Effect of Energy Integration on Safety Indexes of Suspension PVC Production Process
Abstract
1. Introduction
2. Materials and Methods
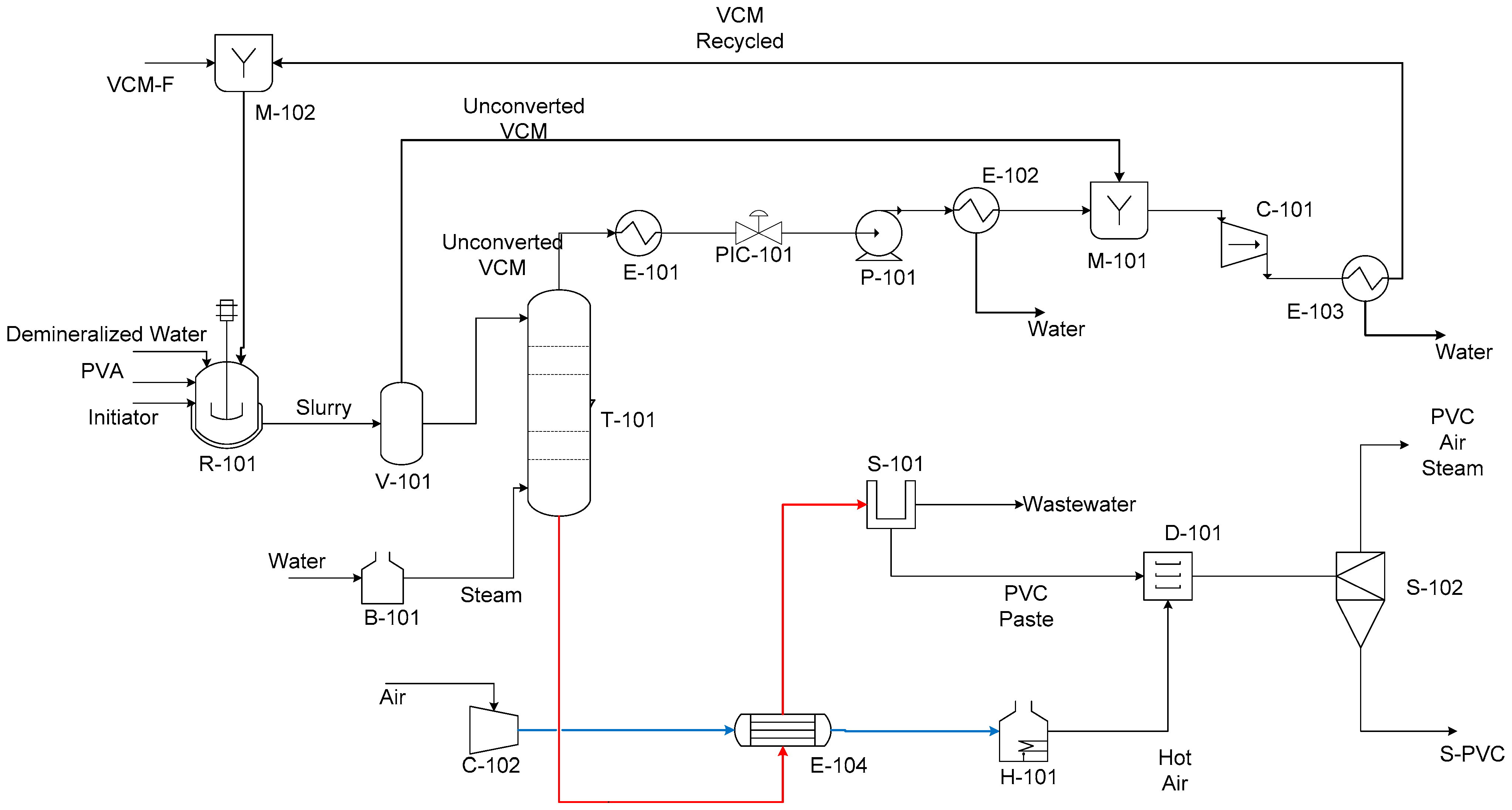
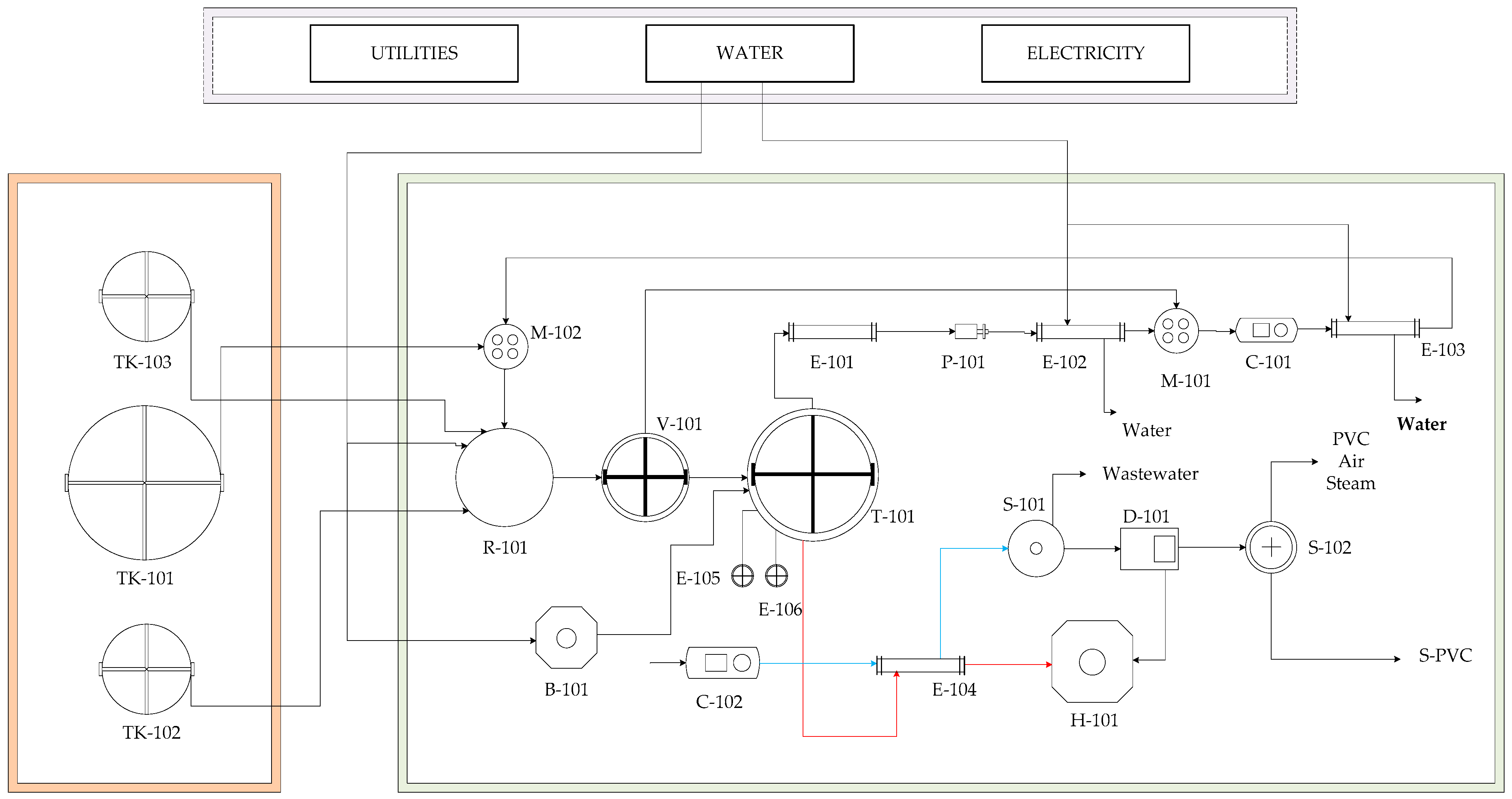
2.1. Process Description
2.2. Energy Integration Using Aspen Energy AnalyzerTM V14
2.3. Process Safety Evaluation
2.4. Chemical Index of Inherent Safety ()
2.5. Inherent Process Safety Index ()
3. Results and Discussion
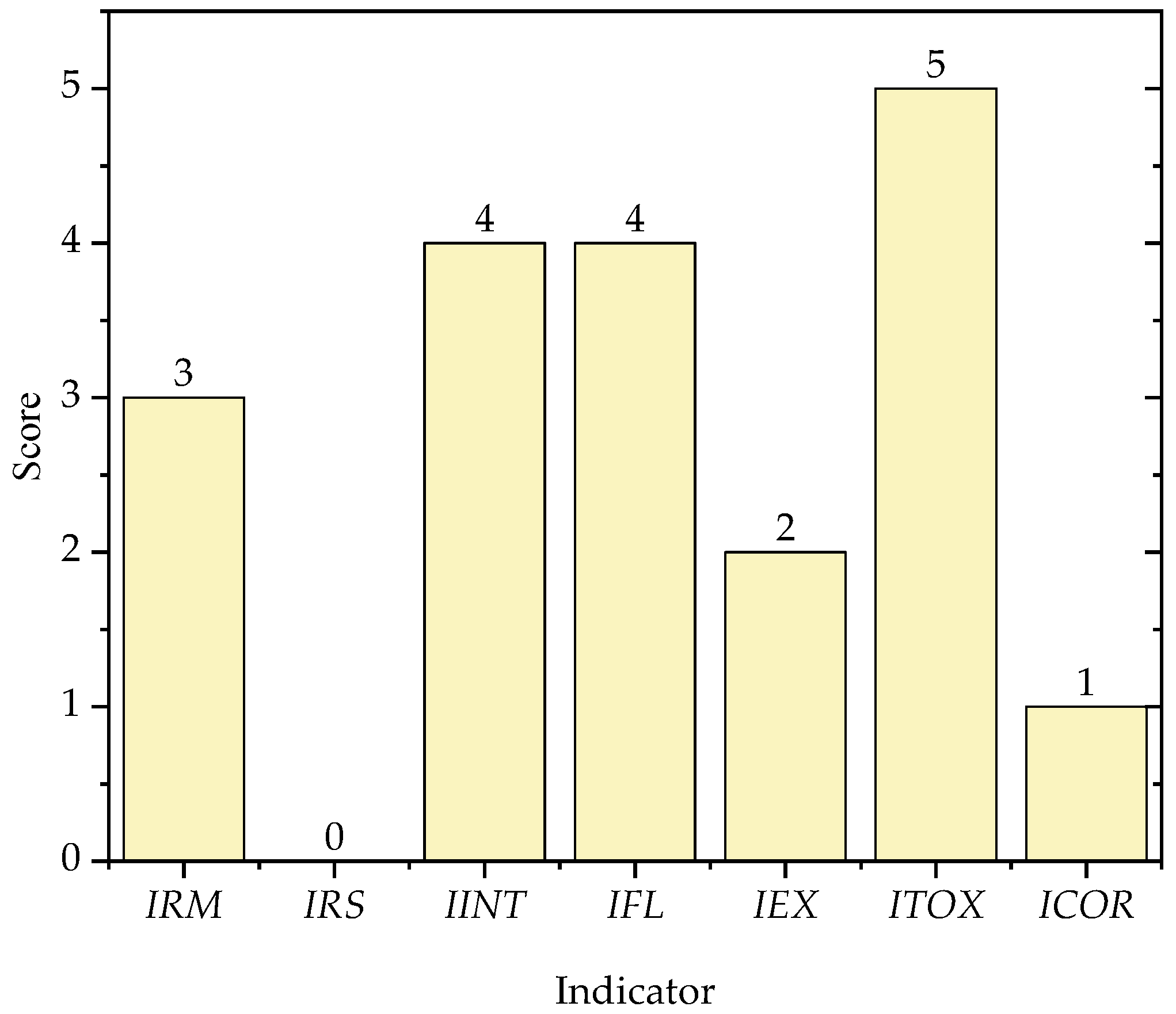
3.1. Contribution of Chemical Process Indicators
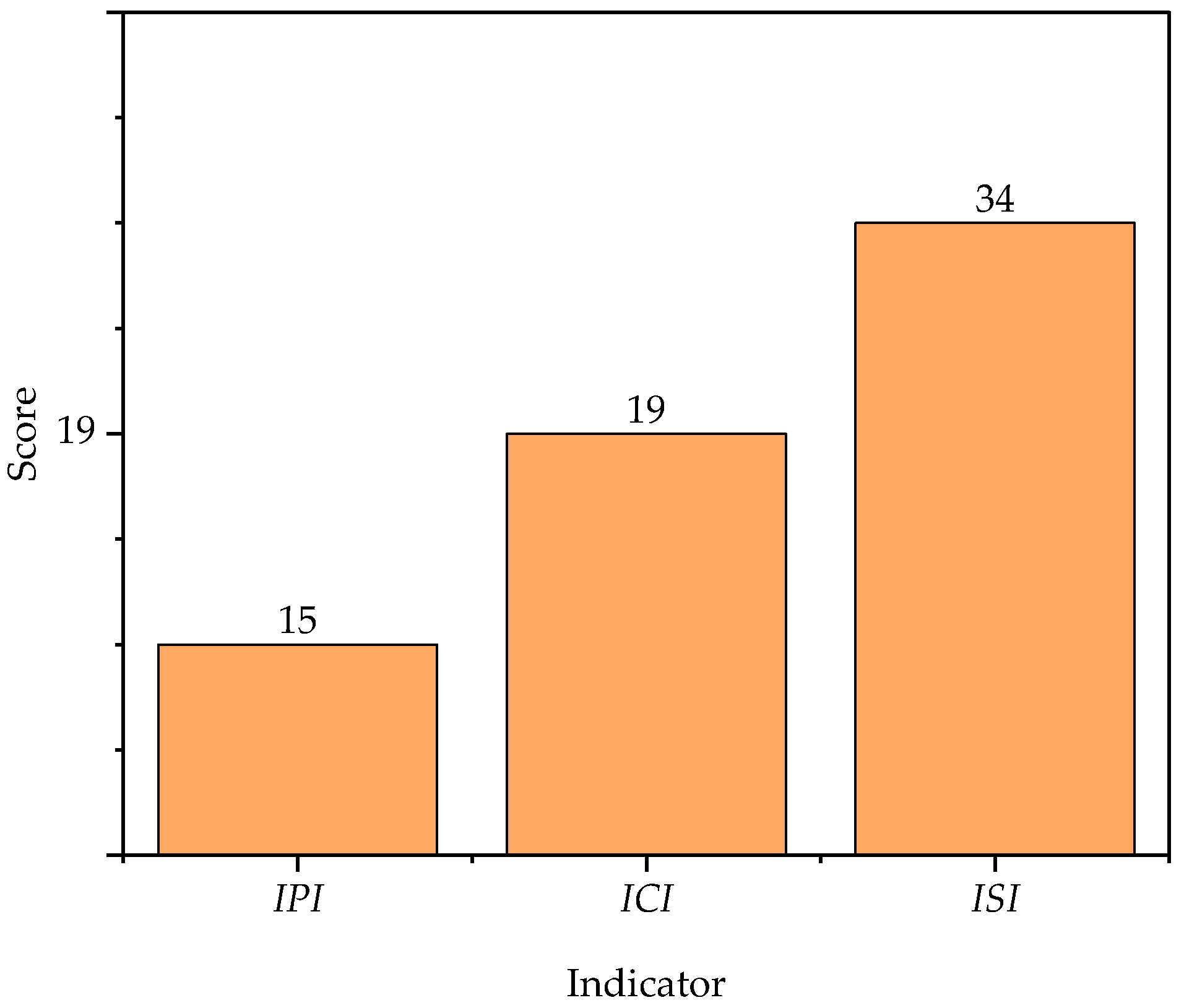
3.2. Contribution of Process Safety Indicator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ISI | Inherent safety index |
| Inherent safety index by chemicals | |
| Inherent process safety index | |
| Main reaction heat subindex, J/g | |
| Secondary reaction heat subindex, J/g | |
| Chemical interactions subindex | |
| Flammability subindex, °C | |
| Explosiveness subindex, % | |
| Toxicity subindex, ppm | |
| Corrosivity subindex | |
| Inventory subindex, t/h | |
| Temperature subindex, °C | |
| Pressure subindex, bar | |
| Equipment safety subindex | |
| Safe structure level subindex | |
| VCM | Vinyl chloride monomer |
| PVC | Polyvinyl chloride |
| PVA | Polyvinyl alcohol |
References
- Witlox, H.W.; Fernandez, M.; Harper, M.; Stene, J. Modelling and validation of atmospheric expansion and near-field dispersion for pressurised vapour or two-phase releases. J. Loss Prev. Process Ind. 2017, 48, 331–344. [Google Scholar] [CrossRef]
- Hong, Y.; Pasman, H.J.; Sachdeva, S.; Markowski, A.S.; Mannan, M.S. A fuzzy logic and probabilistic hybrid approach to quantify the uncertainty in layer of protection analysis. J. Loss Prev. Process Ind. 2016, 43, 10–17. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Kiss, A.A. Pinch point analysis. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 35, pp. 525–564. [Google Scholar] [CrossRef]
- Bolt, H.M. Vinyl Chloride—A Classical Industrial Toxicant of New Interest. Crit. Rev. Toxicol. 2005, 35, 307–323. [Google Scholar] [CrossRef]
- Nawaz, W.; Linke, P.; Koç, M. Safety and sustainability nexus: A review and appraisal. J. Clean. Prod. 2019, 216, 74–87. [Google Scholar] [CrossRef]
- González-Delgado, Á.D.; Aguilar-Vásquez, E.; Ramos-Olmos, M. Chemical and Process Inherent Safety Analysis of Large-Scale Suspension Poly(Vinyl Chloride) Production. Chemengineering 2023, 7, 76. [Google Scholar] [CrossRef]
- Park, S.; Xu, S.; Rogers, W.; Pasman, H.; El-Halwagi, M.M. Incorporating inherent safety during the conceptual process design stage: A literature review. J. Loss Prev. Process Ind. 2020, 63, 104040. [Google Scholar] [CrossRef]
- Mendivil-Arrieta, A.; Aguilar-Vasquez, E.A.; Diaz-Perez, J.M.; Ramos-Olmos, M.; Gonzaléz-Delgado, Á.D. Energy Integration and WEP Technical Evaluation of a Large-Scale PVC Production Process. Sci 2025, 7, 41. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Hashim, H.; Hassim, M.H. A graphical method for assessing inherent safety during research and development phase of process design. J. Loss Prev. Process Ind. 2016, 42, 59–69. [Google Scholar] [CrossRef]
- Chan, I.; Alwi, S.R.W.; Hassim, M.H.; Manan, Z.A.; Klemeš, J.J. Heat Exchanger Network Design Considering Inherent Safety. Energy Procedia 2014, 61, 2469–2473. [Google Scholar] [CrossRef]
- Dunjó, J.; Fthenakis, V.; Vílchez, J.A.; Arnaldos, J. Hazard and operability (HAZOP) analysis. A literature review. J. Hazard. Mater. 2010, 173, 19–32. [Google Scholar] [CrossRef]
- Marhavilas, P.K.; Filippidis, M.; Koulinas, G.K.; Koulouriotis, D.E. The integration of HAZOP study with risk-matrix and the analytical-hierarchy process for identifying critical control-points and prioritizing risks in industry—A case study. J. Loss Prev. Process Ind. 2019, 62, 103981. [Google Scholar] [CrossRef]
- Sano, K.; Koshiba, Y.; Ohtani, H. Risk assessment and risk reduction of an acrylonitrile production plant. J. Loss Prev. Process Ind. 2020, 63, 104015. [Google Scholar] [CrossRef]
- Evangelista Benites, G.; Loyola Carranza, W.; Ramírez Ruiz, R.; Aguilar Rojas, P. Optimización de redes de intercambiadores de calor en endulzamiento de gas natural vía tecnología pinch. Sciéndo Ingenium 2017, 12, 167–184. [Google Scholar]
- Arunthavanathan, R.; Sajid, Z.; Amin, T.; Tian, Y.; Khan, F.; Pistikopoulos, E. Process safety 4.0: Artificial intelligence or intelligence augmentation for safer process operation? AIChE J. 2024, 70, e18475. [Google Scholar] [CrossRef]
- Azizi, A. Applications of Artificial Intelligence Techniques in Industry 4.0; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Zhang, F.; Jan, N. Design and Implementation of Industrial Design and Transformation System Based on Artificial Intelligence Technology. Math. Probl. Eng. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Aguilar-Vásquez, E.; Ramos-Olmos, M.; González-Delgado, Á.D. A Joint Computer-Aided Simulation and Water-Energy-Product (WEP) Approach for Technical Evaluation of PVC Production. Sustainability 2023, 15, 8096. [Google Scholar] [CrossRef]
- Mondal, S.K.; Uddin, M.R.; Paul, P.; Deb, A.; Azad, A. Minimization of the Process Loss in Condensate Fractionation Plant. Procedia Eng. 2014, 90, 524–529. [Google Scholar] [CrossRef]
- Kletz, T.A. Inherently safer design: The growth of an idea. Process Saf. Prog. 1996, 15, 5–8. [Google Scholar] [CrossRef]
- Kletz, T.A. Inherently safer plants. Plant Oper. Prog. 1985, 4, 164–167. [Google Scholar] [CrossRef]
- Heikkilä, A.M. Inherent Safety in Process Plant Design. An Index-Based Approach; VTT Technical Research Centre of Finland: Espoo, Finland, 1999. [Google Scholar]
- Cui, X.; Gui, X.; Hu, J.; Liu, J.; Zhou, R.; Gong, Z.; Li, W.; Yang, Y.; Dong, Y. Thermal safety and overall kinetics for methyl methacrylate solution polymerization via on-line process analytical technology. Thermochim. Acta 2023, 730, 179620. [Google Scholar] [CrossRef]
- Tian, W.; Wu, H.; Liu, Z.; Liu, B.; Cui, Z. Intelligent prediction of incipient fault in vinyl chloride production process based on deep learning. J. Clean. Prod. 2024, 472, 143474. [Google Scholar] [CrossRef]
- Bagheri, M.; Borhani, T.N.G.; Zahedi, G. Estimation of flash point and autoignition temperature of organic sulfur chemicals. Energy Convers. Manag. 2012, 58, 185–196. [Google Scholar] [CrossRef]
- Giurcan, V.; Razus, D.; Mitu, M.; Oancea, D. Prediction of flammability limits of fuel-air and fuel-air-inert mixtures from explosivity parameters in closed vessels. J. Loss Prev. Process Ind. 2015, 34, 65–71. [Google Scholar] [CrossRef]
- Lynch, H.N.; Prueitt, R.L.; Goodman, J.E. Critique of the ACGIH 2016 derivation of toluene diisocyanate Threshold Limit Values. Regul. Toxicol. Pharmacol. 2018, 97, 189–196. [Google Scholar] [CrossRef]
- Chong, N.S.; Abdulramoni, S.; Patterson, D.; Brown, H. Releases of Fire-Derived Contaminants from Polymer Pipes Made of Polyvinyl Chloride. Toxics 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.D.; Faroon, O.; Jones, D.; Lumpkin, M.H.; Stickney, J.; Citra, M.J. Toxicological Profile for Vinyl Chloride; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles: Atlanta, GA, USA, 2006. [Google Scholar]
- O’Mara, M.M.; Grider, L.B.; Daniel, R.L. Combustion Products from Vinyl Chloride Monomer. Am. Ind. Hyg. Assoc. J. 1971, 32, 153–156. [Google Scholar] [CrossRef]
- Veysi, A.; Roushani, M.; Najafi, H. Synthesis and evaluation of CuNi-MOF as a corrosion inhibitor of AISI 304 and 316 stainless steel in 1N HCl solution. Heliyon 2025, 11, e41296. [Google Scholar] [CrossRef]
- Fàbregas i Oller, O.; Marín Merrouni, N.; Sánchez Martínez, A.; Sánchez Cano, S.; Serrano i Vallvé, M. Planta de producción de cloruro de vinilo. 2018. Available online: https://ddd.uab.cat/record/199194 (accessed on 1 June 2025).
- U.S. Chemical Safety and Hazard Investigation Board. Investigation Report—Vinyl Chloride Monomer Explosion (5 Dead, 3 Injured, and Community Evacuated); U.S. Chemical Safety and Hazard Investigation Board: Washington, DC, USA, 2007.
- Aquino-Gaspar, H.M.; Díaz-Ovalle, C.O.; López-Molina, A.; Conde-Mejía, C.; Valenzuela-Gómez, L.M. Incident analysis of the “Pajaritos” petrochemical complex. J. Loss Prev. Process Ind. 2021, 70, 104404. [Google Scholar] [CrossRef]
- Ogle, R.A.; Megerle, M.V.; Morrison, D.R.; Carpenter, A.R. Explosion caused by flashing liquid in a process vessel. J. Hazard. Mater. 2004, 115, 133–140. [Google Scholar] [CrossRef]
- Hernández, J.G.S.; Gómez-Castro, F.I.; Romero-Izquierdo, A.G.; Conde-Mejía, C.; López-Molina, A. Partial energy integration between biofuels production processes: Effect on costs, CO2 emissions and process safety. Process Saf. Environ. Prot. 2022, 159, 918–930. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.; Liu, Z.; Hao, L.; Wei, H. Synergy of electrification and energy efficiency improvement via vapor recompression heat pump and heat exchanger network to achieve decarbonization of extractive distillation. Sep. Purif. Technol. 2022, 293, 121065. [Google Scholar] [CrossRef]
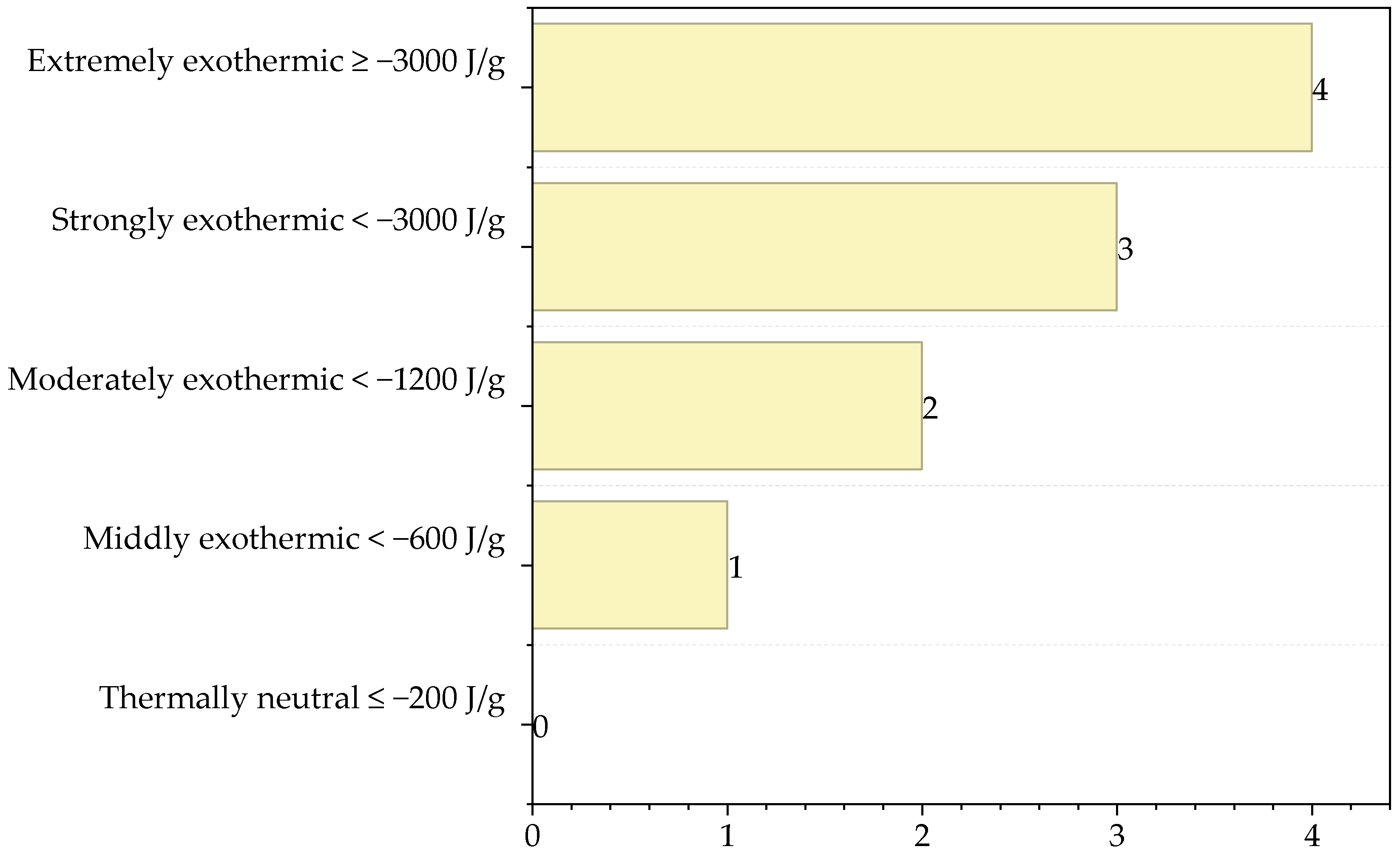



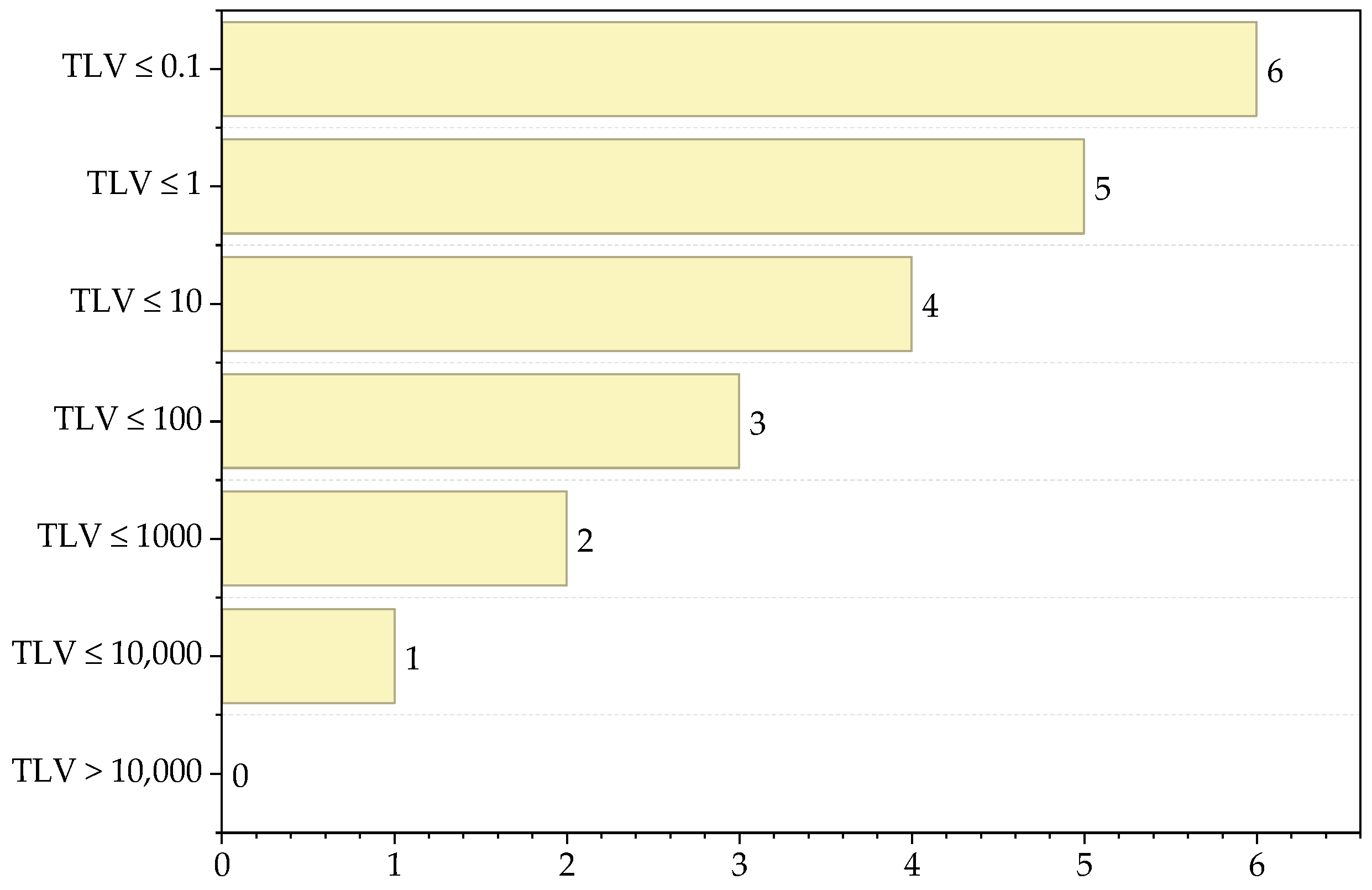
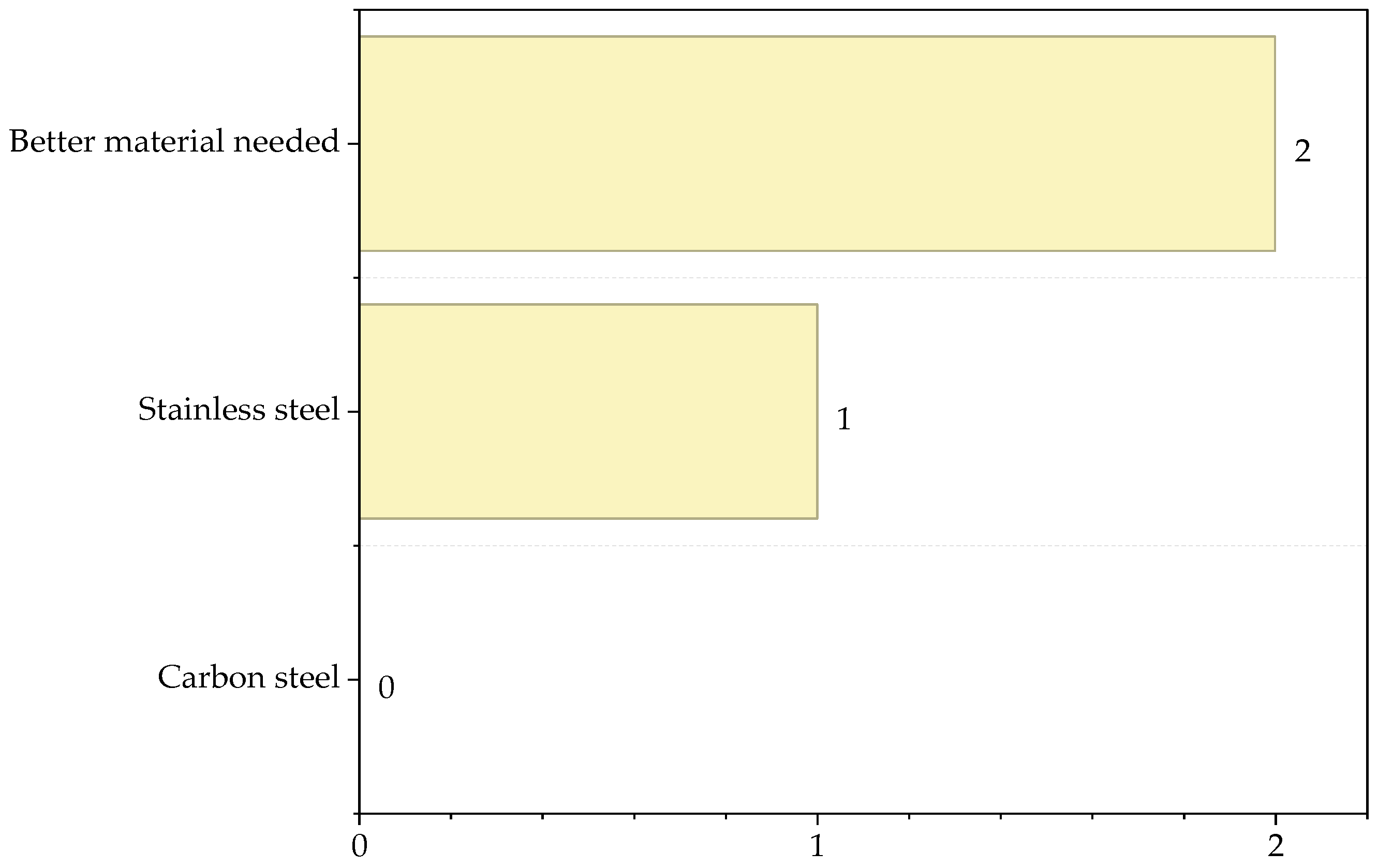
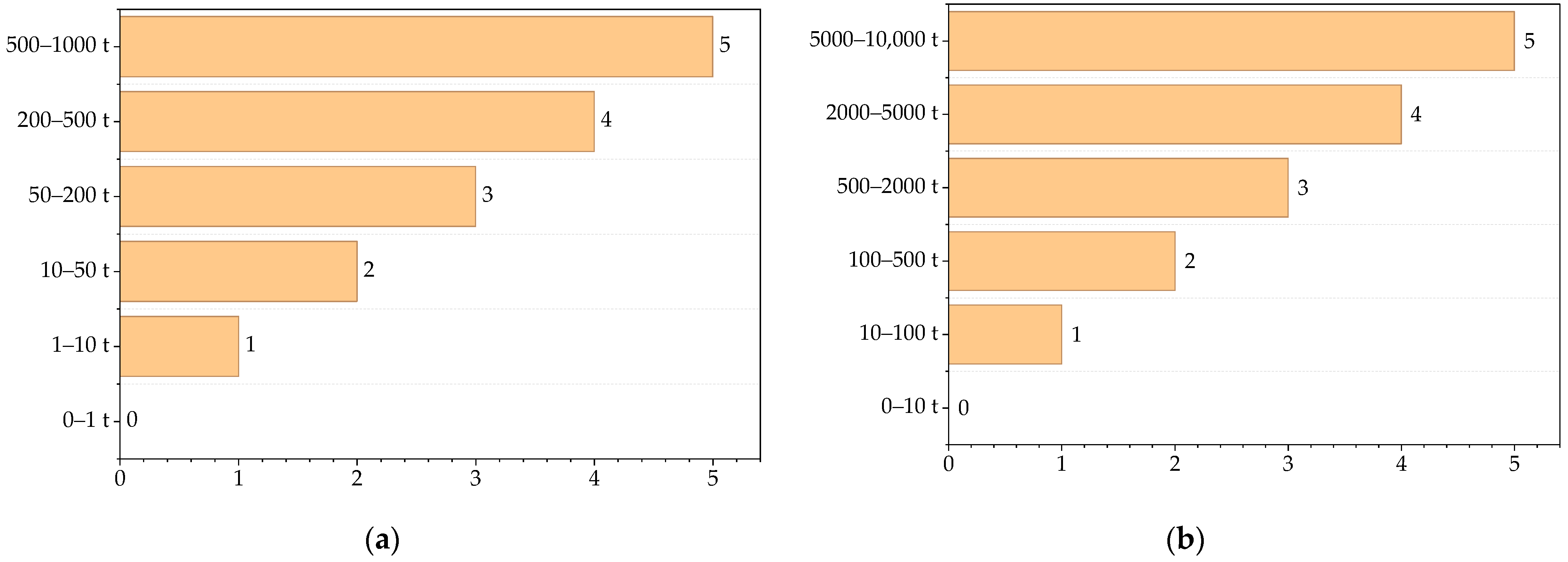

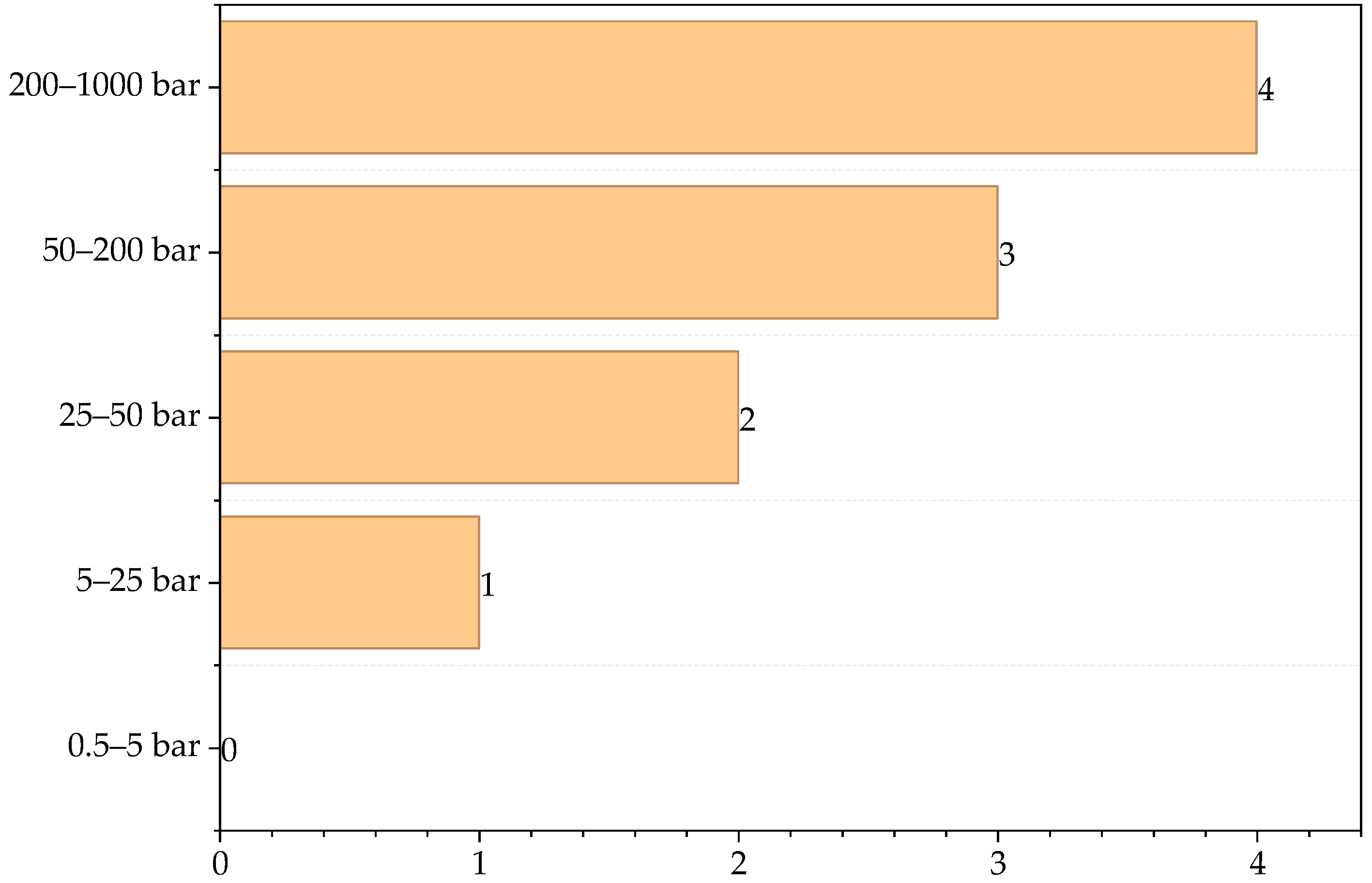


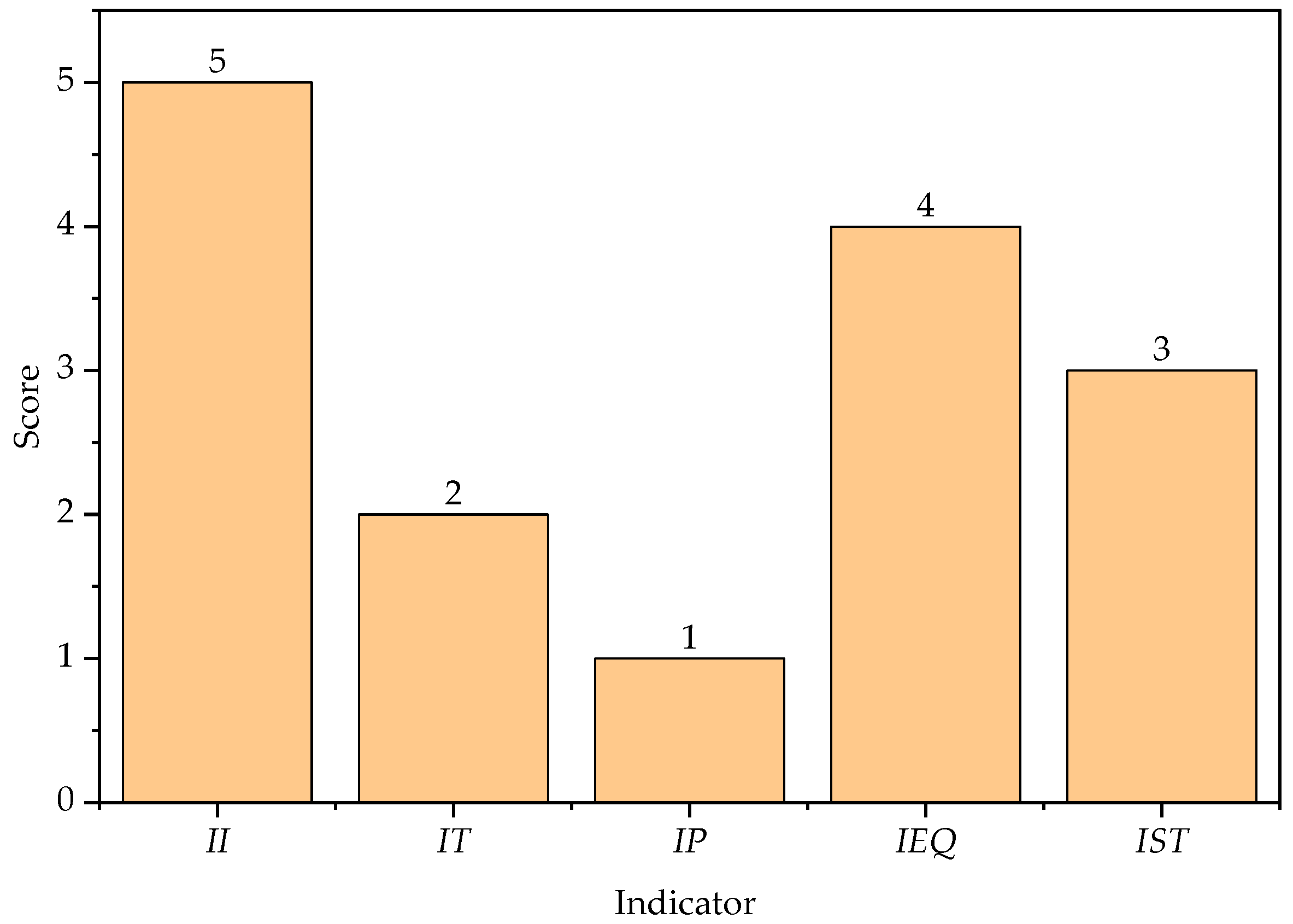
| Substance | Type of Interaction | Score |
|---|---|---|
| VCM | Explosion | 4 |
| Does not interact | 0 | |
| Does not interact | 0 | |
| PVC | Explosion | 4 |
| PVA | Formation of toxic gas 1 | 2 |
| Does not interact | 0 | |
| Maximum score | 4 | |
| Substance | Flash Point (°C)/Boiling Point (°C) | Flammability Type | Score |
|---|---|---|---|
| VCM | −78 | Highly flammable | 4 |
| - | Non-flammable | 0 | |
| - | Non-flammable | 0 | |
| PVC | - | - | 0 |
| PVA | 300 | Fuel | 1 |
| - | Non-flammable | 0 | |
| Maximum score | 4 | ||
| Substance | (UEL-LEL) % | Score |
|---|---|---|
| VCM | 29.40 | 2 |
| Non-explosive | 0 | |
| Non-explosive | 0 | |
| PVC | - | 0 |
| PVA | - | 0 |
| Non-explosive | 0 | |
| Maximum score | 2 | |
| Substance | TLV (ppm) | Score |
|---|---|---|
| VCM | 1 | 5 |
| Non-toxic | 0 | |
| Non-toxic | 0 | |
| PVC | 1 | 5 |
| PVA | - | 0 |
| Non-toxic | 0 | |
| Maximum score | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendivil-Arrieta, A.; Diaz-Pérez, J.M.; González-Delgado, Á.D. Effect of Energy Integration on Safety Indexes of Suspension PVC Production Process. Processes 2025, 13, 2926. https://doi.org/10.3390/pr13092926
Mendivil-Arrieta A, Diaz-Pérez JM, González-Delgado ÁD. Effect of Energy Integration on Safety Indexes of Suspension PVC Production Process. Processes. 2025; 13(9):2926. https://doi.org/10.3390/pr13092926
Chicago/Turabian StyleMendivil-Arrieta, Antonio, Juan Manuel Diaz-Pérez, and Ángel Darío González-Delgado. 2025. "Effect of Energy Integration on Safety Indexes of Suspension PVC Production Process" Processes 13, no. 9: 2926. https://doi.org/10.3390/pr13092926
APA StyleMendivil-Arrieta, A., Diaz-Pérez, J. M., & González-Delgado, Á. D. (2025). Effect of Energy Integration on Safety Indexes of Suspension PVC Production Process. Processes, 13(9), 2926. https://doi.org/10.3390/pr13092926








