Abstract
This study presents the results of an investigation into the effectiveness of microwave (MW) treatment (1) as a preconditioning method for technogenic raw materials (2) to enhance zinc (3) leaching (4) efficiency. Selective dielectric heating facilitates phase transformations (5), converting sphalerite (ZnS) into zinc oxide (ZnO), which exhibits significantly improved acid leachability. The response surface methodology (RSM) was utilized to evaluate critical operational variables, encompassing sulfuric acid concentration, leaching period, slurry density, and thermal conditions. The obtained results confirm the potential of MW treatment in hydrometallurgical processes, offering a sustainable and energy-efficient alternative for processing technogenic raw materials.
1. Introduction
The depletion of high-grade ores and the growing need for sustainable resource management have driven extensive research into alternative sources of metals. In response, modern metallurgical practices have increasingly focused on processing industrial waste, low-grade ores, and unconventional mineral resources. Industrialized nations have achieved utilization rates of industrial waste as high as 70–80%, whereas countries like Kazakhstan, despite being rich in mineral resources, face challenges in efficiently processing technogenic raw materials due to limited technologies and specialized equipment [1,2,3,4,5].
One of the most pressing concerns in the mining and metallurgical sectors is the vast accumulation of industrial waste, including technogenic stockpiles, tailings, and byproducts such as clinker from zinc production. These materials contain valuable elements, including rare earth metals, gold, silver, copper, and zinc, which are of significant economic and industrial importance [6,7,8,9,10]. Recent advancements in pyrometallurgical and hydrometallurgical techniques have enabled the extraction of these valuable metals, yet challenges such as high energy consumption, carbon emissions, and processing inefficiencies persist [11,12,13,14,15].
A notable trend in metallurgical innovation is the exploration of seabed mineral deposits and deep-sea mining for critical metals, including cobalt, nickel, and rare earth elements. Researchers have investigated solid-state metallization and ammonium sulfate roasting to selectively recover metals from oceanic crusts and polymetallic nodules [16,17,18,19]. These approaches hold promise for reducing dependence on terrestrial mining while addressing the increasing demand for strategic materials.
In the Commonwealth of Independent States (CIS), annual mineral extraction surpasses 3.5 billion cubic meters, with waste generation reaching 1.5 billion cubic meters. In light of diminishing ore quality and the increasing prevalence of intractable raw materials, adopting thorough processing strategies for low-grade and intricate mineral deposits has emerged as both an economic necessity and a scientific priority [20,21,22,23,24].
Among the most significant technogenic waste materials, zinc-bearing clinker warrants particular attention due to its potential for valuable metal recovery. Pyrometallurgical processes such as the Waelz process, which operates at 1100–1300 °C using carbonaceous reducing agents, remain a dominant method for processing these materials. However, these processes produce substantial CO2 emissions and have high operational costs, making them less viable for high-silica zinc-bearing raw materials [25,26,27,28,29].
In response to these challenges, novel processing techniques have been developed, including the use of microwave irradiation as a pre-treatment method. This technology leverages selective dielectric heating to enhance the reactivity and extractability of target elements, leading to improved leaching kinetics and overall process efficiency. Studies have demonstrated that microwave-assisted processing significantly enhances metal recovery rates while reducing energy consumption and environmental impact [30,31,32,33,34].
The integration of advanced processing technologies, including sorption techniques for niobium extraction, acid leaching for tailings processing, and hydrometallurgical methods for deep-sea mineral extraction, offers a promising path toward more sustainable and economically viable metal recovery [35,36,37,38,39]. These innovations not only enhance resource efficiency but also play a pivotal role in diminishing the buildup of industrial waste and mitigating the ecological impact of mining and metallurgical activities.
Given the urgent need for improved waste management and metal recovery, further research into innovative extraction technologies, including hybrid approaches combining pyrometallurgical and hydrometallurgical processes, is crucial. By leveraging emerging techniques and optimizing existing methods, the metallurgical industry can achieve greater efficiency, sustainability, and economic resilience in the face of depleting high-grade ore reserves.
The primary aim of this investigation is to explore the effects of microwave (MW) treatment on phase transition dynamics and zinc extraction from technogenic raw materials. Particular attention is devoted to examining the structural modifications in these substances triggered by selective dielectric heating, which augments the metal’s solubility.
The study concentrates on refining the parameters of the leaching process through the application of response surface methodology (RSM) to determine the optimal conditions for enhancing zinc recovery. The findings derived from this research could provide a foundation for devising energy-efficient and ecologically sound techniques for the treatment of technogenic raw materials.
2. Materials and Methods
2.1. Materials
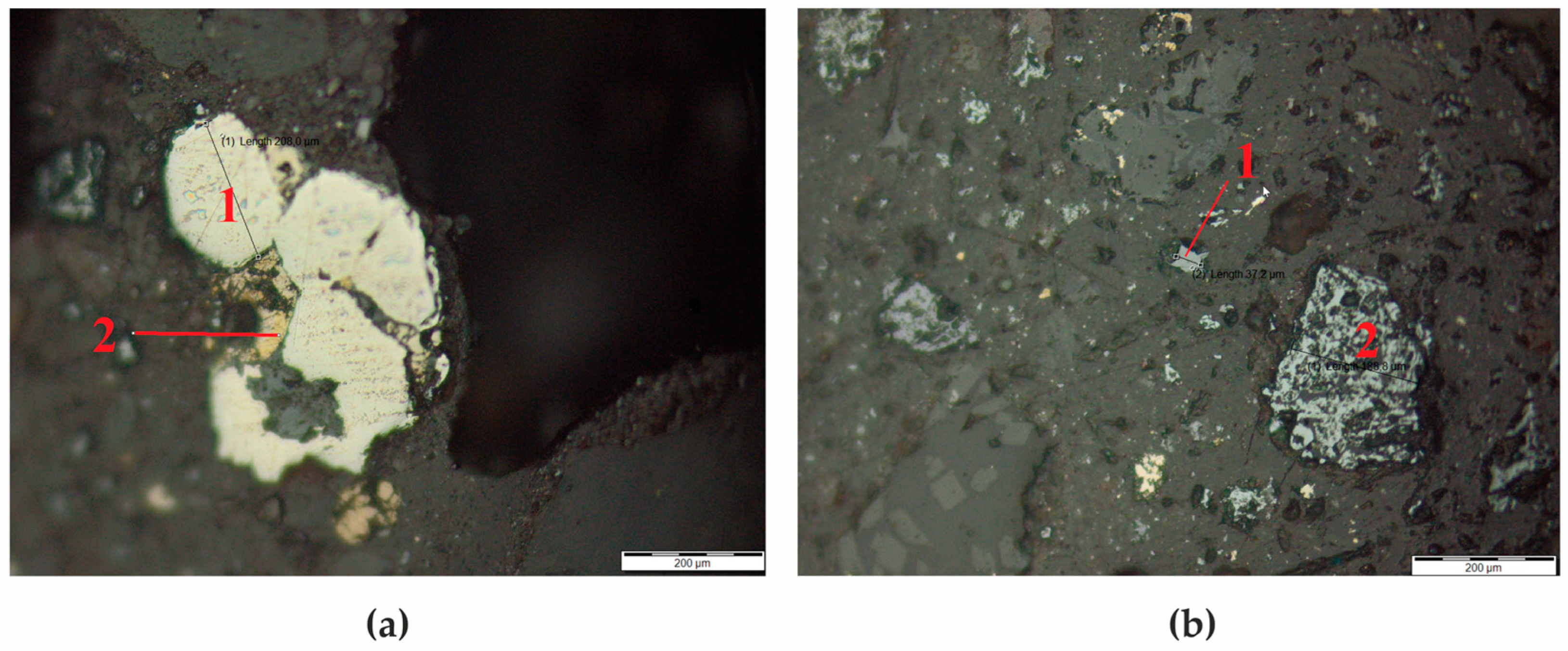
The subject of this investigation was technogenic raw material, specifically zinc production residue in the form of clinker. Throughout the research, the distinctive structural features and particle size distribution of the constituent minerals within the clinker were elucidated (Figure 1). Several prominent minerals were pinpointed, including sphalerite (ZnS), present as scarce fine-grained entities, chalcopyrite (CuFeS2), frequently co-occurring with pyrite (FeS2) and sphalerite, alongside hematite (Fe2O3) and carbonaceous inclusions exhibiting diverse sizes and morphological characteristics.
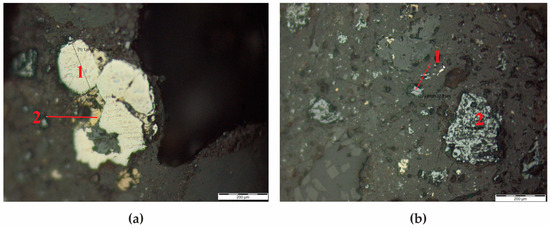
Figure 1.
(a) Pyrite (1) and Chalcopyrite (2); (b) Sphalerite (1) and hematite (2).
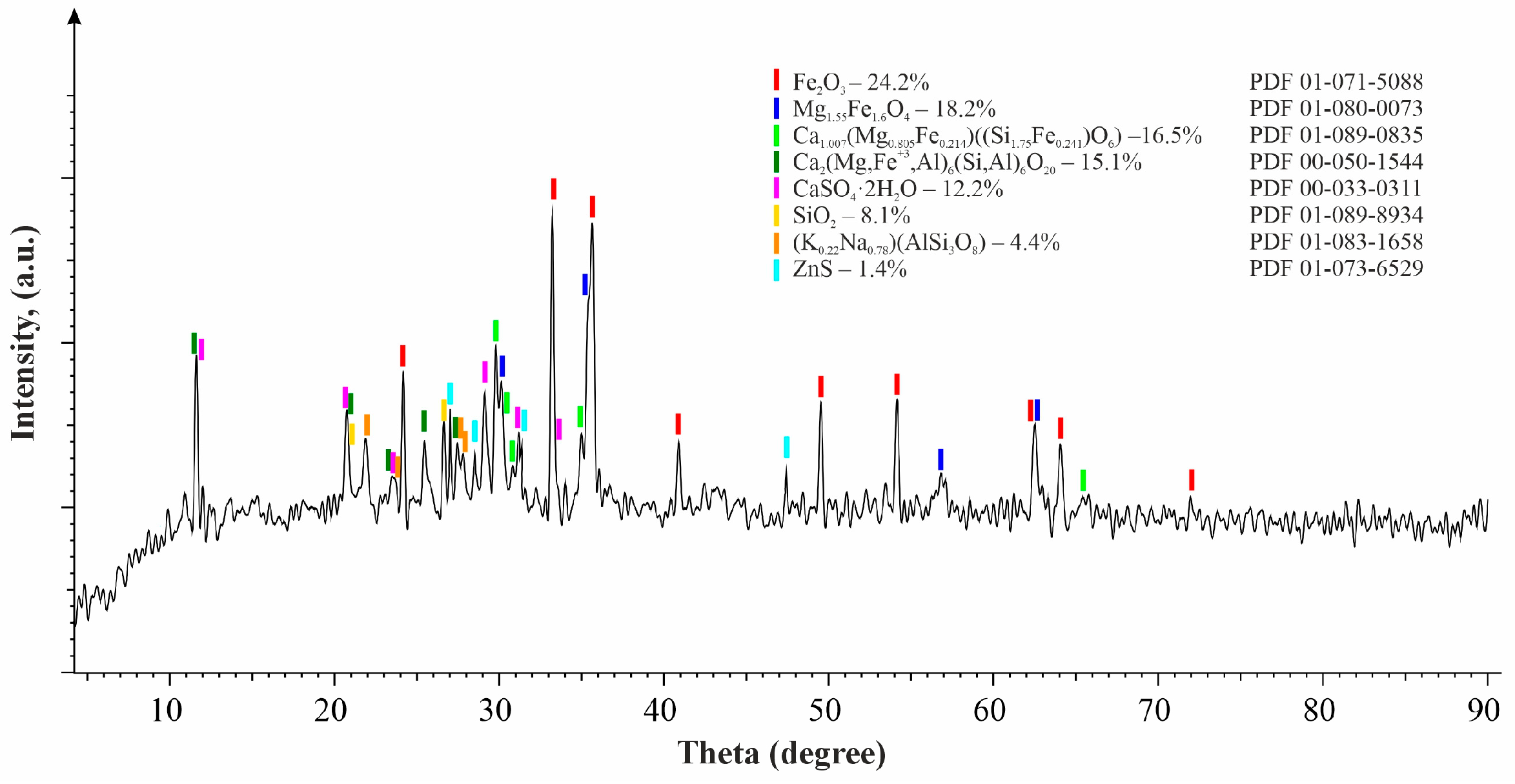
X-ray fluorescence (XRF) examination of the clinker disclosed a substantial presence of iron (37.53%), calcium (3.81%), silicon (4.58%), oxygen (41.64%), copper (1.04%), and zinc (exceeding 1.2%), along with various additional elements, as outlined in Table 1. Moreover, X-ray diffraction (XRD) analysis discerned hematite (Fe2O3—24.2%) and magnesium iron oxide (Mg1.55Fe1.6O4—18.2%) as the predominant phase constituents within the clinker. Additional phase compositions are elaborated in Table 2 and depicted in Figure 2.

Table 1.
Elemental Composition of Clinker via XRF Analysis. (reprinted for Ref. [40]).

Table 2.
Findings from the X-ray phase characterization of the clinker.
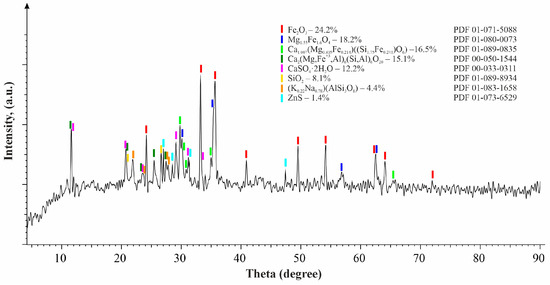
Figure 2.
X-ray diffraction pattern of the clinker (reprinted from Ref. [40]).
An in-depth investigation of the clinker’s composition facilitates a deeper comprehension of its chemical properties, which is crucial for determining the most effective processing methodologies. The outcomes of X-ray fluorescence (XRF) analysis provided the foundation for subsequent experimental studies, concentrating on the effects of microwave (MW) irradiation on the structural and phase transformations within the clinker, the infiltration of reagents into its framework, and the significant enhancement of leaching and extraction efficiency for valuable constituents.
2.2. Analytical Methodologies
This investigation utilizes sophisticated analytical methodologies to assess the phase composition of clinker and to clarify the mechanisms underlying its phase transitions. Mineralogical analysis was performed using an OLYMPUS BX51 optical microscope (Olympus, Tokyo, Japan), a high-resolution research-grade instrument designed for petrographic investigations. This microscope is equipped with an advanced UIS2 optical system, enabling high-contrast imaging and multiple observation modes, including brightfield, polarized light, and differential interference contrast (DIC). These capabilities facilitate the precise identification of mineral phases, grain morphology, and textural relationships within the clinker samples. The phase constitution of the specimens was analyzed employing a Bruker D8 Advance X-ray diffractometer (Bruker, Ettlingen, Germany), which provides high-resolution diffraction data for accurate phase identification and structural analysis of the material. The elemental makeup of the specimens was evaluated utilizing an Axios 1 kW wavelength-dispersive X-ray fluorescence spectrometer (PANalytical, Almelo, The Netherlands), which enables precise quantitative and qualitative determination of elemental content in various materials through high-sensitivity spectral analysis. Data analysis and interpretation were performed using SuperQ5 software (Omnian 37), which guarantees precise calibration, quantification, and evaluation of elemental profiles through the implementation of sophisticated correction algorithms and extensive spectral modeling. The surface microstructure of the specimens was scrutinized employing a JXA-8230 electron probe microanalyzer (JEOL, Tokyo, Japan), which enables high-accuracy elemental mapping and quantitative microanalysis of materials by harnessing an electron beam to produce characteristic X-rays. The elemental composition of the samples was analyzed using an Optima 8300DV inductively coupled plasma optical emission spectrometer (PerkinElmer, Inc., Waltham, MA, USA), which provides high sensitivity and accuracy for multi-element detection through advanced plasma technology and dual-view capability.
2.3. Optimal Experimental Design
To optimize the retrieval of zinc from clinker, this investigation employed an advanced statistical approach, specifically response surface methodology (RSM) coupled with a central composite design (CCD). The principal factors explored included thermal conditions, sulfuric acid strength, extraction time, and slurry consistency (liquid-to-solid proportion). The equation is expressed as follows:
where y signifies the anticipated zinc recovery, b0 represents the intercept term, bᵢ denotes the coefficients for linear effects, bᵢᵢ indicates the coefficients for quadratic effects, bᵢⱼ reflects the coefficients for interaction effects among variables, and k corresponds to the total number of parameters.
To streamline the experimental framework and data assessment, Design Expert 7.0 (Stat-Ease, Inc., Minneapolis, MN, USA) was employed. This tool enabled the meticulous construction of a second-order polynomial model, guaranteeing exceptional predictive precision and statistical robustness.
The most advantageous process conditions were discerned via regression analysis, with an emphasis on thermal conditions, acid strength, extraction duration, and slurry density. The coded values and their associated experimental ranges for the central composite design (CCD) are presented below (Table 3):

Table 3.
Factor Levels and Codes for the Central Composite Design (CCD).
By implementing this experimental strategy, the study successfully refined the process parameters, leading to a more efficient and reproducible approach for maximizing zinc extraction from clinker.
2.4. Experimental Method
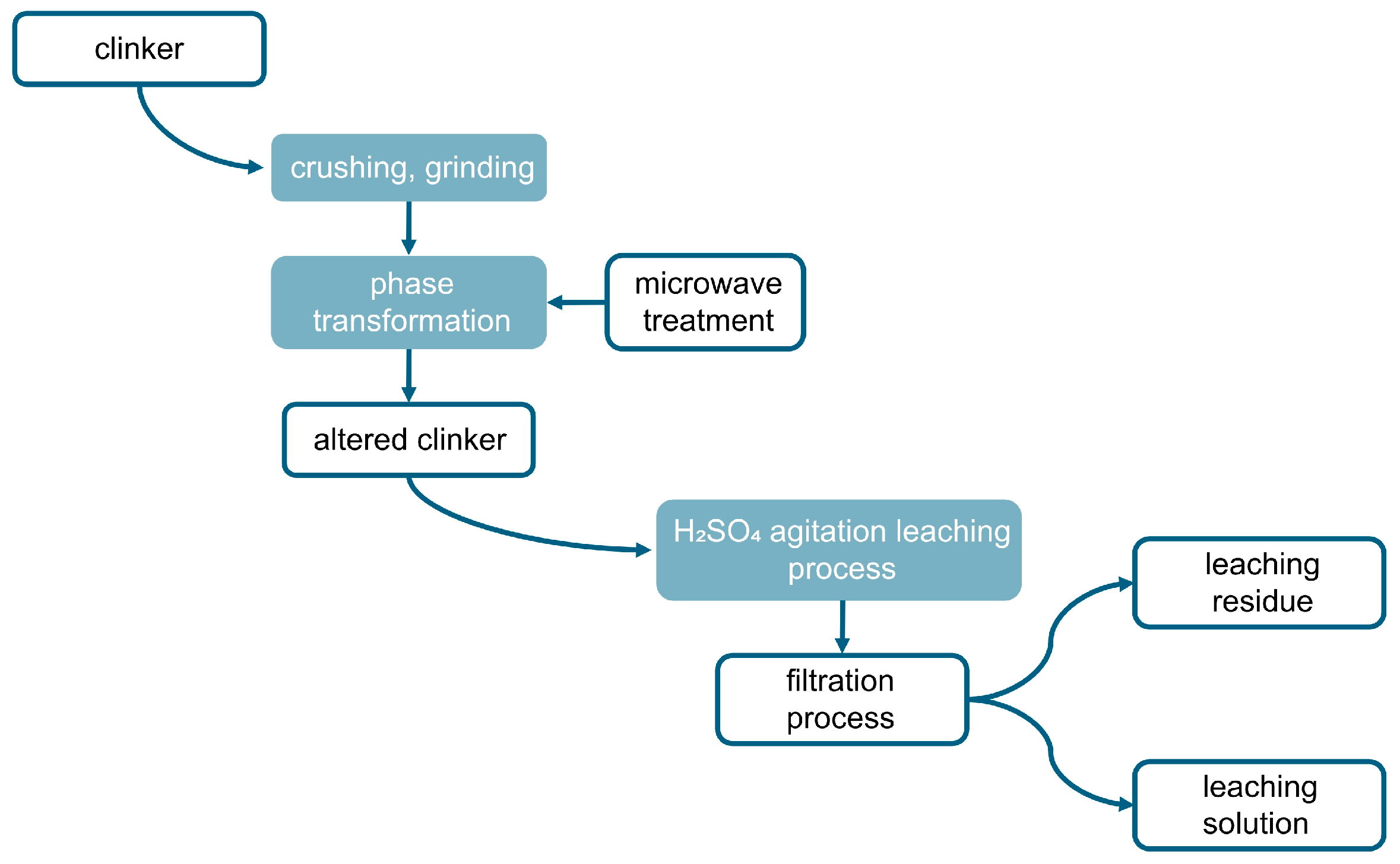
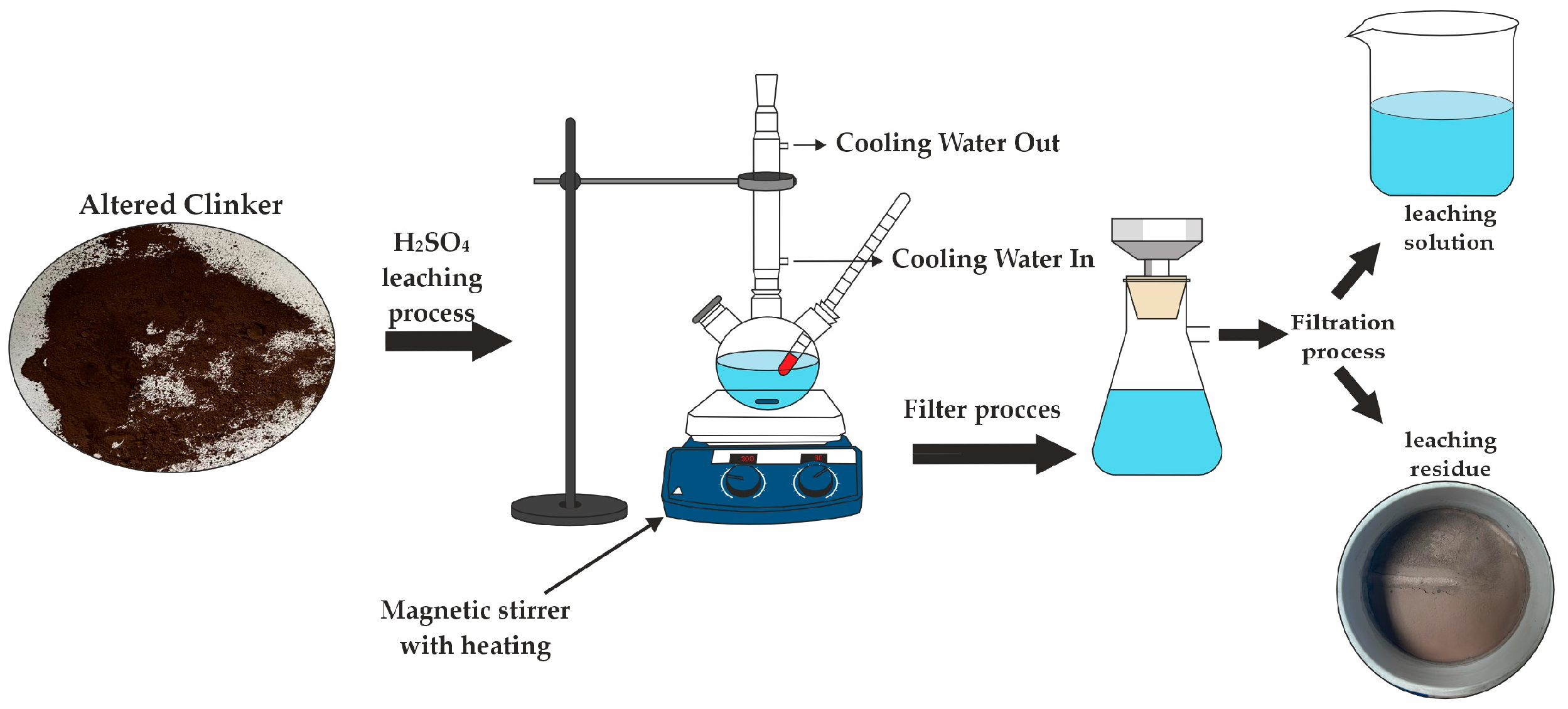
The process of extracting zinc from clinker encompasses three primary phases: pulverization of the clinker to attain 90% of particles finer than 0.071 mm, microwave-facilitated phase alteration, and subsequent leaching. This methodology comprises multiple intricate steps, as outlined in Figure 3. The procedure commences with specimen preparation, succeeded by roasting of the clinker within a high-temperature microwave reactor, specifically the “ENERGY K-50” system (Ust-Kamenogorsk, Kazakhstan), which operates at 915 MHz with a capacity of 25 kW. This apparatus is distinguished by its substantial power output, operational consistency, and superior efficiency [41,42]. Following microwave processing, the clinker is subjected to the leaching phase, as illustrated in Figure 4.
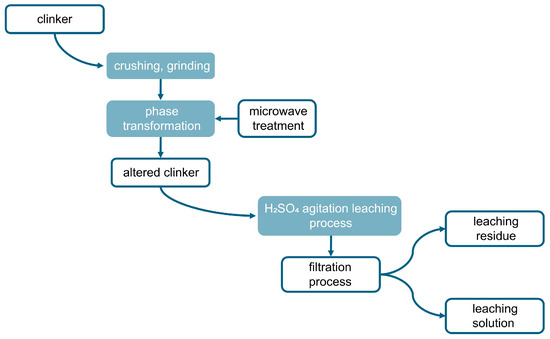
Figure 3.
A schematic representation of the experimental procedure.
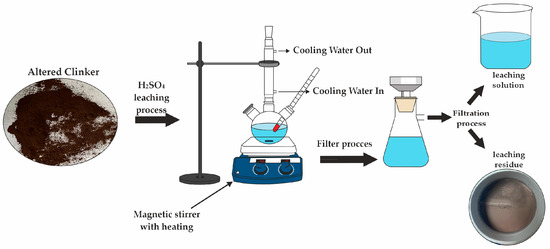
Figure 4.
A schematic representation of the leaching process experimental setup.
In our experiments, the microwave generator was set to a power of 20 kW, which ensured stable heating of the clinker to 600 °C within 5–7 min. This power setting was selected based on a series of preliminary trials, which confirmed its effectiveness in achieving uniform and rapid heating with minimal thermal gradients. The total volume of the processed material was 0.001 m3 (1 L), and the system’s throughput for dry powder reached 300 kg/h with a final material moisture content of no more than 0.5%.
To evaluate the energy efficiency, we calculated the specific energy consumption. The total energy consumption of the “ENERGY K-50” system at 20 kW over 5–7 min (an average of 6 min, or 360 s) was as follows:
The mass of the processed clinker per cycle (with a volume of 0.001 m3 and a clinker density of 4100 kg/m3) was as follows:
Thus, the specific energy consumption per 1 kg of clinker was as follows:
This value is lower than that of conventional pyrometallurgical methods, such as the Waelz process (typically 3–5 MJ/kg), confirming the energy efficiency of microwave treatment.
Additionally, the “ENERGY K-50” system is equipped with a waveguide and a rotating dielectric retort, ensuring uniform microwave distribution via the “traveling wave” method. The control system automatically adjusted the process parameters, including power and temperature, to maintain stable heating conditions.
In the course of the experimentation, Origin Pro 9.8.0.200 software was utilized for generating graphical representations and conducting data evaluation. The efficiency of zinc leaching was determined using the following equation:
where denotes the zinc leaching efficiency (%); represents the zinc proportion in the original clinker (%); indicates the zinc proportion in the post-leaching residue (%); signifies the mass of the initial clinker (g); and corresponds to the mass of the residue after leaching (g).
Throughout the trials, critical operational variables such as reaction temperature, sulfuric acid strength, extraction duration, and slurry density were meticulously observed. Four experimental series were conducted under consistent conditions, as indicated by an asterisk in Table 4. To ensure the robustness of the findings, each test was replicated a minimum of three times, with the mean values of the acquired data employed for subsequent analysis.

Table 4.
Experimental Parameters and Their Ranges for Leaching Studies.
3. Results and Discussion
3.1. Phase Alteration Induced by Microwave Irradiation
Drawing upon insights from our prior investigation [43], where we thoroughly assessed phase transitions in clinker across a range of temperatures, we established the optimal parameters for microwave roasting: a temperature of 600 °C sustained for 5–7 min. These conditions facilitate the most effective conversion of zinc-bearing phases into zinc oxide (ZnO), as governed by the reaction:
ZnS + 1.5O2 = ZnO + SO2
In the present research, these parameters were implemented for clinker processing. Microwave roasting at 600 °C for a duration of 5–7 min proficiently transforms sphalerite into zinc oxide, as depicted in Figure 5.
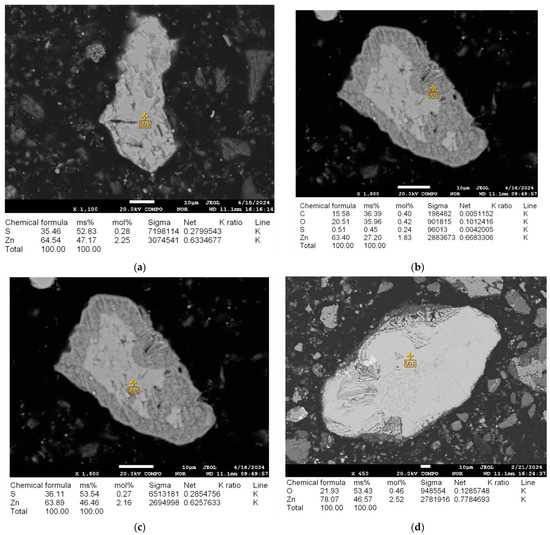
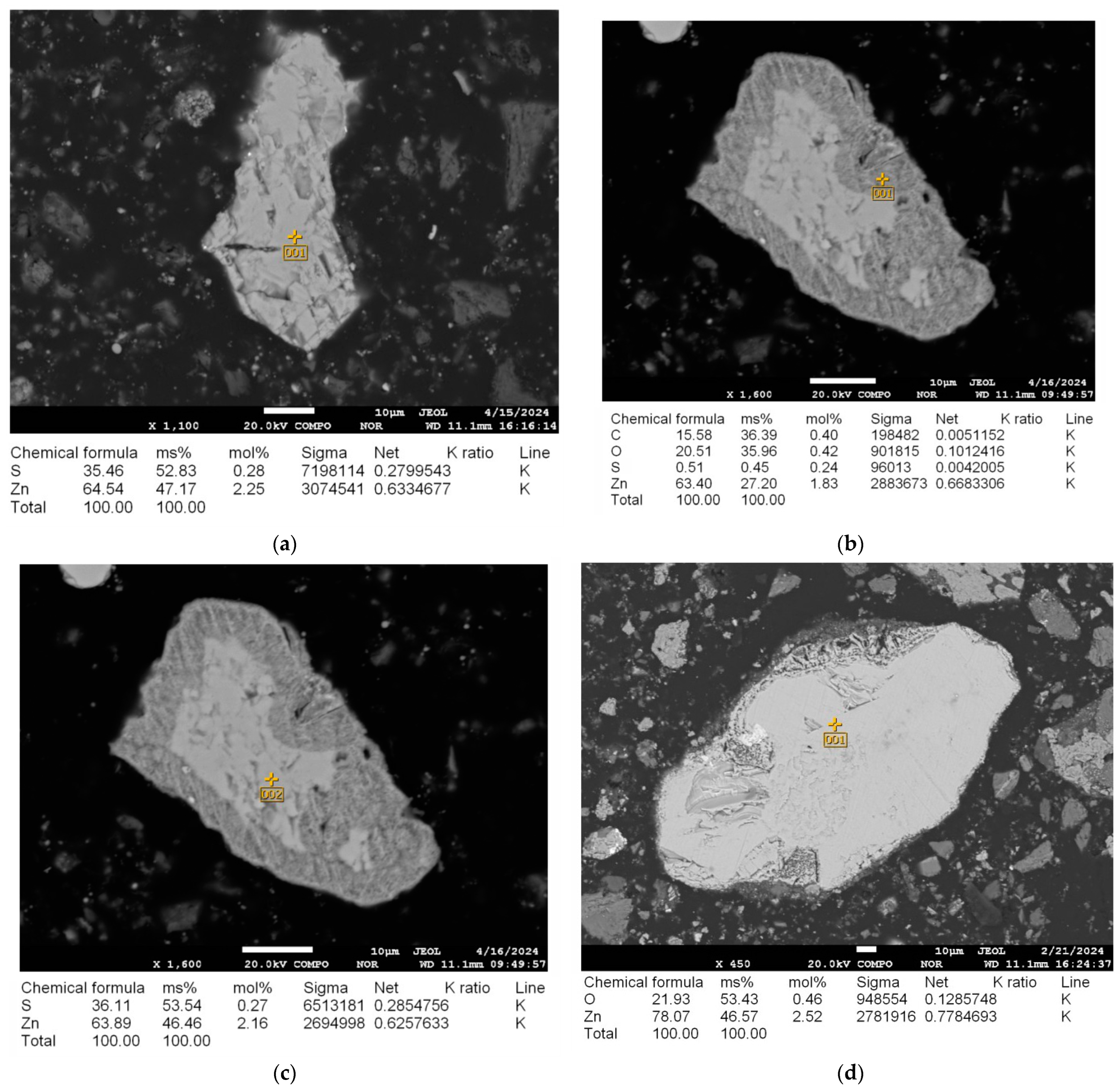
Figure 5.
(a) Microstructure of the initial sample and energy-dispersive analysis of sphalerite prior to microwave irradiation at 25 °C; (b,c) Microstructure and energy-dispersive analysis of sphalerite after microwave irradiation at 600 °C for 3–4 min; (d) Microstructure and energy-dispersive analysis of sphalerite after microwave irradiation at 600 °C for 5–7 min.
The microwave roasting procedure elicits profound alterations in both the chemical composition and microstructural properties of sphalerite. In its pristine condition, before exposure to microwave irradiation (Figure 5a), sphalerite comprises 35.46% sulfur and 64.54% zinc. Following thermal treatment at 600 °C for 3–4 min (Figure 5b), the sulfur proportion on the external surface diminishes to 0.51%, whereas the zinc proportion stabilizes at 63.40%. Simultaneously, the appearance of oxygen (20.51%) indicates the onset of oxidation processes and the partial transformation of sphalerite into zinc oxide (ZnO). However, the analysis of the internal region of the same sample (Figure 5c) reveals a higher sulfur content (36.11%) and zinc content (63.89%), which closely corresponds to the original composition (Figure 5a). This indicates that the material’s transformation remains partial at this point. However, in the specimen processed with microwave heating at 600 °C for a duration of 5–7 min (as shown in Figure 5d), a nearly complete shift in sphalerite to zinc oxide is apparent. This is corroborated by an increased oxygen presence of 21.93% and a zinc concentration elevated to 78.07%.
To further confirm the phase transformations, a comparative analysis of the elemental and phase composition of the clinker before and after microwave treatment was conducted. XRF analysis before treatment (see Table 1) showed a sulfur (S) content of 0.807%, zinc (Zn) content of 1.217%, and oxygen (O) content of 41.644%. After microwave treatment at 600 °C for 5–7 min, the sulfur content decreased to 0.112% (due to the oxidation of ZnS to ZnO with the release of SO2), the zinc content increased to 1.392% (due to the concentration of zinc in the form of ZnO), and the oxygen content increased to 43.215% (due to the formation of oxides). XRD analysis before treatment (see Table 2) indicated that sphalerite (ZnS) constituted 1.4% of the clinker’s phase composition. After treatment, the sphalerite content decreased to 0.2%, while the zincite (ZnO) content increased to 1.3%. Additionally, a reduction in gypsum (CaSO4·2H2O) content from 12.2% to 10.8% was observed due to dehydration (CaSO4·2H2O → CaSO4 + 2H2O). The contents of other phases, such as hematite (Fe2O3) and quartz (SiO2), remained nearly unchanged (24.2% to 24.0% and 8.1% to 8.0%, respectively), confirming the selectivity of microwave heating, which primarily affects sulfide minerals like sphalerite due to their higher values.
The findings substantiate that the most favorable conditions for microwave roasting (600 °C, sustained for 5–7 min) facilitate the complete transformation of sphalerite into zinc oxide, a pivotal element for optimizing the treatment of this substance. These alterations align with the principles of selective dielectric heating, whereby sulfide minerals exhibiting elevated dielectric loss factors () undergo preferential conversion, thereby improving subsequent hydrometallurgical operations. The observed carbon inclusions result from carbon accumulation induced by the electron beam during electron probe microanalysis, with the carbon originating from the adhesive carbon tape employed in preparing the samples.
3.2. Statistical Evaluation
Table 5 presents the outcomes of the analysis of variance (ANOVA) applied to the response surface model for the zinc leaching procedure from clinker.

Table 5.
ANOVA Results for the Quadratic Response Surface Model.
Analysis of variance (ANOVA) is a fundamental statistical method used to evaluate the significance of factors and their interactions within a response model. In this study, ANOVA was applied to a quadratic response surface model to assess the influence of technological parameters—time, concentration, pulp density, and temperature—on zinc extraction. The statistical assessment incorporated essential metrics, including the sum of squares, degrees of freedom (df), mean square, F-value, and p-value. This evaluation sought to identify the factors exerting a substantial influence on the process and to assess the extent to which the model accurately represents the experimental observations.
The results confirmed the statistical significance of the model, as indicated by the F-value of 4.39 and a p-value of less than 0.0001. These values suggest a high reliability of the model, with a probability of obtaining such results by chance being less than 0.1%. The quadratic term A2 (p = 0.0013) demonstrated a nonlinear influence of time on zinc extraction, emphasizing the need to consider second-order effects. Among the primary factors, only temperature (D) was statistically significant (p = 0.0319), while significant interactions were observed for AD (p = 0.0029), BD (p = 0.0380), and CD (p = 0.0476), suggesting synergistic effects between these parameters.
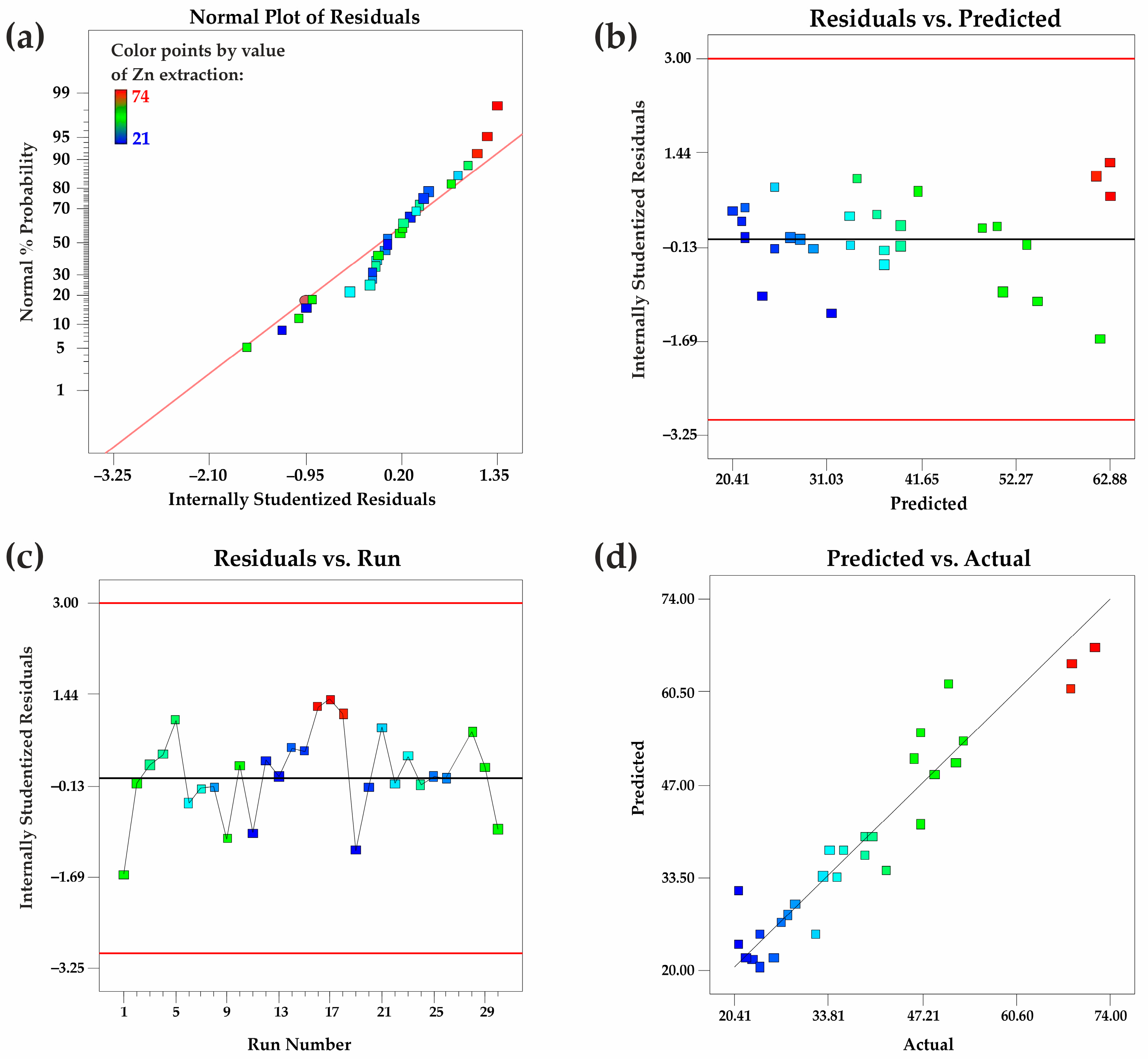
To further refine the model, insignificant variables were eliminated, resulting in a simplified regression equation with a 95% confidence level. The derived equation accounts for the key influencing factors, demonstrating their individual and interactive effects on zinc leaching efficiency. Diagnostic plots were used to evaluate the model’s adequacy, revealing that most experimental data points align along the diagonal axis, indicating minimal deviations and strong predictive accuracy. Additionally, an analysis of residuals confirmed that they follow a normal distribution, further validating the model’s robustness.
An assessment of the model’s lack of fit resulted in a p-value of 0.1661, suggesting an acceptable level of model misfit and confirming its adequacy for response prediction. The limited scatter of residuals near zero in diagnostic visualizations further reinforces the dependability of the quadratic model in representing the empirical findings. These results suggest that the model proficiently elucidates the interplay among critical operational variables and zinc recovery efficiency, rendering it an instrumental resource for predictive analysis and process enhancement.
To enhance confidence in the results obtained using RSM and ANOVA, the model was validated using two approaches.
- (1)
- Comparison of Predicted vs. Actual Values: The “Predicted vs. Actual Values” plot (see Figure 6d) shows that the predicted zinc recovery values closely correlate with the actual values, as the data points are positioned near the diagonal line. To quantify the model’s accuracy, the coefficient of determination (R2) was calculated as 0.803, indicating a high degree of fit between the model and experimental data. Additionally, the Mean Absolute Percentage Error (MAPE) was computed:
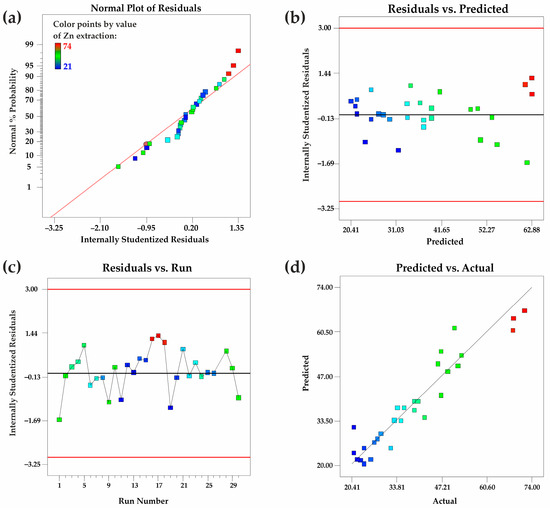 Figure 6. (a) Normal probability distribution of internally studentized residuals; (b) graphical representation of internally studentized residuals versus predicted outcomes; (c) depiction of internally studentized residuals in relation to experimental run sequence; (d) juxtaposition of forecasted outcomes alongside observed values.
Figure 6. (a) Normal probability distribution of internally studentized residuals; (b) graphical representation of internally studentized residuals versus predicted outcomes; (c) depiction of internally studentized residuals in relation to experimental run sequence; (d) juxtaposition of forecasted outcomes alongside observed values.
- (2)
- Cross-Validation: For further validation, the Leave-One-Out Cross-Validation (LOOCV) method was applied using Design Expert 7.0 software. In this method, each experimental result was sequentially excluded from the dataset, and the model was retrained on the remaining data to predict the excluded value. The average prediction error from LOOCV was 5.2%, which is consistent with the MAPE and supports the model’s reliability. Additionally, the adjusted was calculated, which accounts for the number of predictors in the model and confirms its generalizability.
Additionally, the model’s credibility was substantiated through empirical validation by implementing the optimal conditions forecasted by RSM. The model anticipated a zinc recovery rate of 74.0% under the ideal parameters, which included a sulfuric acid strength of 140 g/dm3, a slurry density of 20%, an extraction period of 2 h, and a thermal setting of 80 °C. Experimental confirmation produced an actual zinc recovery of 72.6%, differing from the projected figure by a mere 1.4%, a discrepancy well within the acceptable experimental error threshold of less than 2%. These outcomes affirm the resilience and precision of the formulated model, underscoring its practical utility in real-world applications.
The residual analysis of the quadratic response surface model for zinc extraction confirms its adequacy and reliability. The normal probability plot (a) indicates that residuals follow a normal distribution. The residuals vs. predicted values plot (b) suggests homoscedasticity, showing no systematic variance trends. The residuals vs. run number plot (c) confirms the independence of residuals, indicating no time-related bias. The predicted vs. actual values plot (d) demonstrates strong model accuracy, as data points align closely with the reference line. These findings validate the model’s suitability for predicting zinc extraction efficiency.
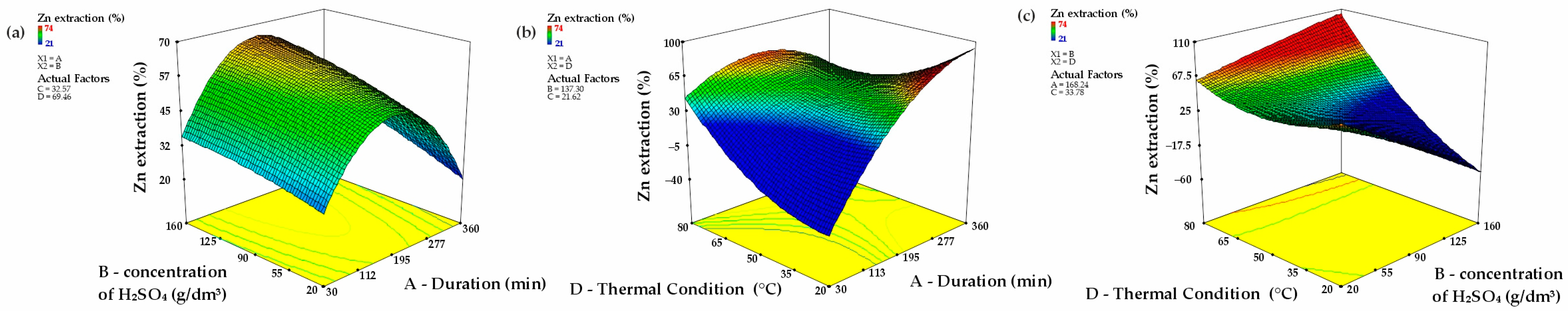
The assessment of how experimental factors influence zinc recovery efficiency (A: Duration; B: Concentration of H2SO4; C: Slurry Density; D: Thermal Condition) is depicted in Figure 7, underscoring significant trends. All four parameters substantially affect the zinc leaching process from clinker, with Thermal Condition proving to be the most critical factor.
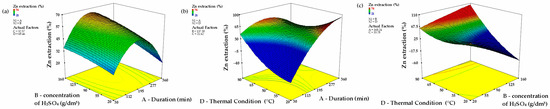
Figure 7.
Three-dimensional response surfaces (with other parameters maintained at their central levels), illustrating the combined effects of B and A (a); D and A (b); D and B (c) (A—leaching duration, B—sulfuric acid concentration, D—temperature).
Interaction Among Variables
The regression model-derived coefficients for variables A, B, C, and D provide a quantitative measure of their influence on zinc recovery. The evaluation indicates that, aside from thermal condition, all other factors negatively impact the leaching efficiency. The order of significance among these operational parameters is delineated as follows:
Thermal Condition (D) > Duration (A) > Concentration of H2SO4 (B) > Slurry Density (C).
Three-dimensional response surface representations, constructed using the quadratic model, yield critical insights into the interactions among these pivotal factors and their effects on zinc recovery.
Figure 7a portrays the interplay between Duration (A) and Concentration of H2SO4 (B), revealing that an increase in both factors improves zinc retrieval. However, at higher H2SO4 concentrations, a saturation threshold is reached, leading to a leveling off of the response. Contour diagrams substantiate that Duration plays a commanding role, particularly in the early reaction phases, underscoring its substantial effect on process kinetics.
Figure 7b delineates the association between Duration (A) and Thermal Condition (D). The response surface representation confirms that Thermal Condition has the most significant impact on zinc recovery. A notable enhancement in extraction efficiency is observed with rising thermal levels, especially during prolonged durations. Even at shorter time spans, elevated thermal conditions markedly improve the process, emphasizing their crucial role in boosting leaching performance.
Figure 7c investigates the joint effect of Concentration of H2SO4 (B) and Thermal Condition (D). The response surface illustrations disclose a synergistic relationship, where concurrent increases in both variables result in higher zinc recovery rates. Nevertheless, Thermal Condition remains the dominant factor, as evidenced by the steeper gradient along its axis.
These findings affirm that Thermal Condition (D) is the primary determinant of leaching efficiency, followed by Duration (A) and Concentration of H2SO4 (B). Based on F-values and significant interactions, the hierarchy of factor influence is established as follows: AD > AC > BD > AB.
The optimal operational parameters were established by evaluating the boundary values of each interacting variable, enabling the identification of the most efficient conditions for zinc extraction. Employing Design Expert software for modeling and optimization, the optimal settings were determined to be a sulfuric acid strength of 140 g/dm3, a slurry density of 20%, an extraction duration of 2 h, and a thermal condition of 80 °C.
Under these refined conditions, the forecasted zinc recovery rate stands at 74.0%, with the model’s desirability index reaching 0.967, which underscores its exceptional precision and dependability.
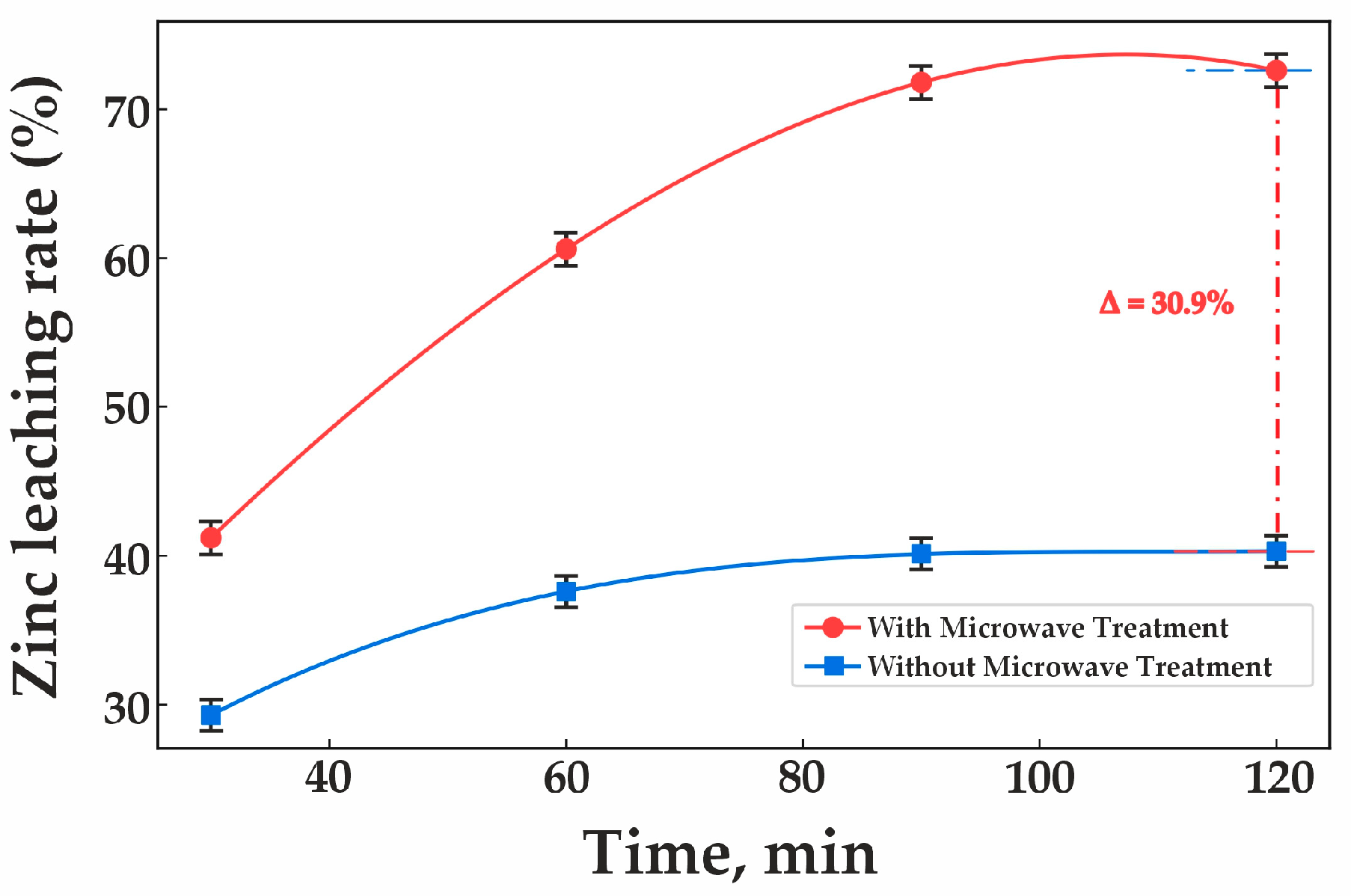
Experimental validation conducted under these optimal conditions demonstrated that microwave (MW) treatment significantly enhances zinc extraction during clinker leaching. As shown in Figure 8, zinc recovery with MW treatment reaches 72.6%, whereas without MW treatment, the recovery rate is only 41.7%. The figure also illustrates a substantial increase in zinc recovery with MW treatment compared to the non-treated sample. In contrast, without MW treatment, zinc extraction remains low and increases only marginally.
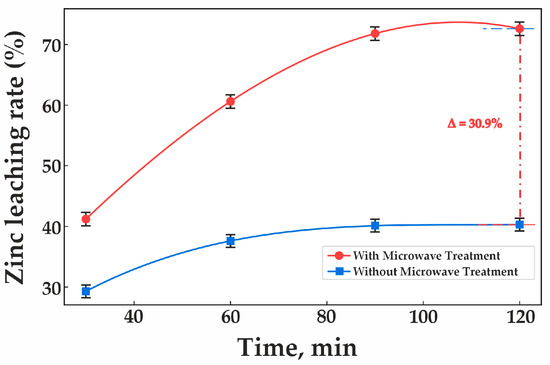
Figure 8.
Effect of microwave roasting on clinker leaching.
4. Conclusions
This investigation has showcased the efficacy of microwave-assisted thermal processing as a novel strategy for improving the leaching efficiency of zinc from technogenic raw materials. The application of microwave irradiation has been shown to induce selective phase transformations, significantly improving the solubility of zinc-bearing compounds in acidic solutions. The experimental results confirm that optimizing key parameters such as sulfuric acid concentration, temperature, pulp density, and leaching duration allows for a more efficient and sustainable hydrometallurgical process.
By employing response surface methodology (RSM) alongside a central composite design (CCD), this research has delivered an in-depth comprehension of the interrelationships among operational variables. Statistical evaluation substantiates that thermal condition is a critical determinant in enhancing leaching performance, while microwave processing augments zinc retrieval by altering the structural characteristics of the raw material. The formulated predictive model has exhibited exceptional precision, reinforcing its suitability for large-scale industrial applications.
Furthermore, microwave treatment enables targeted phase modifications that enhance reagent penetration, reduce processing time, and lower energy consumption compared to conventional thermal treatment methods. The refined conditions established in this research have resulted in a substantial enhancement of zinc retrieval, achieving a rate of 72.6%, which underscores the promise of this method for treating refractory raw materials.
Subsequent studies should focus on conducting a thorough techno-economic evaluation of microwave-assisted processing, considering both cost-efficiency and industrial feasibility. Exploring alternative reagents and further refining leaching parameters could additionally elevate metal recovery efficiencies. Moreover, integrating machine learning algorithms for process optimization and conducting life cycle assessments will provide a deeper understanding of the sustainability and industrial applicability of this technology.
Author Contributions
Conceptualization, B.K., K.S. and A.B.; methodology, K.S. and S.S.; software, G.M., K.S. and N.T.; validation, Z.B. and A.B.; formal analysis, D.K., S.S. and N.T.; investigation, B.K. and D.K.; resources, B.K., K.S. and T.O.; data curation, D.K., Z.B. and A.B.; writing—original draft preparation, Z.B. and S.S.; writing—review and editing, Z.B., B.K., A.B. and D.K.; visualization, D.K., T.O. and Z.B.; supervision, Z.B. and S.S.; project administration, B.K.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported financially by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan under Grant No. AR 19675985.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current Technologies for Recovery of Metals from Industrial Wastes: An Overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Ultarakova, A.A.; Yessengaziyev, A.M.; Kuldeyev, E.I.; Kassymzhanov, K.K.; Uldakhanov, O.K. Processing of Titanium Production Sludge with the Extraction of Titanium Dioxide. Metalurgija 2021, 60, 411–414. [Google Scholar]
- Panichkin, A.; Wieleba, W.; Kenzhegulov, A.; Uskenbayeva, A.; Mamaeva, A.; Imbarova, A.; Kvyatkovskii, S.; Kasenova, B. Effect of thermal treatment of chromium iron melts on the structure and properties of castings. Mater. Res. Express 2023, 10, 086502. [Google Scholar] [CrossRef]
- Ospanov, K.; Smailov, K.; Nuruly, Y. Patterns of Non-Traditional Thermodynamic Functions ΔrG0/n and ΔfG0(Averaged) Changes for Cobalt Minerals. Chem. Bull. Kazakh Natl. Univ. 2020, 96, 22–30. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Tussupbayev, N.K.; Abdykirova, G.Z.; Koizhanova, A.K.; Fischer, D.Y.; Baltabekova, Z.A.; Samenova, N.O. Evaluation of the Efficiency of Using an Oxidizer in the Leaching Process of Gold-Containing Concentrate. Processes 2024, 12, 973. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Ma, R.; Li, Y.; Zhao, H.; Wang, H.; Jiang, S. An Innovative Method for the Efficient and Selective Extraction of Co, Ni, Cu, and Mn from Oceanic Cobalt-Rich Crusts by Ammonium Sulfate Roasting: Behavior, Roasting Kinetics and Mechanism. Miner. Eng. 2024, 207, 108543. [Google Scholar] [CrossRef]
- Zhao, F.; Jiang, X.; Wang, S.; Feng, L.; Li, D. The Recovery of Valuable Metals from Ocean Polymetallic Nodules Using Solid-State Metalized Reduction Technology. Minerals 2019, 10, 20. [Google Scholar] [CrossRef]
- Sakellariadou, F.; González, F.J.; Hein, J.R.; Rincón-Tomás, B.; Arvanitidis, N.; Kuhn, T. Seabed Mining and Blue Growth: Exploring the Potential of Marine Mineral Deposits as a Sustainable Source of Rare Earth Elements (MaREEs) (IUPAC Technical Report). Pure Appl. Chem. 2022, 94, 329–351. [Google Scholar] [CrossRef]
- Wegerer, S.; Semel, M.D.; Weixler, L. Vertical Exploration Approach for Seafloor Massive Sulfide Deposits. In Offshore Technology Conference; OTC: Columbus, OH, USA, 2023; ISBN 978-1-61399-974-5. [Google Scholar] [CrossRef]
- Balaram, V. Deep-Sea Mineral Deposits as a Future Source of Critical Metals, and Environmental Issues—A Brief Review. Miner. Miner. Mater. 2023, 2, 5. [Google Scholar] [CrossRef]
- Nath, S.; Singh, K.K.; Tangjang, S.; Das, S. Industrial Solid Wastes and Environment: An Overview on Global Generation, Implications, and Available Management Options. In Springer Water; Springer: Berlin/Heidelberg, Germany, 2024; pp. 221–246. [Google Scholar] [CrossRef]
- Varjani, S. Trends in Mitigation of Industrial Waste: Global Health Hazards, Environmental Implications and Waste Derived Economy for Environmental Sustainability. Sci. Total Environ. 2022, 811, 152357. [Google Scholar] [CrossRef]
- Bazarbayeva, S.M. Dataset on Industrial Waste Compositions in West Kazakhstan and Conditions for Processing Them into Construction Materials. Data Brief 2024, 54, 110265. [Google Scholar] [CrossRef] [PubMed]
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Amanzholova, L.; Mishra, B.; Abdikerim, B.; Yessimova, D. Modification of Natural Minerals with Technogenic Raw Materials. Metals 2022, 12, 1907. [Google Scholar] [CrossRef]
- Dosmukhamedov, N.; Zholdasbay, E.; Argyn, A. Integrated Chlorination Technology for Producing Alumina and Silica from Ash-Slag Waste of the TPP of Kazakhstan. J. Mater. Res. Technol. 2022, 23, 1435–1446. [Google Scholar] [CrossRef]
- Zhanikulov, N.N.; Sapargaliyeva, B.; Agabekova, A.B.; Alfereva, Y.O.; Syrlybekkyzy, S.; Nurshakhanova, L.K.; Nurbayeva, F.K.; Sabyrbaeva, G.S.; Kozlov, P.T.; Kolesnikova, O.G. Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement. J. Compos. Sci. 2023, 7, 226. [Google Scholar] [CrossRef]
- Jandieri, G. Increasing the Efficiency of Secondary Resources in the Mining and Metallurgical Industry. J. S. Afr. Inst. Min. Metall. 2023, 123, 1–8. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Fediuk, R.; Kolesnikova, O.; Zhanikulov, N.; Zhakipbayev, B.; Kuraev, R.; Akhmetova, E.; Shal, A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials 2022, 15, 324. [Google Scholar] [CrossRef]
- Rahmati, S.; Adavodi, R.; Hosseini, M.R.; Veglio’, F. Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals 2024, 14, 605. [Google Scholar] [CrossRef]
- Ultarakova, A.; Karshyga, Z.; Lokhova, N.; Yessengaziyev, A.; Kassymzhanov, K.; Mukangaliyeva, A. Studies of Niobium Sorption from Chloride Solutions with the Use of Anion-Exchange Resins. Processes 2023, 11, 1288. [Google Scholar] [CrossRef]
- Ultarakova, A.; Karshyga, Z.; Lokhova, N.; Yessengaziyev, A.; Kassymzhanov, K.; Mukangaliyeva, A. Studies on the Processing of Fine Dusts from the Electric Smelting of Ilmenite Concentrates to Obtain Titanium Dioxide. Materials 2022, 15, 8314. [Google Scholar] [CrossRef]
- Abdulvaliev, R.A.; Surkova, T.Y.; Baltabekova, Z.A.; Yessimova, D.M.; Stachowicz, M.; Smailov, K.M.; Dossymbayeva, Z.D.; Ainur, B. Effect of Amino Acids on the Extraction of Copper from Sub-Conditional Raw Materials. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2024, 335, 50–58. [Google Scholar] [CrossRef]
- Walke, S.; Mandake, M.B. Optimization of Chemical Engineering Processes in the Mining and Metal Industry: A Review. J. Mines Met. Fuels 2024, 2023, 240–251. [Google Scholar] [CrossRef]
- Kuandykova, A.; Taimasov, B.; Potapova, E.; Sarsenbaev, B.; Kolesnikov, A.; Begentayev, M.; Kuldeyev, E.; Dauletiyarov, M.; Zhanikulov, N.; Amiraliyev, B.; et al. Production of Composite Cement Clinker Based on Industrial Waste. J. Compos. Sci. 2024, 8, 257. [Google Scholar] [CrossRef]
- Toshkodirova, R.E.; Abdurakhmonov, S. Processing of Clinker—Technogenic Waste of Zinc Production. Univers. Tech. Sci. 2020, 11, 78–81. [Google Scholar]
- Trebukhov, S.; Volodin, V.; Nitsenko, A.; Burabaeva, N.; Ruzakhunova, G. Recovery of Zinc from the Concentrate of Domestic Waste Processing by Vacuum Distillation. Metals 2022, 12, 703. [Google Scholar] [CrossRef]
- Lobanov, V.G.; Kolmachikhina, O.B.; Polygalov, S.E.; Khabibulina, R.E.; Sokolov, L.V. Features of the Presence of Precious Metals in the Zinc Production Clinker. Russ. J. Non-Ferr. Met. 2022, 63, 594–598. [Google Scholar]
- Kiprono, N.R.; Kawalec, A.; Klis, B.; Smolinski, T.; Rogowski, M.; Kalbarczyk, P.; Samczynski, Z.; Norenberg, M.; Ostachowicz, B.; Adamowska, M.; et al. Radiation Techniques for Tracking the Progress of the Hydrometallurgical Leaching Process: A Case Study of Mn and Zn. Metals 2024, 14, 744. [Google Scholar] [CrossRef]
- Maltrana, V.; Morales, J. The Use of Acid Leaching to Recover Metals from Tailings: A Review. Metals 2023, 13, 1862. [Google Scholar] [CrossRef]
- Wang, S.; Gao, F.; Li, B.; Liu, Y.; Deng, T.; Zhang, Y.; Chen, W. Clinkerization of Carbonatable Belite–Melilite Clinker Using Solid Waste at Low Temperature. Constr. Build. Mater. 2024, 418, 135357. [Google Scholar] [CrossRef]
- Zhidebekkyzy, A.; Temerbulatova, Z.; Amangeldiyeva, B.; Sakhariyeva, A. Towards a Circular Economy: An Analysis of Kazakhstani Case. J. Econ. Res. Bus. Adm. 2023, 143, 16–32. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, G. Recent Status of Production, Administration Policies, and Low-Carbon Technology Development of China’s Steel Industry. Metals 2024, 14, 480. [Google Scholar] [CrossRef]
- Diaz, F.; Sommerfeld, M.; Hovestadt, G.; Latacz, D.; Friedrich, B. Lessons Learned from Attempts at Minimising CO2 Emissions in Process Metallurgy—Pyrolysed Secondary Raw Materials, Bio-Coke, and Hydrogen as Alternative Reducing Agents. Proceedings 2024. [Google Scholar] [CrossRef]
- Harvey, J.-P.; Courchesne, W.; Vo, M.D.; Oishi, K.; Robelin, C.; Mahue, U.; Leclerc, P.; Al-Haiek, A. Greener Reactants, Renewable Energies and Environmental Impact Mitigation Strategies in Pyrometallurgical Processes: A Review. MRS Energy Sustain. 2022, 9, 212–247. [Google Scholar] [CrossRef]
- Hamidi, A.; Nazari, P.; Shakibania, S.; Rashchi, F. Microwave Irradiation for the Recovery Enhancement of Fly Ash Components: Thermodynamic and Kinetic Aspects. Chem. Eng. Process. 2023, 191, 109472. [Google Scholar] [CrossRef]
- Lin, S.; Li, K.; Yang, Y.; Gao, L.; Omran, M.; Guo, S.; Chen, J.; Chen, G. Microwave-Assisted Method Investigation for the Selective and Enhanced Leaching of Manganese from Low-Grade Pyrolusite Using Pyrite as the Reducing Agent. Chem. Eng. Process. 2021, 159, 108209. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-Assisted Leaching—A Review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Stojković, M.; Ristić, M.; Đolić, M.; Perić Grujić, A.; Onjia, A. Recovery of Rare Earth Elements from Coal Fly and Bottom Ashes by Ultrasonic Roasting Followed by Microwave Leaching. Metals 2024, 14, 371. [Google Scholar] [CrossRef]
- Fang, X.; Peng, Z.; Yin, T.; Rao, M.; Li, G. Microwave Treatment of Copper–Nickel Sulfide Ore for Promotion of Grinding and Flotation. Metals 2024, 14, 565. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Baltabekova, Z.; Smailov, K. Harnessing Microwave Technology for Enhanced Recovery of Zinc from Industrial Clinker. Metals 2024, 14, 699. [Google Scholar] [CrossRef]
- Ye, L.; Peng, Z.; Tian, R.; Tang, H.; Zhang, J.; Rao, M.; Li, G. A novel process for highly efficient separation of boron and iron from ludwigite ore based on low-temperature microwave roasting. Powder Technol. 2022, 410, 117848. [Google Scholar] [CrossRef]
- Ma, A.; Zheng, X.; Gao, L.; Li, K.; Omran, M.; Chen, G. Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment. Metals 2021, 11, 1922. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, L.; Yang, K. Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore. Superalloys 2023, 13, 356. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).