Scalability and Performance of Iron–Carbon Micro-Electrolysis with Hydrogen Peroxide for Textile Wastewater Treatment
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Batch Experiments—Micro-Electrolysis Treatment
2.3. Micro-Electrolysis Treatment in Continuous Flow Experiments
2.4. Iron–Carbon Micro-Electrolysis Coupled with a Hydrogen Peroxide Process for Textile Wastewater Treatment
2.5. Pilot Scale Test
2.6. Analytical Methods
2.7. Modeling by the Response Surface Method
2.8. Determination of the Reaction Intermediates
3. Results and Discussion
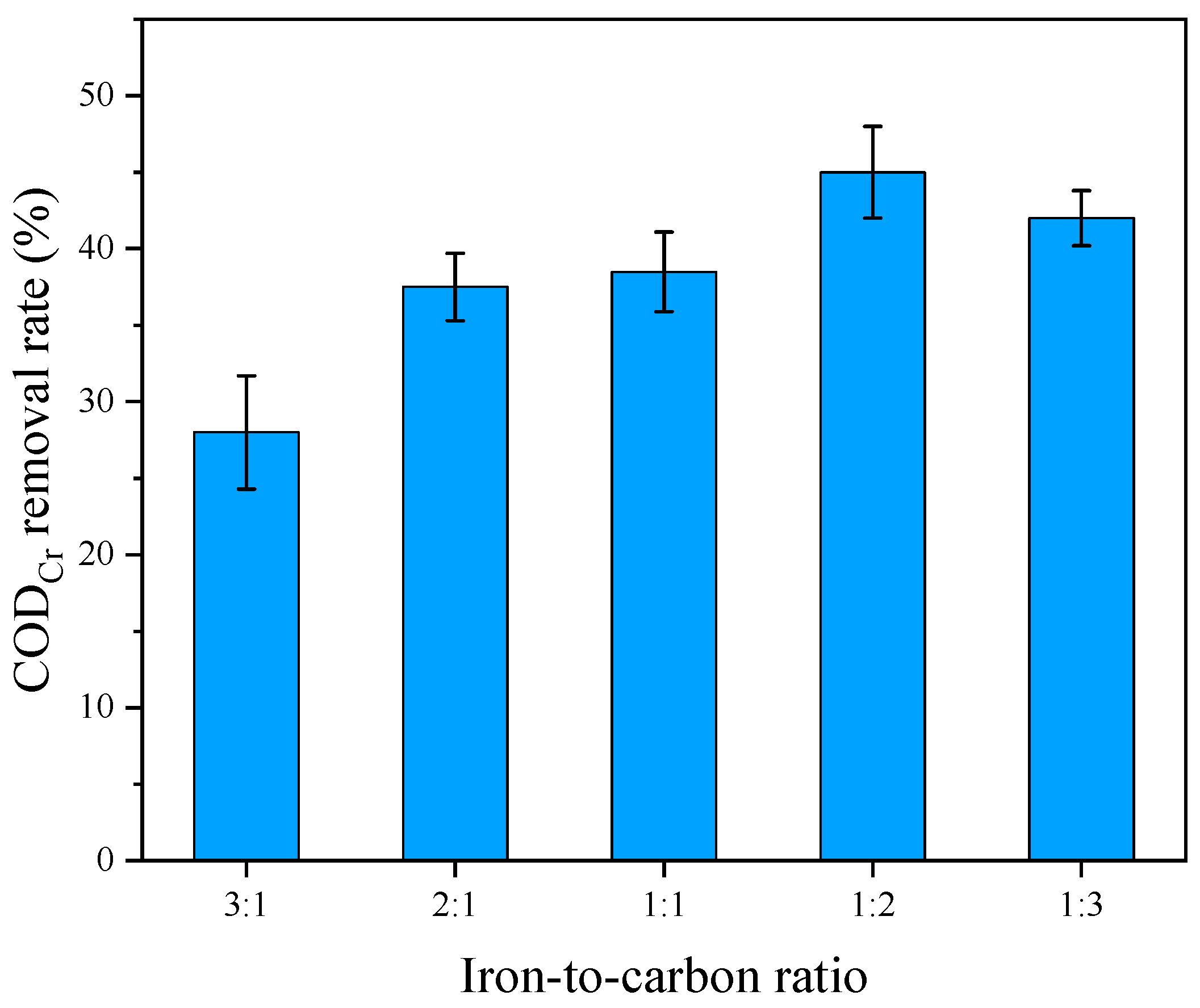
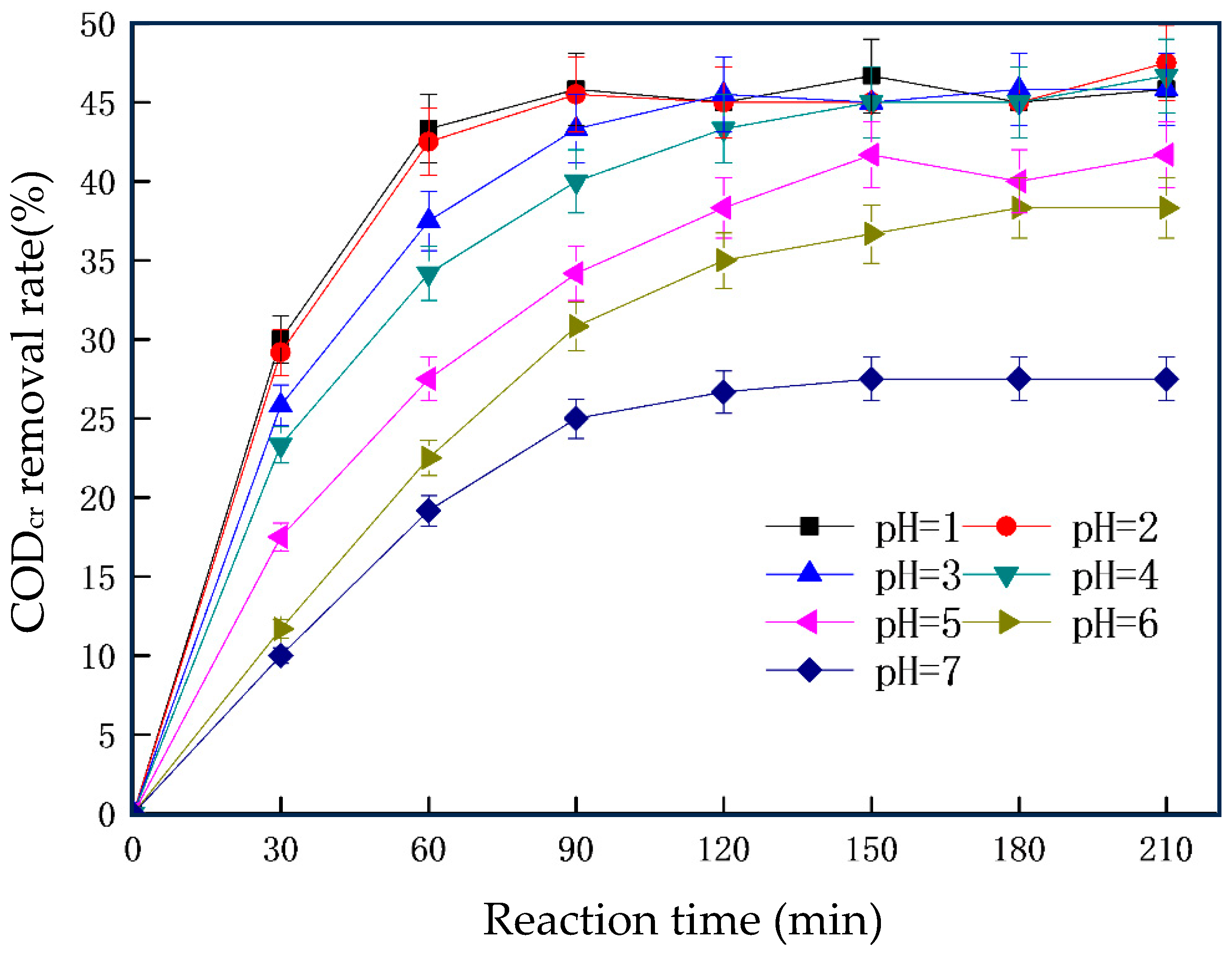
3.1. Single-Factor Experiment of the Iron–Carbon Micro-Electrolysis Reaction
3.2. Response Surface Analysis of Iron–Carbon Micro-Electrolysis
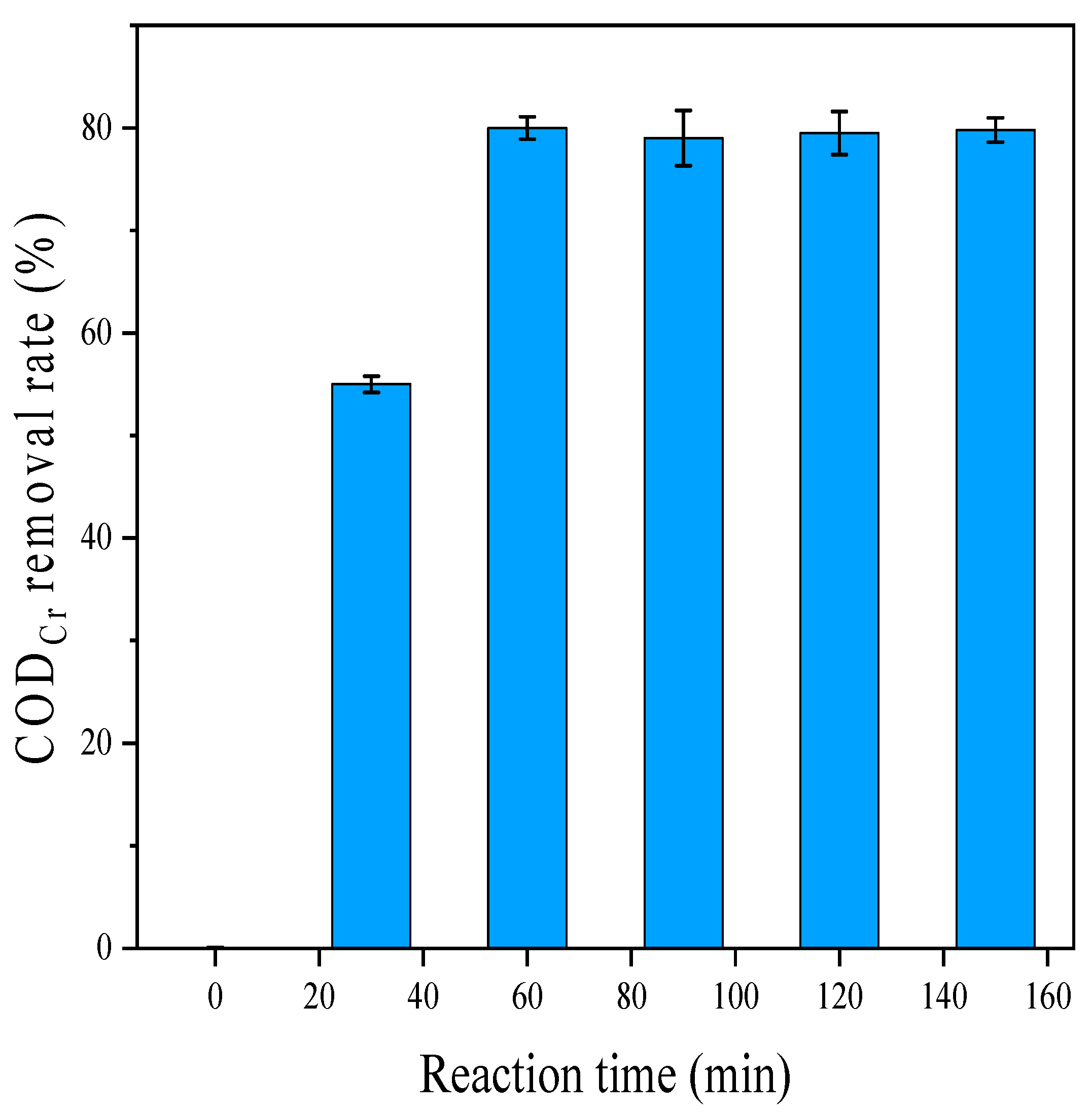
3.3. Single-Factor Experiment of Iron–Carbon Micro-Electrolysis Coupled with a Hydrogen Peroxide Reaction
3.4. Response Surface Analysis of Iron–Carbon Micro-Electrolysis Coupled with a Hydrogen Peroxide Reaction
3.5. Potential Degradation Pathways
3.6. Pilot-Scale Test
3.6.1. Process Steps
3.6.2. Process Operation Efficiency
3.6.3. Economic Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leal, T.W.; Lourenco, L.A.; Scheibe, A.S.; de Souza, S.M.A.G.U.; de Souza, A.A.U. Textile wastewater treatment using low-cost adsorbent aiming the water reuse in dyeing process. J. Environ. Chem. Eng. 2018, 6, 2705–2712. [Google Scholar]
- Madan, S.; Madan, R.; Hussain, A. Performance evaluation study of effluent treatment plant of polyester textile dyeing industry: A case study of SIDCUL, Haridwar, Uttarakhand, India. Int. J. Environ. Waste Manag. 2024, 34, 105–118. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Rahman, M.; Tabassum, Z. Biotechnological Approach to Treat Textile Dyeing Effluents: A Critical Review Analysing the Practical Applications. Text. Leather Rev. 2024, 7, 124–152. [Google Scholar]
- Liu, X.; Wang, J. Decolorization and degradation of various dyes and dye-containing wastewater treatment by electron beam radiation technology: An overview. Chemosphere 2024, 351, 141255. [Google Scholar]
- GB 4287-2012; Discharge Standards of Water Pollutants for Dyeing and Finishing of Textile Industry. Ministry of Ecological Environment, General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2012.
- Yaseen, D.A.; Scholz, M. Treatment of synthetic textile wastewater containing dye mixtures with microcosms. Environ. Sci. Pollut. Res. 2018, 25, 1980–1997. [Google Scholar] [CrossRef]
- Sultana, Z.; Ali, M.E.; Uddin, M.S.; Haque, M.M. Study on implementation of effluent treatment plants for safe environment from textile waste. J. Res. Environ. Sci. Toxicol. 2013, 2, 9–16. [Google Scholar]
- Guvenc, S.Y.; Can-Güven, E.; Çifçi, D.İ.; Varank, G. Biodegradability enhancement and sequential treatment of real chemical industry wastewater by chemical coagulation and Electro-Fenton processes. Chem. Eng. Process.-Process Intensif. 2023, 194, 109598. [Google Scholar]
- Sharma, A.; Syed, Z.; Brighu, U.; Gupta, A.B.; Ram, C. Adsorption of textile wastewater on alkali-activated sand. J. Clean. Prod. 2019, 220, 23–32. [Google Scholar]
- Wang, Y.-N.; Wang, R. Reverse osmosis membrane separation technology. In Membrane Separation Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–45. [Google Scholar]
- Zhang, T.; Zheng, W.; Wang, Q.; Wu, Z.; Wang, Z. Designed strategies of nanofiltration technology for Mg2+/Li+ separation from salt-lake brine: A comprehensive review. Desalination 2023, 546, 116205. [Google Scholar]
- Qiu, Y.; Depuydt, S.; Ren, L.-F.; Zhong, C.; Wu, C.; Shao, J.; Xia, L.; Zhao, Y.; Van der Bruggen, B. Progress of ultrafiltration-based technology in ion removal and recovery: Enhanced membranes and integrated processes. ACS EST Water 2023, 3, 1702–1719. [Google Scholar]
- Ma, Z.; Chang, H.; Liang, Y.; Meng, Y.; Ren, L.; Liang, H. Research progress and trends on state-of-the-art membrane technologies in textile wastewater treatment. Sep. Purif. Technol. 2023, 333, 125853. [Google Scholar]
- da Silva, L.T.V.; Romero, F.B.; Amazonas, A.A.; Ribeiro, J.P.; Neto, E.F.A.; de Oliveira, A.G.; do Nascimento, R.F. Polishing of treated textile effluent using combined electrochemical oxidation and ozonation technique. Desalination Water Treat. 2023, 310, 50–58. [Google Scholar]
- Tuncer, N.; Sönmez, G. Removal of COD and color from textile wastewater by the Fenton and UV/H2O2 oxidation processes and optimization. Water Air Soil. Pollut. 2023, 234, 70. [Google Scholar]
- Bhapkar, A.R.; Bhame, S. A review on ZnO and its modifications for photocatalytic degradation of prominent textile effluents: Synthesis, mechanisms, and future directions. J. Environ. Chem. Eng. 2024, 12, 112553. [Google Scholar]
- Di, Ç. Decolorization of methylene blue and methyl orange with Ag doped TiO2 under UV-A and UV-Visible conditions: Process optimization by response surface method and toxicity evaluation. Glob. Nest J. 2016, 18, 371. [Google Scholar]
- Tao, N.C.; Le Luu, T. Different behaviours of biologically textile wastewater treatment using persulfate catalyzed electrochemical oxidation process on Ti/BDD and Ti/SnO2-Nb2O5 anodes. Environ. Eng. Res. 2023, 28, 220555. [Google Scholar]
- Wei, S.; Jia, L.; Tan, J.; Zhang, J.; Guo, Z.; Hu, Z.; Dai, P.; Wu, H. Iron-carbon micro-electrolysis simultaneously enhanced nutrient and heavy metal removal in constructed wetlands for purifying polluted groundwater with variable hydraulic loadings. Chem. Eng. J. 2023, 470, 144367. [Google Scholar]
- Bou-Abdallah, F. The iron redox and hydrolysis chemistry of the ferritins. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 719–731. [Google Scholar]
- Kremer, M. Mechanism of the Fenton reaction: Evidence for a new intermediate. Phys. Chem. Chem. Phys. 1999, 1, 3595–3605. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Zhou, W.; Rajic, L.; Chen, L.; Kou, K.; Ding, Y.; Meng, X.; Wang, Y.; Mulaw, B.; Gao, J.; Qin, Y. Activated carbon as effective cathode material in iron-free Electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption. Electrochim. Acta 2019, 296, 317–326. [Google Scholar] [PubMed]





| Level | Parameters | ||
|---|---|---|---|
| A—IS Dose (g/L) | B—Mass Ratio of Carbon to Iron | C—pH | |
| −1 | 20 | 1 | 3 |
| 0 | 25 | 2 | 4 |
| 1 | 30 | 3 | 5 |
| Level | Parameters | ||
|---|---|---|---|
| A—pH | B—H2O2 Dose (mg/L) | C—Reaction Time (Min) | |
| −1 | 2 | 100 | 90 |
| 0 | 3 | 130 | 120 |
| 1 | 4 | 160 | 150 |
| Serial Number | Time of Peak (min) | Organic Matter | Molecular Weight | Area of Peak (%) |
|---|---|---|---|---|
| 1 | 13.179 | 2, 4, 7, 9-tetramethyl-5-decynne 4, 7-diol | 226 | 1.72 |
| 2 | 15.066 | 1, 2, 3, 4-tetrahydrocyclopentadiene and [b] indole | 157 | 8.43 |
| 3 | 16.541 | 2-amino-4-methylbenzothiazole | 164 | 45.37 |
| 4 | 17.054 | phthalate esters | 278 | 0.92 |
| 5 | 17.356 | methyl palmitate | 270 | 0.85 |
| 6 | 17.995 | palmitic acid | 256 | 21.78 |
| 7 | 18.759 | methyl stearate | 298 | 0.7 |
| 8 | 18.868/19.143 | cyclopentyl methyl methylphosphonic acid | 178 | 2.91/9.82 |
| 9 | 19.429 | octadecylamide | 283 | 2.07 |
| 10 | 19.805 | 1, 4-butanediol diglycidyl ether | 202 | 0.74 |
| 11 | 20.863 | oleate amide | 281 | 4.69 |
| Serial Number | Time of Peak (min) | Organic Matter | Molecular Weight | Area of Peak (%) |
|---|---|---|---|---|
| 1 | 11.538 | Ethyl 3-hydroxyhexanoate | 160 | 4.82 |
| 2 | 13.156 | O-toluene isothiocyanate | 149 | 11.09 |
| 3 | 15.074 | 2-Phenylpyrimidine | 156 | 8.82 |
| 4 | 17.049/17.776 | Diisobutyl phthalate; Dibutyl phthalate | 278 | 6.46/1.61 |
| 5 | 17.983 | Palmitic acid | 256 | 21.59 |
| 6 | 18.831/19.115 | Cyclopentyl methyl methyl phosphate | 178 | 8.71/7.21 |
| 7 | 19.422 | Octadecylamide | 283 | 6.75 |
| 8 | 20.842 | Oleate amide | 281 | 17.54 |
| Serial Number | Time of Peak (min) | Organic Matter | Molecular Weight | Area of Peak (%) |
|---|---|---|---|---|
| 1 | 10.874 | 2-(2-hydroxypropoxy)-1-propanol | 134 | 3.93 |
| 2 | 12.633 | 1,1′[(1-methyl-1, 2-ethyl subunit) di (oxygen)] di-(2-propanol) | 192 | 5.01 |
| 3 | 13.647 | 2-methyl-2, 4-pentanediol | 118 | 2.25 |
| 4 | 15.086 | 1-[2-(2-methoxy-1-methylethoxy)-1-methylethoxy]-2-propanol | 206 | 4.18 |
| 5 | 17.049 | diisobutyl phthalate | 278 | 3.11 |
| 6 | 17.948 | palmitic acid | 256 | 47.33 |
| 7 | 19.355 | octadecanoic acid | 284 | 8.79 |
| 8 | 20.849 | oleate amide | 281 | 21.89 |
| Items | Doses (ton/m3)/Equipment Quantity (Actual Work Quantity) | Unit Price (CNY/Ton) | Cost (CNY/m3) | Cost (USD/m3) |
|---|---|---|---|---|
| H2SO4 (98%) | 1.55 × 10−5 | 580 | 0.00900 | 0.001261 |
| IS | 9 × 10−6 | 2000 | 0.01800 | 0.002526 |
| H2O2 (30%) | 1.7 × 10−4 | 1000 | 0.17000 | 0.023834 |
| NaOH | 1.43 × 10−8 | 2100 | 0.00003 | 0.000004 |
| Al2(SO4)3 | 8.57 × 10−5 | 2100 | 0.18000 | 0.025236 |
| Sewage Pump | 2 (1) | 0.35750 | 0.050121 | |
| Air Pump | 2 (1) | 0.48750 | 0.068347 | |
| Chemical Dosing Pump | 8 (4) | 0.05700 | 0.007991 | |
| Total | 1.27903 | 0.179320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Wang, M.; Cui, W.; Zhang, H. Scalability and Performance of Iron–Carbon Micro-Electrolysis with Hydrogen Peroxide for Textile Wastewater Treatment. Processes 2025, 13, 970. https://doi.org/10.3390/pr13040970
Lu H, Wang M, Cui W, Zhang H. Scalability and Performance of Iron–Carbon Micro-Electrolysis with Hydrogen Peroxide for Textile Wastewater Treatment. Processes. 2025; 13(4):970. https://doi.org/10.3390/pr13040970
Chicago/Turabian StyleLu, Hongxiu, Meng Wang, Wei Cui, and He Zhang. 2025. "Scalability and Performance of Iron–Carbon Micro-Electrolysis with Hydrogen Peroxide for Textile Wastewater Treatment" Processes 13, no. 4: 970. https://doi.org/10.3390/pr13040970
APA StyleLu, H., Wang, M., Cui, W., & Zhang, H. (2025). Scalability and Performance of Iron–Carbon Micro-Electrolysis with Hydrogen Peroxide for Textile Wastewater Treatment. Processes, 13(4), 970. https://doi.org/10.3390/pr13040970





