Performance Evaluation and Kinetic Analysis of an Iron Ore as Oxygen Carrier in Chemical Looping Combustion
Abstract
1. Introduction
2. Experimental Section
2.1. Material
2.2. Experimental Setup and Procedures
2.3. Experimental Data Processing
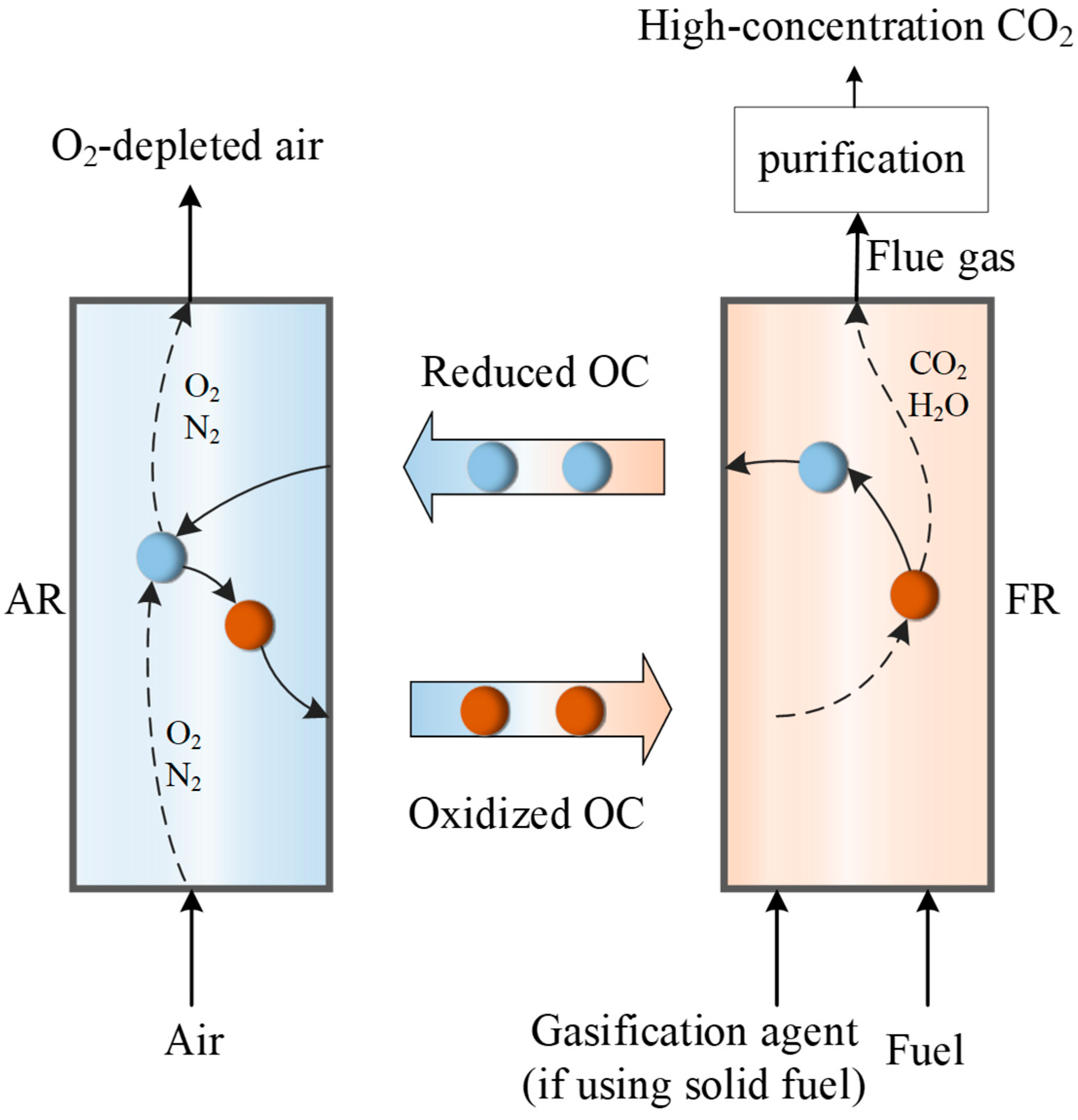
3. CLC Performance of the Iron Ore as Oxygen Carrier
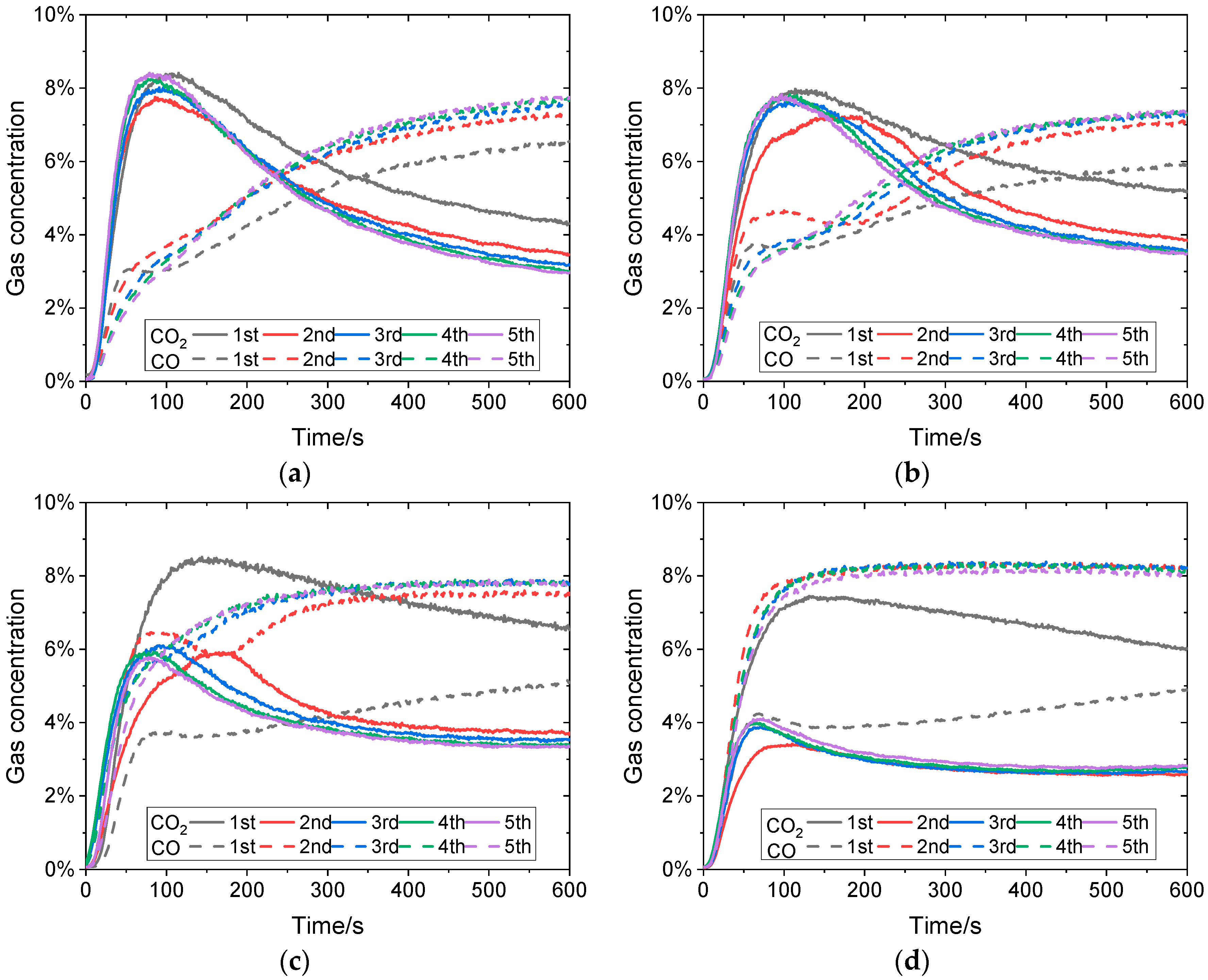
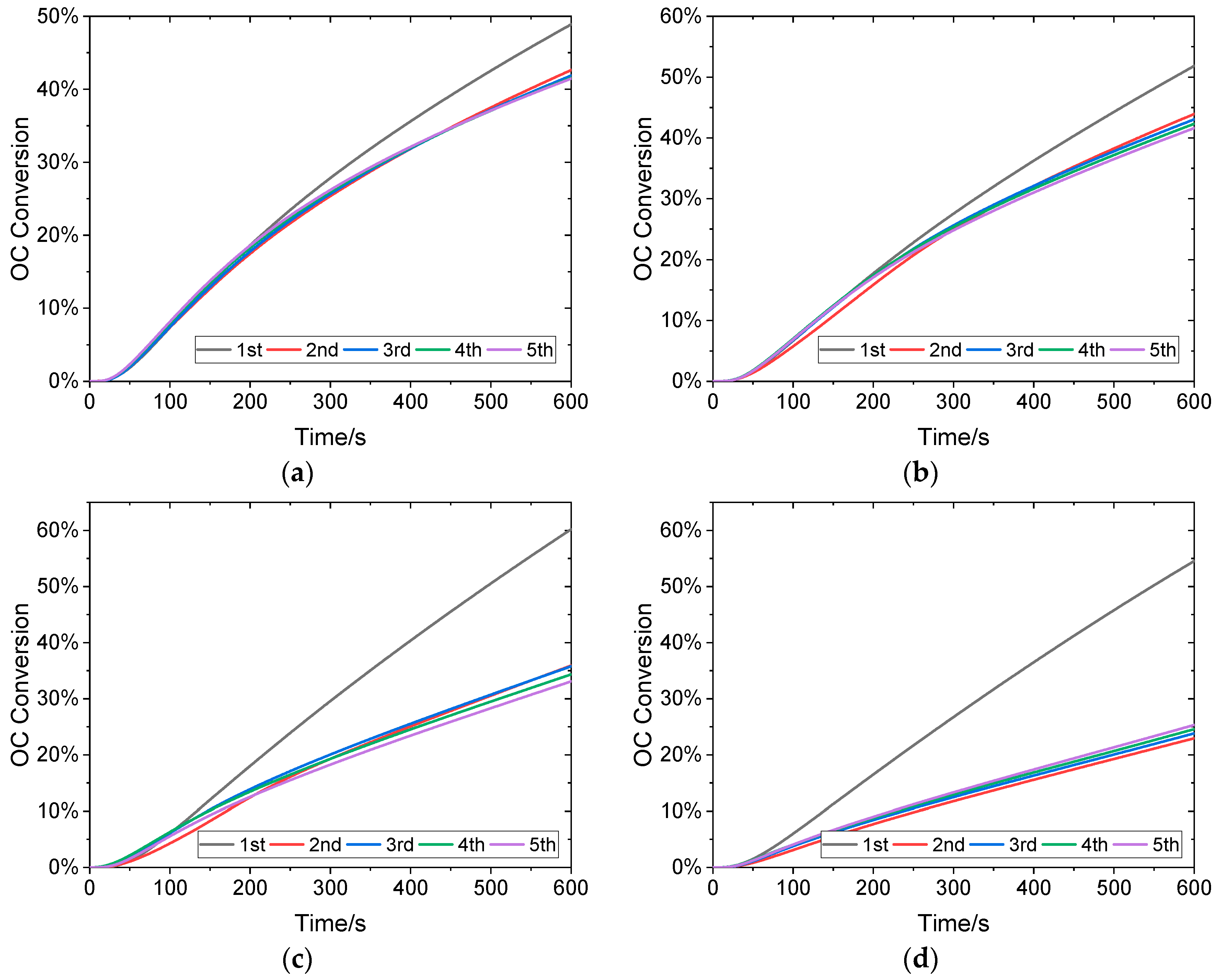
3.1. Effects of Reaction Temperature
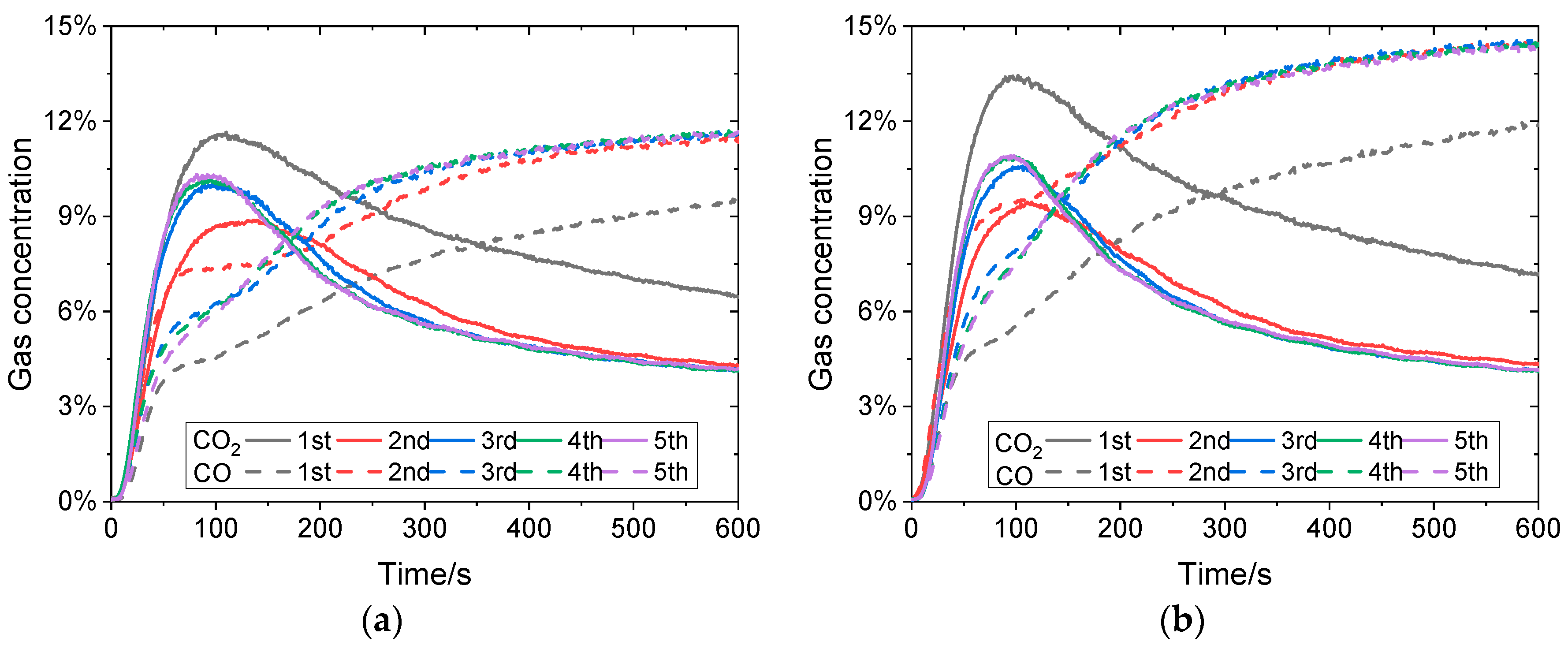
3.2. Effects of CO Concentration
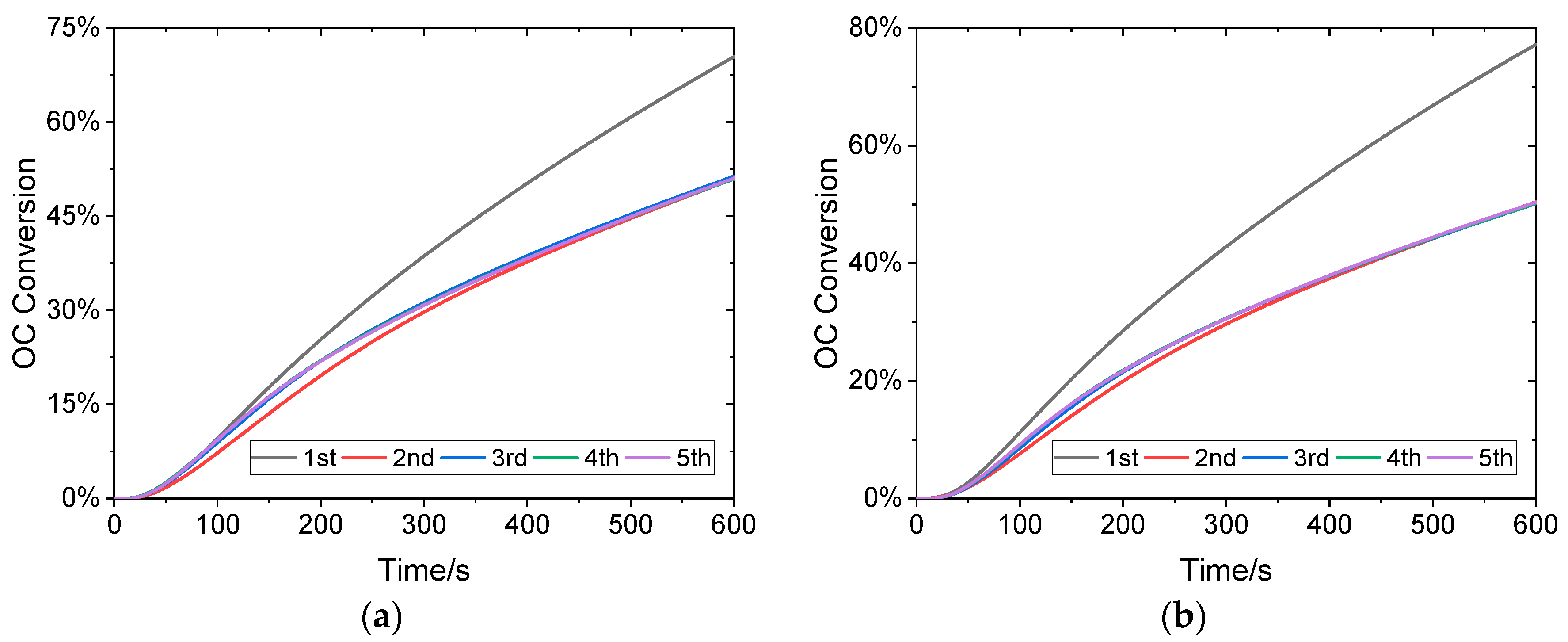
3.3. Self-Activation of Sintered OC
4. Kinetic Analysis
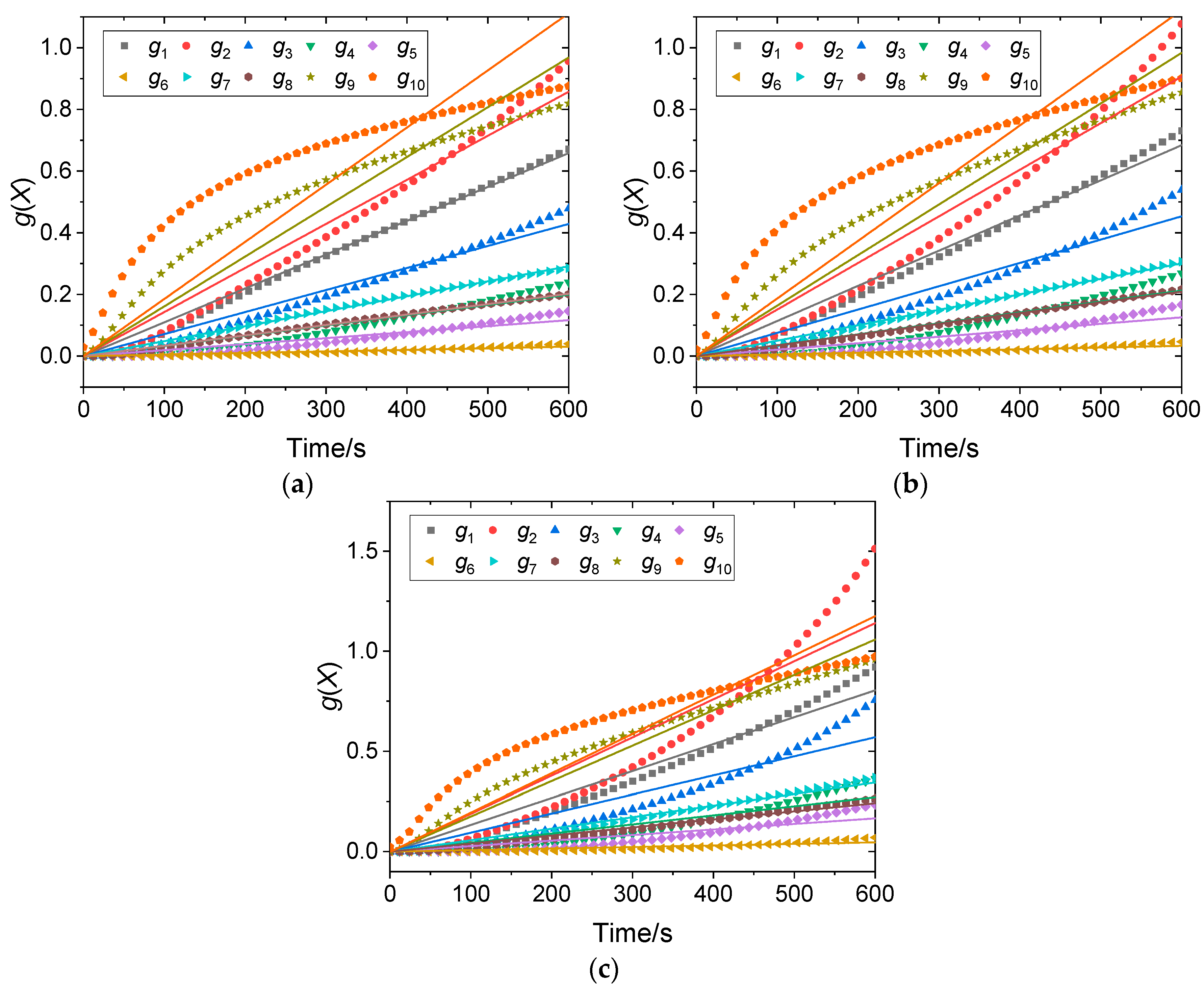
4.1. Model Fitting Method
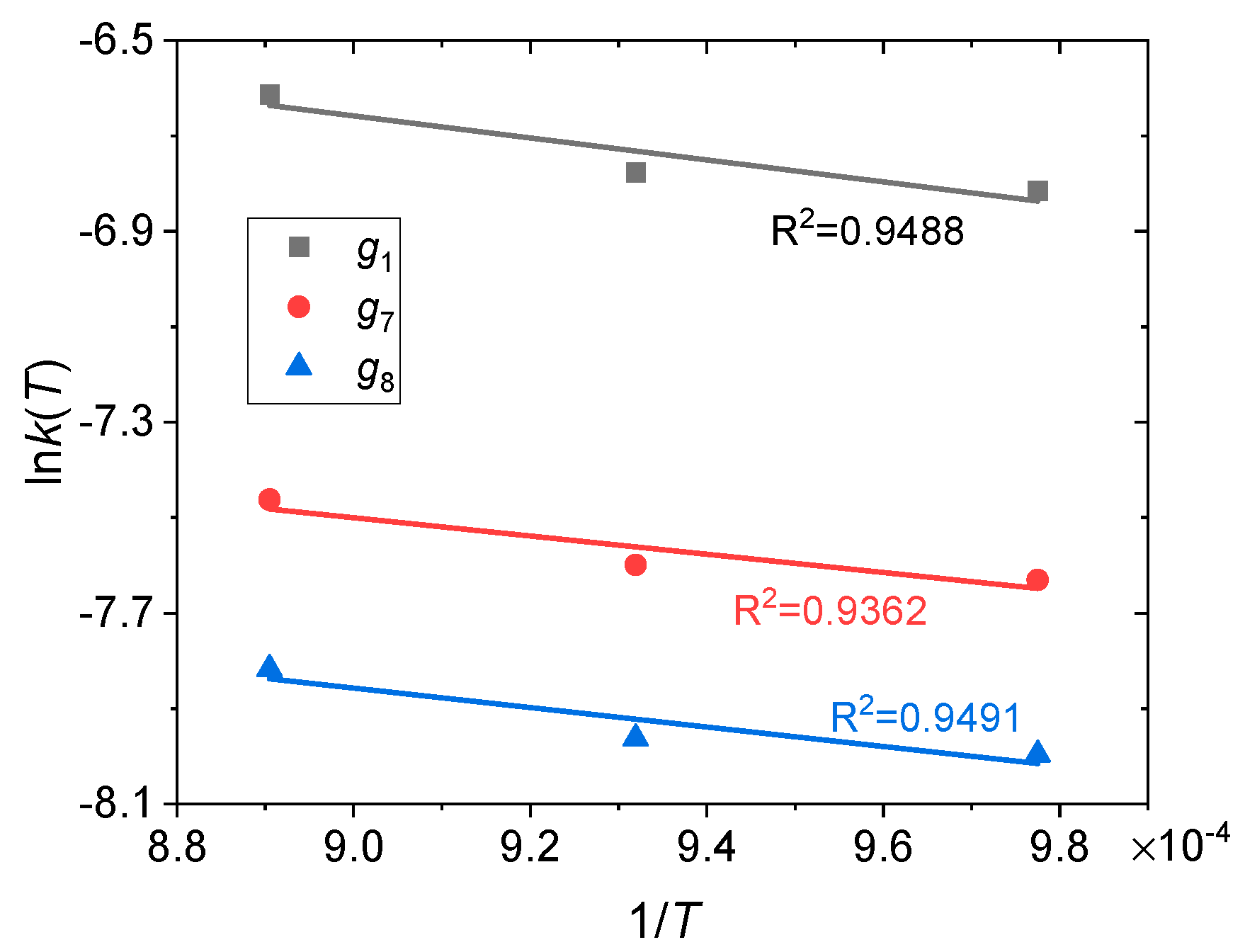
4.2. Determination of Kinetic Parameters
5. Conclusions
- (1)
- Increasing the reaction temperature from 750 °C to 850 °C enhances the initial OC conversion, whereas operating at 900 °C instigates swift potential sintering, as indicated by a pronounced drop in CO2 yield.
- (2)
- High CO concentration not only expedites the initial conversion but also intensifies sintering. This reaffirms the imperative for meticulous control over reduction depth.
- (3)
- A notable self-activation effect was noted in sintered OC during prolonged cycling at 900 °C with 20% CO, which led to a gradual increase in conversion from 32.07% to 37.33% over 15 cycles.
- (4)
- Kinetic modeling confirms that the reduction of this OC adheres to a contracting volume model or first-order kinetic mechanism, characterized by an apparent activation energy of 15.93–19.13 kJ mol−1.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seneviratne, S.I.; Rogelj, J.; Séférian, R.; Wartenburger, R.; Allen, M.R.; Cain, M.; Millar, R.J.; Ebi, K.L.; Ellis, N.; Hoegh-Guldberg, O.; et al. The many possible climates from the Paris Agreement’s aim of 1.5 °C warming. Nature 2018, 558, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Boon, J. Sorption-enhanced reactions as enablers for CO2 capture and utilisation. Curr. Opin. Chem. Eng. 2023, 40, 100919. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.S. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef]
- Matthews, H.D.; Wynes, S. Current global efforts are insufficient to limit warming to 1.5 °C. Science 2022, 376, 1404–1409. [Google Scholar] [CrossRef]
- Tollefson, J. Earth breaches 1.5 °C climate limit for the first time: What does it mean? Nature 2025, 637, 769–770. [Google Scholar] [CrossRef]
- International Energy Agency. Global Energy Review 2025. Available online: www.iea.org/reports/global-energy-review-2025/co2-emissions (accessed on 8 August 2025).
- Hong, F.; Qi, Y.; Yang, Z.; Yu, L.; Guang, X.; Diao, J.; Sun, B.; Liu, H. Recent advances of CO2 hydrogenation to methanol. DeCarbon 2025, 8, 100111. [Google Scholar] [CrossRef]
- Wang, F.; Guan, D.; Wu, C.; Zhang, X.; Wang, G. Numerical study on induction heating enhanced methanol steam reforming for hydrogen production. DeCarbon 2024, 6, 100075. [Google Scholar] [CrossRef]
- Dou, B.; Song, Y.; Liu, Y.; Feng, C. High temperature CO2 capture using calcium oxide sorbent in a fixed-bed reactor. J. Hazard. Mater. 2010, 183, 759–765. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in carbon capture technologies: A review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgaragy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Kong, Z.; Shao, Y.; Jin, B. Auto-thermal operation and optimization of coal-fueled separated gasification chemical looping combustion in a pilot-scale unit. Chem. Eng. J. 2020, 383, 123159. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A. Chemical-looping combustion: Status and research needs. Proc. Combust. Inst. 2019, 37, 4303–4317. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; Diego, L.F.; García-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Jin, B. Spatiotemporal statistical characteristics of multiphase flow behaviors in fuel reactor for separated-gasification chemical looping combustion of solid fuel. Chem. Eng. J. 2021, 412, 128575. [Google Scholar] [CrossRef]
- Lyngfelt, A. 11—Oxygen carriers for chemical-looping combustion. In Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture; Woodhead Publishing: Cambridge, UK, 2015; pp. 221–254. [Google Scholar] [CrossRef]
- Mendiara, T.; Pérez, R.; Abad, A.; Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Low-Cost Fe-Based Oxygen Carrier Materials for the iG-CLC Process with Coal. 1. Ind. Eng. Chem. Res. 2012, 51, 16216–16229. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, Y.; Lin, X.; Wang, B.; Lyu, Q. Research Progress and Perspectives of Solid Fuels Chemical Looping Reaction with Fe-Based Oxygen Carriers. Energy Fuels 2022, 36, 13956–13984. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Driessche, I.V.; Snijkers, F.; Verberckmoes, A. Optimization of spray dried attrition-resistant iron based oxygen carriers for chemical looping reforming. Chem. Eng. J. 2017, 309, 824–839. [Google Scholar] [CrossRef]
- Abad, A.; Gayán, P.; García-Labiano, F.; Diego, L.F.; Izquierdo, M.T.; Mendiara, T.; Adánez, J. Relevance of oxygen carrier properties on the design of a chemical looping combustion unit with gaseous fuels. Greenh. Gases Sci. Technol. 2023, 13, 125–143. [Google Scholar] [CrossRef]
- Cheng, D.; Yong, Q.; Zhao, Y.; Gong, B.; Zhang, J. Study on the Interaction of the Fe-Based Oxygen Carrier with Ashes. Energy Fuels 2020, 34, 9796–9809. [Google Scholar] [CrossRef]
- Liu, X.; Bu, H.; Zou, G.; Wu, X.; Zheng, C.; Ma, J.; Zhao, H. Industrial-Scale Preparation of Biore Oxygen Carriers for Chemical Looping Combustion via a Hydroforming Method. Energy Fuels 2024, 38, 6156–6170. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, W.; Wei, G.; Liu, X.; Lin, S.; Li, J.; Li, D.; Meng, Q.; Nie, L.; et al. Demonstration of a 5-MWth Chemical Looping Combustion Unit Fueled by Lignite. Engineering 2025, in press. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Jin, B. Thermodynamic evaluation and modelling of an auto-thermal hybrid system of chemical looping combustion and air separation for power generation coupling with CO2 cycles. Energy 2021, 236, 121431. [Google Scholar] [CrossRef]
- Ströhle, J.; Orth, M.; Epple, B. Design and operation of a 1MWth chemical looping plant. Appl. Energy 2014, 113, 1490–1495. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Use of Ores and Industrial Products as Oxygen Carriers in Chemical-Looping Combustion. Energy Fuels 2009, 23, 2307–2315. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Driessche, I.V.; Snijkers, F.; Verberckmoes, A. Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Liu, F.; Liu, J.; Yang, Y. Review on the Theoretical Understanding of Oxygen Carrier Development for Chemical-Looping Technologies. Energy Fuels 2022, 36, 9373–9384. [Google Scholar] [CrossRef]
- Adánez, J.; Cuadrat, A.; Abad, A.; Gayán, P.; Diego, L.F.; García-Labiano, F. Ilmenite Activation during Consecutive Redox Cycles in Chemical-Looping Combustion. Energy Fuels 2010, 24, 1402–1413. [Google Scholar] [CrossRef]
- Ksepko, E.; Babinski, P.; Evdou, A.; Nalbandian, L. Studies on the redox reaction kinetics of selected, naturally occurring oxygen carrier. J. Therm. Anal. Calorim. 2016, 124, 137–150. [Google Scholar] [CrossRef][Green Version]
- Lu, X.; Rahman, R.A.; Lu, D.Y.; Ridha, F.N.; Duchesne, M.A.; Tan, Y.; Hughes, R.W. Pressurized chemical looping combustion with CO: Reduction reactivity and oxygen-transport capacity of ilmenite ore. Appl. Energy 2016, 184, 132–139. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; Cuadrat, A.; García-Labiano, F.; Gayán, P.; Diego, L.F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 689–702. [Google Scholar] [CrossRef]
- Su, M.; Ma, J.; Tian, X.; Zhao, H. Reduction kinetics of hematite as oxygen carrier in chemical looping combustion. Fuel Process. Technol. 2017, 155, 160–167. [Google Scholar] [CrossRef]
- Su, M.; Zhao, H.; Tian, X.; Zhang, P.; Du, B.; Liu, Z. Intrinsic Reduction Kinetics Investigation on a Hematite Oxygen Carrier by CO in Chemical Looping Combustion. Energy Fuels 2017, 31, 3010–3018. [Google Scholar] [CrossRef]
- Ksepko, E.; Babiński, P.; Nalbandian, L. The redox reaction kinetics of Sinai ore for chemical looping combustion applications. Appl. Energy 2017, 190, 1258–1274. [Google Scholar] [CrossRef]
- Khakpoor, N.; Mostafavi, E.; Mahinpey, N.; Siegler, H.D.H. Oxygen transport capacity and kinetic study of ilmenite ores for methane chemical-looping combustion. Energy 2019, 169, 329–337. [Google Scholar] [CrossRef]
- Mendiara, T.; Abad, A.; de Diego, L.; García-Labiano, F.; Gayán, P.; Adánez, J. Reduction and oxidation kinetics of Tierga iron ore for Chemical Looping Combustion with diverse fuels. Chem. Eng. J. 2019, 359, 37–46. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Jin, B.; Zhao, J.; Sun, C.; Snape, C.E. Experimental Evaluation of a Chinese Sulfur-Containing Lean Iron Ore as the Oxygen Carrier for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2016, 55, 428–435. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Shao, Y.; Jin, Z.; Jin, B. Reactivity of a Chinese lean iron ore as oxygen carrier: Kinetics and characterization. Thermochim. Acta 2018, 670, 114–122. [Google Scholar] [CrossRef]
- Lyngfelt, A. Chemical Looping Combustion: Status and Development Challenges. Energy Fuels 2020, 34, 9077–9093. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhang, S.; Kong, Z.; Jin, Z.; Shao, Y.; Jin, B. Test Operation of a Separated-Gasification Chemical Looping Combustion System for Coal. Energy Fuels 2018, 32, 11411–11420. [Google Scholar] [CrossRef]


| OC Types | Active Compositions | Test Fuel | Reactor | Apparent Activation Energy | Reaction Order | Reference |
|---|---|---|---|---|---|---|
| Kryvbas iron ore | 84.86% Fe2O3 | CH4 | TGA | 42.0 kJ mol−1 | 1.98 | [31] |
| Canadian ilmenite | 55.8% Fe2O3 32.6% TiO2 | CO | TGA | 115 kJ mol−1 | 0.67 | [32] |
| Norwegian ilmenite | 11.2% Fe2O3 54.6% Fe2TiO5 | H2 CO CH4 | TGA | 109.2 kJ mol−1 113.3 kJ mol−1 165.2 kJ mol−1 | 1 | [33] |
| Chinese hematite | 66% Fe2O3 | CO | TGA | 110.75 kJ mol−1 | 1.5 | [34] |
| Chinese hematite | 66% Fe2O3 | CO | Fluidized bed reactor | 74.48 kJ mol−1 | 1 | [35] |
| Ilmenite | 46.4% Fe2O3 | CH4 | TGA | 62.4 kJ mol−1 | 0.52 | [36] |
| Canadian ilmenite | 10.4% Fe2O3 + 30% Fe2TiO5 | CH4 | TGA | 106.7 kJ mol−1 | 0.7 | [37] |
| Spanish iron ore | 76.5% Fe2O3 | H2 CO CH4 | TGA | 81.1 kJ mol−1 76.1 kJ mol−1 257 kJ mol−1 | 1 | [38] |
| Lean iron ore | 35.21% Fe2O3 10.0% CaSO4 | CH4 CO | TGA | 62 kJ mol−1 56 kJ mol−1 | 0.5 | [39] |
| Chinese iron ore | 44.16% Fe2O3 | CH4 | TGA | 157.5kJ mol−1 126.9 kJ mol−1 | 1 2 | [40] |
| Components | Fe2O3 | SiO2 | Al2O3 | CaO | MgO | Others |
|---|---|---|---|---|---|---|
| Weight | 41.34% | 32.17% | 11.85% | 3.77% | 1.47% | 9.40% |
| Kinetic Model | f(X) | g(X) |
|---|---|---|
| Kinetics-order models | f1 = 1 − X | g1 = −ln(1 − X) |
| f2 = (1 − X)2 | g2 = (1 − X)−1 − 1 | |
| f3 = (1 − X)3 | g3 = [(1-X)−2 − 1]/2 | |
| Diffusion model | f4 = 1/(2X) | g4 = X2 |
| f5 = 1/[−ln(1 − X)] | g5 = (1 − X)ln(1 − X) + X | |
| f6 = (3/2)(1 − X)2/3[1 − (1 − X)1/3] | g6 = [1 − (1 − X)1/3]2 | |
| Contraction model | f7 = 2(1 − X)1/2 | g7 = 1 − (1 − X)1/2 |
| f8 = 3(1 − X)2/3 | g8 = 1 − (1 − X)1/3 | |
| Nucleation model | f9 = 2(1 − X)[−ln(1 − X)]1/2 | g9 = [−ln(1 − X)]1/2 |
| f10 = 3(1 − X)[−ln(1 − X)]2/3 | g10 = [−ln(1 − X)]1/3 |
| T (°C) | g1 | g2 | g3 | g4 | g5 | g6 | g7 | g8 | g9 | g10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 750 | 0.9933 | 0.9714 | 0.9714 | 0.9071 | 0.8879 | 0.8655 | 0.9949 | 0.9951 | 0.8289 | 0.3878 |
| 800 | 0.9835 | 0.9461 | 0.9461 | 0.8776 | 0.8545 | 0.828 | 0.9924 | 0.9902 | 0.8843 | 0.5104 |
| 850 | 0.9580 | 0.8913 | 0.8913 | 0.8433 | 0.8129 | 0.7774 | 0.9785 | 0.9727 | 0.9453 | 0.6787 |
| Model | g1 | g7 | g8 |
|---|---|---|---|
| Apparent activation energy | 19.13 kJ mol−1 | 15.93 kJ mol−1 | 16.94 kJ mol−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, C.; Liang, Q.; Li, Q.; He, M.; Shen, X.; Wang, H.; Wang, M.; Wang, X. Performance Evaluation and Kinetic Analysis of an Iron Ore as Oxygen Carrier in Chemical Looping Combustion. Processes 2025, 13, 2949. https://doi.org/10.3390/pr13092949
Tao C, Liang Q, Li Q, He M, Shen X, Wang H, Wang M, Wang X. Performance Evaluation and Kinetic Analysis of an Iron Ore as Oxygen Carrier in Chemical Looping Combustion. Processes. 2025; 13(9):2949. https://doi.org/10.3390/pr13092949
Chicago/Turabian StyleTao, Congxi, Qian Liang, Qingmei Li, Minghai He, Xuhui Shen, Hao Wang, Ming Wang, and Xudong Wang. 2025. "Performance Evaluation and Kinetic Analysis of an Iron Ore as Oxygen Carrier in Chemical Looping Combustion" Processes 13, no. 9: 2949. https://doi.org/10.3390/pr13092949
APA StyleTao, C., Liang, Q., Li, Q., He, M., Shen, X., Wang, H., Wang, M., & Wang, X. (2025). Performance Evaluation and Kinetic Analysis of an Iron Ore as Oxygen Carrier in Chemical Looping Combustion. Processes, 13(9), 2949. https://doi.org/10.3390/pr13092949







