Abstract
This study reports the synthesis of high cis-1,4 polybutadiene with a narrow molecular weight distribution (Đ < 2.0) by coordinative chain transfer polymerization (CCTP) using a homogeneous ternary NdV3/diisobutyl aluminum hydride (DIBAH)/dimethyldichlorosilane (Me2SiCl2) Ziegler–Natta catalyst system. The polymerization parameters, notably the monomer-to-initiator ratio ([M]/[Nd]) and the halogen-to-initiator ratio ([Cl]/[Nd]), were systematically varied to define the CCTP operational window. It was found that CCTP conditions are achieved only at low [M]/[Nd] ratios (<2500) and intermediate [Cl]/[Nd] ratios between 1.0 and 2.0, facilitating the production of polymers with molecular weights below 32 kDa and narrow dispersity. Increasing these ratios beyond these thresholds potentially induces the formation of insoluble, hyper-halogenated catalytic species and increases medium viscosity, which significantly broadens the molecular weight distribution (Đ > 4.0) and impairs CCTP control. These findings challenge previous assumptions that higher halogen concentrations are necessary for CCTP, thereby providing important mechanistic insights for tuning active species and achieving improved polymer architecture. The work demonstrates a viable pathway to control polymer microstructure and molecular weight in neodymium-based CCTP, which is critical for design of high-performance elastomeric materials.
1. Introduction
The production of polydienes, particularly polybutadiene, with a high molecular weight, narrow molecular weight distribution (Đ), and high cis-1,4 microstructure is a persistent challenge in elastomer manufacturing. Materials with these properties exhibit excellent mechanical and thermal behavior, including enhanced wear resistance, low rolling resistance, high elasticity at low temperatures, fatigue resistance, improved adhesion, and crack flexibility, making them indispensable for high-performance tire applications [1,2,3]. However, no existing synthetic method has been able to simultaneously achieve all these desired physicochemical properties in polybutadiene.
Conventional polymerization methods present trade-offs: anionic polymerization produces polymers with narrow molecular weight distributions but typically low cis-1,4 content (~50%), whereas Ziegler–Natta catalysis with neodymium, titanium, cobalt, or nickel-based systems allows control of geometric isomerism (cis or trans) and achieves high stereoselectivity (>90% cis-1,4) in industrial polydiene production; however, the synthesized polymers are characterized by broad molecular weight distributions (Đ > 3) [4,5,6].
Coordinative chain transfer polymerization (CCTP) has emerged as a promising strategy to overcome these limitations, enabling precise control over molecular weight, dispersity, and microstructure in conjugated diene polymerization. During the kinetic criteria for CCTP, the growing polymer chain propagated (PL) from the active species is able to undergo chain transfer to CTA via a transmetalation process to give rise to a dormant species (PS) with the macromolecular chain attached to the main group metal (see Figure 1). It is essential to recognize that the fulfillment of these kinetic requirements heavily depends on the type and concentration of the chain transfer agent employed [5,7].
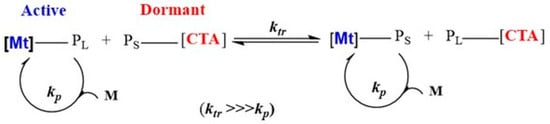
Figure 1.
Coordinative chain transfer polymerization mechanism.
In neodymium-based Ziegler–Natta catalytic systems, two general configurations exist: (a) binary systems and (b) ternary systems. Ternary systems are distinguished by their homogeneous nature and high catalytic activity in non-polar solvents. They typically comprise a tri-carboxylate or tri-alkoxide metal complex, an alkylaluminum co-catalyst (often a trialkyl- or dialkylaluminum hydride), which also serves as the chain transfer agent and scavenger, and a halogen source that activates the catalyst by halogenating the metal species [7,8,9,10,11]. For this reason, to reach the conditions for CCTP status is not a trivial issue. Since 2010, when Zhang et al. [12] reported the isoprene CCTP using a ternary Ziegler–Natta catalyst based on neodymium, different research groups have reported polymerization of both butadiene and isoprene under CCTP conditions [13,14,15,16,17] using Ziegler–Natta catalytic systems based on rare earth metal. However, in all cases, the polybutadienes and polyisoprenes synthetized have been characterized by their low molecular weight (<50,000 g/mol); this is mainly attributed to the [M]/[Nd] and [CTA]/[Nd] ratios used to achieve the CCTP condition, which, in principle, only allow one to obtain low-molecular-weight polymers.
In this work, we explore the polymerization of 1,3-butadiene utilizing a ternary Ziegler–Natta catalyst system based on neodymium versatate (NdV3), diisobutylaluminum hydride (DIBAH), and dimethyldichlorosilane (Me2SiCl2), focusing on the influence of key polymerization parameters to elucidate their roles in coordinative chain transfer polymerization (CCTP). Our objective is to understand how varying the [Cl]/[Nd], [CTA]/[Nd], and [M]/[Nd] ratios affects polymer molecular weight, dispersity, and microstructure, with the aim of achieving precise control over these critical polymer properties. By systematically operating at low-to-moderate [Cl]/[Nd] ratios, traditional [CTA]/[Nd] ratios (20–30), and a range of elevated [M]/[Nd] ratios (≥2500), we uncover the operational window that supports well-controlled polymer growth while delineating the boundaries where deviations from ideal CCTP behavior begin to manifest. This approach challenges existing assumptions about conventional [Cl]/[Nd] thresholds for CCTP and provides novel kinetic and mechanistic insights into the interplay between catalyst composition and polymer architecture within neodymium ternary catalyst systems.
2. Materials and Methods
Materials. 1,3-Butadiene (Sigma Aldrich, St. Louis, MI, USA) with a purity of 98% and was purified additionally before use by passing it through a packed column with a mixture of 50/50 w/w of aluminum oxide and molecular sieve. Cyclohexane (Química Delta, Ciudad de México, México) with a purity of 99.5% and was refluxed over lithium aluminum hydride for 4 h in a nitrogen atmosphere and subsequently distilled over metallic sodium. Afterward, it was refluxed over metallic sodium for 4 h and finally distilled and stored in stainless steel containers with 20 L capacity in a nitrogen atmosphere. The neodymium versatate was obtained from Solvay as solution in hexane 0.54 mol/L, and was used as received. Diisobutylaluminum hydride solution 1.0 M in hexane (Sigma Aldrich, St. Louis, MI, USA) was used as received. Dimethyldichlorosilane (98%) (Sigma Aldrich, St. Louis, MI, USA) was diluted in cyclohexane purified to a 0.222 mol/L concentration.
Polymerization. All manipulations were carried out under a dry nitrogen atmosphere in cyclohexane solution in a stainless-steel reactor with 2 L capacity with mechanical stirring and an internal coil cooling system (Delta reactor, Saltillo, México). A detailed polymerization procedure is described as a typical example.
The cyclohexane solvent and 1,3-butadiene monomer were introduced at the stainless-steel reactor using a glass column feed system under nitrogen atmosphere as shown in Figure 2. The stainless-steel reactor was heated at reaction temperature with an electrical heating jacket and stirred at 80 rpm to reach stable temperature before adding the catalytic system. The catalytic system was prepared in an glove box (Labmaster 130, MBraun, Gaithersburg, MD, USA)in an oxygen- and moisture-free glass ampoule capped with a rubber septum with 30 mL capacity. The addition order of the different components of the catalytic system at the ampule was (1) DIBAH, (2) NdV3, and (3) Me2SiCl2, according to the experimental design shown in Table 1 using syringes to handle oxygen- and moisture-sensitive substances, and was stirred for 30 min at room temperature before being added via syringes to the stainless steel reactor to initiate polymerization. Once the catalytic system was added, the pressure of the reactor increased to 80 psi using nitrogen.
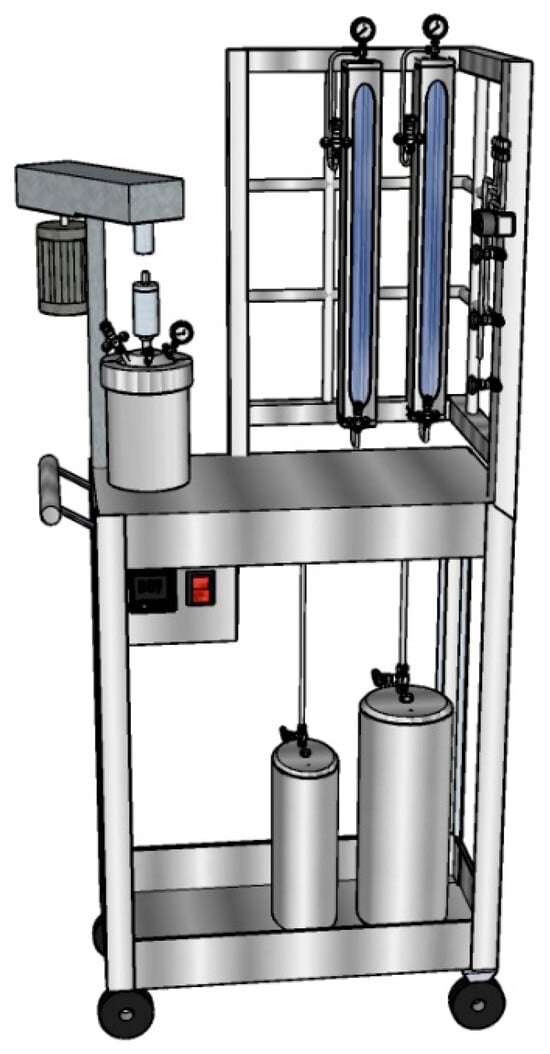
Figure 2.
Schematic diagram of the reaction system.

Table 1.
Experimental design.
Different samples were taken from the mass reaction via syringes and quenched with methanol, and conversion was determined gravimetrically. Polymerization was quenched by adding 1.0 mL acidified methanol containing pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionate) (Irganox 1010, Mississauga, ON, Canada) as a stabilizer. The polymer samples were washed with methanol repeatedly and finally dried in a vacuum oven at 50 °C to a constant weight.
Characterization. The number-average molecular weight (Mn), weight-average molecular weight (Mw), and molecular weight distribution (Ð) of polymers were measured at 28 °C by gel permeation chromatography (GPC) in an Agilent Technologies (Santa Clara, CA, USA) model PL-GPC 50 equipped with a mix column of 5 µm and refraction index detector calibrated with polystyrene standards. Tetrahydrofuran was used as eluent at a flow rate of 1.0 mL/min. The microstructure of polymers was determined by 1H and 13C NMR recorded on a JEOL (Tokyo, Japan) 400 MHz spectrometer in CDCl3 at room temperature.
3. Results and Discussion
3.1. Effect of [Cl]/[Nd] Ratio
The initial stage of this study aimed to identify the optimal [Cl]/[Nd] ratio that maximizes polymerization rate while evaluating its influence on molecular weight, dispersity, and microstructure of the resulting polybutadiene. Additionally, the number of growing polymer chains per neodymium center (Np) was determined using Equation (1), where [M]0 and [Nd]0 correspond to the initial concentration of monomer and neodymium catalysts, respectively. Mmonomer is the molar mass of monomer, and is the number-average molecular weight corrected by a factor of 0.5 to account for calibration with polystyrene standards [14,18,19]. The data in Table 2 summarize the experiments varying the [Cl]/[Nd] ratio from 0.7 to 2.6, with Figure 3a,b illustrating the conversion progression and its linearized behavior over time, respectively.

Table 2.
Effect of the [Cl]/[Nd] ratio over butadiene polymerization kinetics and molar mass properties.
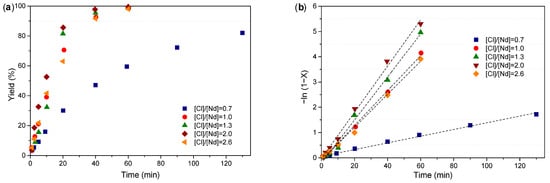
Figure 3.
(a) Evolution of the conversion with time at different [Cl]/[Nd] ratios; (b) linear form of the conversion–time behavior at different [Cl]/[Nd] ratios.
The polymerization rate is given by Equation (2); considering [M]o and [Nd] constants, the propagation constant (kp) is obtained by Equations (3) and (4).
- = conversion degree;
- [Nd] = concentration of all Nd species;
- t = polymerization time.
The chain transfer rate constant was estimated by solving the function relation between the polymerization degree (PD) and polymerization time expressed by Equation (5) in order to obtain Equation (6) where [Nd]0, [Al]0, and [M]0 are the initial concentrations of neodymium catalyst, Al(i-Bu)2H, and monomer, respectively; kp is the rate constant of propagation, and is the polymerization time [20].
Table 2 reveals that the polymerization rate reaches its maximum at a [Cl]/[Nd] ratio of 2.0. Notably, the number of polymer chains propagated per active species decreases significantly with increasing [Cl]/[Nd] (Figure 4a). This trend reflects the progressive formation of higher halogenated neodymium species, which transition from sub-halogenated, homogeneous, and soluble forms to hyper-halogenated, heterogeneous, and insoluble species within the reaction medium [11,21,22,23]. Consequently, this induces the formation of polymers with higher molecular weight and broader dispersity (Ð), as demonstrated in Figure 4b.
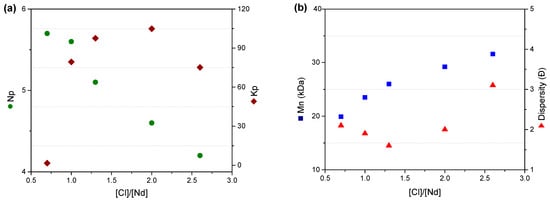
Figure 4.
(a) Effect of the [Cl]/[Nd] ratio on the number of polymer chains grown by active species and polymerization rate constant; (b) effect of the [Cl]/[Nd] ratio on the average molecular weight (Mn) and dispersion (Ð).
The broadened and multimodal molecular weight distributions observed (Figure 5) are consistent with previous literature [24]. Heterogeneous species are characterized by their high activity and short lifetime, giving rise to broad and multimodal molecular weight distribution curves as shown in Figure 5, where the high molecular weight fraction is the result of these polymer chains growing into short-lived active species. The results obtained in these experiments indicate that to work in the CCTP regime with the objective to obtain polymers with narrow Ð requires the use of a low [Cl]/[Nd] ratio regardless of whether these relationships do not allow for obtaining a high content of cis-1,4 micro-structures, as shown in Table 2.
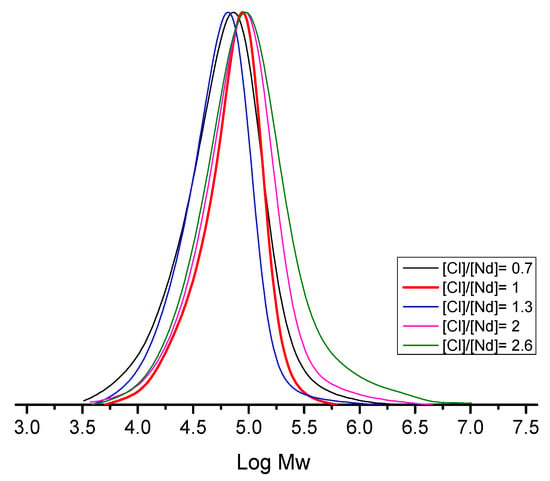
Figure 5.
Effect of the [Cl]/[Nd] ratio on the average molecular weight (Log Mw) of polybutadiene.
The [Cl]/[Nd] ratios employed in our experiments are notably lower than those commonly reported in the literature for achieving the CCTP regime during polymerization of butadiene and isoprene with the homogeneous NdV3/DIBAH/Me2SiCl2 catalytic system, where typical values range from 2.0 to 3.0 or higher [12,13,14,16,17]. Interestingly, our results indicate that at a [Cl]/[Nd] ratio of 1.0, the highest number of polymer chains initiated per active species (Np), was observed. This suggests that at this relatively low halogen-to-neodymium ratio, the halogenation level of the active catalytic centers is optimal, enabling the lowest dispersity (Ð) values alongside the highest polymerization rate and cis-1,4 microstructure content. Furthermore, increasing the [Cl]/[Nd] ratio leads to polybutadienes with progressively higher molecular weights (Figure 5), which is attributed to the formation of species with enhanced catalytic activity due to greater halogenation. However, it is critical to note that these elevated molecular weights primarily result from polymer chains grown on heterogeneous, hyper-halogenated species [17], which ultimately become insoluble in the reaction medium. This insolubility contributes to broad and multimodal molecular weight distributions, a phenomenon previously associated with the formation of NdCl3 during 1,3-butadiene polymerization [25].
3.2. Effect of [Al]/[Nd] Ratio
To assess whether increasing the concentration of the chain transfer agent (CTA) in the catalytic system effectively modulates the chain transfer rate and consequently controls the dispersity of the synthesized polymers, a series of polymerization experiments were conducted at three different [Al]/[Nd] ratios (20, 25, and 30) at 70 °C with an elevated monomer-to-neodymium ratio [M]/[Nd]. The results are compiled in Table 3.

Table 3.
Effect of the [Al]/[Nd] ratio over butadiene polymerization kinetics and molar mass properties.
As the [Al]/[Nd] ratio increases from 20 to 30, a marked increase in the number of active polymers (Np) from 3.6 to 8.5 was observed, indicating greater generation of initiating species. However, the increase in [Al] combined with [M]/[Nd] = 4000 favors the formation of species that do not fully promote CCTP conditions, as reflected by the decrease in the chain transfer constant (ktr) from 104,266.8 to 30,044.5 L/mol·s. Under these conditions, the system begins to behave more like traditional coordination polymerization, showing high monomer conversion and consistently high cis-1,4 microstructure content, which are characteristic of this type of polymerization. Nevertheless, this combination of higher Np and lower ktr allows for maintaining relatively narrow dispersities (Ð = 2.4 at [Al]/[Nd] = 30), demonstrating that a certain degree of control over polymer mini-construction and effective polymerization is still preserved.
This behavior reflects an increase in the number of polymer chains initiated per active species (Np = 3.6 → 8.5) and is accompanied by changes in the propagation (kp) and chain transfer (ktr) constants, highlighting the balance between chain growth and transfer events. The decrease in ktr values with increasing CTA concentration is attributed to the greater competition in the reversible chain reaction that exists between CTA species and active centers (Np) [26].
Figure 6a,b demonstrate that increasing the [Al]/[Nd] ratio accelerates the monomer conversion, exhibiting a pseudo-linear trend characteristic of first-order kinetics. The observed reduction in number-average molecular weight (Mn) is attributed to the excess diisobutylaluminum hydride (DIBAH), which enhances chain transfer by generating a larger number of polymer chains initiated by active species. This indicates not only shorter polymer chains but also an increase in the number of catalytic centers participating in initiation. Overall, higher [Al]/[Nd] ratios improve catalyst productivity and polymerization rate while favoring chain transfer over propagation, resulting in a more controlled molecular weight distribution (Figure 7).
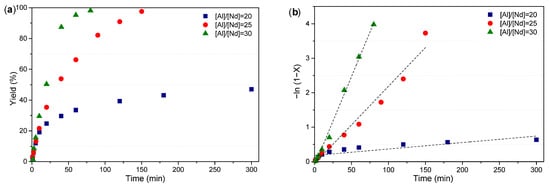
Figure 6.
(a) The evolution of conversion in relation to reaction time, and (b) the linear form of the conversion–time, determined similarly as in the experiments carried out at different [Al]/[Nd] ratios.
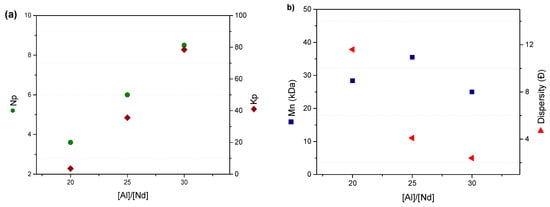
Figure 7.
(a) Effect of the ratio of [Al]/[Nd] on the number of polymer chains growing by active species and polymerization rate constant; (b) effect of the ratio of [Al]/[Nd] on the average molecular weight (Mn) and dispersion (Ð).
An additional notable observation is the markedly lower propagation rate constant (kp) in experiment Bd-6 compared to Bd-2, despite having identical [Cl]/[Nd] and [Al]/[Nd] ratios but differing in reaction temperature and a higher [M]/[Nd] ratio. This behavior is attributed to the increased presence of impurities at elevated monomer concentrations, which leads to greater consumption of DIBAH as a scavenger rather than as an activator or chain transfer agent. This interpretation is supported by GPC data (Figure 8), where the polybutadiene synthesized at the lowest [Al]/[Nd] ratio of 20 exhibits bimodal molecular weight distribution, the highest molecular weights, and the broadest dispersity (Ð). These features reflect a lower number of active centers and fewer reversible chain transfer reactions. Conversely, increasing the [Al]/[Nd] ratio to 30 significantly increases kp and the number of polymer chains per active species (Np), thereby reducing both Mn and Ð.
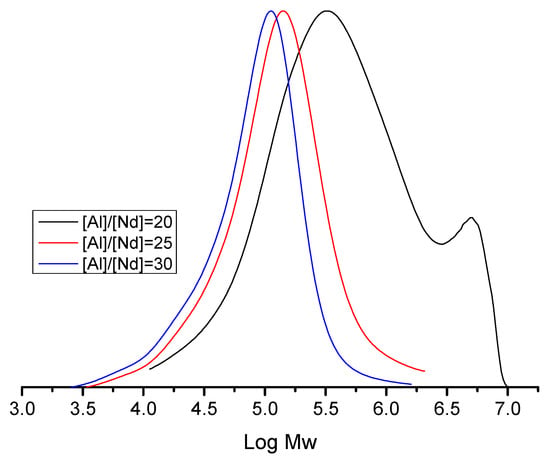
Figure 8.
Effect of the [Al]/[Nd] ratio on the average molecular weight (Log Mw) of polybutadiene.
Finally, it is also important to mention that in the experiments carried out at [M]/[Nd] = 4000, T = 70 °C, [Cl]/[Nd] = 1.0, and increasing the ratio of [Al]/[Nd] from 20 to 30, it was not possible to achieve the CCTP condition, since in none of the experiments was it possible to reach Ð values lower than 2.0. However, the experimental results show a tendency of Ð to decrease with an increasing [Al]/[Nd] ratio. This trend suggests that the CCTP condition also indirectly depends on the [M]/[Nd] ratio, since at higher monomer concentrations, a greater amount of cocatalysts will be consumed as a scavenger of the reaction medium.
3.3. Effect of [M]/[Nd] Ratio
The experiments conducted with increasing [M]/[Nd] ratios (Table 4) clearly demonstrate the pronounced effects on monomer yield, as well as molecular weight and kinetic properties. The observed decline in yield at higher monomer concentrations is attributed to a decreased number of active species present in the reaction medium. According to Equation (1), the polymerization rate depends directly on both the concentration of active species [Nd] and the initial monomer concentration [M]o. The reduced yield values, which appear to be governed primarily by the availability of active species [Nd], are explained by deactivation phenomena affecting these species, likely resulting from an increased level of impurities introduced with higher monomer concentrations. This deactivation phenomenon is supported by analyzing the behavior in the first 60 min of the reaction (Figure 9b). As can be observed in experiments Bd-9 and Bd-10 ([M]/[Nd] = 4000 and 7000 respectively), the linearity in the −ln (1 − x) vs. reaction time graph is lost after the first 60 min. This phenomenon, coupled with the low conversions obtained after long reaction periods (5 and 6 h, respectively), indicates a gradual deactivation of the active species during the course of the reaction.

Table 4.
Effect of the [M]/[Nd] ratio over butadiene polymerization kinetics and molar mass properties.
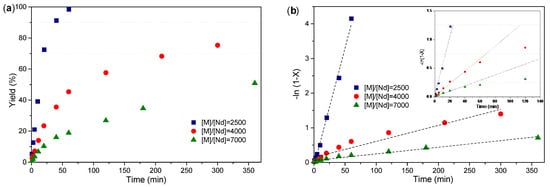
Figure 9.
(a,b) show the evolution of conversion with time and the linear form of the conversion–time, respectively, determined similarly as in the experiments carried out at different [M]/[Nd] ratios.
Regarding the kinetic parameters kp and ktr, the data indicate that although the number of active species decreases with rising monomer concentration, the polymerization rate—which depends on both kp and the initial monomer concentration—is primarily driven by [M]o. The escalation in ktr observed at higher monomer levels supports the conclusion that diminished competition in reversible chain transfer events between active species and the chain transfer agent (CTA) occurs under these conditions (Figure 10) [27].
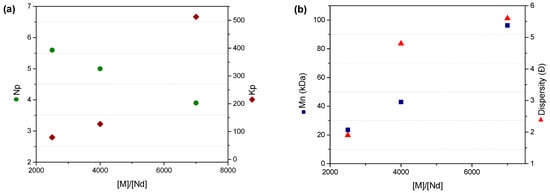
Figure 10.
(a) Effect of the ratio of [M]/[Nd] on the number of polymer chains growing by active species and polymerization rate constant; (b) effect of the ratio of [M]/[Nd] on the average molecular weight (Mn) and dispersion (Ð).
This interpretation is corroborated by GPC analysis (Figure 11) and the corresponding Mn values, which reveal a notable increase in Ð alongside an increase in molecular weight. These observations suggest a competitive interplay between deactivation of active centers and reversible polymerization and chain transfer reactions.
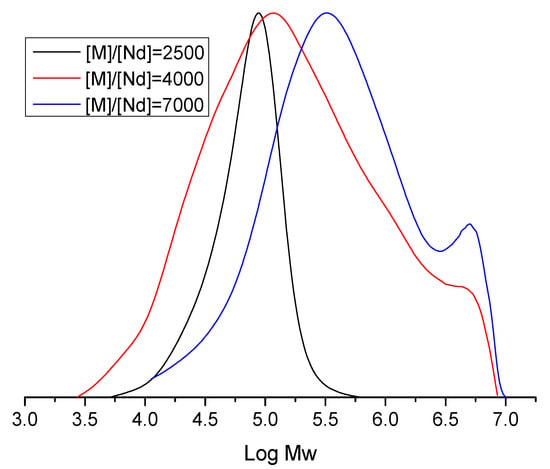
Figure 11.
Effect of the [M]/[Nd] ratio on the average molecular weight (Log Mw) of polybutadiene.
4. Conclusions
1,3-Butadiene was successfully polymerized via coordinative chain transfer polymerization (CCTP) using a ternary catalytic system composed of neodymium versatate (NdV3), diisobutylaluminum hydride [Al(i-Bu)2H], and dimethyldichlorosilane (Me2SiCl2). The catalytic system demonstrated high efficiency, consistently achieving polymer yields exceeding 80%.
Our findings establish that optimal CCTP conditions require the precise control of component ratios. A low [Cl]/[Nd] ratio is essential for achieving narrow dispersity (Ð < 2.0), while a ratio of [Cl]/[Nd] = 2.0 yields the maximum polymerization rate. However, increasing this ratio beyond optimal levels promotes the formation of insoluble, hyper-halogenated species characterized by high catalytic activity but short lifetimes, resulting in broad and multimodal molecular weight distributions that compromise the inherent advantage of CCTP.
The [Al]/[Nd] ratio significantly influences both polymerization kinetics and molecular weight control. Increasing this ratio from 20 to 30 markedly enhanced the monomer conversion and polymerization rate while simultaneously reducing both number-average molecular weight (Mn) and dispersity. This behavior reflects the dual role of DIBAH as both a chain transfer agent and cocatalyst, where higher concentrations favor chain transfer events over propagation.
Conversely, elevated [M]/[Nd] ratios proved detrimental to CCTP performance, resulting in decreased yields and increased values of both Mn and dispersity. This deterioration is attributed to the deactivation of active species by impurities introduced at higher monomer concentrations, highlighting the importance of maintaining low monomer-to-catalyst ratios for optimal performance.
Overall, the study demonstrates that effective CCTP conditions for synthesizing polybutadiene with controlled molecular weight (Mn < 35 kDa) and narrow molecular weight distribution (Ð < 2.0) can be achieved within a defined and narrow operational window: specifically, low [M]/[Nd] ratios (<2500) combined with optimized [Cl]/[Nd] ratios (1.0–2.0). Exceeding these critical thresholds leads to significant dispersity broadening and loss of the CCTP regime, underscoring the delicate balance required for maintaining controlled polymerization.
Author Contributions
Conceptualization, T.C., L.V., H.R.L.G. and R.D.d.L.; data curation, I.M., J.A.D.E. and J.L.G.Z.; formal analysis, J.L.G.Z. and T.C.; funding acquisition, H.R.L.G. and R.D.d.L.; investigation, J.L.G.Z.; methodology, I.M., M.R.L. and J.A.D.E.; project administration, H.R.L.G. and R.D.d.L.; resources, L.V. and R.D.d.L.; visualization, L.V. and H.R.L.G.; writing—original draft, H.R.L.G., J.L.G.Z. and T.C.; writing—review and editing, L.V., H.R.L.G. and R.D.d.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Secretariat of Science, Humanities, Technology, and Innovation (SECIHTI, Mexico) under project No. A1-S-34241, and by the Centro de Investigación en Química Aplicada (CIQA) through internal project 6752.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We acknowledge Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI) for the financial support provided for the completion of Córdova’s postdoctoral fellowship. We appreciate the technical support of Myrna Salinas Hernández, Ricardo Mendoza, and Maricela García for their technical support in chemical and thermal characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, H.; Tang, M.; Hao, Y.; Zhou, Z.; Sun, D.; Yu, P.; Wu, Y. Novel Polybutadiene Rubber with Long Cis-1,4 and Syndiotactic Vinyl Segments (CVBR) for High Performance Sidewall of All-Steel Giant off-the-Road Tire. Compos. B Eng. 2024, 275, 111349. [Google Scholar] [CrossRef]
- Méndez-Hernández, M.L.; Rivera-Armenta, J.L.; Páramo-García, U.; Corona Galvan, S.; García-Alamilla, R.; Salazar-Cruz, B.A. Synthesis of High Cis-1,4-BR with Neodymium for the Manufacture of Tires. Int. J. Polym. Sci. 2016, 2016, 7239540. [Google Scholar] [CrossRef]
- Mitina, I.I.; Mikhailov, S.I.; Fomina, A.A. New-Generation, High-Performance Tyre Rubbers: The Present and Future Market for Skd-Nd (Polybutadiene Rubber on a Neodymium Catalyst) and Dssk (Solution-Polymerisation Styrene–Butadiene Rubber). Int. Polym. Sci. Technol. 2013, 40, 5–9. [Google Scholar] [CrossRef]
- Scoti, M.; De Stefano, F.; Zanchin, G.; Leone, G.; De Rosa, C.; Ricci, G. Synthesis, Structure, and Properties of Poly(Isoprene)s of Different Constitutions and Configurations from Catalysts Based on Complexes of Nd, Co, and Fe. Macromolecules 2023, 56, 4629–4638. [Google Scholar] [CrossRef]
- Alexandro Valencia López, L.; Javier Enríquez-Medrano, F.; Maldonado Textle, H.; Soriano Corral, F.; Ricardo López González, H.; St Thomas, C.; Hernández Gámez, F.; Luis Olivares Romero, J.; Enrique Díaz de León Gómez, R. The Influence of Co-Catalyst in the Polymerization of 1,3-Butadiene Catalyzed by Neodymium Chloride Tripentanolate. J. Mex. Chem. Soc. 2016, 60, 141–147. [Google Scholar] [CrossRef]
- Niu, Q.; He, A.; Si, C.; Liu, G.; Xiao, M.; Chen, G.; Jin, M. Influence of Titanium Loading of TiCl4/MgCl2 Ziegler-Natta Catalyst on Trans-1,4 Polymerization of Isoprene. Polymer 2024, 307, 127317. [Google Scholar] [CrossRef]
- Guo, H.; Bi, J.; Wang, J.; Zhang, X.; Jiang, S.; Wu, Z. Correlation of Alkylaluminum Cocatalyst in Nd-Based Ternary Catalyst with the Polymerization Performance of Isoprene. Polymer 2017, 119, 176–184. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Windisch, H.; Obrecht, W. Polymerization of 1,3-Butadiene Initiated by Neodymium Versatate/Diisobutylaluminium Hydride/Ethylaluminium Sesquichloride: Kinetics and Conclusions About the Reaction Mechanism. Macromol. Chem. Phys. 2002, 203, 1055–1064. [Google Scholar] [CrossRef]
- Rocha, T.C.J.; Coutinho, F.M.B.; Soares, B.G. Effect of Alkylaluminum Structure on Ziegler-Natta Catalyst Systems Based on Neodymium for Producing High-Cis Polybutadiene. Polym. Bull. 2009, 62, 1–10. [Google Scholar] [CrossRef]
- Fischbach, A.; Perdih, F.; Sirsch, P.; Scherer, W.; Anwander, R. Rare-Earth Ziegler−Natta Catalysts: Carboxylate−Alkyl Interchange. Organometallics 2002, 21, 4569–4571. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-Based Ziegler/Natta Catalysts and Their Application in Diene Polymerization. In Neodymium Based Ziegler Catalysts—Fundamental Chemistry; Nuyken, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–154. ISBN 978-3-540-34811-5. [Google Scholar]
- Fan, C.; Bai, C.; Cai, H.; Dai, Q.; Zhang, X.; Wang, F. Preparation of High Cis-1,4 Polyisoprene with Narrow Molecular Weight Distribution via Coordinative Chain Transfer Polymerization. J. Polym. Sci. A Polym. Chem. 2010, 48, 4768–4774. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, C.; Hu, Y.; Jia, X.; Bai, C.; Zhang, X. Reversible Coordinative Chain Transfer Polymerization of Isoprene and Copolymerization with ε-Caprolactone by Neodymium-Based Catalyst. Polymer 2012, 53, 6027–6032. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Zheng, W.; Guo, J.; Zhang, C.; Zhao, L.; Zhang, H.; Hu, Y.; Bai, C.; Zhang, X. Fully-Reversible and Semi-Reversible Coordinative Chain Transfer Polymerizations of 1,3-Butadiene with Neodymium-Based Catalytic Systems. Polymer 2013, 54, 6716–6724. [Google Scholar] [CrossRef]
- Georges, S.; Touré, A.O.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Copolymerization and Terpolymerization of Conjugated Dienes. Macromolecules 2014, 47, 4538–4547. [Google Scholar] [CrossRef]
- Tang, Z.; Liang, A.; Liang, H.; Zhao, J.; Xu, L.; Zhang, J. Reversible Coordinative Chain Transfer Polymerization of Butadiene Using a Neodymium Phosphonate Catalyst. Macromol. Res. 2019, 27, 789–794. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Liu, H.; Hu, Y.; Zhang, X. Randomly Coordinative Chain Transfer Copolymerization of 1,3-Butadiene and Isoprene: A Highly Atom-Economic Way for Accessing Butadiene/Isoprene Rubber. Ind. Eng. Chem. Res. 2020, 59, 10754–10762. [Google Scholar] [CrossRef]
- Koyama Takahashi, M.F.; de Lima, M.; Polito, W.L. Molecular Weight Determination of HTPB Resins by Vapor Pressure Osmometry (VPO) and Gel Permeation Chromatography (GPC): The Effect of Calibration Standards. Polym. Bull. 1997, 38, 455–460. [Google Scholar] [CrossRef]
- Zinck, P.; Terrier, M.; Mortreux, A.; Visseaux, M. On the Number-Average Molecular Weight of Poly(1,4-Trans Isoprene) Determined by Conventional GPC. Polym. Test. 2009, 28, 106–108. [Google Scholar] [CrossRef]
- Liu, B.; Cui, D. Regioselective Chain Shuttling Polymerization of Isoprene: An Approach To Access New Materials from Single Monomer. Macromolecules 2016, 49, 6226–6231. [Google Scholar] [CrossRef]
- Barbotin, F.; Spitz, R.; Boisson, C. Heterogeneous Ziegler-Natta Catalyst Based on Neodymium for the Stereospecific Polymerization of Butadiene. Macromol. Rapid Commun. 2001, 22, 1411–1414. [Google Scholar] [CrossRef]
- Urazbaev, V.N.; Efimov, V.P.; Sabirov, Z.M.; Monakov, Y.B. Structure of Active Centers, Their Stereospecificity Distribution, and Multiplicity in Diene Polymerization Initiated by NdCl3-Based Catalytic Systems. J. Appl. Polym. Sci. 2003, 89, 601–603. [Google Scholar] [CrossRef]
- Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. Theoretical Study of the Halogen Concentration Effect on the 1,3-Butadiene Polymerization Catalyzed by the Neodymium-Based Ziegler–Natta System. Reactions 2024, 5, 753–764. [Google Scholar] [CrossRef]
- Masliy, A.N.; Akhmetov, I.G.; Kuznetsov, A.M.; Davletbaeva, I.M. DFT and ONIOM Simulation of 1,3-Butadiene Polymerization Catalyzed by Neodymium-Based Ziegler–Natta System. Polymers 2023, 15, 1166. [Google Scholar] [CrossRef]
- Quirk, R.P.; Kells, A.M.; Yunlu, K.; Cuif, J.-P. Butadiene Polymerization Using Neodymium Versatate-Based Catalysts: Catalyst Optimization and Effects of Water and Excess Versatic Acid. Polymer 2000, 41, 5903–5908. [Google Scholar] [CrossRef]
- González-Zapata, J.L.; Enríquez-Medrano, F.J.; López González, H.R.; Revilla-Vázquez, J.; Carrizales, R.M.; Georgouvelas, D.; Valencia, L.; Díaz de León Gómez, R.E. Introducing Random Bio-Terpene Segments to High Cis-Polybutadiene: Making Elastomeric Materials More Sustainable. RSC Adv. 2020, 10, 44096–44102. [Google Scholar] [CrossRef]
- Córdova, T.; Enríquez-Medrano, F.J.; Cartagena, E.M.; Villanueva, A.B.; Valencia, L.; Álvarez, E.N.C.; González, R.L.; Díaz-de-León, R. Coordinative Chain Transfer Polymerization of Sustainable Terpene Monomers Using a Neodymium-Based Catalyst System. Polymers 2022, 14, 2907. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).