Comparison of Statistical Process Control Models for Monitoring the Biological Burden of a Buffer Solution Used as Input to Produce an Attenuated Viral Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Buffer Solution Used in the Production of an Attenuated Viral Vaccine
2.2. Sample Collection for Biological Burden Analysis
2.3. Monitoring the Biological Load of the Buffer Solution
2.4. Statistical Process Controls for Monitoring Biological Load in Buffer Solutions
3. Results and Discussion
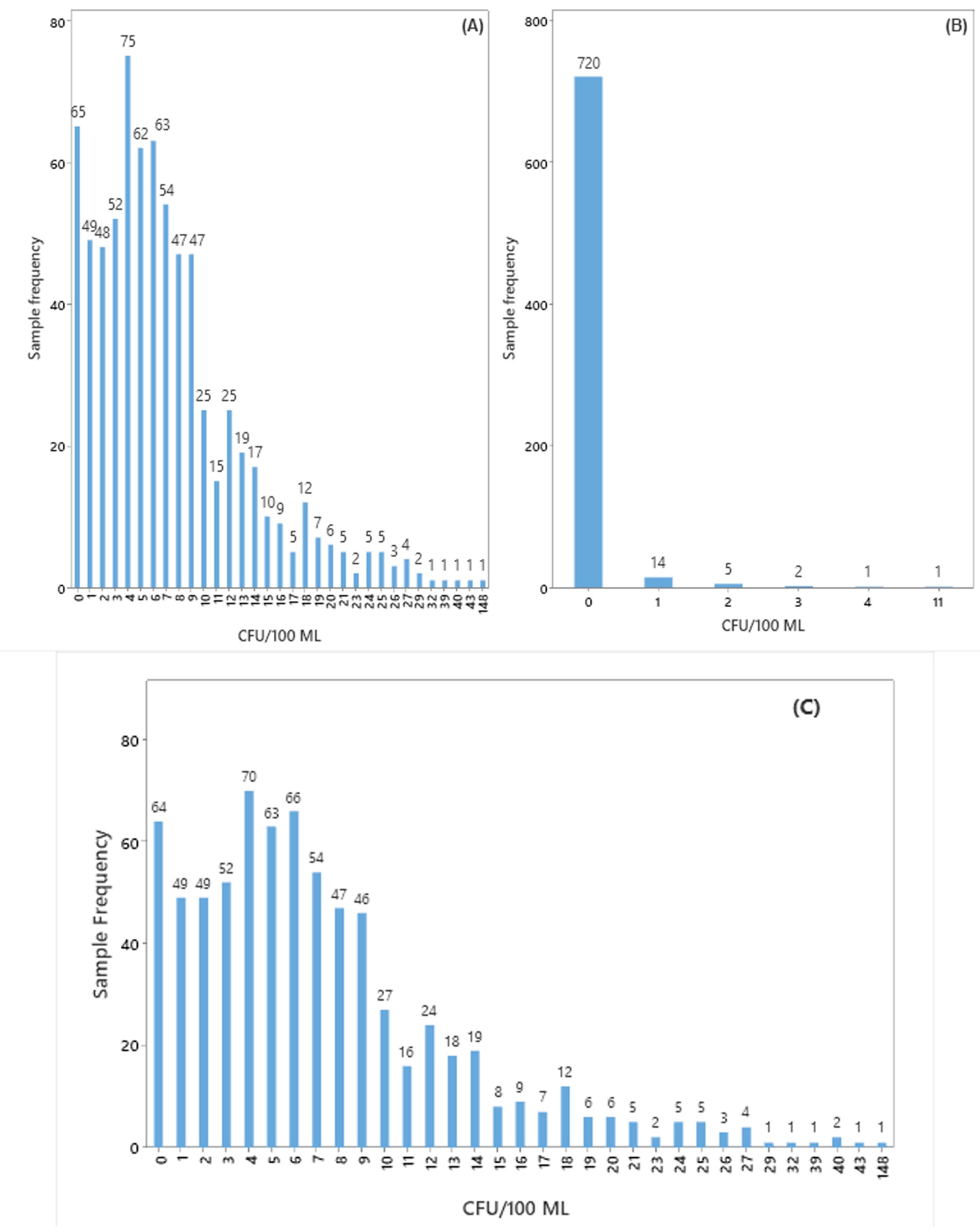
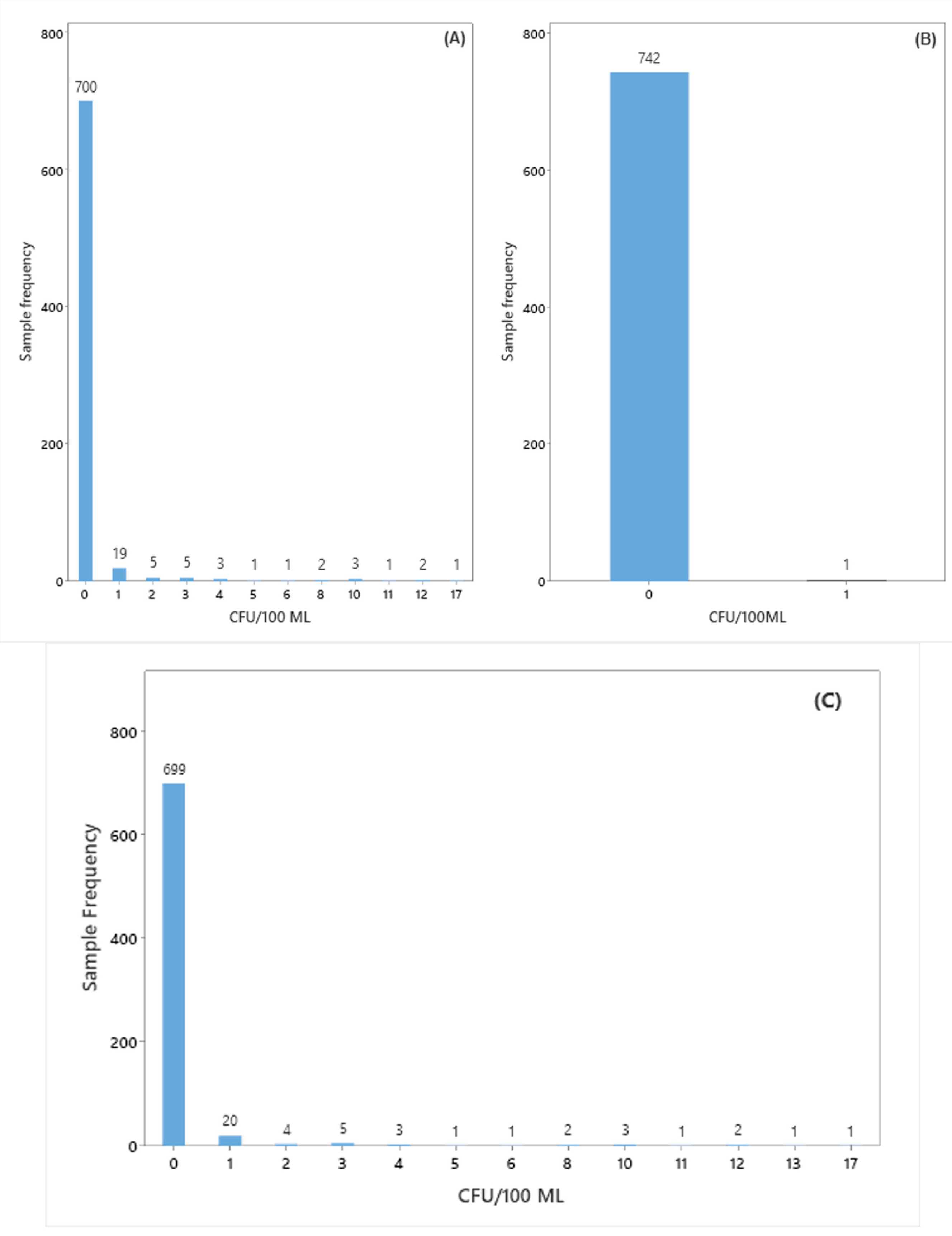
3.1. Identification of Microorganisms Present in the Bioburden of the Buffer Solution
3.2. Statistical Tools Used in Monitoring the Biological Burden of Buffer Solution
3.3. Evaluation of Factors That May Cause Contamination of the Buffer Solution Used in the Formulation of an Attenuated Viral Vaccine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nascimento, J.M.R.; Santos, M.R.; Quintilio, M.S.V. Quality control in the pharmaceutical industries. Rev. JRG Estud. Acad. 2022, 5, 43–55. [Google Scholar] [CrossRef]
- Mariani, C.A. Método PDCA e ferramentas da qualidade no gerenciamento de processos industriais: Um estudo de caso. Rev. Adm. Inovação 2005, 2, 110–126. [Google Scholar]
- Sharma, D.; Patel, P.; Shah, M.A. Comprehensive study on Industry 4.0 in the pharmaceutical industry for sustainable development. Environ. Sci. Pollut. Res. Int. 2023, 30, 90088–90098. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.A.N.; Lima, J.R.; Silva, J.L.; Alencar, J.R.B.; Soares-Sobrinho, J.L.; Lima, L.G.; Rolim-Neto, P.J. Application of statistical process control in the pharmaceutical industry. Rev. Basic. Farm. Sci. Apl. 2006, 27, 177–187. [Google Scholar]
- Maciel, T.H.; Branco, G.M.; Werner, L. Cartas de controle multivariadas: Estudo de caso em vinícolas italianas. Cad. IME Sér. Estat. UERJ 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Pérez-Benítez, B.E.; Tercero-Gómez, V.G.; Khakifirooz, M.A. Review on Statistical Process Control in Healthcare: Data-Driven Monitoring Schemes. IEEE Acess 2023, 11, 56248–56272. [Google Scholar] [CrossRef]
- Saidy, C.; Xia, K.; Kircaliali, A.; Harik, R.; Bayoumi, A. The Application of Statistical Quality Control Methods in Predictive Maintenance 4.0: An Unconventional Use of Statistical Process Control (SPC) Charts in Health Monitoring and Predictive Analytics. In Advances in Asset Management and Condition Monitoring; Smart Innovation, Systems and Technologies; Springer SBM: Cham, Switzerland, 2020; Volume 166, pp. 1051–1061. [Google Scholar]
- Kuk, E.; Bobek, S.; Nalepa, G.J. Explainable proactive control of industrial processes. J. Comput. Sci. 2024, 81, 102329. [Google Scholar] [CrossRef]
- Tran, P.H.; Nadi, A.A.; Nguyen, T.H.; Tran, K.D.; Tran, K.P. Application of Machine Learning in Statistical Process Control Charts: A survey and perspective. In Control Charts and Machine Learning for Anomaly Detection in Manufacturing; Springer Series in Reliability Engineering; Springer: Cham, Switzerland, 2021; pp. 7–42. [Google Scholar]
- Ramos, V.B.A.; Costa, L.V.; Pereira, G.R.; Miranda, R.V.S.L.; Valadão, T.B.; Brandão, M.L.L.; da Costa, L.V. Development of a methodology for classifying the criticality of isolated bacterial species in the environmental monitoring of the pharmaceutical industry producing immunobiologicals. In Biosafety and Bioprotection Symposium: High Biological Containment Environments, 1st ed.; Quality Week: From Compliance to Performance, 11; Instituto de Comunicação e Informação Científica e Tecnológica em Saúde (Icict/Fiocruz): Rio de Janeiro, Brazil, 2024; Available online: https://arca.fiocruz.br/items/4bce9071-0d2a-469c-a68c-7761f27f7181 (accessed on 30 October 2024).
- European Medicines Agency. Guideline on the Sterilisation of the Medicinal Product, Active Substance, Excipient and Primary Container; EMA/CHMP/CVMP/QWP/850374/2015; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- National Health Surveillance Agency. Normative Instruction No. 127, of March 30, 2022. Provides for Good Manufacturing Practices Complementary to Biological Inputs and Medicines; Official Gazette of the Union No. 62, Section 1; National Health Surveillance Agency: Brasília, Brazil, 2022. [Google Scholar]
- Mattoso, J.M.V.; Costa, L.V.; Vale, B.A.; Reis, C.M.F.; Andrade, J.M.; Braga, L.M.P.S.; Conceição, G.M.S.; Costa, P.B.M.; Silva, J.B.; Rodrigues, L.A.P.; et al. Quantitative and Qualitative Evaluation of Microorganism Profile Identified in Bioburden Analysis in a Biopharmaceutical Facility in Brazil: Criteria for Classification and Management of Results. PDA J. Pharm. Sci. Technol. 2025, 79, 125–156. [Google Scholar] [CrossRef]
- Bain, D.; Hardy, D.; Bell, B.L.; Forng, R.Y.; Knight, M.; Bawa, A.; Ramsey, S.; Arbesser-Rastburg, C.; Paul, M.; Ton, C.; et al. Microbial Monitoring For Biological Drug Substance Manufacturing: An Industry Perspective. PDA J. Pharm. Sci. Technol. 2015, 69, 451–460. [Google Scholar] [CrossRef]
- Kremer, T.; Patel, R. Correlation between Environmental Monitoring and Product Bioburden. Biomed. Instrum. Technol. 2019, 53, 32–37. [Google Scholar] [CrossRef]
- Santos, E.P. Guide to Good Immunization Practices in Remote, Hard-to-Reach Areas. Brazilian Society of Immunization. 2017. Available online: https://sbim.org.br/images/books/guia-imunizacao-areas-remotas.pdf (accessed on 30 October 2024).
- National Health Surveillance Agency. Normative Instruction No. 35, of August 21, 2019. Provides for Good Manufacturing Practices Complementary to Sterile Medicines; Official Gazette of the Union No. 162, Section 1; National Health Surveillance Agency: Brasília, Brazil, 2019. [Google Scholar]
- European Commission. Rules Governing Medicinal Products in the European Union, Volume 4, EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use, Annex 1—Manufacture of Sterile Medicinal Products; European Commission: Luxembourg, 2022. [Google Scholar]
- National Health Surveillance Agency. Brazilian Pharmacopoeia. <5.5.3.1.2> Counting the Total Number of Mesophilic Microorganisms, 7th ed.; National Health Surveillance Agency: Brasília, Brazil, 2024; pp. 110–111. Available online: https://anvisalegis.datalegis.net/action/UrlPublicasAction.php?acao=abrirAtoPublico&num_ato=00000940&sgl_tipo=RDC&sgl_orgao=ANVISA/MS&vlr_ano=2024&seq_ato=222&cod_modulo=134&cod_menu=1696 (accessed on 30 October 2024).
- United States Pharmaceuticals. National Formulary. In Microbiological Examination of Nonsterile Products: Enumeration Tests; U.S. Pharmacopeia: Rockville, MD, USA, 2022; Chapter 61; Available online: https://www.usp.org/harmonization-standards/pdg/general-methods/microbial-enumeration (accessed on 30 October 2024).
- Tripathi, N.; Zubair, M.; Sapra, A. Gram staining. In StatPearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562156/ (accessed on 14 August 2023).
- MINITAB. Help and How to Improve Quality and Processes with Control Charts, Supporting Topics for Understanding Attributes, Control Charts, Overdispersion and Underdispersion. 2024. Available online: https://support.minitab.com/en-us/minitab/help-and-how-to/quality-and-process-improvement/control-charts/supporting-topics/basics/understanding-control-charts/ (accessed on 30 October 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Simões, A.; Veiga, F.; Vitorino, C. Question-based review for pharmaceutical development: An enhanced quality approach. Eur. J. Pharm. Biopharm. 2024, 195, 114174. [Google Scholar] [CrossRef]
- Rogers, A.; Bullard, K.; Dod, A.; Wang, Y. Bacterial Growth Curve Measurements with a Multimode Microplate Reader. Bio Protoc. 2022, 12, e4410. [Google Scholar] [CrossRef]
- Sandle, T. Improving microbiological assurance for bioburden tests Improving microbiological assurance for bioburden testing. Eur. Pharm. Rev. 2016, 21, 41–44. Available online: https://www.researchgate.net/publication/305114253 (accessed on 30 October 2024).
- Song, M.; Li, Q.; Liu, C.; Wang, P.; Qin, F.; Zhang, L.; Fan, Y.; Shao, H.; Chen, G.; Yang, M. A comprehensive technology strategy for microbial identification and contamination investigation in the sterile drug manufacturing facility—A case study. Front. Microbiol. 2024, 15, 1327175. [Google Scholar] [CrossRef]
- Caldeira, N.G.S.; de Souza, M.L.S.; de Miranda, R.V.S.L.; Costa, L.V.; Forsythe, S.J.; Zahner, V.; Brandão, M.L.L. Characterization by MALDI-TOF MS and 16S rRNA Gene Sequencing of Aerobic Endospore-Forming Bacteria Isolated from Pharmaceutical Facility in Rio de Janeiro, Brazil. Microorganisms 2024, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, D.; Złoch, M.; Pomastowski, P.; Szultka-Młyńska, M. Implications of Sample Preparation Methods on the MALDI-TOF MS Identification of Spore-Forming Bacillus Species from Food Samples: A Closer Look at Bacillus licheniformis, Peribacillus simplex, Lysinibacillus fusiformis, Bacillus flexus, and Bacillus marisflavi. ACS Omega 2023, 8, 34982–34994. [Google Scholar] [PubMed]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Dai, H.; Ma, L.; Xu, Z.; Soteyome, T.; Yuan, L.; Yang, Z. Invited review: Role of Bacillus licheniformis in the dairy industry—Friends or foes? J. Dairy Sci. 2024, 107, 7520–7532. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Cai, M.; Hu, Y.; Ge, C.; Wang, X.; Duan, R. Bacillus licheniformis bloodstream infections associated with oral probiotic administration: Two case reports. Indian J. Med. Microbiol. 2024, 47, 100485. [Google Scholar] [CrossRef]
- Wei, M.P.; Yu, H.; Guo, Y.H.; Cheng, Y.L.; Xie, Y.F.; Yao, W.R. Antibacterial activity of Sapindus saponins against microorganisms related to food hygiene and the synergistic action mode of Sapindoside A and B against Micrococcus luteus in vitro. Food Control 2021, 130, 108337. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Q.; Li, Z.; Kang, Z.; Zhang, L. Kocuria species: An underappreciated pathogen in pediatric patients—A single-center retrospective analysis of 10 years’ experience in China. Diagn. Microbiol. Infect. Dis. 2023, 107, 116078. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Araújo, D.; Oliveira, R.; Silva, B.L.; Castro, J.; Ramos, C.; Matos, F.; Almeida, C.; Silva, S. Antimicrobial resistance patterns of Staphylococcus spp. isolated from clinical specimens of companion animals in Northern Portugal, 2021–2023. Vet. J. 2024, 1, 305. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.C.; Ahmad, F.; Giambiagi-deMarval, M. Staphylococcus haemolyticus: An updated review on nosocomial infections, antimicrobial resistance, virulence, genetic traits, and strategies for combating this emerging opportunistic pathogen. Microbiol. Res. 2024, 282, 127652. [Google Scholar] [CrossRef]
- Sigudu, T.T.; Oguttu, J.W.; Qekwana, D.N. Antimicrobial Resistance of Staphylococcus spp. from Human Specimens Submitted to Diagnostic Laboratories in South Africa, 2012–2017. Microorganisms 2024, 12, 1862. [Google Scholar] [CrossRef]
- Sigudu, T.T.; Oguttu, J.W.; Qekwana, D.N. Prevalence of Staphylococcus spp. from human specimens submitted to diagnostic laboratories in South Africa, 2012–2017. S. Afr. J. Infect. Dis. 2023, 38, 477. [Google Scholar] [CrossRef]
- Lima, J.C. Identificação e avaliação da hemólise e biofilme de Staphylococcus spp. isoladas de uma indústria farmacêutica. In Proceedings of the Reunião Anual da FESBE, Búzios, Brazil, 27–30 August 2023; FeSBE: Búzios, Brazil, 2023. Available online: https://arca.fiocruz.br/items/0db4ff2e-ee75-4b1a-b336-58461fe7801f (accessed on 30 October 2024).
- Vale, B.A.; Lima, J.C.; Souza, P.A.; Miranda, R.V.S.L.; Brandão, M.L.L.; Costa, L.V.; Tom, H.K. Characterization of Staphylococcus hominis strains isolated in an immunobiological pharmaceutical unit. In Proceedings of the Congresso Brasileiro de Microbiologia 2023, Foz do Iguaçu, Brazil, 18–22 October 2023; 145-1. Available online: https://sbmicrobiologia.org.br/32cbm-anais/resumos/R-0145-1.html (accessed on 30 October 2024).
- Loureiro, J.M.P.; Guimarães, R.C.C.; Valadão, T.B.; Miranda, R.V.S.L.; Andrade, J.M.; Costa, I.V.; Brandão, M.L.L. Application of Fourier-Transform Infrared Spectroscopy (FT-IR) for Staphylococcus epidermidis Typing as a Tool for Contamination Control Strategy in a Pharmaceutical Industry Facility. PDA J. Pharm. Sci. Technol. Novemb. 2024, 78, 761–762. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Primo, L.M.D.G.; Franzyk, H.; Hansen, P.R.; Pavan, F.R. Recent advances in the development of antimicrobial peptides against ESKAPE pathogens. Heliyon 2024, 10, e31958. [Google Scholar] [CrossRef]
- Ribeiro, T.A.N.; dos Santos, G.A.; dos Santos, C.T.; Soares, D.C.F.; Saraiva, M.F.; Leal, D.H.S.; Sachs, D. Eugenol as a promising antibiofilm and anti-quorum sensing agent: A systematic review. Microb. Pathog. 2024, 196, 106937. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Attili, A.R.; De Martino, L. Acinetobacter baumannii: Its Clinical Significance in Human and Veterinary Medicine. Pathogens 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Idris, F.N.; Nadzir, M.M. Multi-drug resistant ESKAPE pathogens and the uses of plants as their antimicrobial agents. Arch. Microbiol. 2023, 205, 115. [Google Scholar] [CrossRef]
- Vasconcellos, L.; Silva, S.V.; da Costa, L.V.; de Miranda, R.V.d.S.L.; dos Reis, C.M.F.; Braga, L.M.P.S.; Silva, C.; Conceição, G.; Mattoso, J.; Silva, I.B.; et al. Phenotypical and molecular characterization of Acinetobacter spp. isolated from a pharmaceutical facility. Lett. Appl. Microbiol. 2023, 76, ovad101. [Google Scholar] [CrossRef]
- Gordon, O.; Goverde, M.; Pazdan, J.; Staerk, A.; Roesti, D. Comparison of Different Calculation Approaches for Defining Microbiological Control Levels Based on Historical Data. PDA J. Pharm. Sci. Technol. 2015, 69, 383–398. [Google Scholar] [CrossRef]
- Sousa, B.K. Análise de Diagnóstico em Modelos de Regressão Bell. Master’s Thesis, Centro de Ciências Exatas e da Terra, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2023. [Google Scholar]
- Eissa, M.E. Variable and Attribute Control Charts in Trend Analysis of Active Pharmaceutical Components: Process Efficiency Monitoring and Comparative Study. Exp. Med. 2018, 1, 31–44. [Google Scholar]
- Eissa, M.E. Application of Laney control chart in assessment of microbiological quality of oral pharmaceutical filterable products. Bangladesh J. Sci. Ind. Res. 2017, 52, 239–246. [Google Scholar] [CrossRef][Green Version]
- Montgomery, D.C. Introduction to Statistical Quality Control, 7th ed.; LTC: Rio de Janeiro, Brazil, 2016; p. 572. [Google Scholar]
- Moon, J. An Investigation into the Use of Laney U Chart as a Visual Schedule Tracker to Graphically Monitor the Schedule Performance Index. J. Eng. Proj. Prod. Manag. 2020, 10, 35–41. [Google Scholar][Green Version]
- Mora, J.G.M.; Guzmán, B.A.F.; Suárez, J.H.; León, J.J.M. Aplicación del Control Estadístico de Procesos: Un caso de estudio. Cienc. Lat. Rev. Científica Multidiscip. 2025, 9, 440–462. [Google Scholar] [CrossRef]
- Castellares, F.; Ferrari, S.L.P.; Lemonte, A.J. On the Bell distribution and its associated regression model for count data. Appl. Math. Model. 2018, 56, 172–185. [Google Scholar] [CrossRef]
- Boaventura, L.L.; Ferreira, P.H.; Fiaccone, R.L.; Ramos, P.L.; Louzada, F. New statistical process control charts for overdispersed count data based on the Bell distribution. An. Acad. Bras. Cienc. 2023, 95, e20200246. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.; Miranda, R.; Santos, M.; Costa, L.; Silva, R.; Miranda, C.; Boas, M.; Brandão, M. Evaluation of Fourier-Transform Infrared Spectroscopy as a rapid method to type Stenotrophomonas maltophilia strains isolated from pharmaceutical industry. In Annals of the Symposium: Building Pathways to Accelerate the Development of the National Technological Innovation Ecosystem; Institute of Technology in Immunobiologicals: Rio de Janeiro, Brazil, 2024; p. 123. [Google Scholar]

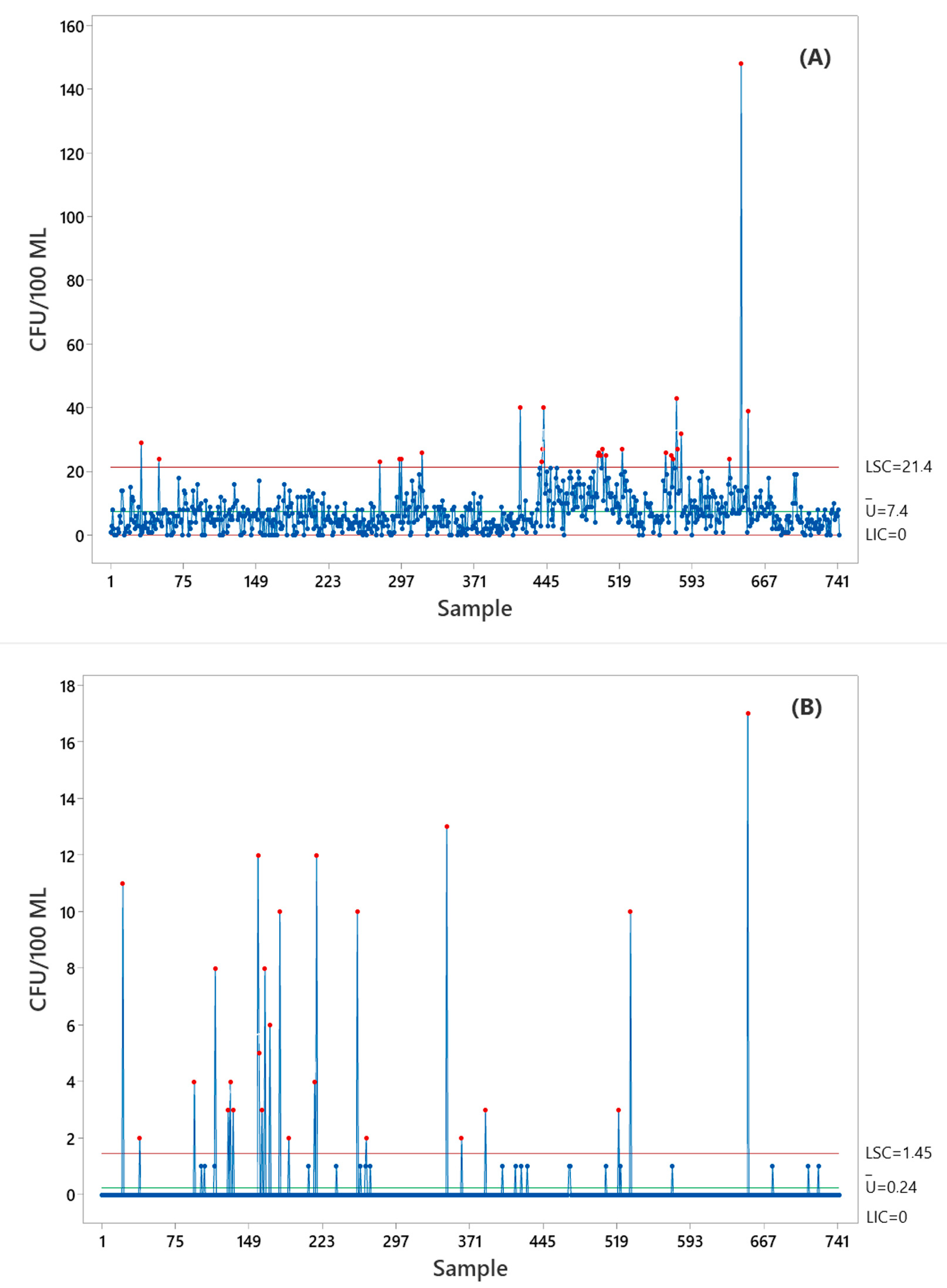
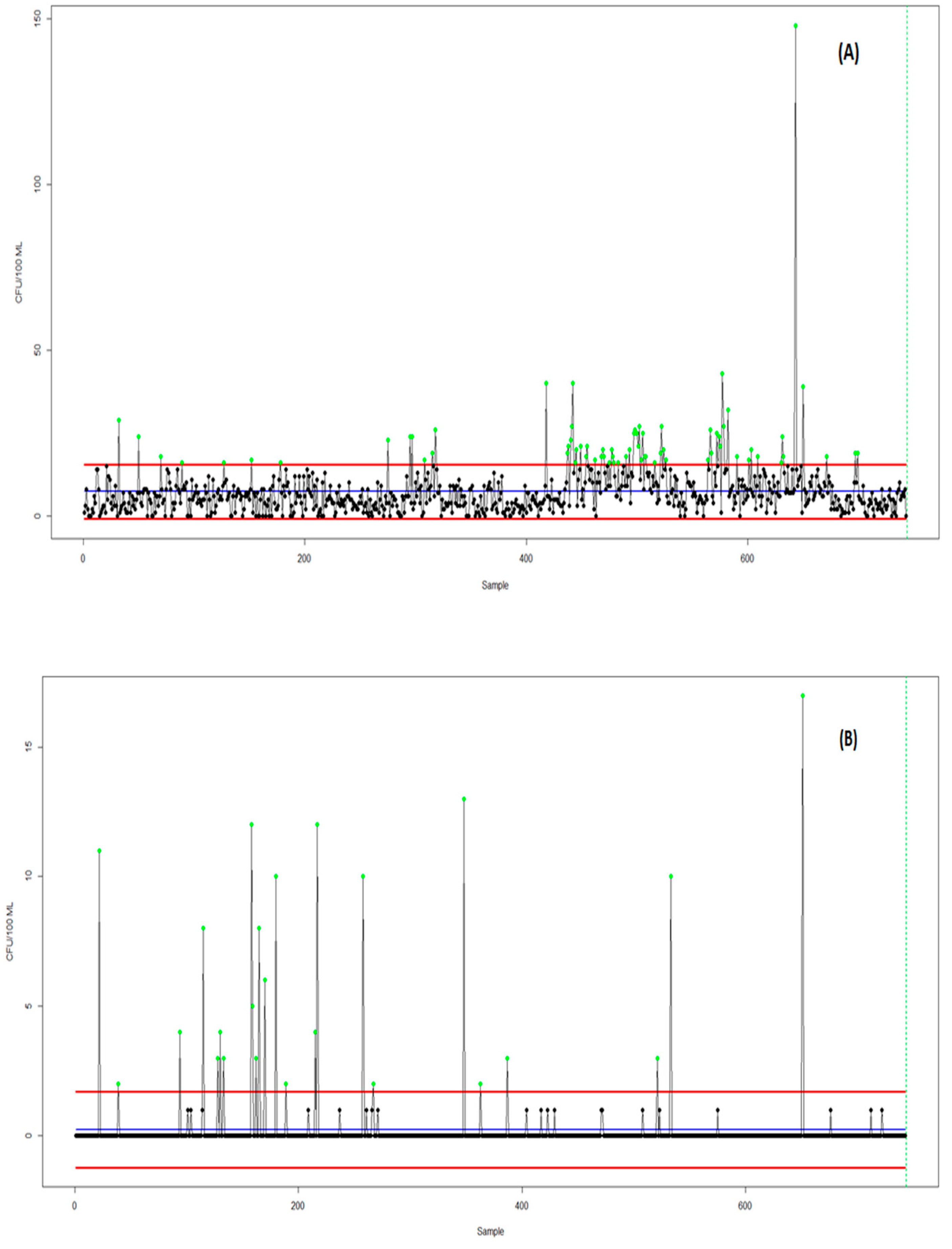
| Criteria | Laney U-Chart | Bell Distribution Chart |
|---|---|---|
| Number of Points | 743 | 743 |
| Mean (CL) | 7.38 | 7.38 |
| Empirical Dispersion (φ) | 8.90 (strong overdispersion) | (modeled by the dispersion parameter) |
| σ_z (Laney) | 1.72 | — |
| Predicted Variance | — | 54.44 |
| Outlier | 26 (3.5%) | 6 (0.8%) |
| Residuals—Minimum | −1.58 | −1.00 |
| Residuals—Median | −0.29 | −0.19 |
| Residuals—Maximum | 30.08 | 19.06 |
| Shapiro–Wilk (normalidade) | p < 0.0001 → rejects normality | p < 0.0001 → rejects normality |
| Ljung–Box (independence) | p < 2.2 × 10−16 → significant autocorrelation | p < 2.2 × 10−16 → significant autocorrelation |
| χ2/gL (Pearson coefficient) | 8.90 | 1.21 |
| MAR (mean standardized residual) | 1.02 | 0.65 |
| AIC (information criterion) | — | 4862.1 (compared to Poisson, reference = 6811.1) |
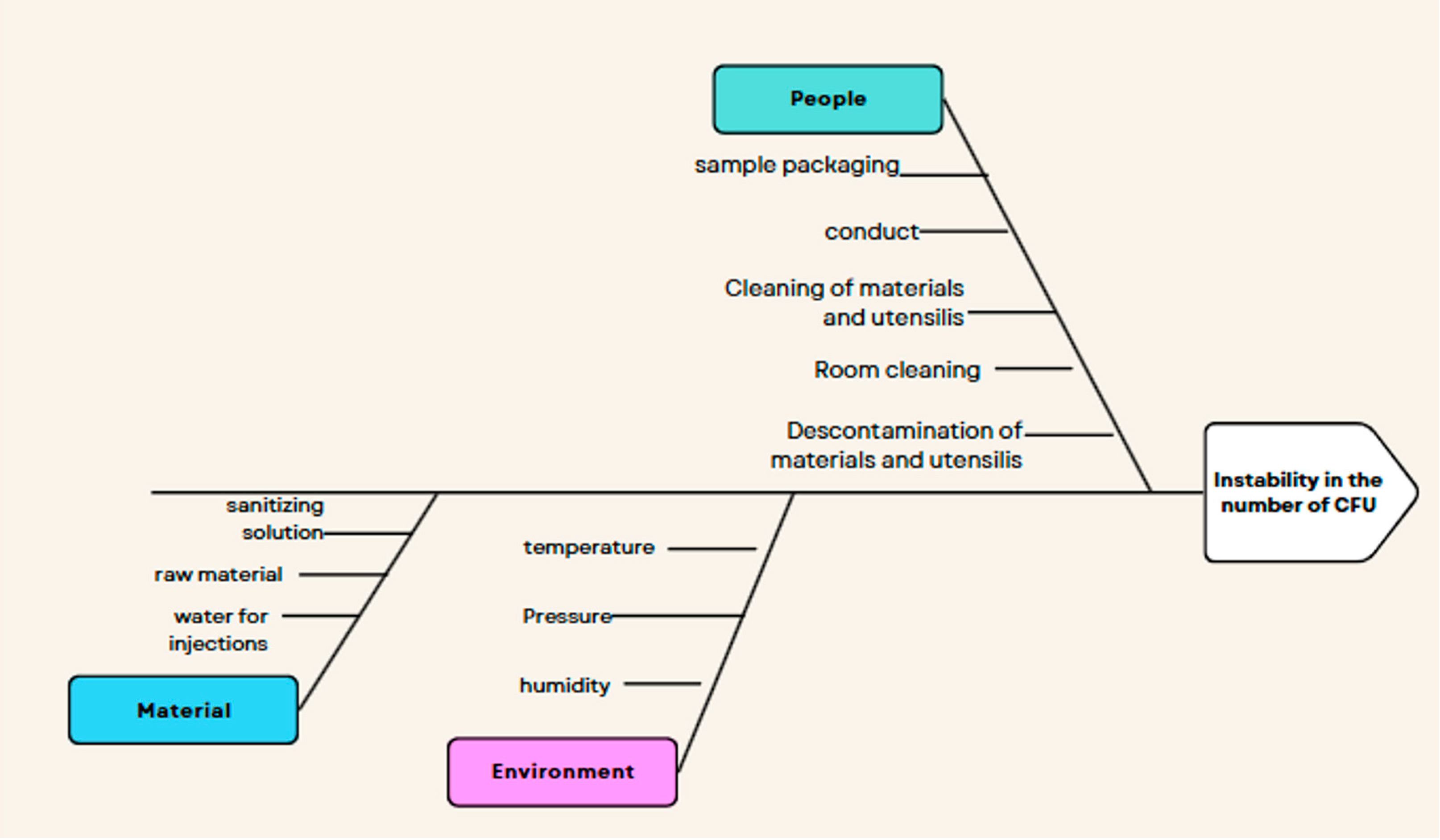
| Factors | Impacts |
|---|---|
| Labor |
|
| Raw materials |
|
| Storage and/or packaging of the buffer solution |
|
| Sanitizing solution |
|
| Water for injections (WFI) |
|
| Cleaning of the preparation room |
|
| Decontamination of materials and utensils |
|
| Room pressure |
|
| Relative humidity of the environment |
|
| Room temperature |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattoso, J.M.V.; Conceição, G.M.S.d.; Silva, A.P.R.d.; Miranda, P.V.P.; Rodrigues, L.d.A.P.; Brandão, M.L.L.; dos Anjos, J.P. Comparison of Statistical Process Control Models for Monitoring the Biological Burden of a Buffer Solution Used as Input to Produce an Attenuated Viral Vaccine. Processes 2025, 13, 2917. https://doi.org/10.3390/pr13092917
Mattoso JMV, Conceição GMSd, Silva APRd, Miranda PVP, Rodrigues LdAP, Brandão MLL, dos Anjos JP. Comparison of Statistical Process Control Models for Monitoring the Biological Burden of a Buffer Solution Used as Input to Produce an Attenuated Viral Vaccine. Processes. 2025; 13(9):2917. https://doi.org/10.3390/pr13092917
Chicago/Turabian StyleMattoso, Josiane Machado Vieira, Greice Maria Silva da Conceição, Ana Paula Roque da Silva, Paulo Vinicius Pereira Miranda, Letícia de Alencar Pereira Rodrigues, Marcelo Luiz Lima Brandão, and Jeancarlo Pereira dos Anjos. 2025. "Comparison of Statistical Process Control Models for Monitoring the Biological Burden of a Buffer Solution Used as Input to Produce an Attenuated Viral Vaccine" Processes 13, no. 9: 2917. https://doi.org/10.3390/pr13092917
APA StyleMattoso, J. M. V., Conceição, G. M. S. d., Silva, A. P. R. d., Miranda, P. V. P., Rodrigues, L. d. A. P., Brandão, M. L. L., & dos Anjos, J. P. (2025). Comparison of Statistical Process Control Models for Monitoring the Biological Burden of a Buffer Solution Used as Input to Produce an Attenuated Viral Vaccine. Processes, 13(9), 2917. https://doi.org/10.3390/pr13092917