminium alloys in alkaline media. Electrochim. Acta 2018, 289, 175–192. [Google Scholar] [CrossRef]
- Mansfeld, F. The use of electrochemical techniques for the investigation and monitoring of microbiologically influenced corrosion and its inhibition—A review. Mater. Corros. 2003, 54, 489–502. [Google Scholar] [CrossRef]
- Moradi, M.; Song, Z.; Yang, L.; Jiang, J.; He, J. Effect of marine Pseudoalteromonas sp. on the microstructure and corrosion behaviour of 2205 duplex stainless steel. Corros. Sci. 2014, 84, 103–112. [Google Scholar] [CrossRef]
- Beech, I.B.; Campbell, S.A. Accelerated low water corrosion of carbon steel in the presence of a biofilm harbouring sulphate-reducing and sulphur-oxidising bacteria recovered from a marine sediment. Electrochim. Acta 2008, 54, 14–21. [Google Scholar] [CrossRef]
- Xu, D.; Xia, J.; Zhou, E.; Zhang, D.; Li, H.; Yang, C.; Li, Q.; Lin, H.; Li, X.; Yang, K. Accelerated corrosion of 2205 duplex stainless steel caused by marine aerobic Pseudomonas aeruginosa biofilm. Bioelectrochemistry 2017, 113, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Asif, M.; Zhang, G.; Liu, H. The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria. Corros. Sci. 2017, 114, 102–111. [Google Scholar] [CrossRef]
- Moradi, M.; Duan, J.; Ashassi-Sorkhabi, H.; Luan, X. De-alloying of 316 stainless steel in the presence of a mixture of metal-oxidizing bacteria. Corros. Sci. 2011, 53, 4282–4290. [Google Scholar] [CrossRef]
- Aljohani, T.A.; Hayden, B.E. A simultaneous screening of the corrosion resistance of Ni–W thin film alloys. Electrochim. Acta 2013, 111, 930–936. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, J.; Cui, T.; Fan, L.; Chen, X.; Liu, W.; Chang, W.; Du, C.; Zhang, D. Influence of NaCl concentration on microbiologically influenced corrosion of carbon steel by halophilic archaeon Natronorubrum tibetense. Bioelectrochemistry 2021, 140, 107746. [Google Scholar] [CrossRef]
- Lin, C.; Ruan, H. Multi-phase-field modeling of localized corrosion involving galvanic pitting and mechano-electrochemical coupling. Corros. Sci. 2020, 177, 108900. [Google Scholar] [CrossRef]
- Wang, H.; Hu, C.; Hu, X.; Yang, M.; Qu, J. Effects of disinfectant and biofilm on the corrosion of cast iron pipes in a reclaimed water distribution system. Water Res. 2012, 46, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Ting, Y.-P.; Pehkonen, S.O. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros. Sci. 2007, 49, 2159–2176. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Corrosion of X52 pipeline steel in a simulated soil solution with coexistence of Desulfovibrio desulfuricans and Pseudomonas aeruginosa bacteria. Corros. Sci. 2020, 173, 108753. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Denitrification of groundwater using PHBV blends in packed bed reactors and the microbial diversity. Chemosphere 2016, 155, 463–470. [Google Scholar] [CrossRef] [PubMed]












| Reagent | Purity (%) | Dosage (g/L) |
|---|---|---|
| CaCl2 | >99.0 | 0.1 |
| KH2PO4 | >99.0 | 0.5 |
| Na2SO4 | >99.0 | 1.0 |
| MgSO4·7H2O | >99.0 | 2.0 |
| FeSO4 | >99.0 | 0.2 |
| NH4Cl | >99.0 | 1.0 |
| yeast extract | >99.5 | 1.0 |
| sodium lactate | >99.0 | 3.48 |
| Reagent | Purity (%) | Dosage (g/L) |
|---|---|---|
| K2HPO4 | >99.0 | 0.5 |
| KNO3 | >99.0 | 2 |
| CaCl2 | >99.0 | 0.05 |
| MgSO4·7H2O | >99.0 | 1.5 |
| seignette salt | >99.0 | 20 |
| Group | SRB | DNB | Glutaral | KNO3 |
|---|---|---|---|---|
| contrast | − | − | − | − |
| SRB | + | − | − | − |
| DNB | − | + | − | − |
| SRB+DNB | + | + | − | − |
| KNO3 | + | + | − | + |
| glutaral | + | + | + | − |
| glutaral+KNO3 | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Guo, Y.; Wen, C.; Duan, M.; Lan, G. A Combined Glutaraldehyde and Denitrifying Bacteria Strategy for Enhanced Control of SRB-Induced Corrosion in Shale Gas Infrastructure. Processes 2026, 14, 334. https://doi.org/10.3390/pr14020334
Guo Y, Wen C, Duan M, Lan G. A Combined Glutaraldehyde and Denitrifying Bacteria Strategy for Enhanced Control of SRB-Induced Corrosion in Shale Gas Infrastructure. Processes. 2026; 14(2):334. https://doi.org/10.3390/pr14020334
Chicago/Turabian StyleGuo, Yu, Chongrong Wen, Ming Duan, and Guihong Lan. 2026. "A Combined Glutaraldehyde and Denitrifying Bacteria Strategy for Enhanced Control of SRB-Induced Corrosion in Shale Gas Infrastructure" Processes 14, no. 2: 334. https://doi.org/10.3390/pr14020334
APA StyleGuo, Y., Wen, C., Duan, M., & Lan, G. (2026). A Combined Glutaraldehyde and Denitrifying Bacteria Strategy for Enhanced Control of SRB-Induced Corrosion in Shale Gas Infrastructure. Processes, 14(2), 334. https://doi.org/10.3390/pr14020334






