Electrocoagulation for the Removal of Antibiotics and Resistant Bacteria: Advances and Synergistic Technologies
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Analysis
- The presence of drugs related to the COVID-19 pandemic (antibiotics such as tetracycline and sulfamethoxazole) or the removal of microplastics.
- Coupling of electrochemically assisted coagulation with other treatments such as reverse osmosis, activated carbon, use of membranes, photocatalysis, or biodegradation, in addition to the analysis of generated sludge and coagulant by scanning electron microscopy.
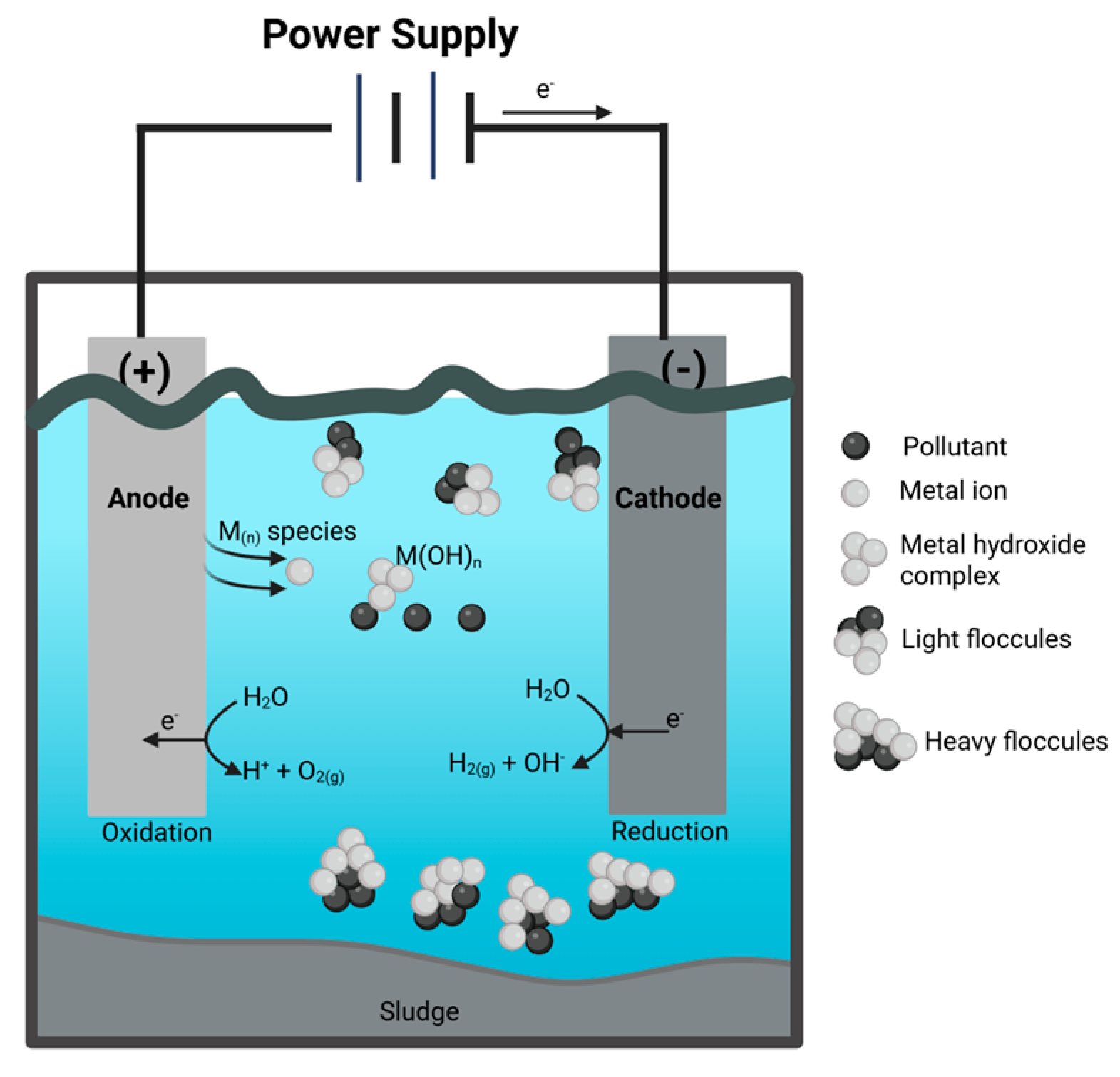
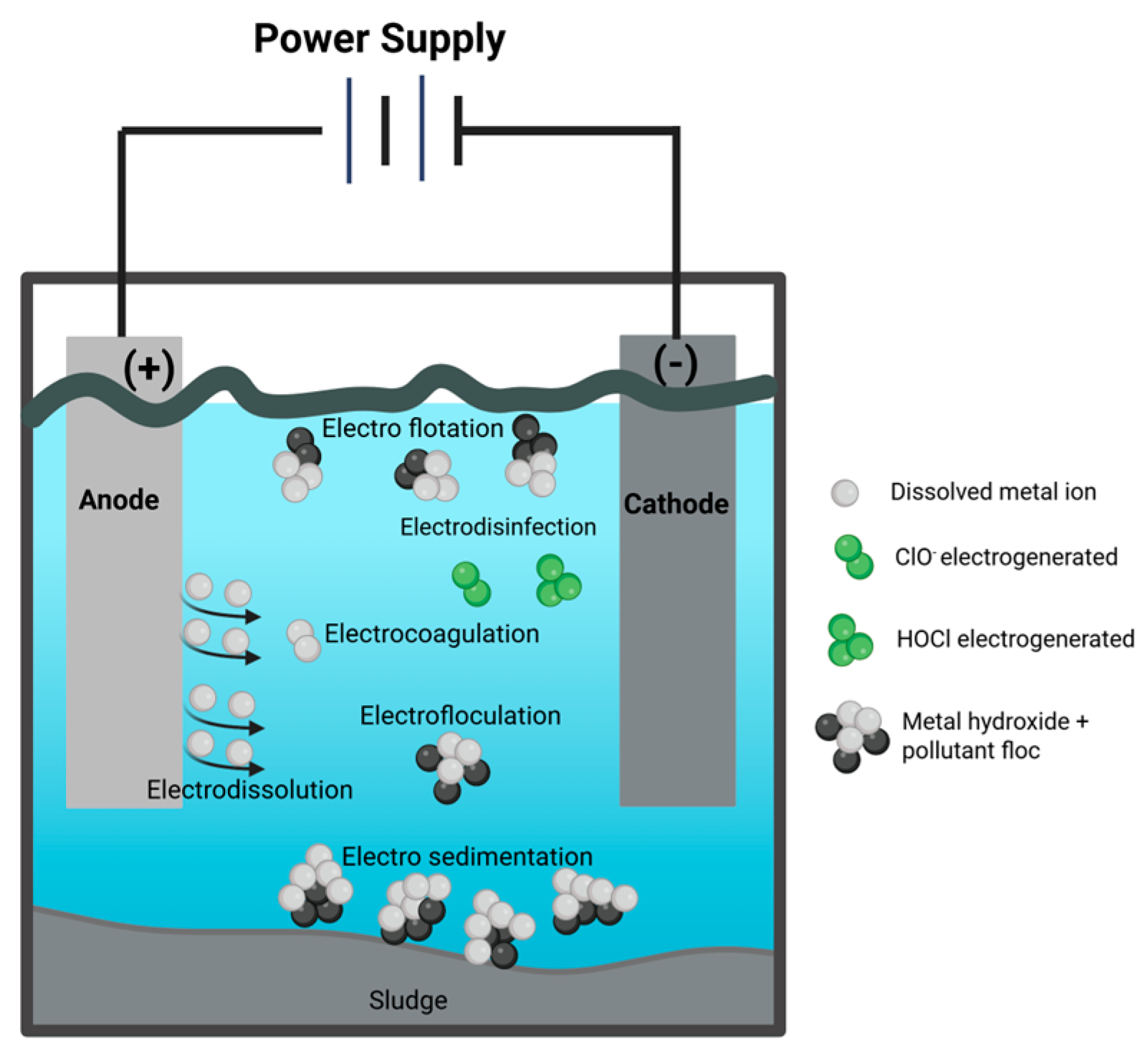
3.2. Basic Principles of Electrocoagulation
- Formation of the electrocoagulant by electrolytic oxidation of the anode metal.
- Destabilization of contaminants and blends.
- Formation of flocs through the aggregation of contaminant particles or the adsorption of these particles by the coagulant.
- Electrodissociation occurs when the electrode dissociates due to the passage of electric charge in the system, generating the necessary force to release metal ions from the solid into the liquid. The dissolution rate is not significantly affected by salinity.
- Electrocoagulation: Starts when the electrocoagulant is formed.
- Electro flocculation: Flocculation of the material to be removed.
- Electro flotation: Generated by the bubbling of gases produced at the electrodes.
- Electro-sedimentation: This is due to the increase in solids that have been removed and their higher density.
- Disinfection: It can occur if sodium chloride is added as an electrolyte or if chlorinated compounds are present in the water.
3.3. Considerations for Water Treatment Application
3.3.1. Material
| Electrode Material | Applicability | Type of Water | Target Pollutant | Pollutant Load | Removal | Findings | References |
|---|---|---|---|---|---|---|---|
| Al/Al Fe/Fe Fe/Al | Wastewater treatment | Real | COD of pharmaceutical compounds | 7692 mgL−1 | 92.3% | The best combination is Fe/Al | [37] |
| SS and Al | Disinfection | Synthetic WW | E. coli | 5 × 105 UFC/100 mL | 100% | Iron hydroxide was a better inactivant | [40] |
| Al CS | Disinfection of wastewater effluents | Spiked water | E. coli | 5 × 105– 4 × 107 UFC/100 mL | More than 5log10 | Selection of material depends on the initial pH | [41] |
| Al/Fe | HWW | Real spiked water | CFZ | 0.423 mgL−1 | 94.6% | Able to remove cefazolin | [42] |
| Al Fe | Effluents with the formation of recalcitrant metal complexes | Synthetic spiked WW | TC TC:Ni | 15 mgL−1 | TC: Fe: 99.3% Al: 99.8% TC-N: 1:1: 100% 1:2: 99.6% | The ratio TC:Ni influences the removal efficiency. | [38] |
| Carbon steel anode | Effluents with the formation of recalcitrant metal complexes | Synthetic spiked WW | TC TC:Cu | 24.05 mgL−1 | TC-100% TOC-80.2% Cu2+-8.1% | Promising to remove TC-Cu complexes | [42] |
| Al and SS 304 anodes | WW | Real spiked wáter | AMX TMP | 10 mgL−1 | Al: AMX 21.52% AMX + TMP 7.84% SS 304: TMP 13.10% | Determination of corrosion velocities | [43] |
| Al Fe | GW | Real | COD COT E. coli | COD (460 mgL−1) COT (185 mgL−1) E. coli- (2−2.8 × 103 CFU/100 mL) | EC + O3: Al: COD 65% Fe: COD 85.8% TOC 71.14% E. coli 85.42% EC (Fe) +O3 + UV: COD 95.65% COT 87.35% E. coli 96.88% | Better removals with the iron electrode | [44] |
| Al And LCS Anodes | MWW | Spiked synthetic | AMP DOX STZ Tylosin | 50 mgL−1 each | AMP 3.6 ± 3.2% DOX ~100% STZ 3.3 ± 0.4% Tylosin 3.1 ± 0.3% | DOX was the only antibiotic effectively removed | [17] |
| Fe Al | WW | Simulated | TC COT | 0.05 mmolL−1 | Fe: TC-99.6% COT-79.8% Al: TC-97% COT-77% | The iron electrode showed higher performance. | [45] |
| For bath water: Al/Al For laundry water: Al/Fe (mild steel) | GW | Bath water (synthetic) Laundry water (synthetic and real spiked water) | E. coli BOD | Synthetic bath water: BOD 159 mgL−1 Synthetic laundry water: BOD 243 mgL−1 Spiked real laundry water: E. Coli: 105.6 CFUmL−1 | Bath water: BOD-51.8% Laundry water: E. coli: ≥6.1 log reduction | Al/Al and Al/Fe had the best results | [46] |
| Al/N doped porous carbon loaded with Co/Fe sites (N-Co/Fe-PC) | WW containing copper and antibiotics | Synthetic | Cu CIP COT | 20 mgL−1 | Cu-99.69% CIP-96.40% COT-83.62% | The cathode material withstands up to 6 times of reuse. Loss of 15%. | [47] |
| Fe/carbon felt, Al, SS cathodes | DWW | Real | E. coli | 392 ± 100 × 106 MPN mL−1 | 99.99% | Optimal combination: an iron anode and a carbon felt cathode. | [48] |
| Al/Al (non-insulated and Insulated) | PWW | Synthetic | CFX | 30.16 mgL−1 34.26 mgL−1 | 88.21% 81.73% | Insulated electrodes reduce the dispersion of electrical current, | [49] |
| Fe/Fe | RW | Simulated | E. coli Total coliforms Enterococci Phages | Total Coliforms 5 log(MNP/100 mL) E. coli 4.5 log(MNP/100 mL) Enterococci 3.5 log(MNP/100 mL) Somatic Coliphage: 3.5 log(MNP/100 mL) | E. coli: 1.7 log Total coliforms: 1.5 log Enterococci: 1.0 log Phages: 2.0 log | Fe/Fe combination was the best option. | [50] |
3.3.2. Electrode Distance
| Electrode Material (Anode/Cathode) | Electrode Spacing (cm) | Applicability | Type of Water | Target Pollutant | Pollutant Load | Removal | References |
|---|---|---|---|---|---|---|---|
| Al/Al | 1.0 | WW | Synthetic | CPX | 50 mgL−1 | 98.48% | [52] |
| Al/Fe | 2.0 | WW with recalcitrant metal complexes | Synthetic | TC TC:Cu | 15 mgL−1 | TC: >99% TC:Cu: 1:1-100% 1:2-99.6% | [38] |
| Fe/Fe | 3.0 | PWW | Synthetic | MZN | 21.6 mgL−1 | 100% | [53] |
| Steel/Steel | 0.5 | WW | Real | E. coli | - | 96% | [54] |
| Al anode | 0.5 | PW | Synthetic | E. coli | 105 UFCL−1 | 100% | [55] |
| Al (chitosan as adsorbent) | 1.0 | HWW | Synthetic | CFZ | 60 mgL−1 | 100% | [56] |
| Fe/Fe | 1.58 | HWW | Simulated | CIP | 60 mgL−1 | 100% | [57] |
| Fe/Fe | 1.25 | HWW | Real | AZM | - | 92.3% | [58] |
| Al/Al | 1.0 | DCWW | Synthetic and real | CIP | Synthetic sample: 32.5 mg L−1 Real sample: 154 ± 6 μg L−1 | >88.00% | [59] |
| Fe/Fe | 1.5 | DCWW | Simulated | CAP | 30 mgL−1 | PSPC-EC: 98.85% DC-EC: 98.28% APC-EC: 98.36% | [60] |
| Perforated SS sheets | 0.5 | WW | Synthetic | CIP LVX | 25 mgL−1 | CIP: 93.47% LVX: 88% CIP:LVX: 1:1- 93.0:91.8% 1:4- 90.10:96.10% 4:1- 96.30%:92.97% | [61] |
| Al/Pt | 4.0 | SW | Simulated | E. coli K-12 with plasmid RP4 carrying blaTEM, tetR and aphA | 1 × 108 CFUmL−1 | ARB-3.04 log reduction | [62] |
3.3.3. Number of Electrodes
- The amount of metal ions to be supplied in the water matrix. A higher number of electrodes will promote a higher number of metal ions, resulting in better removal of the pollutant load. However, producing an excess of dissolved metals carries risks: it can significantly increase the volume of sludge produced, or, if the metals do not fully react, the treated liquid will retain a high concentration of unwanted metals.
- Electrical requirements: A greater presence of electrodes reduces the amount of energy supplied, since it no longer requires a large force to produce the metal ions. This primarily impacts the economic analysis, as lower energy consumption implies lower costs and makes the EC process more cost-effective.
3.3.4. Voltage Supplied
3.3.5. Current Intensity or Current Density—Which Parameter Is More Appropriate?
| Target Pollutant | Removal | Applicability | Type of Water | Type of Current (I = Electrical Intensity; j = Current Density) | Optimal Value | References |
|---|---|---|---|---|---|---|
| COD of pharmaceutical compounds | 92.3% | Effluents of PWW | Real | I (DC power supply) | 40 mA | [37] |
| Total coliforms E. coli | 2.3 log10 2.35 log10 | Vertical wetland effluent from domestic water | Real | I (DC power supply) | 1300 mA | [67] |
| TC | 99.6% | WW | Simulated | I (DC power supply) | 0.3 mA | [45] |
| TC | 95% | Livestock WW | Synthetic | I (Positive single pulse current, PSPC) | 200 mA | [68] |
| CQ | 95% | PWW | Spiked tap water | j (DC power supply) | 66.89 mAcm−2 | [69] |
| Fecal contamination (total coliforms, fecal coliforms and enterococci) | 100% | WW reuse for agriculture | Real | I (AC power supply) | 500 mA | [70] |
| COD (β-lactam antibiotic derivatives), and TC antibiotics | 75.64% | PWW | Real | j (DC power supply) | 46.83 mAcm−2 | [71] |
| CIP | 98.48% | WW | Synthetic | j (DC power supply) | 1.5 mAcm−2 | [52] |
| E. coli | GW and TW: 2.22–2.53 log10 units Tap water: 3.80 log10 units | GW TW Tap water | Real Real Spiked | j (DC power supply) | 1.0 mAcm−2 | [72] |
| TC TOC TC-Cu | TC-100% TOC-80.2% Cu2+-88.1% | Water with metal–organic complexes | Synthetic spiked | j (DC power supply) | 4.17 × 10−7 mAcm−2 | [42] |
| E. coli Total coliforms | 0% 26.09% | Poultry slaughterhouse WW | Real | j (DC power supply) | Raw water: 20 mAcm−2 Polished water: 30 mAcm−2 | [73] |
| MNZ | 57.30% and 41.70% | Effluent disinfection | Synthetic | j (DC power supply) | 40 Am−2 | [74] |
| CIP TOC | 98% 87% | WW | Synthetic | j (DC power supply) | 22.2 Am−2 | [63] |
| E. coli | 100% | Reclamation of urban TWW | Real | j (DC power supply) | 5–7 Am−2 | [75] |
| AMX COD TOC | Synthetic water: AMX-90.56% COD-65.5% TOC-44.5% Real hospital wastewater: DQO-47.7% TOC-38% | HWW | Synthetic Real | j (DC power supply) | 2.31 mAcm−2 | [76] |
3.3.6. Initial pH
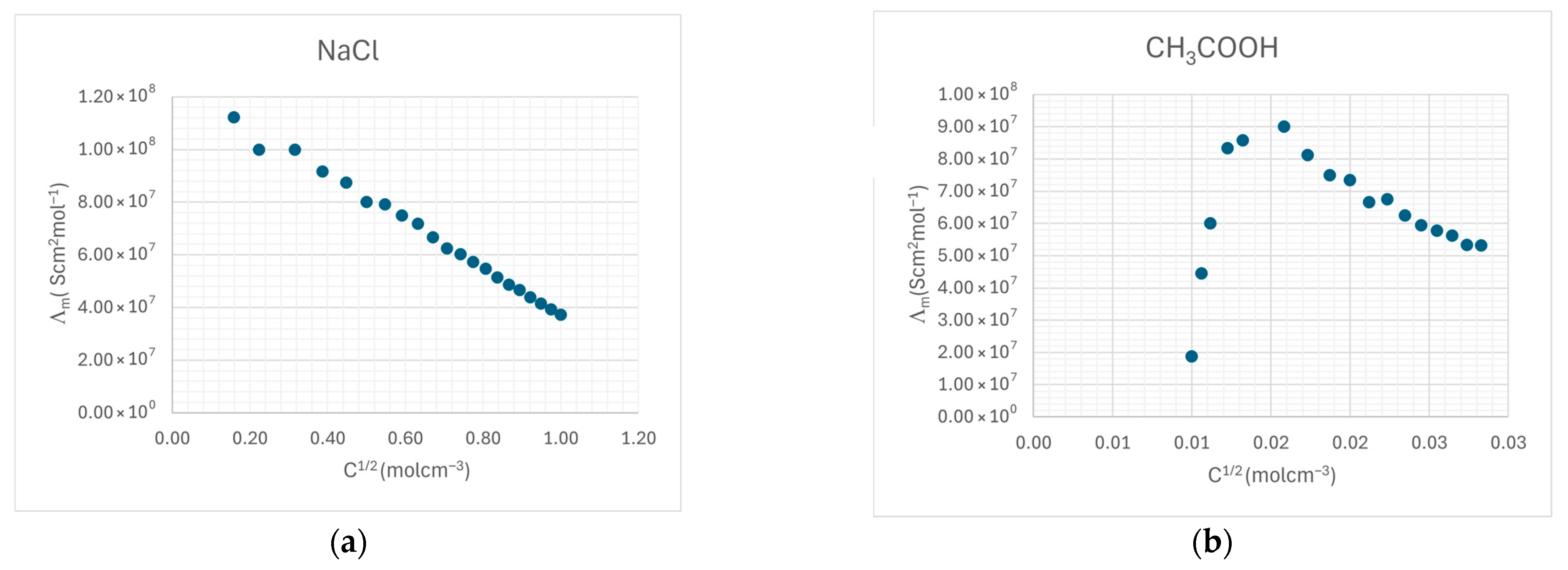
3.3.7. Electrical Conductivity and Molar Conductivity
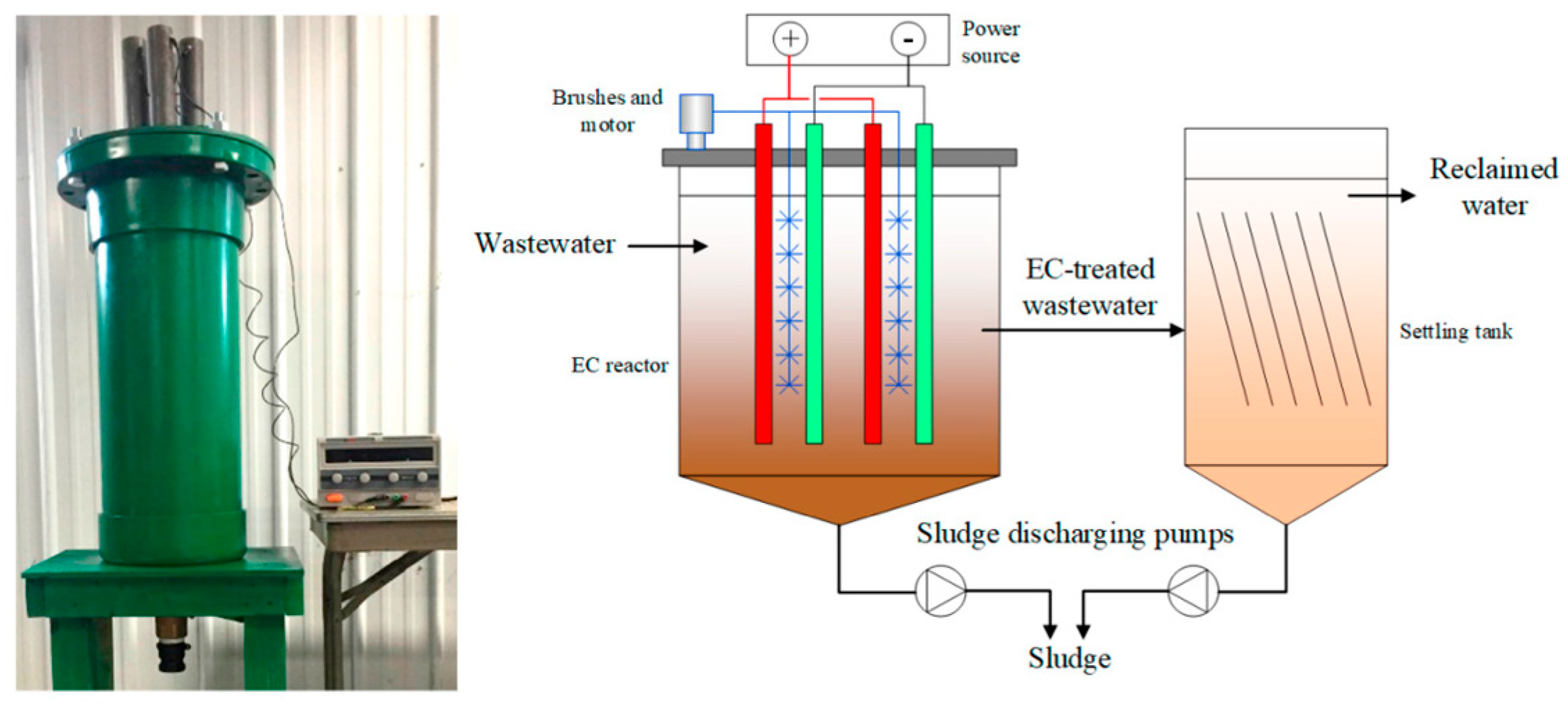
3.3.8. Characteristics of the EC Reactor
- Start with a small-sized reactor (laboratory or pilot scale) to perform the EC tests.
- Since, in most cases, foams are generated that contain contaminants, the use of mechanical paddles at the top of the reactor can be considered to remove the foam formed on the surface.
- A drain cock at the bottom of the reactor for the separation of sediment and treated wastewater.
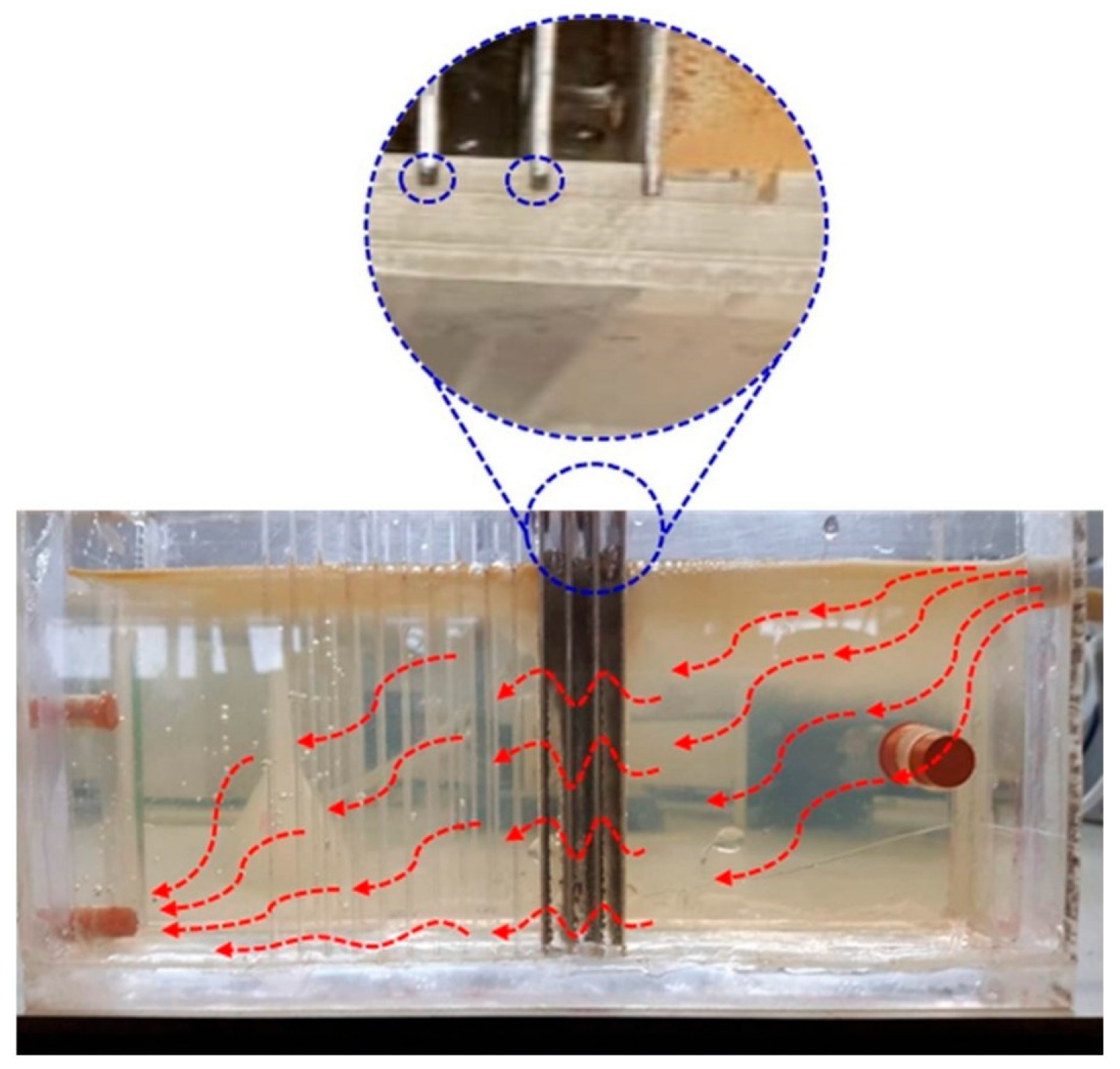
- In continuous flow reactors, excessive turbulence must be avoided at both the inlet and outlet to prevent the floccules from breaking. In this case, the arrangement of the electrodes is a parameter to consider, as authors such as those have perforated the electrodes, which allows for adequate turbulence to favor the flocculation-coagulation process. In this case, the arrangement of electrodes is a parameter to consider, since, for example, in Figure 7, the use of perforated electrodes is illustrated, which allows adequate turbulence to improve the flocculation-coagulation process [54].
- Agitation speed: If it is too fast, it will break the flocs formed. If it is too slow, it may not favor the formation of agglomerates. In the case of antibiotic removal, Table 4 helps define the speed, depending on the reactor volume.
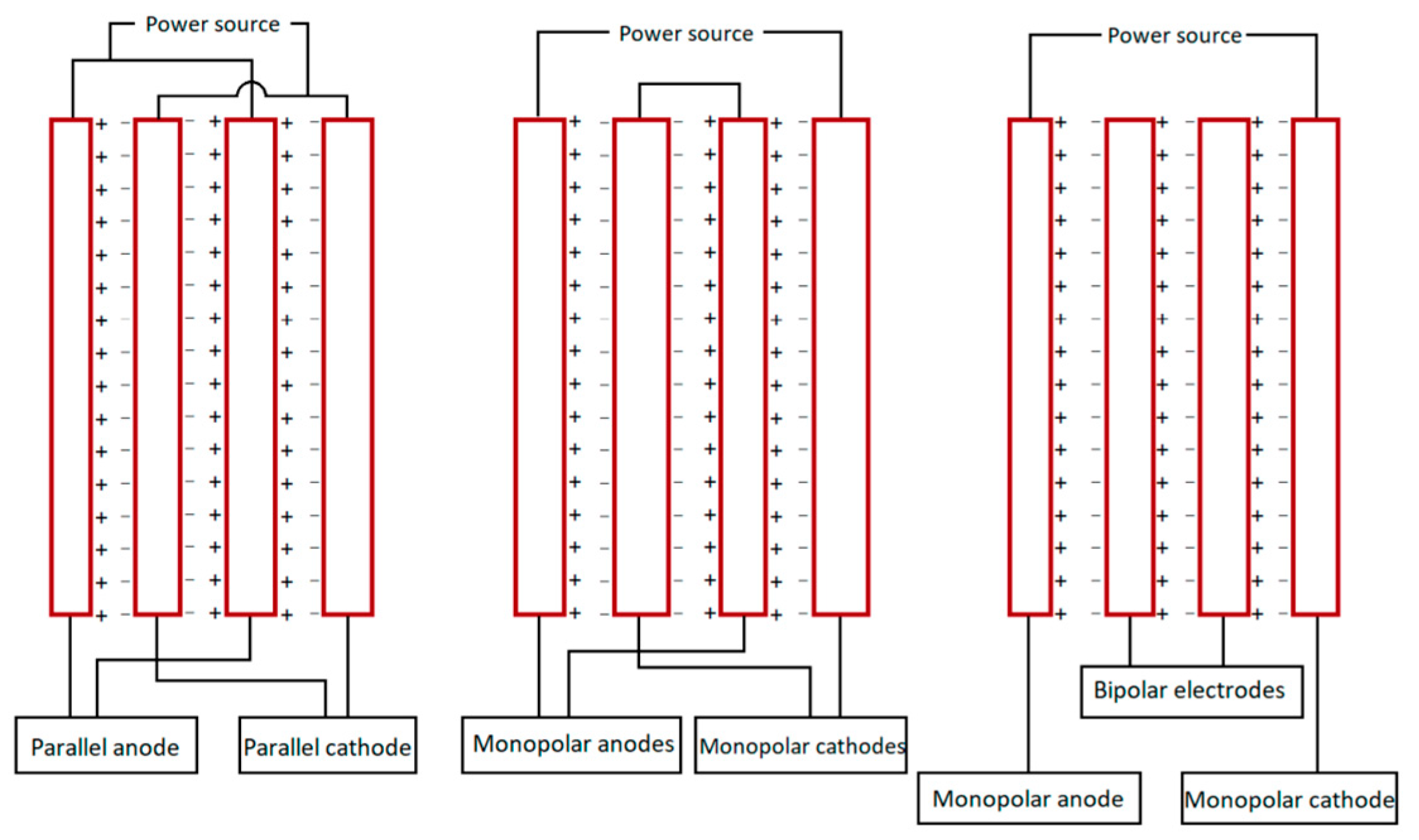
- The configuration and geometry of the electrodes: These are key factors that directly impact treatment performance. The electrode arrangement (e.g., monopolar or bipolar), spacing, orientation, and surface area must be carefully considered, as they significantly influence pollutant removal efficiency, energy consumption, and electrode durability. An optimized electrode setup can enhance current distribution, reduce internal resistance, and limit electrode passivation, contributing to a more efficient, cost-effective, and long-lasting reactor system (Figure 8).
- If possible, design a reactor with two cells (especially if the flow is continuous or a batch process with feedback), where electro-dissociation of the electrode is carried out in one cell and flocculation, coagulation, and sedimentation in the other. The above is to avoid interference in the analysis of the samples taken (Figure 9).
- If the electrocoagulation process is to be coupled with another treatment that is carried out simultaneously, the needs of both methods must be considered.
| Target Pollutant | Volume Range (L) | Stirrer | Speed (rpm) |
|---|---|---|---|
| E. coli, TC, AMX, MZN, total coliforms, OFL, enterococci, clostridium perfringens spores, somatic coliphages and eukaryotes, CIP, ARGs, AMP, DOX, STZ, tylosin and CTX | 0.100–0.250 | Magnetic bar | 70–400 |
| E. coli, AMX, TMP, CAP, MZN, TC, DDBAC, ARGs, Enterococci and phages | 0.250–0.500 | Magnetic bar | 100–600 |
| E. coli, CIP, LVX, ARGs, OFL and CAP | 0.500–1.00 | Magnetic bar | 120–1100 |
| CFZ, TC, E. coli, coliphage ΦX174, CIP and RhB | 1.50–2.00 | ND | 200–1000 |
| CQ and CFX | 2.00–3.00 | ND | 600 |
3.3.9. Energy and Electrode Consumption
3.3.10. EC Operational Costs
3.3.11. Sludge Management
3.3.12. Identification of By-Products and Toxicity Assessment
3.3.13. Combination of Electrocoagulation with Other Treatment Processes
| Type of Integrated System | Technologies Involved | Key Features | Target Pollutant | Removal | Type of Water | References |
|---|---|---|---|---|---|---|
| Hybrid | EC + PHC | Enhances antibiotic removal and promotes the production of hydrogen gas. | CIP | 92.5% | HWW (simulated) | [98] |
| Hybrid | EC + US | The EC provides iron ions that favor removal by adsorption to iron hydroxide and by Fenton reactions. Ultrasound-generated microbubbles attenuate the EC process. | AMX | >80% | PWW (synthetic) | [99] |
| Coupling | EC + EO | EC: Electrodes: Al, SS/titanium mesh Time: 60 min. EO: Electrodes: IrO2-Ta2O5|Ti/titanium mesh I: 10 mA Time: 120 min. Highlight: effectively removes the antibiotic and reduces entrained salts. | AMX | 100% | WW (synthetic) | [84] |
| Coupling | EC + EF | EC is most effective in inactivating intracellular ARGs and bacteria. EF is more effective in inactivating extracellular ARGs. Coupling is more effective in removing both. | ARGs | intracellular ARGs-2.49–3.25 logs extracellular ARGs -3.23–4.38 log | Swine WW (real) | [20] |
| Coupling | VFCW + EC | The coupling enhances phosphorus removal and the effluent has better water quality. | Total coliforms E. coli | 2.37 log10 2.35 log10 | MW effluent (real) | [67] |
| Coupling | EC + O3 | O3 dose: 47.5 mgL−1 Electrodes: Al and Fe | TOC | 85% | GW (real) | [44] |
| Coupling | EC + O3 + UV | EC removes suspended solids, turbidity, and COD O3 and UV disinfect | TOC E. coli | 87% 96% | GW (real) | [44] |
| Hybrid | EC + UA | Time: 10 min. Adsorbent: chitosan + graphene oxide. | OFL | 98% | WW (synthetic) | [97] |
| Hybrid | Ultrasonic + EC | Perforated electrodes that function as baffles minimize the need for stirrers and favor the inactivation of microorganisms. | E. coli | 100% | PW (synthetic) | [55] |
| Coupling | EC + AD | Optimal conditions: pH: 7.8 j: 15.5 mA cm−2 CFZinitial: 60 mg L−1 IED: 1.0 cm Chitosan dosage: 0.7 g L−1 Electrolyte support: NaCL Dose of electrolyte: of 0.07 M Time: 23 min | CFZ | 100% | Hospital wastewater (synthetic) | [56] |
| Hybrid | EC + EF | The iron ion that promotes Fenton reactions, together with the absence of dissolved oxygen in the water, favors the inactivation of microorganisms in both the water and the sludge generated. | E. coli | Generated magnetite: 4.7 log cells GR: 3.2 log cells (both in the absence of DO) | WW (real) | [100] |
| Hybrid | EC + HEF | Enhances the removal of heavy metal-antibiotic complexes from wastewater. | CIP:Cu complexes | Cu-99.69% CIP-96.40% TOC-83.62% | WW with antibiotic and cupper (synthetic) | [47] |
| Coupling | UV disinfection + EC | J: 20 mAcm−2 pHs between 3 and 7 Removal of both intracellular and extracellular ARGs. | sul1, sul2, tetO, and tetX | 1.62 to 2.83 logs | Secondary clarifier effluent (real) | [20] |
| Coupling | IP + EC | Complete elimination of: fecal coliforms, total coliforms, and enterococci, turbidity, COD, PO43−, NH4+, and NO3−. | Fecal coliforms Total coliforms Enterococci | 100% | Wastewater (real) | [70] |
| Hybrid | UV + EC | pH: 7.4 j: mAcm−2 Electrodes: Al and Fe Electrolyte dosis: 1 g Na2SO4/L Time: 40 min. | E. coli | 100% | Grey water (real) | [64] |
| Hybrid | eMBR | Minimizes membrane fouling, and electrochemical processes favor the removal of recalcitrant organic contaminants. | DCF CMZ AMX | 75.25 ± 8.79% 73.84 ± 9.24% 72.12 ± 10.11% | Municipal wastewater (simulated, spiked) | [101] |
| Simultaneous | MBBR combined with EC | Inicial suspended solids: <3000 mgL−1 Contact time: 24 h | AZM | 92.3% | Hospital wastewater (real) | [58] |
| Coupling | EC + UF + chloration | Maximum permissible limits of: Turbidity: <1NTU E. coli: <50 cfumL−1 pH: 6–9 | E. coli, Norovirus Salmonella | E. coli: <50 cfu mL−1 | Dishwashing water (simulated) | [102] |
| Coupling | Ozone + EC | J: 33.2 Am−2 Time: 37.8 min pH: 8.4 Ozone dose: 0.7 gh−1. | SMX | 99.65% | Wastewater (modeling) | [103] |
| Hybrid | EC + Adsorption | It should be considered: initial concentration, pH, current density, retention time, and chitosan dosage. | CTX | 100% | Polluted water (synthetic) | [85] |
| Coupling | EC + EO | EC: Removal: 97.9% Time: 20 min EO: improved by 2.09% Time: 30 min. | E. coli | 99.9% | Domestic wastewater (synthetic) | [48] |
| Coupling | EC + EF | The adsorption of microorganisms on iron hydroxide, the disinfectant effects of electrogenerated chlorinated products, and the Fenton reaction with H2O2 favor the inactivation and disinfection of microorganisms. | E. coli, enterococci, Clostridium perfringens spores, somatic coliphages and eukaryotes (amoebae, flagellates, ciliates and metazoa) | Primary effluent: Heterotrophic bacteria: 3.5 log/mL E. coli: 0.5 log/mL Enterococcus: 1.5 log/mL Clostridium perfringes spores: 2.3 log/mL Coliphages and eukaryotes: 0 log/mL Secondary effluent: Heterotrophic bacteria: 3.5 log/mL E. coli: 0.2 log/mL Enterococcus: 1.7 log/mL Clostridium perfringes spores: 1.7 log/mL Coliphages and eukaryotes: 0 log/ml | Primary and secondary treatment effluent (real) | [104] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Adsorption |
| Al | Aluminum |
| AMP | Ampicilline |
| AMX | Amoxicillin |
| AOP | Advanced oxidation process |
| APC-EC | Alternating pulse current EC |
| ARB | antibiotic-resistant bacteria |
| ARG | antibiotic-resistance genes |
| AZM | Azithromycin |
| BOD | Biological Oxygen Demand |
| CMZ | Carbamazepin |
| CAP | Chloramphenicol |
| CFX | Cephalexin |
| CFZ | Cefazolin |
| CIP | Ciprofloxacin |
| COD | Chemical oxygen demand |
| COT | Carbon organic total |
| CQ | Chloroquine |
| CS | Commercial Steel |
| CTX | Ceftriaxone |
| CWs | Constructed wetlands |
| DCF | Diclofenac |
| DC-EC | Direct current electrocoagulation |
| DCWW | Drug-containing wastewater |
| DDBAC | benzyldimethyldodecylammonium chloride |
| DO | Dissolved oxygen |
| DOX | Doxycycline |
| DWW | Domestic Wastewaters |
| EF | Electro-Fenton |
| eMBR | Electro membrane bioreactor |
| EO | Electro oxidation |
| e.m.f. | Electromotive force (e.m.f.) |
| Fe | Iron |
| GR | Green Rust |
| GW | Greywater |
| HEF | Hetero Electro Fenton |
| HWW | Hospital wastewaters |
| IP | Infiltration-percolation |
| LVX | Levofloxacin |
| MABR | Moving-aerated biofilm reactor |
| MBBR | Moving bed biofilm reactor |
| MZN | Metronidazole |
| MW | Municipal water |
| MWW | Municipal wastewater |
| OFL | Ofloxacin |
| PHC | Photocatalysis |
| PCP-EC | Positive single pulse current Electrocoagulation |
| PW | Polluted waters |
| PWW | Pharmaceutical wastewater |
| RhB | Rhodamine B |
| RSM | Response Statistical Method |
| ROS | Reactive oxygen species |
| RW | Rainwater |
| SS | Stainless Steel |
| SMX | Sulfamethoxazole |
| STZ | Sulfathiazole |
| SW | Storm water |
| TC | Tetraclycline |
| TC:Cu | Tetracycline-Cupper complex |
| TC-Ni | tetracycline -nickel complexes |
| TOC | Total Organic Carbon |
| TMP | Trimethoprim |
| TW | Treated water |
| TWW | Treated wastewater |
| UA | Ultrasonic adsorption |
| UF | Ultrafiltration |
| US | Ultrasound |
| VFCW | Vertical Flow Constructed wetland |
| WW | Wastewater |
References
- Maghsodian, Z.; Sanati, A.M.; Mashifana, T.; Sillanpää, M.; Feng, S.; Nhat, T.; Ramavandi, B. Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review. Antibiotics 2022, 11, 1461. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; An, L.; Xu, X.; Du, W.; Dai, R. A review of antibiotics in surface water and their removal by advanced electrocoagulation technologies. Sci. Total Environ. 2024, 906, 167737. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, T.J.; Keizers, P.H.; Béen, F.; Meijer, J.; Houtman, C.J.; Al Gharib, I.; Molenaar, D.; Hamers, T.; Lamoree, M.H. Identifying antimicrobials and their metabolites in wastewater and surface water with effect-directed analysis. Chemosphere 2023, 320, 138093. [Google Scholar] [CrossRef]
- Liu, C.; Tan, L.; Zhang, L.; Tian, W.; Ma, L. A Review of the Distribution of Antibiotics in Water in Different Regions of China and Current Antibiotic Degradation Pathways. Front. Environ. Sci. 2021, 9, 692298. [Google Scholar] [CrossRef]
- Mahmood, A.R.; Al-Haideri, H.H.; Hassan, F.M. Detection of Antibiotics in Drinking Water Treatment Plants in Baghdad City, Iraq. Adv. Public Health 2019, 2019, 7851354. [Google Scholar] [CrossRef]
- Liu, J.; Deng, W.-J.; Ying, G.-G.; Tsang, E.P.K.; Hong, H.-C. Occurrence and distribution of antibiotics in surface water. Ecotoxicology 2022, 31, 1111–1119. [Google Scholar] [CrossRef]
- Kulik, K.; Lenart-Boroń, A.; Wyrzykowska, K. Impact of Antibiotic Pollution on the Bacterial Population within Surface Water with Special Focus on Mountain Rivers. Water 2023, 15, 975. [Google Scholar] [CrossRef]
- Flach, K.A.; Bones, U.A.; Wolff, D.B.; Silveira, A.d.O.; da Rosa, G.M.; Carissimi, E.; Silvestri, S. Antibiotic resistant bacteria and genes (ARB and ARG) in water and sewage treatment units: A review. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100941. [Google Scholar] [CrossRef]
- Bairán, G.; Rebollar-Pérez, G.; Chávez-Bravo, E.; Torres, E. Treatment processes for microbial resistance mitigation: The technological contribution to tackle the problem of antibiotic resistance. Int. J. Environ. Res. Public Health 2020, 17, 8866. [Google Scholar] [CrossRef]
- Mosaka, T.B.M.; Unuofin, J.O.; Daramola, M.O.; Tizaoui, C.; Iwarere, S.A. Inactivation of antibiotic-resistant bacteria and antibiotic-resistance genes in wastewater streams: Current challenges and future perspectives. Front. Microbiol. 2023, 13, 1100102. [Google Scholar] [CrossRef]
- Castañeda, C.; Gracia, F.J.; Conesa, J.A.; Latorre, B. Geomorphological control of habitat distribution in an intermittent shallow saline lake, Gallocanta Lake, NE Spain. Sci. Total Environ. 2020, 726, 138601. [Google Scholar] [CrossRef]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; La Mastra, C.; Lio, R.M.S.; Barchitta, M.; Agodi, A. The Impact of Wastewater on Antimicrobial Resistance: A Scoping Review of Transmission Pathways and Contributing Factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef]
- Béalu, Z.; Walther, J.; Abusafia, A.; Altmann, K.; Meurer, M.; Gretzschel, O.; Schäfer, M.; Steinmetz, H. Removal of Organic Micropollutants and Microplastics via Ozonation Followed by Granular Activated Carbon Filtration. Environments 2024, 11, 241. [Google Scholar] [CrossRef]
- Ammar, M.; Yousef, E.; Mahmoud, M.A.; Ashraf, S.; Baltrusaitis, J. A Comprehensive Review of the Developments in Electrocoagulation for the Removal of Contaminants from Wastewater. Separations 2023, 10, 337. [Google Scholar] [CrossRef]
- Nidheesh, P.; Gökkuş, Ö. Advances in electrocoagulation process. Chemosphere 2023, 310, 136779. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Jajko, M.; Sobczak, A. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere 2018, 194, 381–389. [Google Scholar] [CrossRef]
- Yoshida, G.; Takeda, N.; Kitazono, Y.; Toyoda, K.; Umetsu, K.; Ihara, I. Removal of tetracycline antibiotics from dairy farm wastewater by electrocoagulation using iron electrodes. J. Water Environ. Technol. 2020, 18, 157–165. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Balderas-Hernández, P.; Bilyeu, B. Electrocoagulation: Fundamentals and prospectives. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 61–76. [Google Scholar] [CrossRef]
- Chen, L.; Gao, S.; Lou, L.; Zhou, Z. Removal of Intracellular and Extracellular Antibiotic Resistance Genes from Swine Wastewater by Sequential Electrocoagulation and Electro-Fenton Processes. Environ. Eng. Sci. 2021, 38, 74–80. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Dong, X.; Shen, C. Removal of Intracellular and Extracellular Antibiotic Resistance Genes in Municipal Wastewater Effluent by Electrocoagulation. Environ. Eng. Sci. 2020, 37, 783–789. [Google Scholar] [CrossRef]
- Huerta, I.S.S.; Morales, G.R.; Hernández, P.B.; Díaz, C.E.B.; Silva, T.B.P.; Pérez, P.Á.; Torres, I.R. Technical Considerations for Designing an Electrocoagulation Reactor for Wastewater Treatment: A Brief Review. Processes 2025, 13, 1679. [Google Scholar] [CrossRef]
- López-Guzmán, M.; Flores-Hidalgo, M.A.; Reynoso-Cuevas, L. Electrocoagulation process: An approach to continuous processes, reactors design, pharmaceuticals removal, and hybrid systems—A review. Processes 2021, 9, 1831. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Ge, R.; Yang, S.; Zhai, Y.; Hua, T.; Ondon, B.S.; Zhou, Q.; Li, F. Microbial electro-Fenton: A promising system for antibiotics resistance genes degradation and energy generation. Sci. Total Environ. 2020, 699, 134160. [Google Scholar] [CrossRef]
- Llanos, J.; Cotillas, S.; Cañizares, P.; Rodrigo, M.A. Electrocoagulation as a key technique in the integrated urban water cycle–A case study in the centre of Spain. Urban Water J. 2017, 14, 650–654. [Google Scholar] [CrossRef]
- Ahmad, A.; Priyadarshini, M.; Das, S.; Ghangrekar, M.M. Electrocoagulation as an efficacious technology for the treatment of wastewater containing active pharmaceutical compounds: A review. Sep. Sci. Technol. 2022, 57, 1234–1256. [Google Scholar] [CrossRef]
- Bajpai, M.; Katoch, S.S.; Nautiyal, A.; Shakya, R.; Sharma, S. Investigation of Electrode Materials Used in Electrocoagulation Process for Wastewater Treatment. In Handbook of Sustainable Materials: Modelling, Characterization, and Optimization; CRC Press: Boca Raton, FL, USA, 2023; pp. 135–150. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z.; Fang, S.; Song, B.; Cao, P.; Liu, H.; Yang, Y. Removal and toxic forecast of microplastics treated by electrocoagulation: Influence of dissolved organic matter. Chemosphere 2022, 308, 136309. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Sheob, M.; Saeed, B.; Khan, S.U.; Shirinkar, M.; Frontistis, Z.; Basheer, F.; Farooqi, I.H. Use of electrocoagulation for treatment of pharmaceutical compounds in water/wastewater: A review exploring opportunities and challenges. Water 2021, 13, 2105. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Lo, S.-L.; Liou, Y.-H.; Hu, C.-Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation-flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Feng, L.; Stuart, M.C.; Adachi, Y. Dynamics of polyelectrolyte adsorption and colloidal flocculation upon mixing studied using mono-dispersed polystyrene latex particles. Adv. Colloid Interface Sci. 2015, 226, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajmi, F.; Al-Marri, M.; Almomani, F. Electrocoagulation Process as an Efficient Method for the Treatment of Produced Water Treatment for Possible Recycling and Reuse. Water 2025, 17, 23. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Cañizares, P.; Jiménez, C.; Martínez, F.; Sáez, C.; Rodrigo, M.A. Study of the electrocoagulation process using aluminum and iron electrodes. Ind. Eng. Chem. Res. 2007, 46, 6189–6195. [Google Scholar] [CrossRef]
- Agostinho, L.C.L.; Nascimento, L.; Cavalcanti, B.F. Water Hardness Removal for Industrial Use: Application of the Electrolysis Process. J. Cancer Sci. Ther. 2021, 460, 460–465. [Google Scholar] [CrossRef]
- Fekete, É.; Lengyel, B.; Cserfalvi, T.; Pajkossy, T. Electrochemical dissolution of aluminium in electrocoagulation experiments. J. Solid State Electrochem. 2016, 20, 3107–3114. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Anji Reddy, M. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process. Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Mustafa, F.S.; Ezugwu, O.N.; Gazi, M. Efficient removal of antibiotic in single and binary mixture of nickel by electrocoagulation process: Hydrogen generation and cost analysis. Chemosphere 2022, 300, 134532. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, Y.; Saeb, K.; Tavana, A.; Rahnavard, A.; Fahimi, F.G. Effective removal of cefazolin from hospital wastewater by the electrocoagulation process. Water Sci. Technol. 2019, 80, 2422–2429. [Google Scholar] [CrossRef]
- Ndjomgoue-Yossa, A.C.; Nanseu-Njiki, C.P.; Kengne, I.M.; Ngameni, E. Effect of electrode material and supporting electrolyte on the treatment of water containing Escherichia coli by electrocoagulation. Int. J. Environ. Sci. Technol. 2015, 12, 2103–2110. [Google Scholar] [CrossRef]
- Ndjomgoue-Yossa, A.C.; Nanseu-Njiki, C.P.; Ngameni, E.; Azaroual, M. Effect of pH on Escherichia coli Removal by Electrocoagulation and Elimination Kinetics after Treatment. J. Chem. 2022, 2022, 5249368. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, W.; Zhang, X.; Si, G.; Zhang, P.; Li, B.; Su, R.; Gao, X. Efficient removal of Tetracycline-Cu complexes from water by electrocoagulation technology. J. Clean. Prod. 2021, 289, 125729. [Google Scholar] [CrossRef]
- Oliveira, J.T.; de Sousa, M.C.; Martins, I.A.; de Sena, L.M.G.; Nogueira, T.R.; Vidal, C.B.; Neto, E.F.A.; Romero, F.B.; Campos, O.S.; do Nascimento, R.F. Electrocoagulation/oxidation/flotation by direct pulsed current applied to the removal of antibiotics from Brazilian WWTP effluents. Electrochim. Acta 2021, 388, 138499. [Google Scholar] [CrossRef]
- Barzegar, G.; Wu, J.; Ghanbari, F. Enhanced treatment of greywater using electrocoagulation/ozonation: Investigation of process parameters. Process. Saf. Environ. Prot. 2019, 121, 125–132. [Google Scholar] [CrossRef]
- Lu, J.B.; Zhang, W.; Zhang, X.T.; Si, J.F.; Zhang, P.; Li, B.X.; Su, R.X.; Gao, X. Performance and mechanism of electrocoagulation process for tetracycline removal from water. Chin. J. Environ. Eng. 2019, 13, 13–14. [Google Scholar] [CrossRef]
- Govindan, K.; McNamara, P.J.; Lavin, J.; Raja, M.; Samuel, M.; Kuru, W.; Smies, A.; Mayer, B.K. Response surface methodology for evaluating electrocoagulation treatment of bath and laundry greywater. J. Water Process. Eng. 2025, 71, 107273. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.; Gu, C.; Wang, C.; Sun, S.; Song, P. MOF-derived N-Co/Fe-PC composite as heterogeneous electro-Fenton catalysis combined with electrocoagulation process for enhanced degradation of Cu-CIP complexes from wastewater. Chem. Eng. J. 2023, 452, 139592. [Google Scholar] [CrossRef]
- Özyonar, F.; Korkmaz, M.U. Sequential use of the electrocoagulation-electrooxidation processes for domestic wastewater treatment. Chemosphere 2022, 290, 133172. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Danesh, S. A sustainable system for decontamination of cephalexin antibiotic using electrocoagulation technology and response surface methodology. Remediat. J. 2023, 33, 365–378. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Olson, N.; Ives, R.; Nshimyimana, J.P.; Rusinek, C.A.; Rose, J.B.; Liao, W. Techno-economic analysis of electrocoagulation on water reclamation and bacterial/viral indicator reductions of a high-strength organic wastewater-anaerobic digestion effluent. Sustainability 2020, 12, 2697. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, T.; Rämö, J.; Lassi, U. Recent Applications of Electrocoagulation in Treatment of Water and Wastewater—A Review. Green Sustain. Chem. 2013, 3, 89–121. [Google Scholar] [CrossRef]
- Majid, R.; Samaka, I.S. Ciprofloxacin Removing from Aqueous Solutions Using Batch Reactor Electrocoagulation Process with Aluminum Electrodes. J. Ecol. Eng. 2023, 24, 364–372. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Dolatabadi, M. Electrochemical treatment of pharmaceutical wastewater through electrosynthesis of iron hydroxides for practical removal of metronidazole. Chemosphere 2018, 212, 533–539. [Google Scholar] [CrossRef]
- Hashim, K.S.; Kot, P.; Zubaidi, S.L.; Alwash, R.; Al Khaddar, R.; Shaw, A.; Al-Jumeily, D.; Aljefery, M.H. Energy efficient electrocoagulation using baffle-plates electrodes for efficient Escherichia coli removal from wastewater. J. Water Process. Eng. 2020, 33, 101079. [Google Scholar] [CrossRef]
- Hashim, K.S.; Ali, S.S.M.; AlRifaie, J.K.; Kot, P.; Shaw, A.; Al Khaddar, R.; Idowu, I.; Gkantou, M. Escherichia coli inactivation using a hybrid ultrasonic–electrocoagulation reactor. Chemosphere 2020, 247, 125868. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Asadipour, A.; Yoosefian, M.; Dolatabadi, M. Improved electrocoagulation process using chitosan for efficient removal of cefazolin antibiotic from hospital wastewater through sweep flocculation and adsorption: Kinetic and isotherm study. Desalination Water Treat. 2017, 92, 160–171. [Google Scholar] [CrossRef]
- Yoosefian, M.; Ahmadzadeh, S.; Aghasi, M.; Dolatabadi, M. Optimization of electrocoagulation process for efficient removal of ciprofloxacin antibiotic using iron electrode; kinetic and isotherm studies of adsorption. J. Mol. Liq. 2017, 225, 544–553. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, S.; Farooqi, I.H.; Ali, I. Performance Evaluation and Parameter Optimization of Moving Bed Biofilm Reactor (MBBR) along with Electrocoagulation for Removal of Azithromycin. Lett. Appl. NanoBioSci. 2022, 11, 3329–3343. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Asadipour, A.; Pournamdari, M.; Behnam, B.; Rahimi, H.R.; Dolatabadi, M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology. Process. Saf. Environ. Prot. 2017, 109, 538–547. [Google Scholar] [CrossRef]
- Zeng, W.; Li, H.; Zhang, W.; Fan, L.; Zhang, C. Removal of chloramphenicol by direct current and pulse current electrocoagula-tion: Implications for energy consumption and sludge reduction. Desalination Water Treat. 2023, 281, 150–162. [Google Scholar] [CrossRef]
- Mohammed, S.J.; M-Ridha, M.J.; Abed, K.M.; Elgharbawy, A.A.M. Removal of levofloxacin and ciprofloxacin from aqueous solutions and an economic evaluation using the electrocoagulation process. Int. J. Environ. Anal. Chem. 2021, 103, 3801–3819. [Google Scholar] [CrossRef]
- Zuo, X.; Zhang, S.; Chen, S.; Sun, H. Stormwater runoff treatment through electrocoagulation: Antibiotic resistant bacteria removal and its transmission risks. Environ. Technol. 2024, 45, 2743–2752. [Google Scholar] [CrossRef]
- Espinoza-Quiñones, F.R.; de Souza, A.R.C.; Módenes, A.N.; Trigueros, D.E.G.; de Pauli, A.R.; de Souza, P.S.C.; Kroumov, A.D. Removal Performance, Antibacterial Effects, and Toxicity Assessment of Ciprofloxacin Treated by the Electrocoagulation Process. Water Air Soil Pollut. 2016, 227, 460. [Google Scholar] [CrossRef]
- Çalışkan, Y.; Öztürk, H.; Bektaş, N.; Yatmaz, H.C. UVA enhanced electrocoagulation comparing Al and Fe electrodes for reclamation of greywater. Sep. Sci. Technol. 2020, 56, 1622–1632. [Google Scholar] [CrossRef]
- Mahmoudnia, A.; Mehrdadi, N.; Baghdadi, M.; Moussavi, G. Simultaneous removal of microplastics and benzalkonium chloride using electrocoagulation process: Statistical modeling and techno-economic optimization. Environ. Sci. Pollut. Res. 2023, 30, 66195–66208. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, S.; Gasmi, A.; Elboughdiri, N.; Aydi, S.S.; Ghernaout, D.; Hannachi, A. Nanofiltration and electrocoagulation treatment of Moorish Bath wastewater for irrigation purposes: Case study of El-Hamma, southeastern Tunisia. J. Water Process. Eng. 2025, 70, 106955. [Google Scholar] [CrossRef]
- Goerck, J.; Wolff, D.B.; de Araújo, R.K.; Decezaro, S.T. Electrocoagulation as post-treatment of the effluent from a vertical flow constructed wetland. Eng. Sanit. Ambient. 2021, 26, 113–121. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, J.; Yang, C.; Hu, Z.; Liu, F.; Zhang, C. Removal of tetracycline from livestock wastewater by positive single pulse current electrocoagulation: Mechanism, toxicity assessment and cost evaluation. Sci. Total Environ. 2022, 810, 151955. [Google Scholar] [CrossRef]
- Elkacmi, R.; Zahnoune, R.; El Amri, R.; Boudouch, O. Application of electrocoagulation process for the removal of chloroquine from an aqueous solution. H2Open J. 2023, 6, 75–87. [Google Scholar] [CrossRef]
- Fitouri, S.; Hecini, L.; Lissir, B.; Bali, M. Urban wastewater treatment by coupling infiltration–percolation and electrocoagulation processes for reuse. Euro-Mediterr. J. Environ. Integr. 2025, 10, 1483–1492. [Google Scholar] [CrossRef]
- Kermet-Said, H.; Moulai-Mostefa, N. Optimization of turbidity and COD removal from pharmaceutical wastewater by electrocoagulation. Isotherm modeling and cost analysis. Pol. J. Environ. Stud. 2015, 24, 1049–1061. [Google Scholar] [CrossRef]
- Sruthi, G.; Ahammed, M.M.; Makwana, A.R. Effect of source water/wastewater quality on bacterial removal during electrocoagulation. Water Sci. Technol. 2018, 77, 1460–1468. [Google Scholar] [CrossRef]
- Potrich, M.C.; Duarte, E.d.S.A.; Sikora, M.d.S.; da Rocha, R.D.C. Electrocoagulation for nutrients removal in the slaughterhouse wastewater: Comparison between iron and aluminum electrodes treatment. Environ. Technol. 2022, 43, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, F.; Du, X.; Zhang, C.; Yang, C.; Offiong, N.-A.; Bi, Y.; Zeng, W.; Ren, H. Removal of metronidazole from wastewater by electrocoagulation with chloride ions electrolyte: The role of reactive chlorine species and process optimization. Sep. Purif. Technol. 2022, 290, 120799. [Google Scholar] [CrossRef]
- Cotillas, S.; Llanos, J.; Moraleda, I.; Cañizares, P.; Rodrigo, M.A. Scaling-up an integrated electrodisinfection-electrocoagulation process for wastewater reclamation. Chem. Eng. J. 2020, 380, 122415. [Google Scholar] [CrossRef]
- Mehrabankhahi, G.K.; Leili, M.; Shokooni, R.; Rahmani, A.; Azarian, G.; Khorram, N.S.; Razavi, M. The Optimization of Electrocoagulation Process Efficiency in the Removal of Amoxicillin Antibiotic and COD from Aqueous Solutions and Hospital Wastewater under Optimal Conditions: A Case Study of Alimoradian Hospital, Nahavand. Sci. J. Kurd. Univ. Med Sci. 2023, 28, 135–155. [Google Scholar] [CrossRef]
- Prieto-García, F.; Callejas-Hernández, J.; Reyes-Cruz, V.E.; Marmolejo-Santillán, Y.; Prieto-Méndez, J. Electrodissolution aluminum electrode during an electrocoagulation acid whey. DYNA 2014, 81, 129–136. [Google Scholar] [CrossRef]
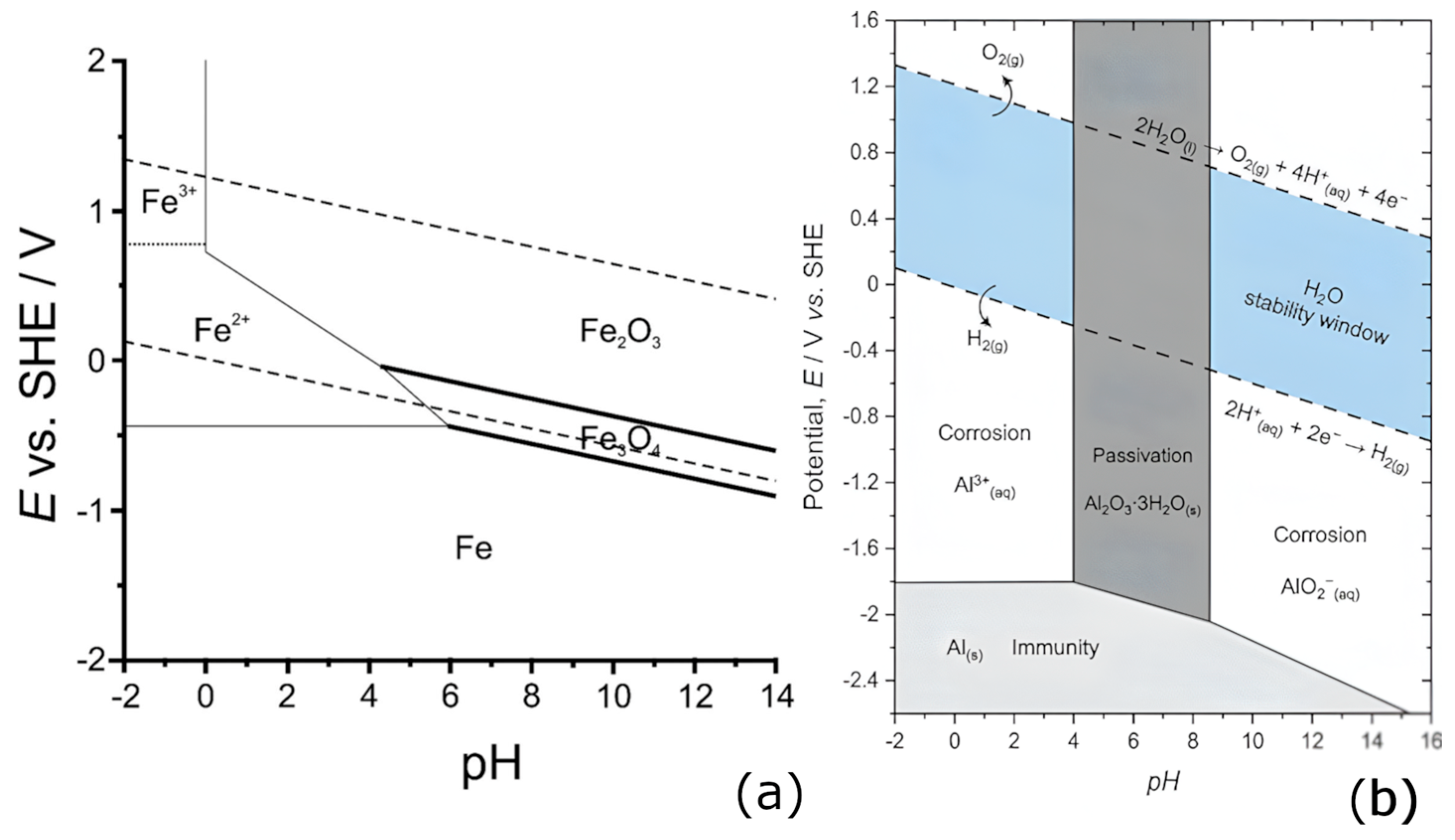
- Perry, S.C.; Gateman, S.M.; Stephens, L.I.; Lacasse, R.; Schulz, R.; Mauzeroll, J. Pourbaix diagrams as a simple route to first principles corrosion simulation. J. Electrochem. Soc. 2019, 166, C3186–C3192. [Google Scholar] [CrossRef]
- Leung, O.M.; Schoetz, T.; Prodromakis, T.; de Leon, C.P. Review—Progress in Electrolytes for Rechargeable Aluminium Batteries. J. Electrochem. Soc. 2021, 168, 056509. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Mridha, M.J.; Ali, Q.A.; Abed, K.M.; Ahmadzadeh, S. Reliable treatment approach for levofloxacin and ciprofloxacin removal from aqueous medium: Process modelling, kinetic and isotherm studies. Desalination Water Treat. 2023, 307, 50–62. [Google Scholar] [CrossRef]
- Golnabi, H.; Matloob, M.; Bahar, M.; Matloob, M.R.; Bahar, M.; Sharifian, M. Investigation of Electrical Conductivity of Different Water Liquids and Electrolyte Solutions. 2009. Available online: https://www.researchgate.net/publication/267413510 (accessed on 4 August 2025).
- Martínez, L. Measuring the conductivity of very dilute electrolyte solutions, drop by drop. Quim. Nova 2018, 41, 814–817. [Google Scholar] [CrossRef]
- JBolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- González-Nava, V.; Bacame-Valenzuela, F.; Reyes-Vidal, Y.; Manríquez, J.; Sepúlveda-Guzmán, S.; Bustos, E. Electrochemical treatment of hemodialysis wastewater including pharmaceutical products. Electrochim. Acta 2023, 437, 141470. [Google Scholar] [CrossRef]
- Noudeh, G.D.; Asdaghi, M.; Noudeh, N.D.; Dolatabadi, M.; Ahmadzadeh, S. Response surface modeling of ceftriaxone removal from hospital wastewater. Environ. Monit. Assess. 2023, 195, 217. [Google Scholar] [CrossRef]
- Gao, T.; Xiao, K.; Zhang, J.; Xue, W.; Wei, C.; Zhang, X.; Liang, S.; Wang, X.; Huang, X. Techno-economic characteristics of wastewater treatment plants retrofitted from the conventional activated sludge process to the membrane bioreactor process. Front. Environ. Sci. Eng. 2022, 16, 49. [Google Scholar] [CrossRef]
- Van Son, T.; Tuyet Mai, V.T. Application of membrane bioreactor (MBR) for antibiotic elimination from wastewater. VNU J. Sci. Earth Environ. Sci. 2024, 40. [Google Scholar] [CrossRef]
- Campo, G.; Cerutti, A.; Zanetti, M.; Ruffino, B. Membrane aerated biological reactors (MABRs) to enhance the biological treatment process at a WWTP. J. Environ. Manag. 2024, 371, 122921. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, D.; Zhang, Y.; Xu, W.; Zhang, C.; Yang, H.; Ru, Q.; Wang, Y.-F.; Ma, H.; Zhu, E.; et al. Development and Application of Membrane Aerated Biofilm Reactor (MABR)—A Review. Water 2023, 15, 436. [Google Scholar] [CrossRef]
- Boševski, I.; Gotvajn, A.Ž. Biotreatability Improvement of Antibiotic-Contaminated Waters: High Efficiency of Direct Ozonation in Comparison to Hydroxyl Radical Oxidation. Acta Chim. Slov. 2023, 70, 65–73. [Google Scholar] [CrossRef]
- Skalska-Tuomi, K.; Kaijanen, L.; Monteagudo, J.M.; Mänttäri, M. Efficient removal of pharmaceuticals from wastewater: Comparative study of three advanced oxidation processes. J. Environ. Manag. 2025, 375, 124276. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, R.N. How effective aerated continuous electrocoagulation can be for tetracycline removal from river water using aluminium electrodes? Chemosphere 2022, 305, 135476. [Google Scholar] [CrossRef]
- Barshahi, P.A.; Zonoozi, M.H.; Saeedi, M. Rifampin removal using electrocoagulation: Optimizing the process and improving the floated sludge dewaterability. Int. J. Environ. Sci. Technol. 2023, 20, 6275–6290. [Google Scholar] [CrossRef]
- Mbacké, M.K.; Sy, A.; Kane, C.; Mbengue, M. Microbial Fuel Cell for the Recovery of Sludge from the Treatment of Effluents by Electrocoagulation. In Innovations and Interdisciplinary Solutions for Underserved Areas; Seeam, A., Ramsurrun, V., Juddoo, S., Phokeer, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 218–232. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation sludge valorization—A review. Resources 2021, 10, 127. [Google Scholar] [CrossRef]
- Mokni, S.; Tlili, M.; Jedidi, N.; Hassen, A. Applicability of electrocoagulation process to the treatment of Ofloxacin and Chloramphenicol in aqueous media: Removal mechanism and antibacterial activity. J. Water Process. Eng. 2022, 49, 103080. [Google Scholar] [CrossRef]
- Suri, A.; Khandegar, V. Exclusion of Pharmaceutical Compounds by UA Assisted EC Process. J. Hazard. Toxic Radioact. Waste 2021, 25, 04021004. [Google Scholar] [CrossRef]
- Pratiwi, R.; Dilla, D.S.; Slamet. Effect of Electric Voltage on Simultaneous Electrocoagulation-Photocatalysis Process in Removal of Ciprofloxacin-Methylene Blue Mixture and Hydrogen Recovery. Asian J. Chem. 2022, 34, 1145–1150. [Google Scholar] [CrossRef]
- Padilla-Robles, B.; Alonso, A.; Martínez-Delgadillo, S.; González-Brambila, M.; Jaúregui-Haza, U.; Ramírez-Muñoz, J. Electrochemical degradation of amoxicillin in aqueous media. Chem. Eng. Process. Process Intensif. 2015, 94, 93–98. [Google Scholar] [CrossRef]
- Qiao, M.; Zhang, J.; Mao, R.; Zhao, X. Inactivation of Escherichia Coli by mixed-valent nanoparticles in-situ generated during Fe electrocoagulation. Water Res. 2023, 247, 120818. [Google Scholar] [CrossRef]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; de Luna, M.D.G.; Belgiorno, V. Control of emerging contaminants by the combination of electrochemical processes and membrane bioreactors. Environ. Sci. Pollut. Res. 2019, 26, 1103–1112. [Google Scholar] [CrossRef]
- Church, J.; Verbyla, M.E.; Lee, W.H.; Randall, A.A.; Amundsen, T.J.; Zastrow, D.J. Dishwashing water recycling system and related water quality standards for military use. Sci. Total Environ. 2015, 529, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Nghia, N.T.; Tuyen, B.T.K.; Quynh, N.T.; Thuy, N.T.T.; Nguyen, T.N.; Nguyen, V.D.; Tran, T.K.N. Response Methodology Optimization and Artificial Neural Network Modeling for the Removal of Sulfamethoxazole Using an Ozone–Electrocoagulation Hybrid Process. Molecules 2023, 28, 5119. [Google Scholar] [CrossRef]
- Anfruns-Estrada, E.; Bruguera-Casamada, C.; Salvadó, H.; Brillas, E.; Sirés, I.; Araujo, R.M. Inactivation of microbiota from urban wastewater by single and sequential electrocoagulation and electro-Fenton treatments. Water Res. 2017, 126, 450–459. [Google Scholar] [CrossRef]
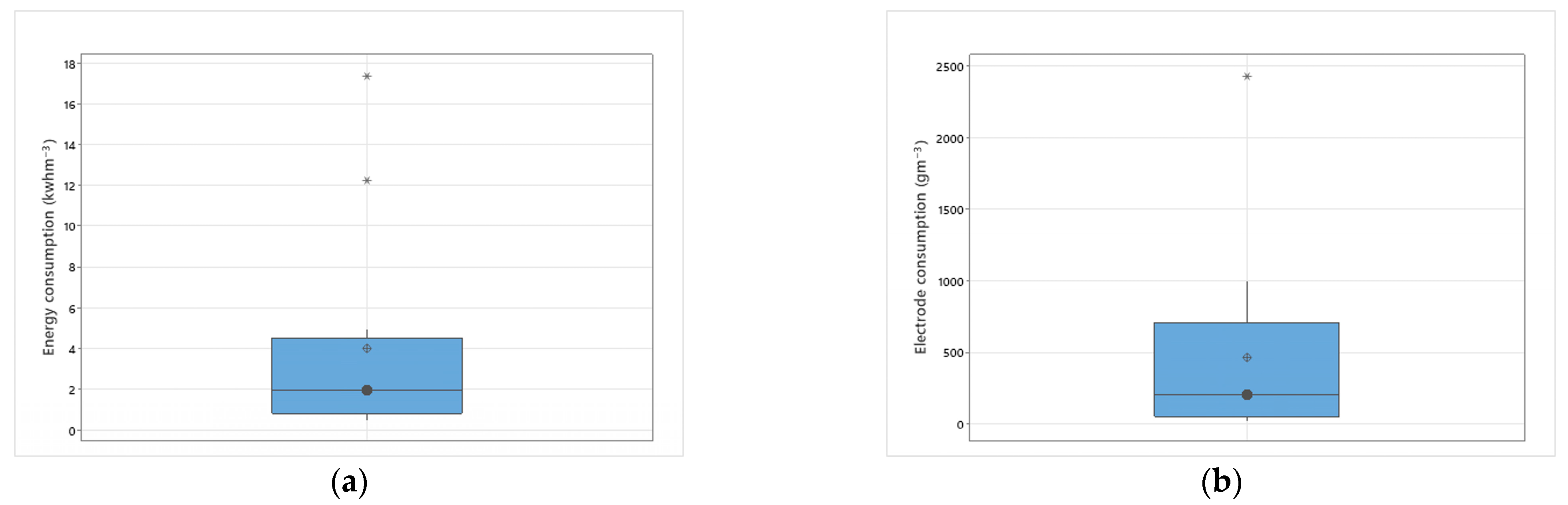
| Technology | Cost (USD$m−3) | Energy Consumption (kWhm−3) | Antibiotic Removal Efficiency | Microorganism Removal Efficiency |
|---|---|---|---|---|
| Activated sludge | 2.71 | 0.4 | 30–70% | 89% |
| MBR | 3.41 | 0.57 | 49.7 (SMX) | 93.30% |
| MABR | NR | ~0.8 | ~96% (TOC) | 85% |
| Ozonation | ~4.37–7.78 | 0.37 | 10–29% (TOC) | >99% |
| EC | 0.011–4.13 | 0.52–17.2 | 80–98% | 67–100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Flores, L.S.; Torres, E. Electrocoagulation for the Removal of Antibiotics and Resistant Bacteria: Advances and Synergistic Technologies. Processes 2025, 13, 2916. https://doi.org/10.3390/pr13092916
Pérez-Flores LS, Torres E. Electrocoagulation for the Removal of Antibiotics and Resistant Bacteria: Advances and Synergistic Technologies. Processes. 2025; 13(9):2916. https://doi.org/10.3390/pr13092916
Chicago/Turabian StylePérez-Flores, Laura Sol, and Eduardo Torres. 2025. "Electrocoagulation for the Removal of Antibiotics and Resistant Bacteria: Advances and Synergistic Technologies" Processes 13, no. 9: 2916. https://doi.org/10.3390/pr13092916
APA StylePérez-Flores, L. S., & Torres, E. (2025). Electrocoagulation for the Removal of Antibiotics and Resistant Bacteria: Advances and Synergistic Technologies. Processes, 13(9), 2916. https://doi.org/10.3390/pr13092916






