Abstract
To address the challenge of low iron extraction efficiency from boiling furnace pyrite cinder (BPC), a significant secondary iron resource posing environmental risks due to massive stockpiling in China, this study investigated the kinetics and reactivity regulation of an oxalic acid-sulfuric acid hybrid leaching system to overcome the inertness and diffusion barriers of hematite. Single-factor experiments and Response Surface Methodology (RSM) optimization were employed to determine optimal leaching parameters (time, temperature, liquid–solid ratio, H2SO4 concentration) under constant stirring (400 r/min) and BPC–oxalic acid ratio (50:1). Shrinking core kinetic modeling, complemented by SEM-EDS/XRD residue characterization, elucidated the dissolution mechanism. Results showed a maximum iron leaching rate of 94.7% at 90 °C, 40 wt% H2SO4, an L/S ratio of 5 mL/g, and a time of 7 h. Kinetics transitioned from liquid-film diffusion control (Ea = 76.9 kJ/mol) below 70 °C to mixed interfacial reaction/internal diffusion control (Ea = 32.4 kJ/mol) above 80 °C. Highly concentrated acid conditions (50% H2SO4) reduced efficiency by >20% due to oxalate protonation, CaSO4 pore occlusion, and increased viscosity. RSM confirmed temperature-dominated kinetics and acid concentration-governed thermodynamics, with no synergy under combined high-temperature/high-acidity conditions. This optimized process enables efficient iron recovery from refractory BPC using minimal reagent consumption.
1. Introduction
Boiling furnace pyrite cinder (BPC), a byproduct generated during sulfuric acid production through high-temperature pyrite roasting [1], is primarily composed of hematite, calcium sulfate hydrates, silica, alumina, and various silicates, with iron content ranging from 30% to 60% [2,3,4]. This iron-rich residue additionally contains trace metal elements, including Mg, Cu, Cr, Pb, Mn, Zn, Ni, and Ag [5,6]. China’s expanding sulfuric acid industry currently produces approximately 12 million tons of BPC annually [7,8,9], resulting in accumulated stockpiles exceeding 100 million tons. The current practice of stockpiling or landfilling this material not only occupies substantial land resources but also poses significant environmental risks through heavy metal contamination of soil, water, and air [10,11,12], while simultaneously wasting valuable metallic resources.
Current valorization strategies for BPC encompass several major applications, including iron production [3,13], cement additives [14], iron-based pigments [15,16], construction materials [17], and photocatalyst development [18,19]. Notably, BPC also contains significant quantities of valuable non-ferrous metals—such as copper, nickel, cobalt, and zinc—presenting considerable potential for comprehensive recovery and utilization [20]. For instance, Jiang et al. [8] demonstrated efficient recovery of up to 82% copper and 99% cobalt from high-sulfur BPC via selective acid leaching. In a complementary approach, Hong et al. [21] achieved extraction rates of 86.15% for copper and 79.61% for cobalt using a novel process based on phase reconstruction through reductive roasting followed by acid leaching. Furthermore, Han et al. [22] developed a chlorination-volatilization method to recover valuable metals from iron-rich pyrite cinder, highlighting the diversity of techniques available for metal reclamation from this secondary resource. However, with increasing depletion of high-grade iron ores, developing efficient BPC iron recovery methods has become imperative. Conventional hydrometallurgical approaches using diluted strong acids (e.g., sulfuric, hydrochloric, or phosphoric acid) demonstrate varying effectiveness: Zhang et al. [23] achieved 89.44% iron leaching using 30% HCl at 90 °C, while Jiang et al. [8] reported 98.1% extraction with 3 mol/L phosphoric acid at 110 °C. Other studies show more moderate efficiencies: 50% with 55% H2SO4 [24] and 71% with 30% HCl [25]. Alternative methods like oxalic acid leaching can reach 91.48% extraction [26], and reductive roasting-magnetic separation processes such as suspension magnetization roasting (SMR) technology have achieved 95.05% recovery by converting hematite to magnetite [27]. These methods nevertheless present significant limitations: mineral acids (HCl, H2SO4, H3PO4) struggle with hematite’s chemical inertness; oxalic acid systems require excessive reagent due to Fe(III)-oxalate precipitation above pH 1.5 [28]; and roasting processes are energy-intensive. Recent advances in oxalic acid-assisted leaching systems [26,28,29], particularly the work by Yang et al. [29] demonstrating 95.7% iron extraction using 20% oxalic acid with 50% sulfuric acid, highlight the potential of hybrid approaches. However, fundamental understanding remains lacking regarding (1) kinetic regime transitions during leaching and (2) acid synergy mechanisms in BPC processing. We hypothesize that an optimized oxalic-sulfuric acid hybrid system can overcome hematite’s kinetic barriers through dual mechanisms: (1) oxalate complexation destabilizing Fe-O bonds and (2) sulfuric acid maintaining acidic conditions to prevent oxalate precipitation while enhancing diffusion, with optimal performance at ~40 wt% H2SO4 concentration, balancing proton activity and oxalate stability.
To address these limitations, this study systematically investigates BPC iron dissolution through an integrated approach that combines microstructural evolution analysis (SEM-EDS/XRD) to correlate phase transformations with leaching stages, shrinking core kinetic modeling with statistical validation, and Response Surface Methodology (RSM) to decode multivariate parameter interactions. The findings provide both theoretical insights into iron dissolution mechanisms from refractory BPC and practical guidelines for industrial implementation, achieving 94.7% iron recovery with minimized reagent consumption.
2. Materials and Methods
2.1. Materials
The boiling furnace pyrite cinder was sourced from Wengfu (Group) Co., Ltd., Guiyang, China, with an iron content of 51.10 wt%. The chemical composition of the BPC is detailed in Table 1. SEM-EDS analysis of BPC (Figure 1a,b) reveals a dense, highly consolidated microstructure. A continuous Fe- and O-rich matrix indicates the dominance of iron oxides, while discrete angular particles consisting solely of Si and O correspond to inert quartz (SiO2). Rod-like structures with strong Ca-S co-localization suggest the presence of gypsum (CaSO4). This compact morphology is anticipated to pose significant intraparticle diffusion resistance during leaching. XRD patterns (Figure 1c) exhibit distinct hematite diffraction peaks at 33.2° (104) and 35.6° (110). This confirms iron predominantly exists as Fe2O3. Additional characteristic peaks correspond to quartz (SiO2) at 20.8° (100) and 26.6° (011), while gypsum (CaSO4) appears at 25.6° (020). Consequently, the iron in BPC is anticipated to exhibit low leachability due to the chemical inertness of hematite, diffusion limitations imposed by the compact structure, and potential encapsulation effects by impurity minerals (SiO2/CaSO4). The XRD pattern shows dominant peaks for SiO2 despite its lower chemical abundance (Table 1). This is a common feature in pyrite cinders, resulting from the high crystallinity of quartz versus the often poorly crystalline or amorphous structure of the iron oxide phases.

Table 1.
Chemical composition of BPC.
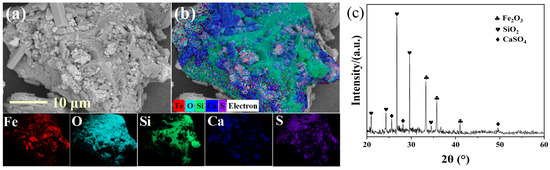
Figure 1.
SEM-EDS (a,b) and XRD (c) of BPC.
2.2. Leaching Method
All high-temperature leaching experiments were conducted under reflux conditions (Figure 2). A mixture containing 10.0 g of BPC and oxalic acid at a fixed mass ratio of 50:1 (optimized in preliminary tests to ensure excess complexing capacity produced cost-effectively) was placed in a 500 mL borosilicate glass round-bottom flask. Sulfuric acid solutions of predetermined concentrations (20–50 wt%) were then added to achieve liquid-to-solid (L/S) ratios ranging from 5 to 15 mL/g. The reaction system was heated in an oil bath (60–98 °C) under continuous magnetic stirring (200–500 r/min). Upon completion of the predetermined leaching duration, the slurries were centrifuged and filtered. The residue was rinsed three times with deionized water to minimize iron loss, followed by vacuum drying at 80 °C. For parametric studies investigating the effects of L/S ratio, H2SO4 concentration, stirring speed, and temperature on iron leaching rate, the mass ratio of BPC to oxalic acid is 50:1 unless otherwise specified.
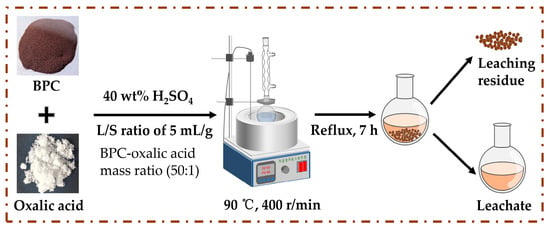
Figure 2.
Experimental process of iron recovery from BPC.
2.3. Response Surface Methodology Design
BPC and oxalic acid were mixed at a mass ratio of 50:1. The leaching process conditions for iron from PC were optimized using a Central Composite Design (CCD) within the RSM framework, under a stirring speed of 400 r/min. Four variables were selected for the leaching experiments: reaction time (A, h), reaction temperature (B, °C), L/S ratio (C, mL/g), and sulfuric acid concentration (D, wt%). Iron leaching rate, defined as the response value X (%), served as the optimization criterion. Based on the CCD method of RSM, a four-factor, three-level experimental optimization scheme was designed. The experimental design matrix is presented in Table 2.

Table 2.
Factor and parameter coding values for response surface tests.
2.4. Analytical Methods
The chemical composition of samples was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, ICAP7400, Thermo Fisher, Waltham, MA, USA). Morphologies of raw materials and leach residues were characterized using scanning electron microscopy (SEM, ZEISS, GeminiSEM 300, Jena, Germany) coupled with energy-dispersive X-Ray spectroscopy (EDS, OXFORD Xplore, Oxford Instruments, Abingdon, UK). Mineral phases were identified by X-Ray diffraction (XRD, Rigaku Ultima IV, Rigaku Corporation, Tokyo, Japan). Total iron content in leachates was quantified through potassium permanganate titration following reduction with titanium trichloride. Iron leaching rate (X) was calculated using Equation (1) [23,24]:
where c is the concentration of potassium dichromate standard titrant (mol/L); V is the volume of potassium dichromate titrant consumed by the sample solution (mL); V0 is the volume of potassium dichromate titrant consumed by the blank solution (mL); 55.85 is the relative atomic mass of iron (g/mol); m is the mass of the sample (g); WFe is the iron content of the sample (%).
X = c × (V − V0) × 55.85 × 100%/(m × WFe)
All leaching experiments were conducted in triplicate, and the reported iron leaching rates represent the mean values. The goodness of fit for kinetic models was evaluated using the coefficient of determination (R2).
3. Results and Discussion
3.1. Influence of Several Factors on Iron Leaching Rate
The leaching of iron from BPC was studied under different conditions. The effects of stirring speed, leaching temperature, sulfuric acid concentration, and L/S ratio on the leaching rate of iron were discussed. In addition, the characteristics of leaching slag under different leaching times were analyzed to investigate the leaching process of iron.
3.1.1. Effect of Stirring Speed and Leaching Temperature
When the leaching was controlled by liquid film diffusion, the stirring speed had a great influence on the solid–liquid leaching reaction [30,31]. The effect of stirring speed on iron leaching was first evaluated to assess potential external diffusion limitations. As expected for a diffusion-influenced process, the leaching efficiency increased markedly with agitation intensity up to 400 r/min, beyond which no significant improvement was observed (Figure 3a). This indicates that external liquid-film diffusion resistance was effectively eliminated at stirring speeds ≥ 400 r/min, allowing uniform dispersion of particles and sufficient reagent access to the solid–liquid interface [32]. Therefore, a stirring speed of 400 r/min was selected for all subsequent experiments to ensure mass transfer was not rate-limiting.
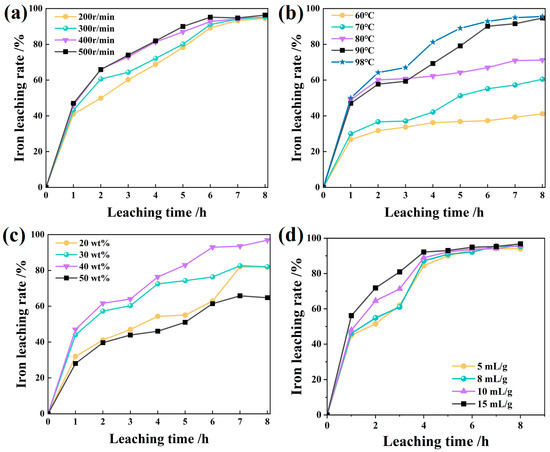
Figure 3.
Influence of leaching time (a), leaching temperature (b), H2SO4 concentration (c), and liquid-solid ratio (d) on leaching rate of iron.
The influence of temperature on leaching kinetics was profound, as illustrated in Figure 3b. The iron extraction efficiency increased substantially from 60 to 90 °C, reflecting the strongly temperature-dependent nature of the process. This enhancement can be attributed to a combination of factors: reduced solution viscosity improving ion mobility, increased molecular kinetic energy accelerating the surface chemical reaction, and potentially enhanced diffusion through the product layer [33,34,35]. The absence of a statistically significant improvement beyond 90 °C suggests that the reaction approaches its maximum intrinsic rate at this temperature, or that other limiting factors (such as reagent decomposition or product layer formation) become dominant. Consequently, 90 °C was selected as the optimal temperature for subsequent experiments.
3.1.2. Effect of Sulfuric Acid Concentration and L/S Ratio
The sulfuric acid concentration demonstrated a pronounced and non-linear impact on leaching performance, with a clear optimum observed at 40 wt% (Figure 3c). The initial enhancement in iron extraction with increasing acid concentration (20 to 40 wt%) is consistent with improved proton availability for hematite dissolution. However, the significant decline in efficiency at hyper-acidic conditions (50 wt%) reveals a critical shift in the governing mechanism.
We attribute this inhibition to a synergistic combination of factors: protonation of the oxalate anions, reducing the availability of active complexing ligands [36,37]; precipitation of CaSO4, which occludes surface pores and creates diffusion barriers; and increased solution viscosity, limiting ion mobility [38]. Consequently, 40 wt% H2SO4 was identified as the optimal concentration, effectively balancing proton activity with oxalate complexation capability.
The liquid-to-solid (L/S) ratio primarily influenced the initial leaching kinetics but not the final extraction yield (Figure 3d). The results show that during the initial 4 h leaching phase, efficiency increased significantly with L/S elevation from 5 to 15 mL/g. Beyond this period, further increases in L/S ratio yielded no significant improvement. Crucially, all L/S ratios achieved equivalent terminal leaching efficiencies (94%) upon completion of the 7 h reaction. Consequently, the minimum effective ratio of 5 mL/g was selected for subsequent operations to minimize lixiviant consumption while maintaining process efficacy.
3.2. Optimization of the Leaching Process
3.2.1. Model Establishment and Analysis of Variance
Based on the outcomes of single-factor experiments, a four-factor, three-level response surface analysis was conducted using the Box–Behnken design module in Design Expert (Version 8.0.6) to investigate the effects of leaching time (A), temperature (B), L/S ratio (C), and sulfuric acid concentration (D) on the BPC leaching process. The experimental data were fitted using a quadratic polynomial regression model, yielding the following equation relating the predicted iron leaching yield (X) to the factors A, B, C, and D:
X = −551.482 − 64.967 × A + 12.733 × B + 11.714 × C + 908.361 × D + 0.7 × A × B − 0.84 × A × C − 27.35 × A × D − 0.044 × B × C − 1.45 × B × D + 3.2 × C × D + 2.07 × A × A − 0.086 × B × B − 0.312 × C × C − 765.37 × D × D
The ANOVA for the quadratic model (Table 3) confirmed its high statistical significance (F-value = 79.13, p < 0.0001). Among the factors, temperature (B) and sulfuric acid concentration (D) were identified as the most significant, with extremely low p-values (p < 0.0001), indicating their dominant roles in governing the leaching kinetics and thermodynamics, respectively. In contrast, leaching time (A) and liquid-to-solid ratio (C) had negligible linear effects (p > 0.72), suggesting their influence is more complex and mediated through interaction terms.

Table 3.
ANOVA for response surface quadratic model analysis of variance.
Figure 4 presents a comparison between the experimental and predicted leaching efficiencies. The data points for predicted values are evenly distributed along both sides of the y = x line, demonstrating a high degree of concordance between model predictions and experimental results. This close agreement confirms that the quadratic model effectively captures the relationship between the four factors and iron leaching efficiency. Consequently, the validated model can be reliably used to analyze and predict iron leaching rates from BPC within the H2C2O4-H2SO4 system across the experimental domain.
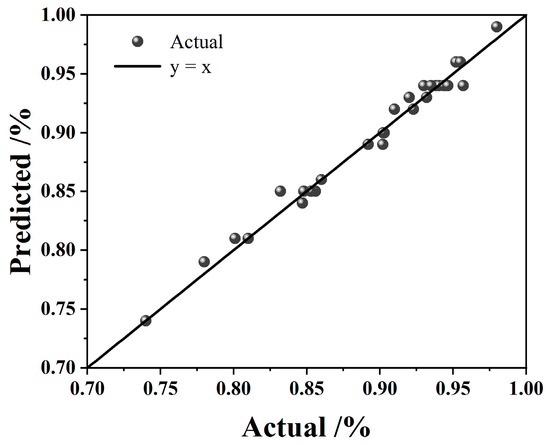
Figure 4.
Comparison of the predicted value with the actual value.
3.2.2. Comparison with Existing Hematite Leaching Processes
To objectively evaluate the efficacy of the optimized H2C2O4-H2SO4 hybrid system, a comparative analysis with reported acid leaching processes for hematite-rich resources is presented in Table 4. As summarized in Table 4, the optimized H2C2O4-H2SO4 hybrid system achieved a high iron leaching efficiency of 94.7% under mild conditions (90 °C, 40 wt% H2SO4), demonstrating clear advantages over both single-acid and other hybrid processes. Single-acid systems require severe conditions: concentrated H2SO4 (55 wt%) leaching reached only 51.2% iron extraction at elevated temperature (110 °C) [24], while HCl (30 wt%) achieved 89.0% at 90 °C but with serious corrosion concerns [23]. Although H3PO4 (3 mol/L) attained 98.1% extraction [8], it demanded both high temperature (110–130 °C) and concentrated acid. Notably, our system matches the performance of a previous H2C2O4-H2SO4 process (95.7% [26]) but with substantially lower oxalic acid dosage (BPC:OA = 50:1 vs. 5:1), offering superior economic potential for scalable iron recovery.

Table 4.
Comparative analysis of iron leaching efficiency from hematite-rich materials using different acid systems.
3.2.3. Response Surface Analysis
Response surface analysis provided critical insights into the interactive effects of key process parameters on iron leaching efficiency. The most significant interaction was observed between leaching time and temperature (AB, Figure 5a). Temperature exhibited kinetic dominance, with leaching efficiencies exceeding 93% at 90 °C regardless of time, while temperatures below 85 °C resulted in consistently low yields (<85%) even with extended duration. This underscores that insufficient thermal energy cannot be compensated by prolonged leaching, as higher temperatures are necessary to overcome the activation barrier and enhance both molecular mobility and interfacial reaction rates.
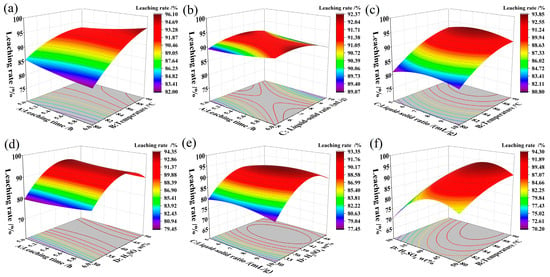
Figure 5.
Response surface diagrams for each leaching factor: (a) factors A and B; (b) factors A and C; (c) factors C and B; (d) factors A and D; (e) factors C and D; (f) factors D and B.
The interactions involving L/S ratio revealed its role in modulating mass transfer rather than determining the ultimate leaching extent. Both time–L/S (AC, Figure 5b) and temperature–L/S (BC, Figure 5c) interactions indicated that low L/S conditions (<6 mL/g) can restrict efficiency by promoting solid accumulation and increasing diffusion resistance. However, elevated temperature (90 °C) partially mitigated these effects by reducing viscosity and improving ion transport. Importantly, all L/S ratios between 5 and 15 mL/g eventually achieved similar final efficiencies under optimal T and t, confirming that L/S influences kinetics rather than thermodynamic limits.
Acid concentration interactions further clarified its thermodynamic role. The time–acid concentration plot (AD, Figure 5d) showed that ≥40% H2SO4 enabled rapid iron extraction within 5 h, whereas lower concentrations led to slow and incomplete leaching due to insufficient H+ supply. The acid concentration–L/S interaction (CD, Figure 5e) confirmed a unimodal response with a clear optimum at 40% H2SO4, above which efficiency declined due to oxalate protonation and precipitate formation.
Notably, the temperature–acid concentration interaction (BD, Figure 5f) was insignificant, indicating that these factors act through independent mechanisms: temperature primarily enhances kinetics, while acid concentration governs thermodynamics. No synergistic effect was observed under simultaneous high-temperature and high-acidity conditions, likely due to offsetting effects such as oxalic acid decomposition and surface passivation.
In summary, optimization should prioritize temperature (90 °C) and acid concentration (40% H2SO4) as primary controlling parameters. L/S ratio and time are secondary and should be tuned to maximize mass transfer and minimize reagent use, without expecting fundamental improvements in extraction limits.
3.3. Characterization of Leaching Residues
To elucidate microstructural evolution during leaching, residues at different leaching intervals were characterized by scanning electron microscopy (SEM) (Figure 6). At 1–2 h, residues exhibited coarse-grained aggregates with distinct blocky and lamellar morphologies. Progressive particle refinement occurred from 3 to 5 h, manifesting as structural fragmentation, increased dispersion, and accumulation of finer particulates. By 6–8 h, residues transitioned to homogenized microstructures featuring fine-grained porous or flocculent networks. This temporal progression corresponds to distinct leaching mechanisms: initial stage (1–2 h)—limited material dissolution with preserved macrostructures, indicating incipient reaction kinetics; intermediate stage (3–5 h)—accelerated decomposition of primary frameworks accompanied by emerging secondary morphologies; final stage (6–8 h)—reaction kinetics approaching a quasi-steady state with structural homogenization, though localized macropores observed at 8 h may indicate incipient structural collapse owing to over-leaching.
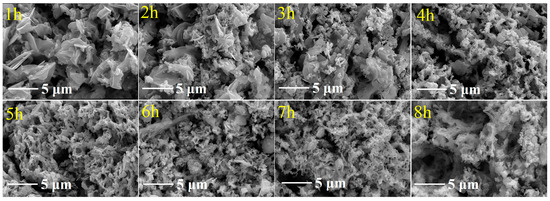
Figure 6.
SEM of leaching residues at different leaching times (leaching conditions: stirring speed of 400 r/min, temperature of 90 °C, L/S ratio of 5:1 mL/g, H2SO4 concentration of 40 wt%); magnification: 20k×.
Figure 7 presents comparative SEM micrographs of leach residues obtained at varying sulfuric acid concentrations (20–50 wt%). Progressive microstructural evolution was observed across the 20–40 wt% range: residues transitioned from consolidated blocky aggregates with limited porosity (20 wt%) to fragmented matrices exhibiting enhanced pore density and interconnectivity (30 wt%). This morphological progression demonstrates acid-concentration-dependent structural degradation, where elevated H2SO4 levels promote both mineral dissolution and pore network development—correlating directly with improved iron leaching kinetics and the ascending efficiency profile. At hyper acidic concentrations (50 wt%), residues developed heterogeneous domains characterized by dense, low-porosity agglomerates. We attribute this transition to rapid surface passivation through CaSO4 precipitation, forming diffusion-limiting barriers that impede acid permeation via pore occlusion, restrict reactive interface accessibility, and ultimately suppress iron extraction efficiency through kinetic inhibition.

Figure 7.
SEM of leaching residue at different H2SO4 concentrations (leaching conditions: leaching time of 7 h, stirring speed of 400 r/min, temperature of 90 °C, L/S ratio of 5:1 mL/g); magnification: 20k×.
Figure 8a presents XRD results for leaching residues at varying leaching durations. Progressive phase evolution was observed: relative peak intensities of hematite (Fe2O3) diminished with leaching time, while those of quartz (SiO2) and gypsum (CaSO4) intensified concomitantly. At 1–2 h, hematite dominated the phase composition, exhibiting prominent reflections at 2θ = 33.33°, 35.85°, 49.51°, 52.40°, 62.56°, and 64.09° (PDF#33-0664). Concurrently, strong quartz peaks appeared at 26.75° (011) and 25.60° (110), with gypsum detected at 24.34° (020) (PDF#33-0311, PDF#37-1496). As leaching progressed from 3 to 7 h, systematic reduction in hematite peak intensities occurred alongside enhancement of quartz signatures, including emerging reflections at 21.05° (101) and 28.81° (100). Gypsum peak intensities similarly increased throughout this period. This phase evolution confirms selective dissolution of hematite during leaching, with concomitant enrichment of residual quartz and gypsum due to their low solubility under acidic conditions.
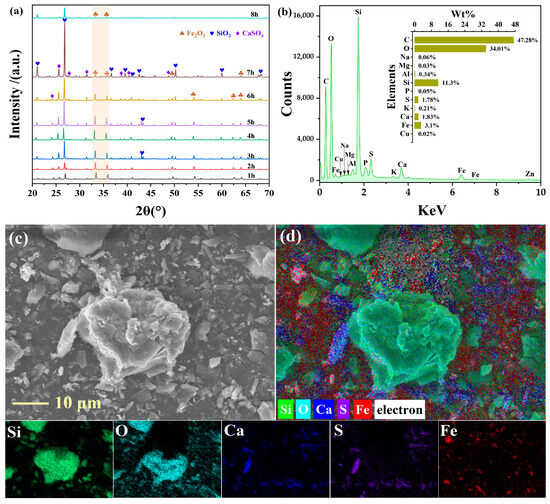
Figure 8.
XRD pattern of the leaching residue (a); SEM-EDS images (b–d).
The SEM-EDS elemental mapping of the residue obtained under optimum leaching conditions (40 wt% H2SO4, 90 °C, 7 h) is presented in Figure 8b–d. The maps clearly show that iron is largely leached out, while silicon (SiO2) remains highly concentrated within distinct, intact particles. This persistence of SiO2 under aggressive acidic conditions is attributed to the kinetic inertness of its highly polymerized crystalline structure [39]. Moreover, the integrity of these Si-rich particles is fully consistent with the strong and sharp quartz diffraction peaks in the XRD pattern of the residue, confirming that quartz comprises the primary inert solid phase after leaching.
3.4. The Kinetics of Iron Leaching
The iron leaching process from BPC particles conforms to the shrinking core model (SCM) for solid–liquid reactions. This model comprises three sequential steps: (1) external diffusion: transport of acid to the particle surface; (2) interfacial chemical reaction: surface reaction at the solid–liquid interface; (3) internal diffusion: acid diffusion through the product layer to the unreacted core surface. These steps are mathematically described by Equations (2)–(4) [40,41], respectively:
where x is the leaching rate of iron; km, kc, k1 are the apparent reaction rate constants of different control models (s−1); t is the leaching time (s).
Liquid-film diffusion control model: 1 − (1 − x)2/3 = kmt
Interfacial chemical reaction control model: 1 − (1 − x)1/3 = kct
Internal diffusion control model: 1 − 3(1 − x)2/3 + 2(1 − x) = kit
To identify the rate-controlling step in iron leaching, experimental data from Figure 3b were fitted to Equations (2)–(4), with results presented in Figure 9a–c and Table 5. As shown in Table 5, the kinetic analysis revealed a distinct temperature-dependent transition in the rate-controlling step. The dominance of liquid-film diffusion at lower temperatures suggests that the process was limited by the transport of lixiviants to the particle surface. In contrast, at higher temperatures (>80 °C), the best fit to both interfacial reaction and internal diffusion models indicates a shift to a mixed control mechanism, where both surface reaction and diffusion through the product layer become important.
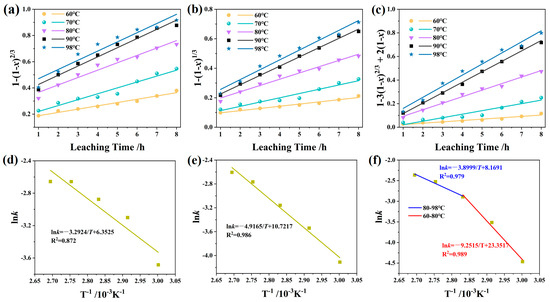
Figure 9.
Leaching kinetics: (a) membrane diffusion control model; (b) interface chemical reaction control model; (c) internal diffusion control model; (d–f) Arrhenius equation: (d) 1 − (1 − x)2/3; (e) 1 − (1 − x)1/3; (f) 1 − 3(1 − x)2/3 + 2(1 − x).

Table 5.
Fitting results of shrinking core models for iron leaching at different temperatures.
To further identify rate-controlling steps, the leaching data (Figure 3b) were fitted using the Johnson–Mehl–Avrami (JMA) model (Equation (5)); the results are shown in Figure S1 and Table S1. The Avrami exponent (n) derived from the JMA model offers deeper insight into the evolution of the rate-limiting mechanisms with increasing temperature [42]. The n reveals a shift in mechanism with temperature: low n-values at 60–70 °C indicate diffusion control, consistent with SCM analysis. As temperature increased to 80–90 °C, n rose to 0.587–0.710, indicating mixed control, where both diffusion and interfacial reaction govern kinetics. At 98 °C (n = 0.747), chemical reaction became dominant, though diffusion resistance from high viscosity and/or pore occlusion remained noticeable.
where x is the iron leaching rate, %; t is the leaching time, h; k is the overall rate constant, h−n; n is the Avrami exponent.
(JMA) model: x = 1 − exp(−k × tn)
The apparent activation energy (Ea) was determined by fitting k (Table 5) and T to the Arrhenius equation (Equation (6)), with results presented in Figure 9d–f and Table S2. The film diffusion control model exhibited poor Arrhenius fitting, indicating a deviation from the single activation energy process due to a transition from film diffusion dominance at lower temperatures to mixed control at elevated temperatures [40]. In contrast, the interfacial reaction model demonstrated excellent correlation, yielding Ea = 40.88 kJ/mol, consistent with values specified in the literature for iron leaching systems (34–90 kJ/mol) [43,44] and falling within the range expected for a chemically controlled reaction, similar to the process identified for hematite dissolution in other studies [45].
where R is the gas constant, A0 is the frequency factor, and T is leaching temperature.
Lnk = −Ea/(RT) + lnA0
The internal diffusion model revealed a distinct inflection at 80 °C (Figure 9f), demonstrating pronounced temperature-dependent kinetic sensitivity. Two linear regimes emerged: (i) a low-temperature regime (60–80 °C) with a higher Ea = 76.91 ± 2.1 kJ/mol (blue line), characteristic of chemically controlled processes; (ii) a high-temperature regime (80–98 °C) with a lower Ea = 32.42 kJ/mol (red line), consistent with diffusion-limited kinetics [41,46]. The high Ea value in the low-temperature regime is indicative of the significant energy required to break down the crystalline hematite structure and is comparable to values reported for the chemical reaction-controlled leaching of other refractory iron oxides [47]. The significantly lower Ea value in the high-temperature regime is highly suggestive of a shift to a diffusion-limited process. This value is remarkably consistent with the activation energy for the diffusion-controlled leaching stage of iron from titanium gypsum in citric acid (16.59 kJ/mol) [48] and aligns with the concept of a product layer creating a resistant barrier, a phenomenon also observed in the leaching of copper tailings [49]. The clear identification of this transition and the quantification of the respective activation energies for a real-world residue like BPC in a hybrid acid system provide a novel and mechanistic insight that extends beyond studies on pure minerals or single-acid systems [45,50].
The pre-exponential factor A0 provides additional insight: A0 = 4.5 × 104 s−1 for the interfacial reaction model is typical for chemically controlled systems [51], while A0 = 3.5 × 103 s−1 for internal diffusion at low temperatures reflects constrained ion mobility. The significantly higher A0 = 1.35 × 1010 s−1 at high temperatures suggests microstructural alterations that enhance ionic transport.
3.5. Thermodynamic Analysis of Iron Speciation and the Role of Oxalic Acid
To elucidate the critical role of oxalic acid in the leaching process, we measured the pH and total Fe3+ concentration in the leachate during the first 10 min of reaction (Figure S2a,b). The results indicate that the pH remained below 0 throughout the 2–10 min period, while the Fe3+ concentration increased gradually. Based on the iron concentrations at different time points, thermodynamic modeling was performed using Hydra/Medusa (32) software to investigate the speciation of dissolved iron under highly acidic conditions (pH < −0.3) (Figure S3). The simulations reveal that under the strong acidity maintained by H2SO4 (pH < 0), iron initially forms the Fe(C2O4)+ complex within the first 4 min, enhancing hematite dissolution through a ligand-promoted reaction; see Equation (7). As the leaching process proceeds, the high proton concentration induces the decomposition of the Fe(C2O4)+ complex, releasing Fe3+; see Equation (8). By 8 min, free Fe3+ becomes the dominant species. This mechanism confirms that oxalic acid acts catalytically—rather than being stoichiometrically consumed—through continuous acid-mediated regeneration. These findings align with the high leaching efficiency achieved at a low oxalate dosage and underscore the synergistic role of H2SO4 in both pH control and complex recycling.
Fe2O3 + 2H2C2O4 + 6H+ → 2Fe(C2O4)+ + 3H2O + 4H+
Fe(C2O4)+ + 2H+ → Fe3+ + H2C2O4
4. Conclusions
This study successfully developed an efficient and environmentally friendly oxalic-sulfuric acid hybrid leaching system for iron recovery from refractory boiling furnace pyrite cinder (BPC). Under optimized conditions (90 °C, 40 wt% H2SO4, L/S ratio of 5 mL/g, stirring speed of 400 r/min, 7 h), a high iron leaching rate of 94.7% was achieved with minimal reagent consumption.
The dissolution mechanism involves oxalate-promoted complexation and acid-driven regeneration: oxalic acid forms soluble Fe(C2O4)+ complexes with Fe3+ on the hematite surface, while sulfuric acid maintains strong acidity to decompose the complex, regenerating oxalic acid and releasing Fe3+, thereby enabling catalytic cycling of oxalate.
Kinetic analysis revealed a transition in the rate-controlling step from liquid-film diffusion control (Ea = 76.9 kJ/mol) at lower temperatures (60–70 °C) to mixed interfacial reaction and internal diffusion control (Ea = 32.4 kJ/mol) at higher temperatures (>80 °C). Highly concentrated H2SO4 (50 wt%) significantly reduced leaching efficiency due to oxalate protonation, CaSO4 precipitation-induced pore blockage, and increased solution viscosity.
Microstructural and mineralogical analyses (SEM-EDS/XRD) confirmed the progressive dissolution of hematite and the enrichment of inert phases such as quartz and gypsum in the residue. RSM optimization further demonstrated that temperature predominantly controls leaching kinetics, while acid concentration governs reaction thermodynamics, with no synergistic effect under simultaneous high-temperature and high-acidity conditions.
The proposed H2C2O4-H2SO4 hybrid process offers a competitive and sustainable alternative for valorizing iron-rich BPC, avoiding the high corrosion, reagent cost, and energy consumption associated with conventional methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13092904/s1, Figure S1: The JMA model at different temperatures; Figure S2: pH (a) and CFe3+ (b) of the leachate at different leaching times (leaching conditions: 90 °C, 40 wt% H2SO4, L/S ratio of 5 mL/g, stirring speed of 400 r/min); Figure S3: Distribution of Fe species; Table S1: Fitting parameters of the Johnson–Mehl–Avrami (JMA) model at different temperatures; Table S2: Fitting parameters of the Arrhenius equation.
Author Contributions
Conceptualization, X.L.; methodology, X.L. and Z.P.; validation, X.L. and Y.Y.; formal analysis, X.L. and Z.P.; investigation, X.L.; resources, Y.Y.; data curation, X.L. and Z.P.; writing—original draft preparation, X.L. and Y.Y.; writing—review and editing, Y.Y.; visualization, Z.P.; supervision, X.L.; project administration, Y.Y.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Key Research and Development Program of Shaanxi Province, China (No. 2023-ZDLSF-63), the National Natural Science Foundation of China (Nos. 42261144749, 52174298, and 52374411), and Xingbao Talent Development Program (2023).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author Yang Yang was employed by the company Wengfu Zijin Chemical Co., Ltd. The author Xiaojiao Li was a PhD candidate at Xi’an Jiaotong University. The author Zhenlin Peng was an undergraduate student at Baoshan University. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- He, B.; Tian, X.; Sun, Y.; Yang, C.; Zeng, Y.; Wang, Y.; Zhang, S.; Pi, Z. Recovery of iron oxide concentrate from high-sulfur and low-grade pyrite cinder using an innovative beneficiating process. Hydrometallurgy 2010, 104, 241–246. [Google Scholar] [CrossRef]
- Tian, C. A novel approach for comprehensive utilization by leaching pyrite cinder with titanium dioxide waste acid by re-sponse surface methodology. ACS Omega 2024, 9, 8510–8519. [Google Scholar] [CrossRef]
- Chen, D.; Guo, H.; Xu, J.; Lv, Y.; Xu, Z.; Huo, H. Recovery of iron from pyrite cinder containing non-ferrous metals using high-temperature chloridizing-reduction-magnetic separation. Metall. Mater. Trans. B 2017, 48, 933–942. [Google Scholar] [CrossRef]
- Pan, W.; Liu, L.; Li, Y.; Jiang, T.; Liu, Y.; Li, Y.; Zhou, J.; Tang, A.; Xue, J. Efficient purification and utilization of pyrite cinder for carbon-coated lithium iron phosphate synthesis. J. Environ. Chem. Eng. 2024, 12, 114720. [Google Scholar] [CrossRef]
- Tiberg, C.; Bendz, D.; Theorin, G.; Kleja, D.B. Evaluating solubility of Zn, Pb, Cu and Cd in pyrite cinder using leaching tests and geochemical modelling. Appl. Geochem. 2017, 85, 106–117. [Google Scholar] [CrossRef]
- Liu, L.; Dong, W.; Niu, M.; Liu, X.; Xue, J.; Tang, A. Fabrication of a confined pyrite cinder-based photo-Fenton catalyst and its degradation performance for ciprofloxacin. J. Mol. Liq. 2022, 360, 119489. [Google Scholar] [CrossRef]
- Dong, T.; Guo, Z.; Zhu, D.; Pan, J.; Ma, W.; Li, S.; Shi, Y. Manufacturing of Fe/C micro-electrolysis materials with the pyrite cinder towards the degradation of high-concentration organic wastewater. J. Environ. Chem. Eng. 2024, 12, 113502. [Google Scholar] [CrossRef]
- Jiang, T.; Tu, Y.; Su, Z.; Lu, M.; Liu, S.; Liu, J.; Gu, F.; Zhang, Y. A novel value-added utilization process for pyrite cinder: Selective recovery of Cu/Co and synthesis of iron phosphate. Hydrometallurgy 2020, 193, 105314. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Liu, W.; Zhang, Z.; Qin, W. Separation of aluminum and lithium in sulfuric acid roasting leachate of overhaul slag by Di-(2-ethylhexyl) phosphoric acid extraction and sulfuric acid strip. Hydrometallurgy 2025, 236, 106521. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, X.; Luo, H.; Deng, J.; Dai, L.; Ju, Z.; Zhu, Z.; Zou, L.; Ji, L.; Li, B.; et al. Release of heavy metals from the pyrite tailings of huangjiagou pyrite mine: Batch experiments. Sustainability 2016, 8, 96. [Google Scholar] [CrossRef]
- Hottenstein, J.D.; Neilson, J.W.; Gil-Loaiza, J.; Root, R.A.; White, S.A.; Chorover, J.; Maier, R.M. Soil microbiome dynamics during pyritic mine tailing phytostabilization: Understanding microbial bioindicators of soil acidification. Front. Microbiol. 2019, 10, 1211. [Google Scholar] [CrossRef]
- Jin, W.; Yang, S.; Tang, C.; Li, Y.; Chang, C.; Chen, Y. Green and short smelting process of bismuth sulphide concentrate with pyrite cinder. J. Clean. Prod. 2022, 377, 134348. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Cai, X.; Fu, J.; Liu, M.; Zhang, P.; Yu, H. The leaching behavior of copper and iron recovery from reduction roasting pyrite cinder. J. Hazard. Mater. 2021, 420, 126561. [Google Scholar] [CrossRef]
- Li, Y.; Xian, Y.; Chen, D.; Zhong, Y.; Wu, X. Process optimization on preparation of composite portland cement with low-grade pyrite cinder. Multipurp. Util. Miner. Resour. 2023, 3, 78–81. [Google Scholar]
- Zamfir, A.; Meghea, A.; Oprea, O.C.; Mihaly, M. Sustainable conversion of acid mine drainage sludge into high-quality ironbased pigments through thermal processing. Process Saf. Environ. Prot. 2025, 198, 107181. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Surkova, T.; Yessimova, D.; Baltabekova, Z.; Abikak, Y.; Abdikerim, B.; Dosymbayeva, Z. Extraction of noble metals from pyrite cinders. ChemEngineering 2023, 7, 14. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Hakkou, R.; Bouamrane, A. A comparative study on the practical use of low sulfide base-metal tailings as aggregates for rendering and masonry mortars. J. Clean. Prod. 2016, 112, 914–925. [Google Scholar] [CrossRef]
- Kerkez, D.; Bečelić-Tomin, M.; Gvoić, V.; Mandić, A.K.; Leovac Maćerak, A.; Tomašević Pilipović, D.; Pešić, V. Pyrite cinder as an effective fenton-like catalyst for the degradation of reactive azo dye: Effects of process parameters and complete effluent characterization. Catalysts 2023, 13, 424. [Google Scholar] [CrossRef]
- Pan, W.; Liu, L.; Liu, Y.; Xu, S.; Zhou, J.; Tang, A.; Xue, J. Construction of a porous pyrite cinder-supported Mn-doped BiFeO3 composite photocatalyst: Confined-space strategy, structure, and visible-light catalytic degradation toward ciprofloxacin. J. Environ. Chem. Eng. 2023, 11, 111355. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Xiao, T.; Chen, Y.; Long, J.; Zhang, G.; Zhang, P.; Li, C.; Zhuang, L.; Li, K. Efficient removal of thallium (I) from wastewater using flower-like manganese dioxide coated magnetic pyrite cinder. J. Clean. Prod. 2019, 353, 867–877. [Google Scholar] [CrossRef]
- Yu, H.; Liang, B.; Song, W.; Zhou, X.; Liu, M.; Zeng, H.; Zhang, H. Recovery of gold and iron oxide from pyrite cinder using reduction roasting, grinding, thiosulfate leaching in the presence of additives and magnetic separation. Hydrometallurgy 2025, 233, 106453. [Google Scholar] [CrossRef]
- Han, P.; Li, Z.; Tu, Y.; Zhao, T.; Wei, L.; Ye, S. Recovery of valuable metals from iron-rich pyrite cinder by chlorination-volatilization method. Min. Metall. Explor. 2024, 41, 345–352. [Google Scholar] [CrossRef]
- Zhang, Y. Study on Preparation of Iron Phosphate from Pyrite Cinder by Hydrochloric Acid Process. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2023. [Google Scholar]
- Zhang, X.; Chen, Y.; Huang, S.; Li, Z.; Zheng, Y.; Chen, Z. Investigation on extracting iron from pyrite cinder by sulfuric acid leaching. Chem. Ind. Eng. Prog. 2003, 22, 165–168. [Google Scholar]
- Li, W.; Hua, T.; Zhou, Q. Optimum technical conditions study of dissolving pyrite cinders by hydrochloric acid. J. Basic Sci. Eng. 2008, 16, 795–801. [Google Scholar]
- Dang, X.; Yang, D.; Wang, B. Mechanism of zinc and iron separation in neutral leaching residue by oxalic acid solution and optimization of the separation condition. Nonferrous Met. Eng. 2024, 14, 96–107. [Google Scholar]
- Li, W.; Wang, S.; Han, Y.; Tang, Z.; Zhang, Y. Recovery of iron from pyrite cinder by suspension magnetization roasting-magnetic separation method: Process optimization and mechanism study. Sep. Purif. Technol. 2024, 332, 125652. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Wang, M.; Wang, H.; Xian, P. Recovery of iron from red mud by selective leach with oxalic acid. Hydrometallurgy 2015, 157, 239–245. [Google Scholar] [CrossRef]
- Yang, B.; Fang, X.; Wang, B.; Dong, Y.; Yang, M. Extraction of iron from pyrite cinder via oxalic acid-intensified leaching method. Chem. Ind. Eng. Prog. 2019, 38, 1552–1560. [Google Scholar] [CrossRef]
- Dreisinger, D.; Abed, N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: Kinetic analysis. Hydrometallurgy 2002, 66, 37–57. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A.; O’Connor, G.M. The kinetics of chalcopyrite leaching in alkaline glycine/glycinate solutions. Miner. Eng. 2019, 135, 118–128. [Google Scholar] [CrossRef]
- Zhang, D.C.; Sun, F.L.; Zhao, Z.W.; Liu, X.H.; Chen, X.Y.; Li, J.T.; He, L.H. Kinetics study on cobalt leaching from cobalt-bearing ternary sulfide in sulfuric acid solution under atmospheric pressure. Trans. Nonferrous Met. Soc. China 2024, 34, 1669–1680. [Google Scholar] [CrossRef]
- Liu, J.L.; Yin, Z.L.; Li, X.H.; Hu, Q.Y.; Liu, W. Recovery of valuable metals from lepidolite by atmosphere leaching and kinetics on dissolution of lithium. Nonferrous Met. Soc. China 2019, 29, 641–649. [Google Scholar] [CrossRef]
- Xing, P.; Wang, J.; Lyu, T.; Zhuang, Y.; Du, X.; Luo, X. Ultrasound-assisted impurity removal from petroleum coke. Sep. Purif. Technol. 2015, 151, 251–255. [Google Scholar] [CrossRef]
- Meng, Q.; Pang, Y.; Chen, Y.; Hao, Y.; Xie, J.; Zhuang, Y.; Xiong, P. Kinetic and mechanism of Ca leaching in the production of high-purity magnesia. JOM 2025, 77, 5777–5789. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Hu, H.; Zhang, T.; Zhou, Y. Dissolution of kaolinite induced by citric, oxalic, and malic acids. J. Colloid Interf. Sci. 2005, 290, 481–488. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.; Yang, M.; Tian, Y.; Yuan, H. Lithium was extracted from lithium-poor clay ores after short calcination by oxalic acid leaching. Particuology 2025, 98, 204–214. [Google Scholar] [CrossRef]
- Amjad, Z.; Koutsoukos, P.G. Evaluation of maleic acid based polymers as scale inhibitors and dispersants for industrial water applications. Desalination 2014, 335, 55–63. [Google Scholar] [CrossRef]
- Lin, C.; Lü, T.; Qi, D.; Cao, Z.; Sun, Y.; Wang, Y. Effects of surface groups on SiO2 nanoparticles on in situ solution polymerization: Kinetics and mechanism. Ind. Eng. Chem. Res. 2018, 57, 15280–15290. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, X.; Han, Y.; Yu, H. Dissolution kinetics and removal mechanism of kaolinite in diasporic bauxite in alkali solution at atmospheric pressure. Nonferrous Met. Soc. China 2019, 29, 2627–2637. [Google Scholar] [CrossRef]
- Valeev, D.; Pankratov, D.; Shoppert, A.; Sokolov, A.; Kasikov, A.; Mikhailova, A.; Salazarconcha, C.; Rodionov, I. Mechanism and kinetics of iron extraction from high silica boehmite–kaolinite bauxite by hydrochloric acid leaching. Nonferrous Met. Soc. China 2021, 31, 3128–3149. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, J.; Gong, Y.; Yang, Y.; Wang, R.; Xu, Z.; Li, J. Acid leaching process for valuable metals in limonitic laterite nickel ore. Nonferr. Met. Sci. Eng. 2024, 15, 274–284. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, X.; Zhao, Z. Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure. Hydrometallurgy 2020, 194, 105353. [Google Scholar] [CrossRef]
- Gok, O.; Anderson, C.G.; Cicekli, G.; Cocen, E.L. Leaching kinetics of copper from chalcopyrite concentrate in nitrous-sulfuric acid. Physicochem. Probl. Miner. Process. 2014, 50, 399–413. [Google Scholar] [CrossRef]
- Vehmaanperä, P.; Salmimies, R.; Häkkinen, A. Thermodynamic and kinetic studies of dissolution of hematite in mixtures of oxalic and sulfuric acid. Min. Metall. Explor. 2021, 38, 69–80. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.C.; Zhang, Q.J.; Zhang, W.M.; Zhang, Q.X. Critical review in adsorption kinetic models. J. Zhejiang Univ.-Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Ji, T.; Yang, B.; Su, S.J.; Ding, S.; Sun, W.Y. Leaching characteristics and kinetics of iron and manganese from iron-rich pyrolusite slag in oxalic acid solution. Sep. Sci. Technol. 2021, 56, 1612–1621. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, H.; Peng, T.; Zhao, D.; Zhang, X. The leaching kinetics of iron from titanium gypsum in a citric acid medium and obtain materials by leaching liquid. Molecules 2023, 28, 952. [Google Scholar] [CrossRef]
- Tao, L.; Wang, L.; Yang, K.; Wang, X.; Chen, L.; Ning, P. Leaching of iron from copper tailings by sulfuric acid: Behavior, kinetics and mechanism. RSC Adv. 2021, 11, 5741–5752. [Google Scholar] [CrossRef]
- Huang, Y.; Mo, W.; Feng, J.; Ren, R.; He, C.; Su, X. Differential leaching behavior of iron and aluminum from Bayer red mud in sulfuric acid/oxalic acid system. Nonferr. Met. (Miner. Process. Sect.) 2023, 2, 34–43. [Google Scholar]
- Gao, G. Research on Leaching Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using an Organic Acid Reductive System. Ph.D. Thesis, Shanghai University, Shanghai, China, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).