Brazilian Amazon Orchids—Part III: Volatile Constituents of Floral Scents from Five Gongora Species and Their Chemometric and Chemotaxonomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Obtaining and Analyzing the Volatile Concentrates
2.3. Multivariate Statistical Analysis
3. Results and Discussion
3.1. Gongora Ruiz & Pav.
3.1.1. Gongora histrionica Rchb.f.

3.1.2. Gongora jauariensis Campacci & J.B.F.Silva

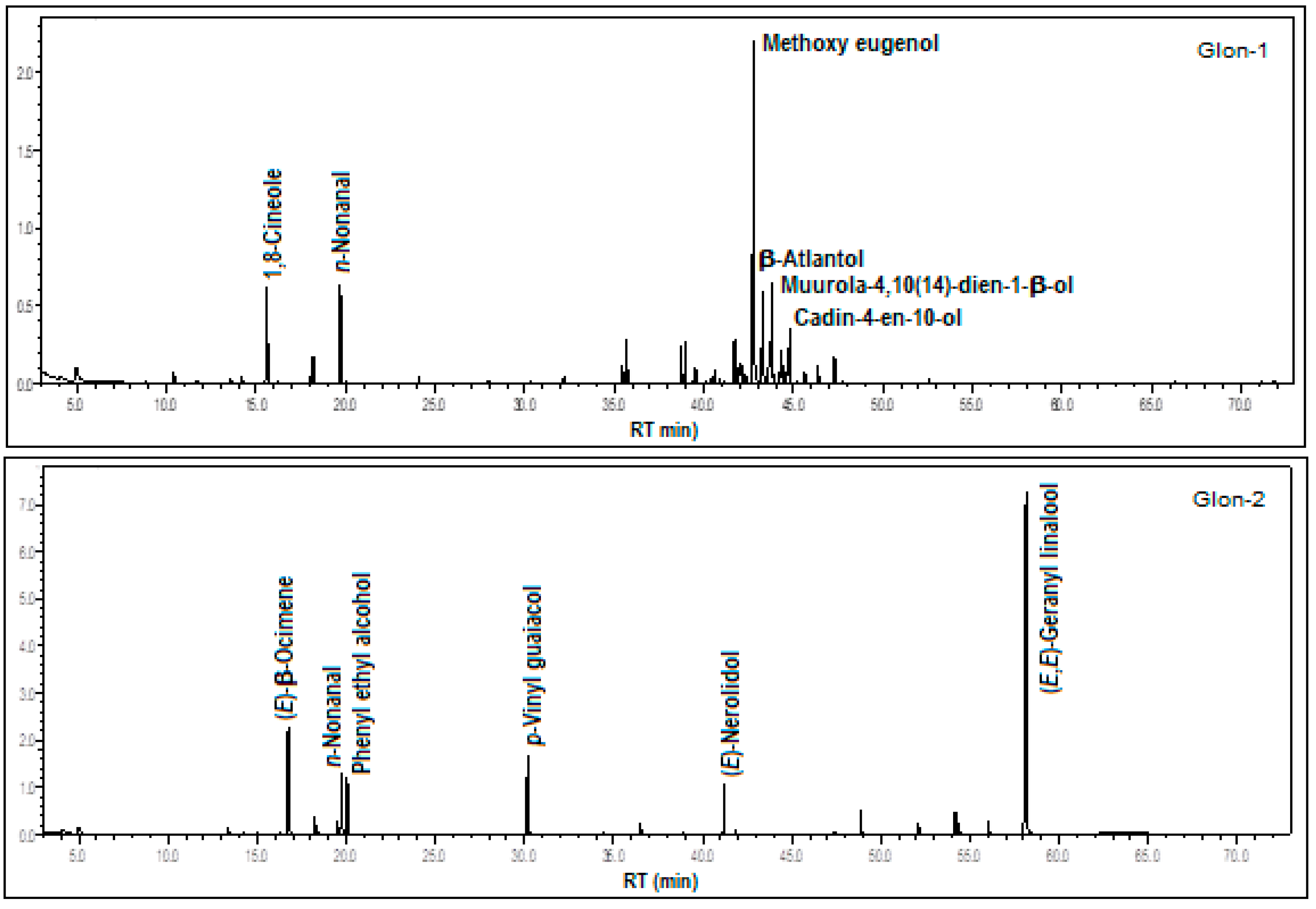
3.1.3. Gongora longiracemosa G.Gerlach & J.B.F.Silva

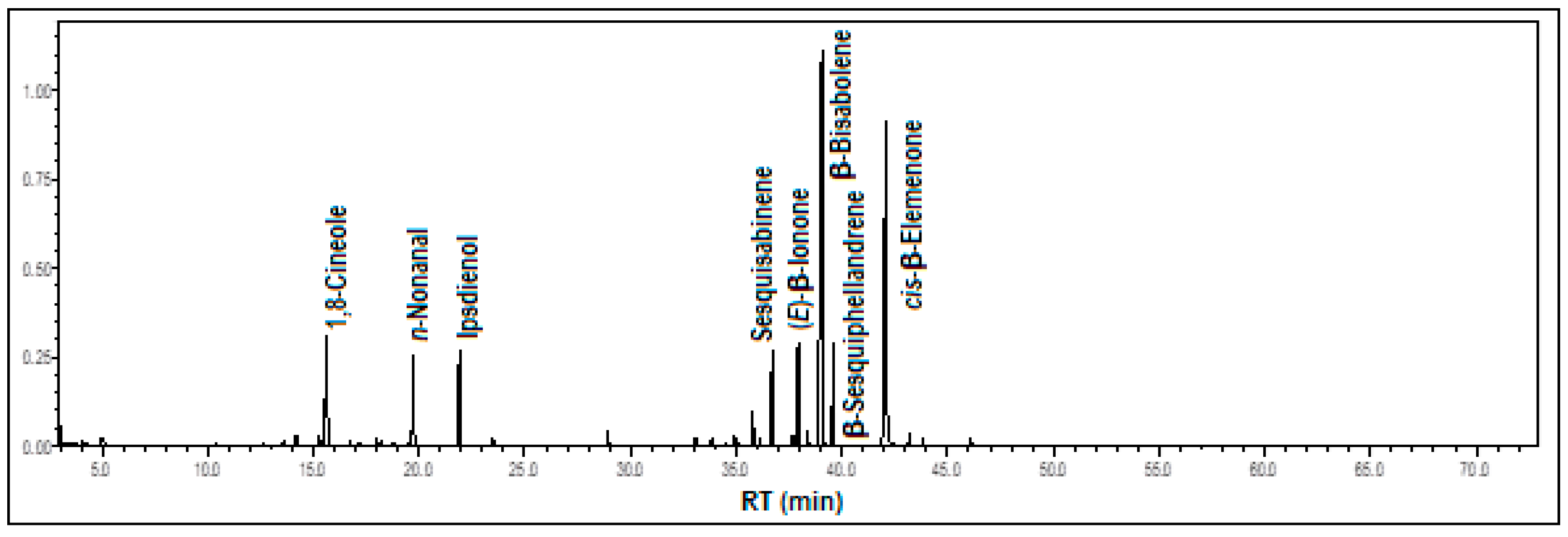
3.1.4. Gongora minax Rchb.f.

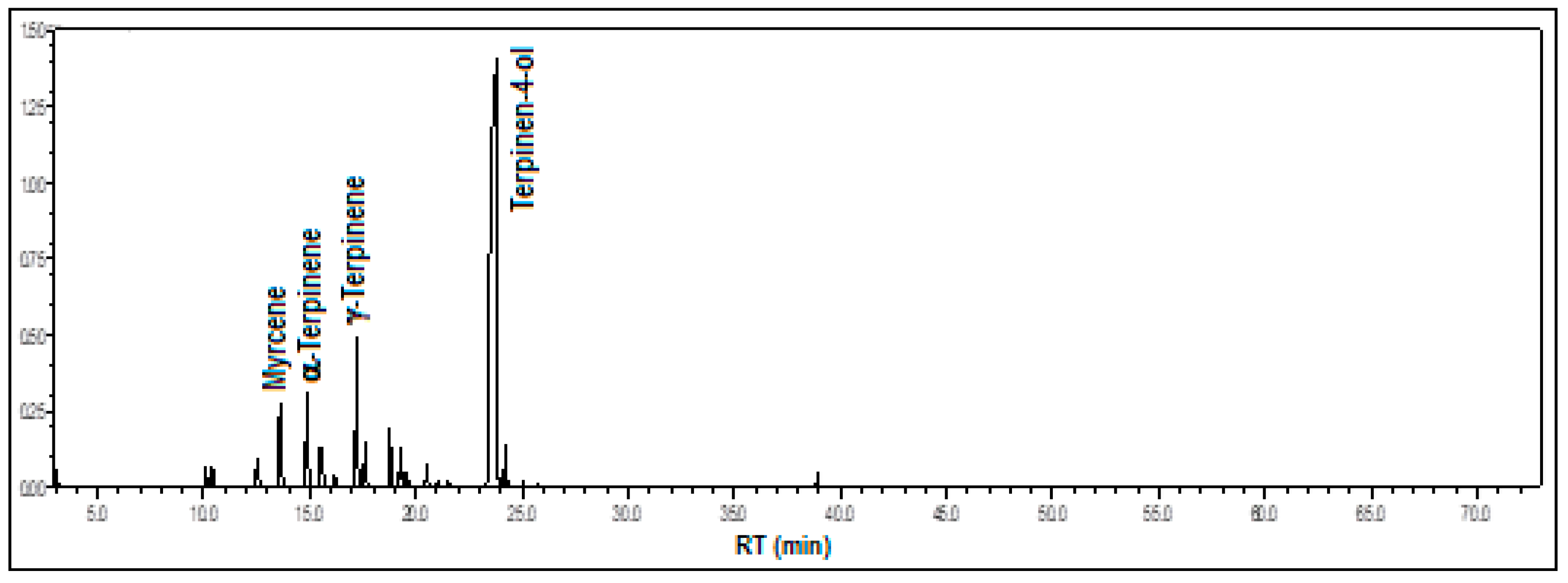
3.1.5. Gongora pleiochroma Rchb.f.

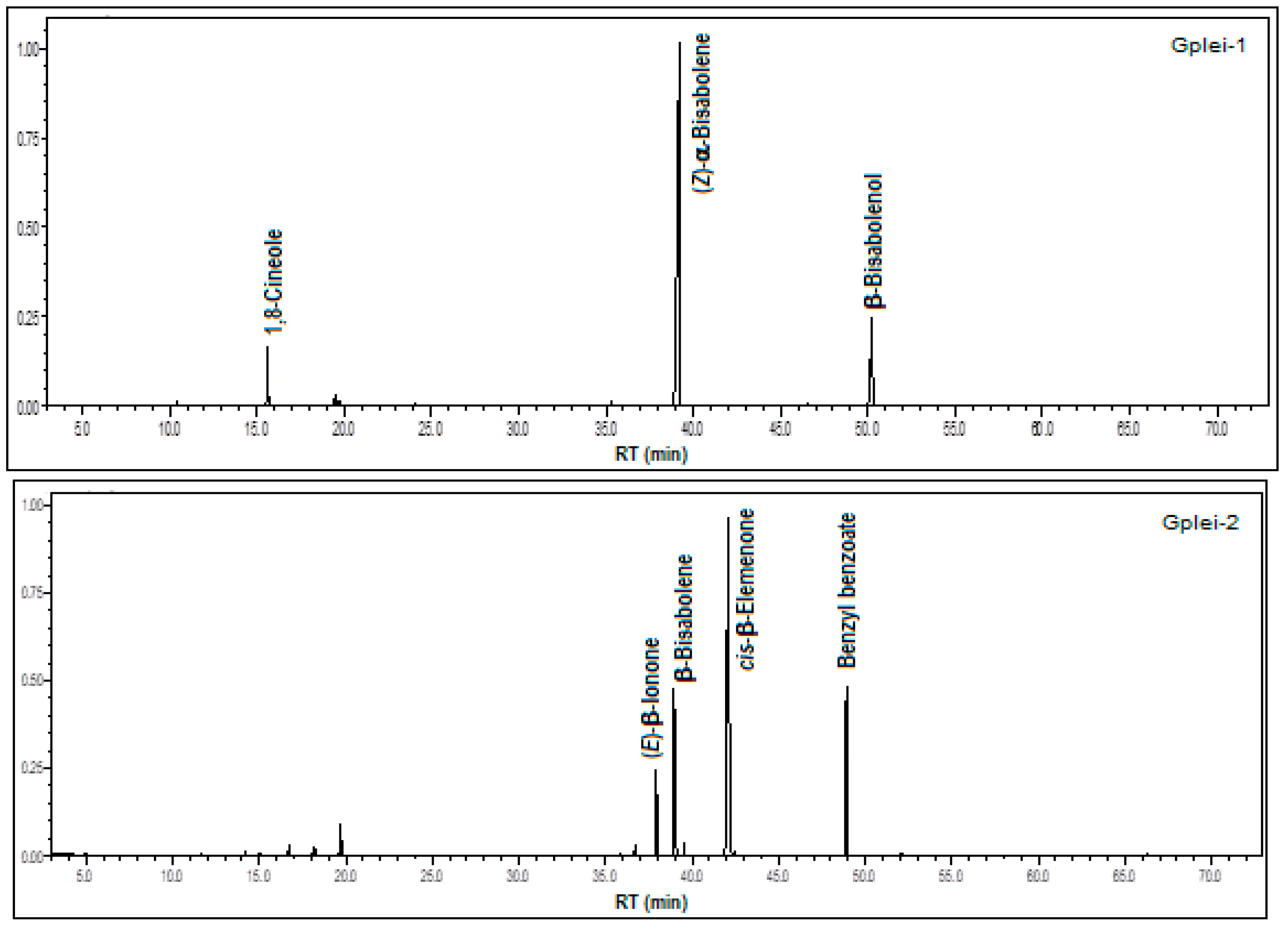
3.2. Floral Scent Chemistry of Gongora
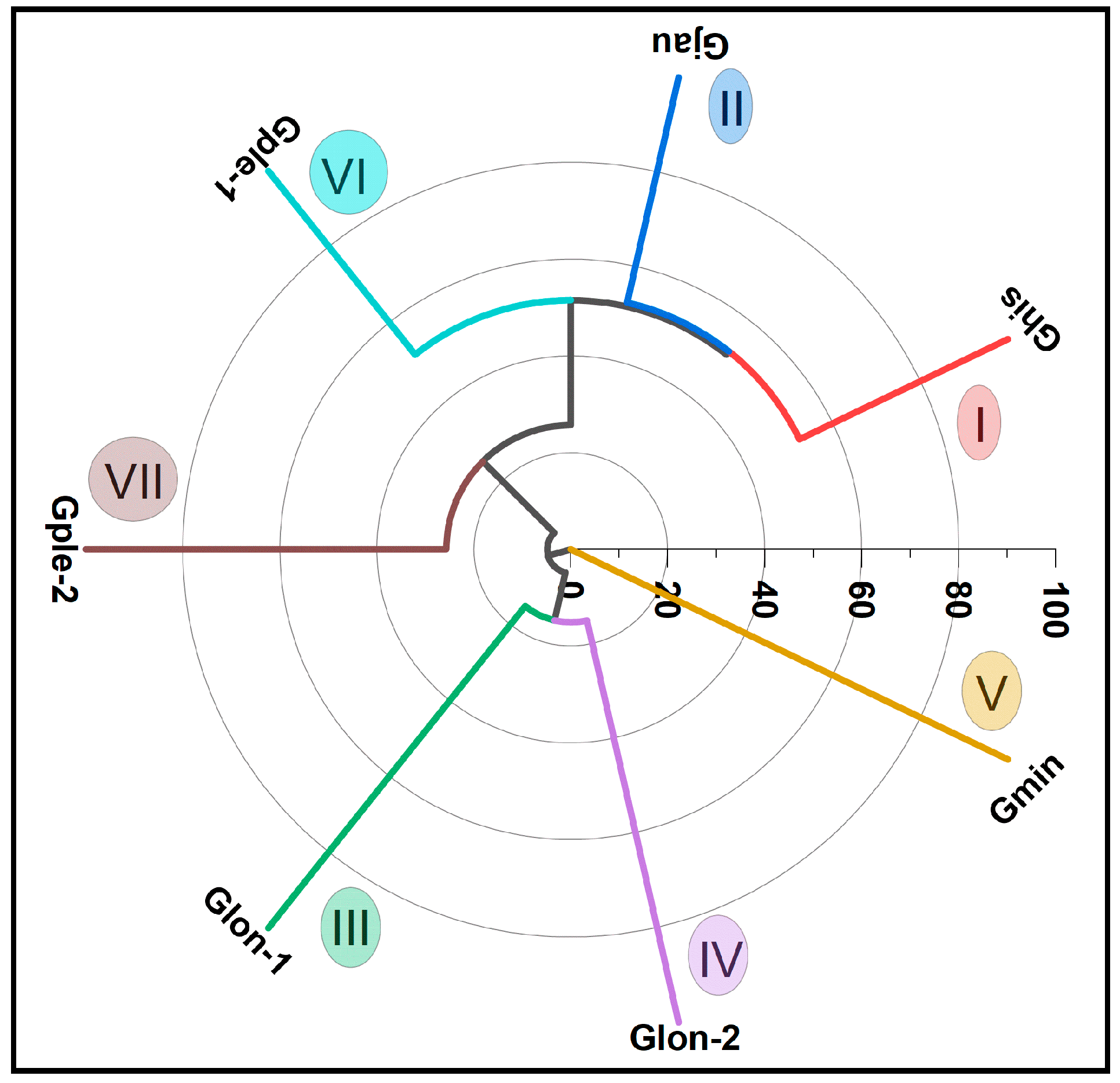
3.3. Gongora Specimens’ Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobson, H.E.M. Relationship between floral fragrance composition and type of pollinator. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Dodson, C.H.; Dressler, R.L.; Hills, H.G.; Adams, R.M.; Williams, N.H. Biologically Active compounds in orchid fragrances. Sciences 1969, 164, 1243–1249. [Google Scholar]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–633. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Kessler, D.; Gase, K.; Baldwin, I.T. Field experiments with transformed plants reveal the sense of floral scents. Science 2008, 321, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Delle-Vedore, R.; Juillet, N.; Bessiere, J.M.; Grison, C.; Barthes, N.; Pailler, T.; Dormont, L.; Schatz, B. Colour-scent associations in a tropical orchid: Three colours but two odours. Phytochemistry 2011, 72, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.Y.; Pan, Z.J.; Hsu, Z.Z.; Yang, Y.P.; Hsu, Y.C.; Chuang, Y.C.; Shih, H.H.; Chen, Y.H.; Tsai, W.C.; Chen, H.H. Research on Orchid Biology and Biotechnology. Plant Cell Physiol. 2011, 52, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. 2014. Available online: www.theplantlist.org/1.1/browse/A/Orchidaceae (accessed on 16 March 2024).
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van Den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Hetherington-Rauth, M.C.; Ramírez, S.R. Evolution and diversity of floral scent chemistry in the euglossine bee-pollinated orchid genus Gongora. Ann. Bot. 2016, 118, 135–148. [Google Scholar] [CrossRef]
- Zimmermann, Y.; Ramírez, S.R.; Eltz, T. Chemical niche differentiation among sympatric species of orchid bees. Ecology 2009, 90, 2994–3008. [Google Scholar] [CrossRef]
- BFG (The Brazilian Flora Group). Flora do Brasil 2020, Jardim Botânico do Rio de Janeiro, Rio de Janeiro. 2021. Available online: https://dspace.jbrj.gov.br/jspui/bitstream/doc/118/5/Flora%202020%20digital.pdf (accessed on 5 July 2024).
- de Vasconcelos, F.M.; Andrade, E.H.A.; Teixeira, L.O.A.; Maia, J.G.S. Volatile Constituents of Floral Scents from Encyclia cordigera (Kunth) Dressler and E. randii (Barb. Rodr.) Porto & Brade (Orchidaceae). J. Braz. Chem. Soc. 2022, 33, 96–101. [Google Scholar] [CrossRef]
- de Vasconcelos, F.M.; Andrade, E.H.A.; Teixeira, L.O.A.; Figueiredo, P.L.B.; Maia, J.G.S. Volatile Constituents from Catasetum (Orchidaceae) Species with Occurrence in the Brazilian Amazon. Plants 2023, 12, 703. [Google Scholar] [CrossRef]
- Likens, S.T.; Nickerson, G.B. Detection of certain hop oil constituents in brewing products. Am. Soc. Brew. Chem. Proc. 1964, 22, 5–13. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2—Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley and Sons Inc.: New York, NY, USA, 2011. [Google Scholar]
- Van de Doll, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Yoshida, D.S.; Rocha, A.E.S.; da Silva, M.F.F. Contribuição à taxonomia de Gongora Ruiz & Pavon (Orchidaceae) no estado do Pará, Brasil. Rev. Ciências Agrárias 2002, 37, 71–82. [Google Scholar]
- Missouri Botanical Garden. Available online: www.tropicos.org/Name/100440555 (accessed on 5 July 2024).
- Barros, F.; Vinhos, F.; Rodrigues, V.T.; Barberena, F.F.V.A.; Fraga, C.N.; Pessoa, E.M.; Foster, W.; Menini Neto, L.; Furtado, S.G.; Nardy, C.; et al. Orchidaceae. In Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brasil, 2015. Available online: https://floradobrasil2015.jbrj.gov.br/FB138888 (accessed on 5 July 2024).
- Barros, F.; Vinhos, F.; Rodrigues, V.T.; Barberena, F.F.V.A.; Fraga, C.N.; Pessoa, E.M.; Foster, W.; Menini Neto, L.; Furtado, S.G.; Nardy, C.; et al. Orchidaceae. In Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brasil, 2015. Available online: https://floradobrasil2015.jbrj.gov.br/FB118162 (accessed on 5 July 2024).
- Casique, J.V.; Andrade, E.H.A.; Dias, A.C.A.A.; Mastroberti, A.A. Novelties in the secretory structures of three species of Gongora (Orchidaceae: Stanhopeinae). Bot. J. Linn. Soc. 2021, 195, 650–670. [Google Scholar] [CrossRef]
- Campacci, M.A.; Bonke, E.; Carr, G.F., Jr.; da Silva, J.B.F.; Campacii, T.V.S.; Laitano, T.L. Coletânea de Orquídeas Brasileiras; Editora Brasil Orquídeas: Taubaté, SP, Brazil, 2021; Volume 17, 688p. [Google Scholar]
- Afonso, E.A.L.; Koch, A.K.; Costa, J.M. Flora preliminar de Orchidaceae no Município de Abaetetuba, Pará, Brasil. Biota Amaz. 2016, 6, 107–118. [Google Scholar] [CrossRef]
- The World Flora Online. Available online: https://worldfloraonline.org/taxon/wfo-0000974442 (accessed on 5 July 2024).
- Missouri Botanical Garden. Available online: www.tropicos.org/Name/23511026 (accessed on 5 July 2024).
- Gerlach, G.; Schill, R. Composition of orchid scents attracting euglossine bees. Bot. Acta 1991, 104, 379–391. [Google Scholar] [CrossRef]
- Kaiser, R. The Scent of Orchids: Olfactory and Chemical Investigations; Givaudan-Roure Research Ltd.: Dübendorf, Switzerland, 1993. [Google Scholar]
- Cancino, A.D.-M.; Damon, A. Fragrance analysis of euglossine bee pollinated orchids from Soconusco, south-east Mexico. Plant Species Biol. 2007, 22, 129–134. [Google Scholar] [CrossRef]
- Nunes, C.E.P.; Gerlach, G.; Bandeira, K.D.O.; Gobbo-Neto, L.; Pansarin, E.R.; Sazima, M. Two orchids, one scent? Floral volatiles of Catasetum cernnum and Gongora bufonia suggest convergent evolution to a unique polination niche. Flora 2017, 232, 207–216. [Google Scholar] [CrossRef]
- Engels, M.E.; Rocha, L.C.F.; Koch, A.K.; Geralch, G. O gênero Gongora (Orchidaceae, Stanhopeinae), no Estado de Mato Grosso, Brasil. Rodriguésia 2020, 71, e03132018. [Google Scholar] [CrossRef]
- Bisrat, D.; Jung, C. Roles of flower scent in bee–flower mediations: A review. J. Ecol. Environ. 2022, 46, 3. [Google Scholar] [CrossRef]
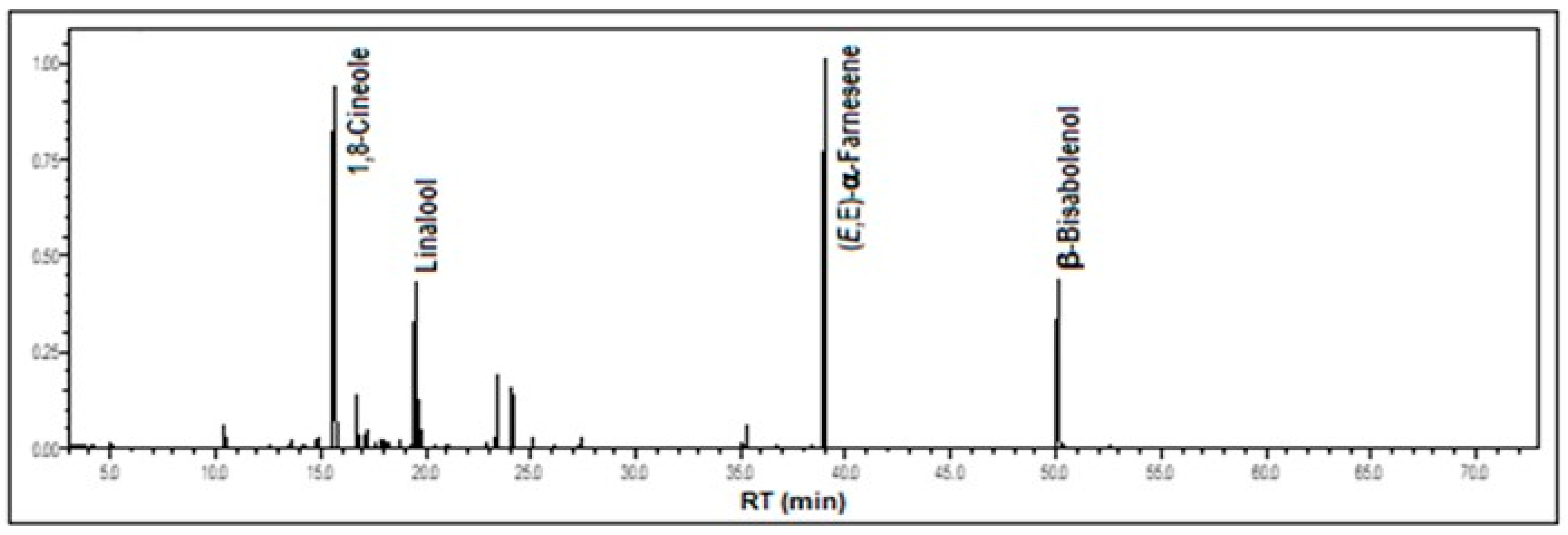

| Constituents (%) | RICalc | RILit | % |
|---|---|---|---|
| n-Octane | 796 | 800 a | 0.4 |
| α-Pinene | 928 | 932 a | 1.9 |
| β-Pinene | 970 | 974 a | 0.2 |
| Myrcene | 989 | 988 a | 0.5 |
| n-Octanal | 1000 | 998 a | 0.2 |
| α-Terpinene | 1013 | 1014 a | 0.6 |
| 1,8-Cineole | 1027 | 1026 a | 27.5 |
| (E)-β-Ocimene | 1047 | 1044 a | 2.9 |
| γ-Terpinene | 1056 | 1054 a | 1.1 |
| cis-Sabinene hydrate | 1063 | 1065 a | 0.3 |
| cis-Linalool oxide (furanoid) | 1069 | 1067 a | 0.5 |
| p-Cresol | 1074 | 1071 a | 0.3 |
| Terpinolene | 1085 | 1086 a | 0.3 |
| trans-Sabinene hydrate | 1094 | 1099 b | 0.2 |
| Linalool | 1099 | 1101 b | 9.8 |
| n-Nonanal | 1102 | 1100 b | 2.5 |
| cis-p-Menth-2-en-1-ol | 1117 | 1118 a | 0.1 |
| 2-vinyl-Anisole | 1128 | 1135 b | 0.1 |
| δ-Terpineol | 1163 | 1162 a | 0.2 |
| Terpinen-4-ol | 1174 | 1174 a | 3.7 |
| α-Terpineol | 1187 | 1186 a | 3.1 |
| trans-Carveol | 1218 | 1215 a | 0.4 |
| Nerol | 1226 | 1227 a | 0.1 |
| Geraniol | 1254 | 1249 a | 0.5 |
| (E)-Caryophyllene | 1418 | 1417 a | 0.2 |
| Undeca-2,4-dien-1 ol | 1424 | 1426 b | 1.0 |
| (E)-β-Farnesene | 1457 | 1454 a | 0.1 |
| α-Zingiberene | 1495 | 1493 a | 0.2 |
| (E,E)-α-Farnesene | 1511 | 1505 a | 29.3 |
| (E)-Nerolidol | 1563 | 1561 a | 0.1 |
| trans-β-Elemenone | 1598 | 1602 a | 0.1 |
| β-Bisabolenol | 1788 | 1789 a | 11.3 |
| Monoterpene hydrocarbons | 7.5 | ||
| Oxygenated monoterpenes | 46.4 | ||
| Sesquiterpene hydrocarbons | 29.8 | ||
| Oxygenated sesquiterpenes | 11.5 | ||
| Benzenoids/Phenylpropanoids | 0.4 | ||
| Fatty acid and derivatives | 4.1 | ||
| Total (%) | 99.7 | ||
| Constituents (%) | RICal | RILit | % |
|---|---|---|---|
| 2-methyl-3-Buten-2-ol acetate | 772 | 771 b | 0.3 |
| n-Octane | 796 | 800 a | 0.7 |
| α-Pinene | 928 | 932 a | 0.1 |
| β-Pinene | 970 | 974 a | 0.1 |
| Myrcene | 989 | 988 a | 0.3 |
| n-Octanal | 1000 | 998 a | 0.5 |
| p-Cymene | 1020 | 1020 a | 0.5 |
| 1,8-Cineole | 1026 | 1026 a | 7.2 |
| (E)-β-Ocimene | 1047 | 1044 a | 0.2 |
| γ-Terpinene | 1056 | 1054 a | 0.2 |
| n-Octanol | 1070 | 1063 a | 0.3 |
| p-Cresol | 1071 | 1072 a | 0.3 |
| Terpinolene | 1085 | 1086 a | 0.1 |
| Linalool | 1098 | 1095 a | 0.1 |
| n-Nonanal | 1102 | 1100 a | 4.2 |
| Ipsdienol | 1145 | 1140 a | 4.1 |
| Terpinen-4-ol | 1174 | 1174 a | 0.1 |
| Naphthalene | 1175 | 1178 a | 0.3 |
| Piperitone | 1250 | 1249 a | 0.1 |
| Safrole | 1285 | 1285 a | 0.6 |
| α-Copaene | 1375 | 1374 a | 0.3 |
| β-Elemene | 1391 | 1389 a | 0.3 |
| Sesquithujene | 1405 | 1405 a | 0.1 |
| cis-α-Bergamotene | 1414 | 1416 a | 0.3 |
| (E)-Caryophyllene | 1418 | 1417 a | 0.3 |
| trans-α-Bergamotene | 1435 | 1432 a | 1.7 |
| (Z)-β-Farnesene | 1443 | 1440 a | 0.3 |
| (E)-β-Farnesene | 1445 | 1452 a | 0.1 |
| α-Humulene | 1452 | 1452 a | 0.1 |
| Sesquisabinene | 1457 | 1457 a | 4.6 |
| γ-Curcumene | 1479 | 1481 a | 0.4 |
| (E)-β-Ionone | 1485 | 1487 a | 4.5 |
| α-Zingiberene | 1495 | 1493 a | 0.6 |
| β-Bisabolene | 1512 | 1508 a | 32.1 |
| (Z)-γ-Bisabolene | 1516 | 1514 a | 0.1 |
| β-Sesquiphellandrene | 1524 | 1521 a | 4.0 |
| (E)-Nerolidol | 1554 | 1561 a | 0.5 |
| cis-β-Elemenone | 1586 | 1589 a | 27.6 |
| Khusimone | 1594 | 1603 b | 0.1 |
| 1,10-di-epi-Cubenol | 1613 | 1618 a | 0.5 |
| α-Acorenol | 1630 | 1632 a | 0.3 |
| β-Bisabolol | 1670 | 1674 a | 0.1 |
| α-Bisabolol | 1685 | 1688 b | 0.1 |
| (Z)-α-trans-Bergamotol | 1688 | 1690 a | 0.3 |
| Monoterpene hydrocarbons | 1.5 | ||
| Oxygenated monoterpenes | 11.9 | ||
| Sesquiterpene hydrocarbons | 45.3 | ||
| Oxygenated sesquiterpenes | 34.0 | ||
| Benzenoids/Phenylpropanoids | 0.6 | ||
| Fatty acid derivatives | 6.3 | ||
| Total (%) | 99.6 | ||
| Constituents (%) | RICal | RILit | Glon-1 | Glon-2 |
|---|---|---|---|---|
| n-Octane | 796 | 800 a | 2.1 | 0.9 |
| α-Pinene | 928 | 932 a | 0.9 | - |
| 6-methyl-5-Hepten-2-one | 984 | 981 a | - | 0.7 |
| 2-pentyl-Furan | 989 | 984 a | 0.3 | |
| n-Octanal | 1000 | 998 a | 0.5 | 0.2 |
| 1,8-Cineole | 1026 | 1026 a | 8.5 | - |
| Benzene acetaldehyde | 1039 | 1036 a | - | 0.1 |
| (E)-β-Ocimene | 1048 | 1044 a | - | 11.4 |
| n-Octanol | 1070 | 1063 a | 0.5 | 0.1 |
| p-Cresol | 1074 | 1071 a | 2.2 | 2.0 |
| Camphenilone | 1078 | 1078 a | - | 0.3 |
| Linalool | 1098 | 1095 a | - | 1.2 |
| n-Nonanal | 1103 | 1100 a | 7.1 | 5.7 |
| Phenyl ethyl alcohol | 1109 | 1106 a | - | 5.7 |
| α-Terpineol | 1187 | 1186 a | 0.3 | - |
| p-vinyl-Guaiacol | 1311 | 1309 a | - | 7.0 |
| Eugenol | 1355 | 11,356 a | 0.4 | - |
| Methyl eugenol | 1403 | 1403 a | - | 0.1 |
| (E)-α-Bergamotene | 1428 | 1432 a | 1.0 | - |
| (γ)-Elemene | 1433 | 1434 a | 3.4 | - |
| Geranyl acetone | 1452 | 1453 a | - | 0.8 |
| α-Cuprenene | 1504 | 1505 a | 2.2 | - |
| Anisyl acetone | 1507 | 1501 b | - | 0.3 |
| (Z)-α-Bisabolene | 1509 | 1506 a | 2.7 | - |
| δ-Cadinene | 1523 | 1522 a | 0.9 | - |
| Occidentalol | 1545 | 1548 b | 0.2 | - |
| β-Vetivenene | 1547 | 1554 a | 1.0 | - |
| Elemicin | 1556 | 1555 a | 0.3 | - |
| (E)-Nerolidol | 1563 | 1561 a | 0.1 | 4.1 |
| Spathulenol | 1577 | 1577 a | 3.0 | - |
| Neryl isovalerate | 1580 | 1582 a | - | 0.2 |
| Caryophyllene oxide | 1583 | 1582 a | 1.7 | - |
| Thujopsan-2-α-ol | 1586 | 1586 a | 1.3 | - |
| Viridiflorol | 1591 | 1592 a | 1.3 | - |
| Methoxy eugenol | 1603 | 1600 b | 27.1 | - |
| β-Atlantol | 1606 | 1608 b | 7.1 | - |
| Eremoligenol | 1626 | 1627 b | 3.1 | - |
| Muurola-4,10(14)-dien-1-β-ol | 1629 | 1630 a | 7.2 | - |
| γ-Eudesmol | 1632 | 1632 b | 0.5 | - |
| Cubenol | 1642 | 1645 a | 2.7 | - |
| α-Muurolol (=Torreyol) | 1646 | 1651 b | 1.3 | - |
| Himachalol | 1650 | 1652 a | 0.5 | - |
| Cadin-4-en-10-ol | 1655 | 1659 b | 4.5 | - |
| Occidentalol acetate | 1676 | 1679 b | 0.6 | - |
| Eudesm-7(11)-en-4-ol | 1695 | 1700 a | 1.2 | - |
| γ-Curcumen-12-ol | 1718 | 1727 b | 1.7 | - |
| (2Z,6E)-Farnesol | 1720 | 1722 a | - | 0.3 |
| Benzyl benzoate | 1758 | 1759 a | - | 2.1 |
| Phenethyl benzoate | 1848 | 1856 b | - | 2.8 |
| (3Z)-Cambrene A | 1969 | 1967 b | - | 1.0 |
| (E,E)-Geranyl linalool | 2034 | 2026 a | - | 52.1 |
| Monoterpene hydrocarbons | 0.9 | 11.4 | ||
| Oxygenated monoterpenes | 11.0 | 4.8 | ||
| Sesquiterpene hydrocarbons | 11.2 | - | ||
| Oxygenated sesquiterpenes | 38.0 | 4.4 | ||
| Diterpene hydrocarbons | - | 1.0 | ||
| Oxygenated diterpenes | - | 52.1 | ||
| Benzenoids/Phenylpropanoids | 27.8 | 17.8 | ||
| Fatty acid and derivatives | 10.5 | 7.6 | ||
| Total (%) | 99.4 | 99.1 | ||
| Constituents (%) | RICal | RILit | % |
|---|---|---|---|
| α-Thujene | 922 | 924 a | 1.0 |
| α-Pinene | 928 | 932 a | 1.2 |
| Sabinene | 968 | 969 a | 1.5 |
| Myrcene | 989 | 988 a | 4.0 |
| α-Phellandrene | 1000 | 1002 a | 0.1 |
| α-Terpinene | 1013 | 1014 a | 4.3 |
| p-Cymene | 1020 | 1020 a | 0.1 |
| Limonene | 1025 | 1024 a | 1.6 |
| 1,8-Cineole | 1026 | 1026 a | 1.0 |
| (Z)-β-Ocimene | 1037 | 1032 | 0.4 |
| (E)-β-Ocimene | 1047 | 1044 a | 0.1 |
| γ-Terpinene | 1056 | 1054 a | 6.3 |
| cis-Sabinene hydrate | 1063 | 1065 a | 2.1 |
| p-Cresol | 1079 | 1071 a | 0.1 |
| Terpinolene | 1085 | 1086 a | 2.2 |
| trans-Sabinene hydrate | 1094 | 1098 a | 1.9 |
| Linalool | 1098 | 1095 a | 0.7 |
| n-Nonanal | 1102 | 1100 a | 0.2 |
| cis-p-Menth-2-en-1-ol | 1118 | 1118 a | 1.1 |
| allo-Ocimene | 1128 | 1128 a | 0.2 |
| trans-p-Menth-2-en-1-ol | 1137 | 1136 a | 0.3 |
| Terpinen-4-ol | 1181 | 1174 a | 67.2 |
| α-Terpineol | 1189 | 1186 a | 1.1 |
| cis-Piperitol | 1193 | 1195 a | 0.2 |
| trans-Piperitol | 1205 | 1207 a | 0.2 |
| Geraniol | 1254 | 1249 a | 0.1 |
| (E,E)-α-Farnesene | 1508 | 1505 a | 0.4 |
| Monoterpene hydrocarbons | 23.0 | ||
| Oxygenated monoterpenes | 76.0 | ||
| Sesquiterpene hydrocarbons | 0.4 | ||
| Fatty acid and derivatives | 0.2 | ||
| Total (%) | 99.6 | ||
| Constituents | RICal | RILit | Gple-1 | Gple-2 |
|---|---|---|---|---|
| n-Hexanal | 796 | 801 a | - | 0.4 |
| α-Pinene | 928 | 932 a | 0.6 | - |
| Myrcene | 989 | 988 a | 0.1 | - |
| n-Octanal | 1000 | 998 a | - | 0.3 |
| p-methyl-Anisole | 1016 | 1015 a | - | 0.2 |
| 1,8-Cineole | 1027 | 1026 a | 5.9 | - |
| (E)-β-Ocimene | 1047 | 1044 a | - | 0.9 |
| n-Octanol | 1070 | 1063 a | - | 0.2 |
| p-Cresol | 1074 | 1071 b | - | 0.8 |
| Linalool | 1098 | 1095 a | 1.0 | 0.1 |
| n-Nonanal | 1102 | 1100 a | 0.5 | 2.5 |
| Terpinen-4-ol | 1174 | 1174 a | 0.1 | - |
| α-Terpineol | 1187 | 1186 a | 0.3 | - |
| Linalool butanoate | 1424 | 1421 a | 0.3 | - |
| trans-α-Bergamotene | 1433 | 1432 a | - | 0.1 |
| Neryl acetone | 1436 | 1434 a | - | 0.1 |
| dihydro-β-Ionone | 1438 | 1434 a | - | 0.1 |
| (E)-β-Farnesene | 1457 | 1454 a | 0.1 | 0.9 |
| γ-Curcumene | 1479 | 1481 a | - | 0.2 |
| (E)-β-Ionone | 1485 | 1487 a | - | 6.5 |
| α-Zingiberene | 1495 | 1493 a | - | 0.1 |
| β-Bisabolene | 1509 | 1505 a | - | 12.4 |
| (Z)-α-Bisabolene | 1512 | 1506 a | 75.6 | 1.3 |
| β-Sesquiphellandrene | 1524 | 1521 a | - | 0.8 |
| (E)-Nerolidol | 1564 | 1561 a | 0.1 | - |
| cis-β-Elemenone | 1587 | 1589 a | - | 55.2 |
| Fokienol | 1594 | 1596 a | - | 0.3 |
| Elemol acetate | 1682 | 1680 a | - | 0.1 |
| (2Z,6Z)-Farnesol | 1700 | 1698 a | 0.2 | - |
| Benzyl benzoate | 1759 | 1759 a | - | 15.6 |
| β-Bisabolenol | 1792 | 1790 a | 14.6 | - |
| Phenyl ethyl octanoate | 1848 | 1846 a | 0.1 | 0.3 |
| Tricosane | 2300 | 2300 a | - | 0.1 |
| Monoterpene hydrocarbons | 0.7 | 0.9 | ||
| Oxygenated monoterpenes | 7.6 | 1.2 | ||
| Sesquiterpene hydrocarbons | 75.7 | 15.8 | ||
| Oxygenated sesquiterpenes | 14.9 | 62.2 | ||
| Benzenoids/Phenylpropanoids | 0.1 | 15.9 | ||
| Fatty acid and derivatives | 0.5 | 3.5 | ||
| Total | 99.5 | 99.5 | ||
| Constituents (%) | Ghis | Gjau | Glon-1 | Glon-2 | Gmin | Gple-1 | Gple-2 |
|---|---|---|---|---|---|---|---|
| 1,8-Cineole | 27.5 | 7.2 | 8.5 | - | - | 5.9 | - |
| (E)-β-Ocimene | - | - | - | 11.4 | - | - | - |
| Myrcene | - | - | - | - | 4.0 | - | - |
| α-Terpinene | - | - | - | - | 4.3 | - | - |
| γ-Terpinene | - | - | - | - | 6.3 | - | - |
| Linalool | 9.8 | - | - | - | - | - | - |
| n-Nonanal | - | 4.2 | 7.1 | 5.7 | - | - | - |
| Ipsidienol | - | 4.1 | - | - | - | - | - |
| Phenyl ethyl alcohol | - | - | - | 5.7 | - | - | - |
| Terpinen-4-ol | - | - | - | - | 67.2 | - | - |
| p-Vinyl guaiacol | - | - | - | 7.0 | - | - | - |
| Sesquisabinene | - | 4.6 | - | - | - | - | - |
| (E)-β-Ionone | - | 4.5 | - | - | - | - | 6.5 |
| (E,E)-α-Farnesene | 29.3 | - | - | - | - | - | - |
| β-Bisabolene | - | 32.1 | - | - | - | - | 12.4 |
| β-Sesquiphellandrene | - | 4.0 | - | - | - | - | - |
| (E)-Nerolidol | - | - | - | 4.1 | - | - | - |
| (Z)-α-Bisabolene | - | - | - | - | - | 75.6 | - |
| cis-β-Elemenone | - | 27.6 | - | - | - | - | 55.2 |
| Methoxy eugenol | - | - | 27.1 | - | - | - | - |
| β-Atlantol | - | - | 7.1 | - | - | - | - |
| Muurola-4,10(14)-dien-1-β-ol | - | - | 7.2 | - | - | - | - |
| Cadin-4-en-10-ol | - | - | 4.5 | - | - | - | - |
| Benzyl benzoate | - | - | - | - | - | - | 15.6 |
| β-Bisabolenol | 11.3 | - | - | - | - | 14.6 | - |
| (E,E)-Geranyl linalool | - | - | - | 52.1 | - | - | - |
| Monoterpene hydrocarbons (MH) | - | - | - | 11.4 | 14.6 | - | - |
| Oxygenated monoterpenes (OM) | 37.3 | 11.3 | 8.5 | - | 67.2 | 5.9 | - |
| Sesquiterpene hydrocarbons (SH) | 29.3 | 40.7 | - | - | - | 75.6 | 12.4 |
| Oxygenated sesquiterpenes (OS) | 11.3 | 32.1 | 18.8 | 4.1 | - | 14.6 | 61.7 |
| Benzenoids/phenylpropanoids (BP) | - | - | 27.1 | 12.7 | - | - | 15.6 |
| Fatty acid and derivatives (FA) | - | 4.2 | 7.1 | 5.7 | - | - | - |
| Oxygenated diterpenes (OD) | - | - | - | 52.1 | - | - | - |
| Total (%) | 77.9 | 88.3 | 61.5 | 86.0 | 81.8 | 96.1 | 89.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vasconcelos, F.M.; Andrade, E.H.A.; de Figueiredo, R.O.; Teixeira, L.O.A.; Figueiredo, P.L.B.; Maia, J.G.S. Brazilian Amazon Orchids—Part III: Volatile Constituents of Floral Scents from Five Gongora Species and Their Chemometric and Chemotaxonomic Analysis. Processes 2025, 13, 2950. https://doi.org/10.3390/pr13092950
de Vasconcelos FM, Andrade EHA, de Figueiredo RO, Teixeira LOA, Figueiredo PLB, Maia JGS. Brazilian Amazon Orchids—Part III: Volatile Constituents of Floral Scents from Five Gongora Species and Their Chemometric and Chemotaxonomic Analysis. Processes. 2025; 13(9):2950. https://doi.org/10.3390/pr13092950
Chicago/Turabian Stylede Vasconcelos, Franciléia M., Eloisa Helena A. Andrade, Raphael O. de Figueiredo, Luiz Otávio A. Teixeira, Pablo Luis B. Figueiredo, and José Guilherme S. Maia. 2025. "Brazilian Amazon Orchids—Part III: Volatile Constituents of Floral Scents from Five Gongora Species and Their Chemometric and Chemotaxonomic Analysis" Processes 13, no. 9: 2950. https://doi.org/10.3390/pr13092950
APA Stylede Vasconcelos, F. M., Andrade, E. H. A., de Figueiredo, R. O., Teixeira, L. O. A., Figueiredo, P. L. B., & Maia, J. G. S. (2025). Brazilian Amazon Orchids—Part III: Volatile Constituents of Floral Scents from Five Gongora Species and Their Chemometric and Chemotaxonomic Analysis. Processes, 13(9), 2950. https://doi.org/10.3390/pr13092950







