Sustainable Bioethylene Production from Lignocellulosic Bioethanol: Performance of Zeolitic Catalysts and Mechanistic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Catalyst Preparation
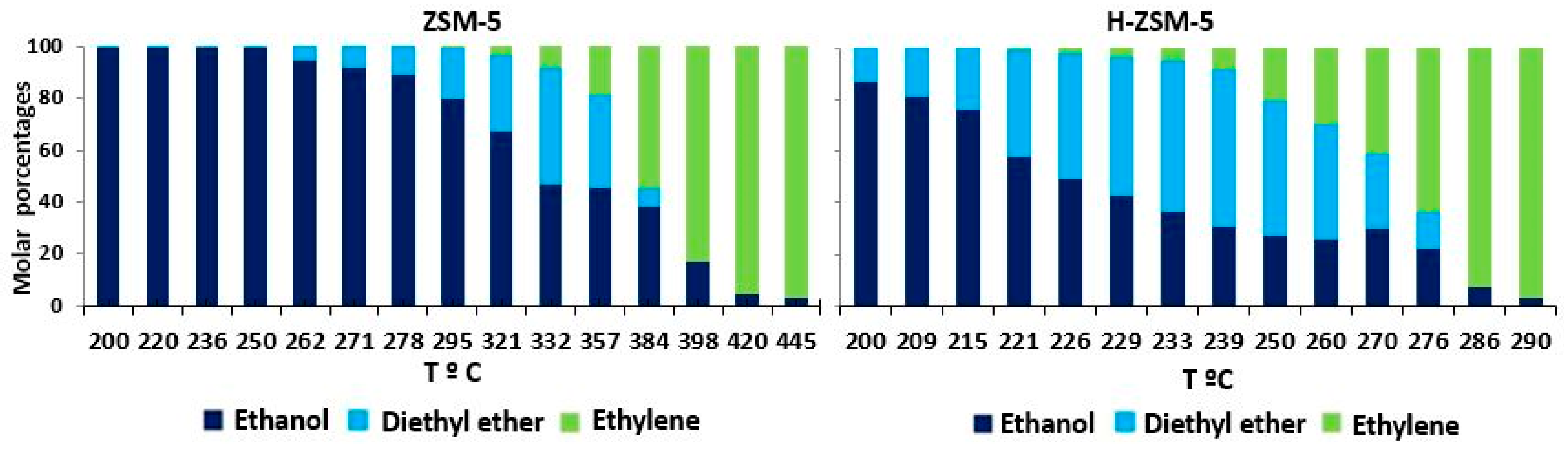
2.3. Catalyst Characterization NH4+Y vs. H-Y and ZSM-5 vs. H-ZSM-5
2.4. Ethanol and 2G Bio-Ethanol Catalytic Dehydration Process
2.5. Reaction Product Characterization
2.6. Computational Methods
3. Results and Discussion
3.1. Catalyst Characterization by Pyridine Desorption Technique and Nitrogen Sorptometry (BET Method)
3.2. Ethanol and 2G Bio-Ethanol Catalytic Dehydration Process
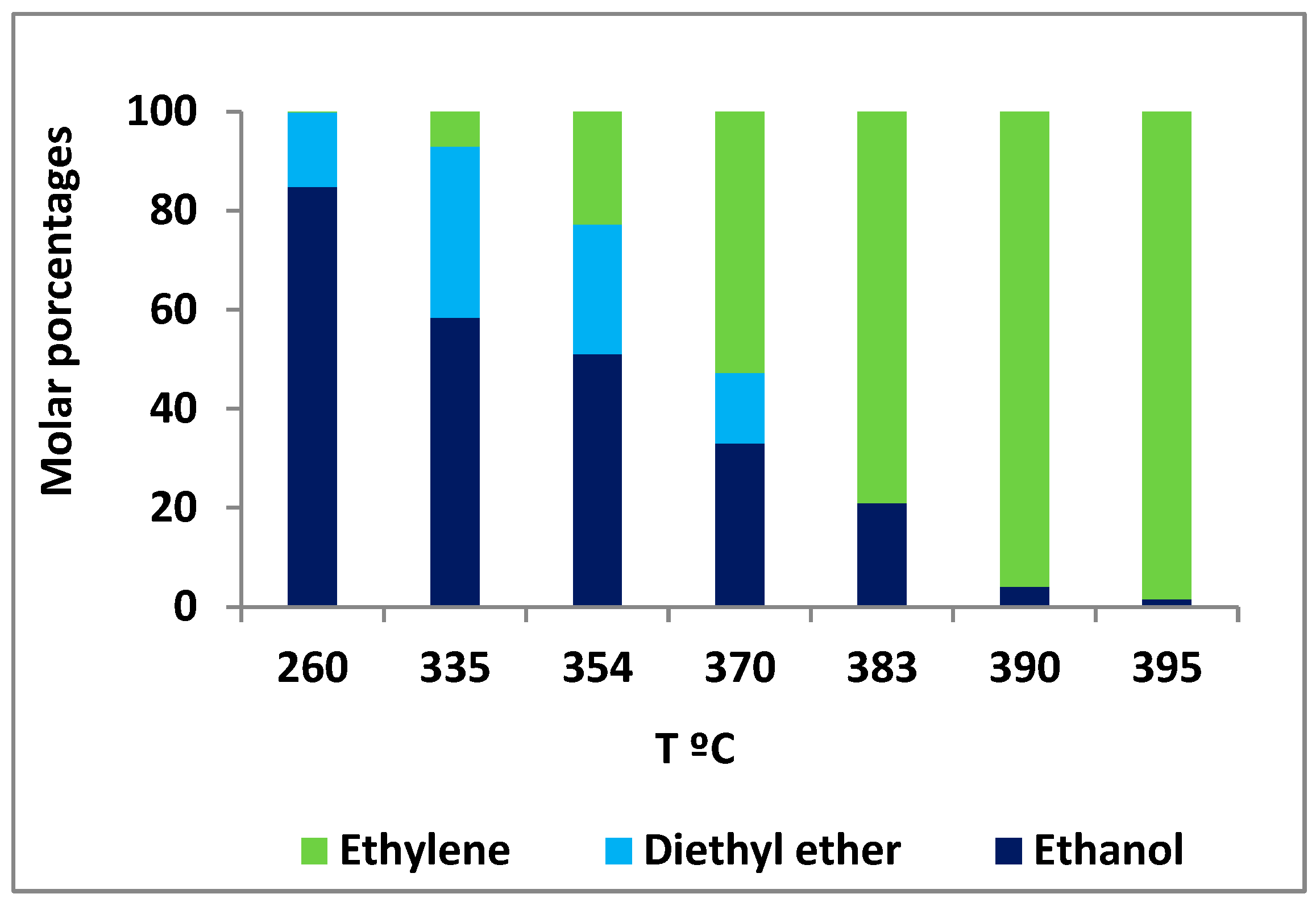
3.2.1. Temperature Effect on the Commercial Ethanol Dehydration Process, Used as Reference
3.2.2. Effects of Reagent Concentration and Weight Hourly Space Velocity (WHSV) in the 2G Bioethanol Dehydration Process
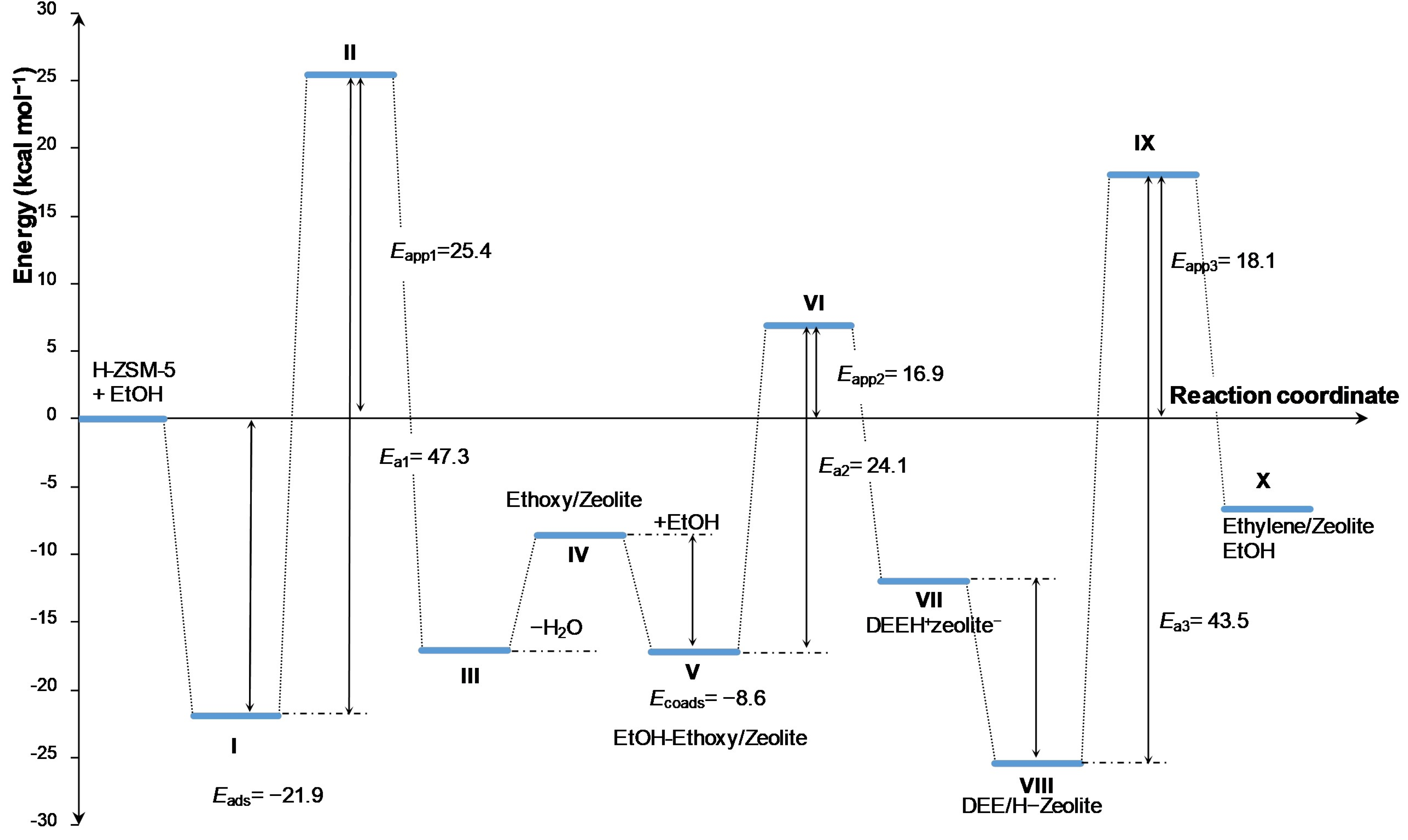
3.3. DFT Study of the Reaction Mechanism for Bioethylene Formation Via Diethyl Ether Intermediate on H-ZSM-5 Zeolite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso-Fariñas, B.; Gallego-Schmid, A.; Haro, P.; Azapagic, A. Environmental Assessment of Thermo-Chemical Processes for Bio-Ethylene Production in Comparison with Bio-Chemical and Fossil-Based Ethylene. J. Clean. Prod. 2018, 202, 817–829. [Google Scholar] [CrossRef]
- Tripodi, A.; Belotti, M.; Rossetti, I. Bioethylene Production: From Reaction Kinetics to Plant Design. ACS Sustain. Chem. Eng. 2019, 7, 13333–13350. [Google Scholar] [CrossRef]
- Zanon-Zotin, M.; Bergman-Fonte, C.; Nogueira Morais, T.; Barbosa Maia, P.L.; Carvalho, L.; Angelkorte, G.; Oliveira Fiorini, A.C.; Rua Rodriguez Rochedo, P.; Portugal-Pereira, J.; Szklo, A.; et al. Unpacking Bio-Based Alternatives to Ethylene Production in Brazil, Europe, and the United States: A Comparative Life Cycle Assessment. J. Clean. Prod. 2023, 428, 139376. [Google Scholar] [CrossRef]
- Melander, A.N.; Qvint, K. Assessing the Sustainability of First Generation Ethanol for Bioethylene Production. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2016. [Google Scholar]
- Eckert, C.; Xu, W.; Xiong, W.; Lynch, S.; Ungerer, J.; Tao, L.; Gill, R.; Maness, P.C.; Yu, J. Ethylene-Forming Enzyme and Bioethylene Production. Biotechnol. Biofuels Bioprod. 2014, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Tripodi, A.; Ramis, G. Hydrogen, Ethylene and Power Production from Bioethanol: Ready for the Renewable Market? Int. J. Hydrogen Energy 2020, 45, 10292–10303. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A Review and Techno-Economical Evaluation. ChemBioEng Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- Gnansounou, E.; Dauriat, A. Techno-Economic Analysis of Lignocellulosic Ethanol: A Review. Bioresour. Technol. 2010, 101, 4980–4991. [Google Scholar] [CrossRef]
- Mendieta, C.M.; Kruyeniski, J.; Felissia, F.E.; Area, M.C. Modelling of the Simultaneous Saccharification and Fermentation for a Pine Sawdust Biorefinery. Fermentation 2022, 8, 130. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.M.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste Biorefineries: Enabling Circular Economies in Developing Countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef]
- Varisli, D.; Dogu, T.; Dogu, G. Ethylene and Diethyl-Ether Production by Dehydration Reaction of Ethanol over Different Heteropolyacid Catalysts. Chem. Eng. Sci. 2007, 62, 5349–5352. [Google Scholar] [CrossRef]
- Dumrongsakda, P.; Ruangpornvisuti, V. Theoretical Investigation of Ethanol Conversion to Ethylene over H-ZSM-5 and Transition Metals-Exchanged ZSM-5. Catal. Lett. 2012, 142, 143–149. [Google Scholar] [CrossRef]
- Sheng, Q.; Ling, K.; Li, Z.; Zhao, L. Effect of Steam Treatment on Catalytic Performance of HZSM-5 Catalyst for Ethanol Dehydration to Ethylene. Fuel Process. Technol. 2013, 110, 73–78. [Google Scholar] [CrossRef]
- Yakovleva, I.S.; Banzaraktsaeva, S.P.; Ovchinnikova, E.V.; Chumachenko, V.A.; Isupova, L.A. Catalytic Dehydration of Bioethanol to Ethylene. Catal. Ind. 2016, 8, 152–167. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wu, H.S. Ethylene Formation from Ethanol Dehydration Using ZSM-5 Catalyst. ACS Omega 2017, 2, 4287–4296. [Google Scholar] [CrossRef]
- Ouayloul, L.; Agirrezabal-Telleria, I.; Paul, S.; Doukkali, M. El Trend and Progress in Catalysis for Ethylene Production from Bioethanol Using ZSM-5. ACS Catal. 2024, 14, 17360–17397. [Google Scholar] [CrossRef]
- Kim, S.; Robichaud, D.J.; Beckham, G.T.; Paton, R.S.; Nimlos, M.R. Ethanol Dehydration in HZSM-5 Studied by Density Functional Theory: Evidence for a Concerted Process. J. Phys. Chem. A 2015, 119, 3604–3614. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Misono, M.; Ono, Y.; Hattori, H. Their Catalytic Properties. In New Solid Acids and Bases; Elsevier: Amsterdam, The Netherlands, 1990; p. 364. ISBN 9780080887555. [Google Scholar]
- Galadima, A.; Muraza, O. Journal of Industrial and Engineering Chemistry Zeolite Catalysts in Upgrading of Bioethanol to Fuels Range Hydrocarbons: A Review. J. Ind. Eng. Chem. 2015, 31, 1–14. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, W.; Li, J.; Lin, S.; Xu, S.; Wei, Y.; Liu, Z. Revealing the Roles of Hydrocarbon Pool Mechanism in Ethanol-to-Hydrocarbons Reaction. J. Catal. 2022, 413, 517–526. [Google Scholar] [CrossRef]
- Iadrat, P.; Yomthong, K.; Rodaum, C.; Pornsetmetakul, P.; Thivasasith, A.; Prasertsab, A.; Fan, X.; Sooknoi, T.; Wattanakit, C. Effects of Zeolite Frameworks and Hierarchical Structures on Catalytic Bioethanol Dehydration: In-Situ DRIFTS and DFT Studies. Fuel 2023, 338, 127208. [Google Scholar] [CrossRef]
- Cardozo, R.E.; Clauser, N.M.; Felissia, F.E.; Area, M.C.; Vallejos, M.E. Design of an Integrated Biorefinery for Bioethylene Production from Industrial Forest Byproducts. Green Chem. 2024, 26, 4092–4102. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Zalazar, M.F.; Paredes, E.N.; Romero Ojeda, G.D.; Cabral, N.D.; Peruchena, N.M. Study of Confinement and Catalysis Effects of the Reaction of Methylation of Benzene by Methanol in H-Beta and H-ZSM-5 Zeolites by Topological Analysis of Electron Density. J. Phys. Chem. C 2018, 122, 3350–3362. [Google Scholar] [CrossRef]
- Gomes, G.J.; Zalazar, M.F.; Arroyo, P.A.; Scremin, F.R.; Costa, M.B.; Bittencourt, P.R.S.; Lindino, C.A.; Peruchena, N.M. Molecular-Level Understanding of the Rate-Determining Step in Esterification Reactions Catalyzed by H-ZSM-5 Zeolite. An Experimental and Theoretical Study. Chem. Sel. 2019, 4, 3031–3041. [Google Scholar] [CrossRef]
- Gomes, G.J.; Zalazar, M.F.; Arroyo, P.A. New Insights into the Effect of the Zeolites Framework Topology on the Esterification Reactions: A Comparative Study from Experiments and Theoretical Calculations. Top. Catal. 2022, 65, 871–886. [Google Scholar] [CrossRef]
- Petelski, A.N.; Peruchena, N.M.; Zalazar, M.F. Acidity of Isomorphic Substituted Zeolites with B, Al and Ga Revisited. ChemPhysChem 2024, 25, e202400080. [Google Scholar] [CrossRef] [PubMed]
- Romero Ojeda, G.D.; Gómez, G.J.; Stival Bittencourt, P.R.; Collins, S.E.; Bosco, M.V.; Peruchena, N.M.; Zalazar, M.F. Comparative Study of Adsorbed Complexes inside Pores and Cavities of Acid Zeolites with Different Topology: Experimental and Theoretical Insights into Confinement Effects. J. Phys. Chem. 2024, 17, 7137–7148. [Google Scholar] [CrossRef]
- Chung, L.W.; Sameera, W.M.C.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.; Liu, F.; et al. The ONIOM Method and Its Applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, version 16; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Hratchian, H.P.; Schlegel, H.B. Theory and applications of computational chemistry the first forty years 2005 Hratchian. In Theory and Applications of Computational Chemistry: The First 40 Years; Dykstra, C.E., Kim, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Madeira, F.F.; Gnep, N.S.; Magnoux, P.; Maury, S.; Cadran, N. Ethanol Transformation over HFAU, HBEA and HMFI Zeolites Presenting Similar Brønsted Acidity. Appl. Catal. A Gen. 2009, 367, 39–46. [Google Scholar] [CrossRef]
- Moon, S.; Chae, H.; Park, M.B. Dehydration of Bioethanol to Ethylene over H-ZSM-5 Catalysts: A Scale-Up Study. Catalysts 2019, 9, 186. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Le Van Mao, R. Conversion of Ethanol in Aqueous Solution over ZSM-5 Zeolites. Appl. Catal. 1990, 58, 119–129. [Google Scholar] [CrossRef]
- Fan, D.; Dai, D.J.; Wu, H.S. Ethylene Formation by Catalytic Dehydration of Ethanol with Industrial Considerations. Materials 2013, 6, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Guo, X.; Liu, M.; Wang, X. High Effective Dehydration of Bio-Ethanol into Ethylene over Nanoscale HZSM-5 Zeolite Catalysts. Catal. Today 2010, 149, 143–147. [Google Scholar] [CrossRef]
- Phung, T.K.; Busca, G. Diethyl Ether Cracking and Ethanol Dehydration: Acid Catalysis and Reaction Paths. Chem. Eng. J. 2015, 272, 92–101. [Google Scholar] [CrossRef]
- Ouayloul, L.; El Doukkali, M.; Jiao, M.; Dumeignil, F.; Agirrezabal-Telleria, I. New Mechanistic Insights into the Role of Water in the Dehydration of Ethanol into Ethylene over ZSM-5 Catalysts at Low Temperature. Green Chem. 2023, 25, 3644–3659. [Google Scholar] [CrossRef]
- Becerra, J.; Quiroga, E.; Tello, E.; Figueredo, M.; Cobo, M. Kinetic Modeling of Polymer-Grade Ethylene Production by Diluted Ethanol Dehydration over H-ZSM-5 for Industrial Design. J. Environ. Chem. Eng. 2018, 6, 6165–6174. [Google Scholar] [CrossRef]






| Catalyst | Brönsted Acidity (mmol Py/g) | Lewis Acidity (mmol Py/g) | ||||
|---|---|---|---|---|---|---|
| 250 °C | 350 °C | 400 °C | 250 °C | 350 °C | 400 °C | |
| NH4+Y | 0.301 | 0.220 | 0.160 | 0.100 | 0.097 | 0.092 |
| H-Y | 0.258 | 0.016 | 0.108 | 0.101 | 0.080 | 0.068 |
| ZSM-5 | 0.545 | 0.386 | 0.323 | 0.013 | 0.008 | 0.008 |
| H-ZSM-5 | 0.321 | 0.272 | 0.231 | 0.049 | 0.050 | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendieta, C.M.; Zalazar, M.F.; Covinich, L.G.; Santori, G.F.; Felissia, F.E.; Area, M.C. Sustainable Bioethylene Production from Lignocellulosic Bioethanol: Performance of Zeolitic Catalysts and Mechanistic Insights. Processes 2025, 13, 2924. https://doi.org/10.3390/pr13092924
Mendieta CM, Zalazar MF, Covinich LG, Santori GF, Felissia FE, Area MC. Sustainable Bioethylene Production from Lignocellulosic Bioethanol: Performance of Zeolitic Catalysts and Mechanistic Insights. Processes. 2025; 13(9):2924. https://doi.org/10.3390/pr13092924
Chicago/Turabian StyleMendieta, Carolina Mónica, María Fernanda Zalazar, Laura Gabriela Covinich, Gerardo Fabián Santori, Fernando Esteban Felissia, and María Cristina Area. 2025. "Sustainable Bioethylene Production from Lignocellulosic Bioethanol: Performance of Zeolitic Catalysts and Mechanistic Insights" Processes 13, no. 9: 2924. https://doi.org/10.3390/pr13092924
APA StyleMendieta, C. M., Zalazar, M. F., Covinich, L. G., Santori, G. F., Felissia, F. E., & Area, M. C. (2025). Sustainable Bioethylene Production from Lignocellulosic Bioethanol: Performance of Zeolitic Catalysts and Mechanistic Insights. Processes, 13(9), 2924. https://doi.org/10.3390/pr13092924











