Investigation of Phase Transformations in Technogenic Raw Materials Under Microwave Treatment for Enhanced Zinc Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methodologies
2.3. Optimal Experimental Design
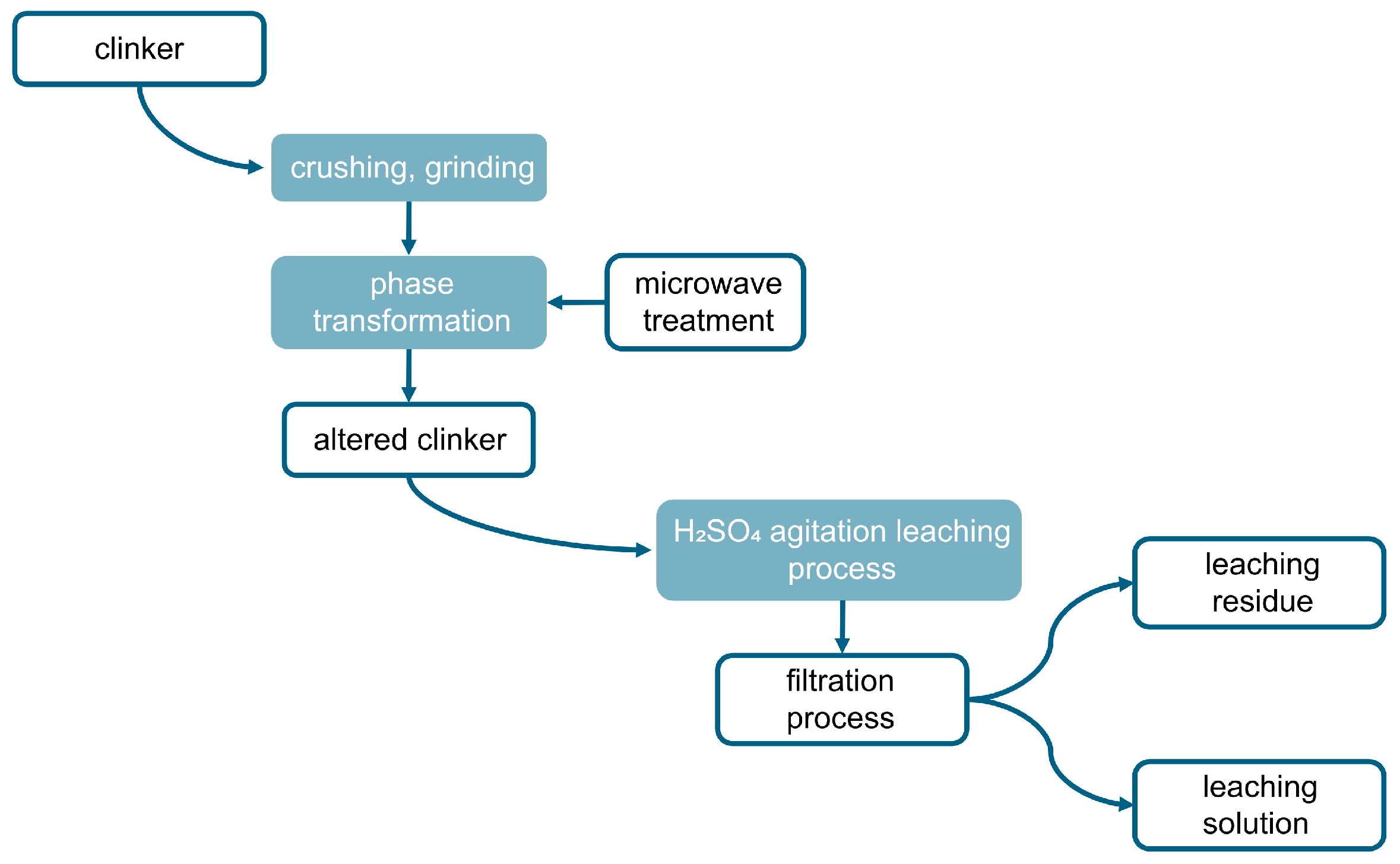
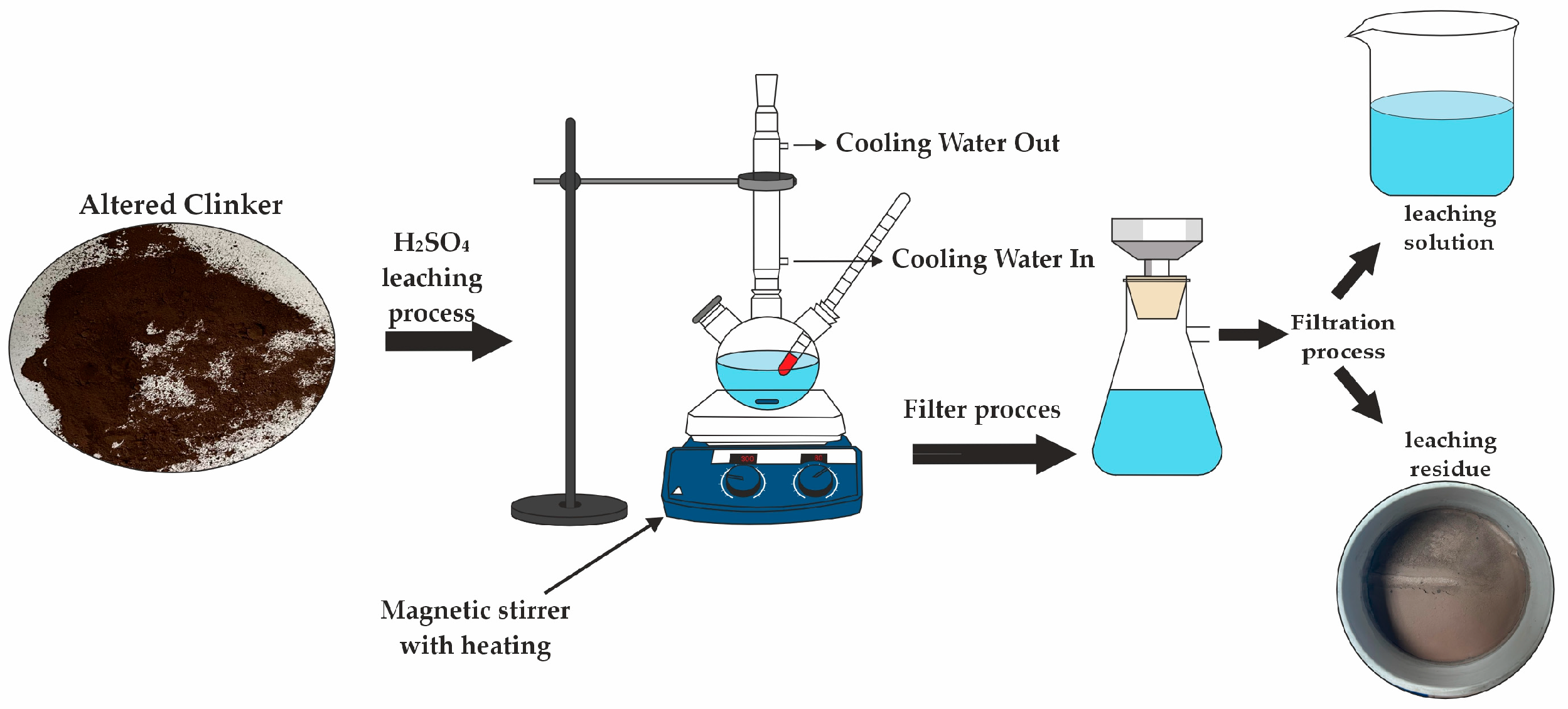
2.4. Experimental Method
3. Results and Discussion
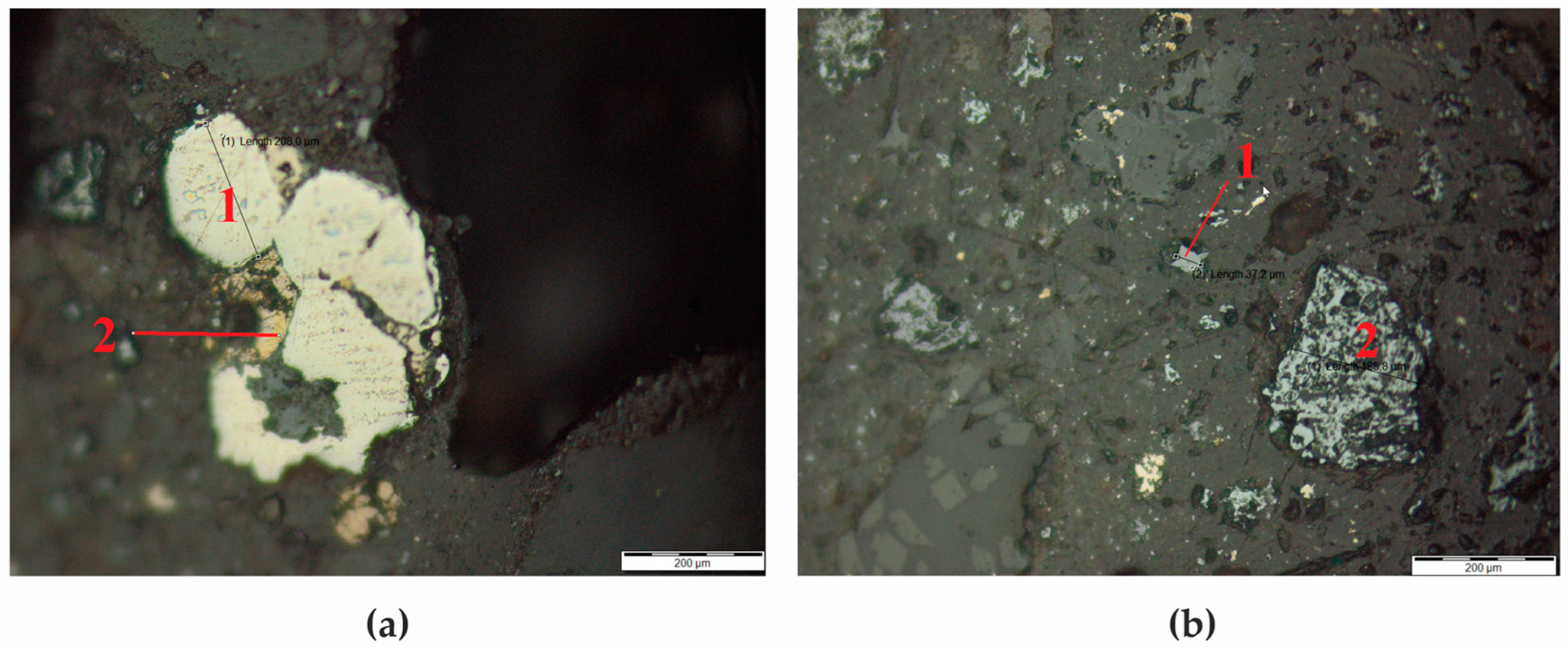
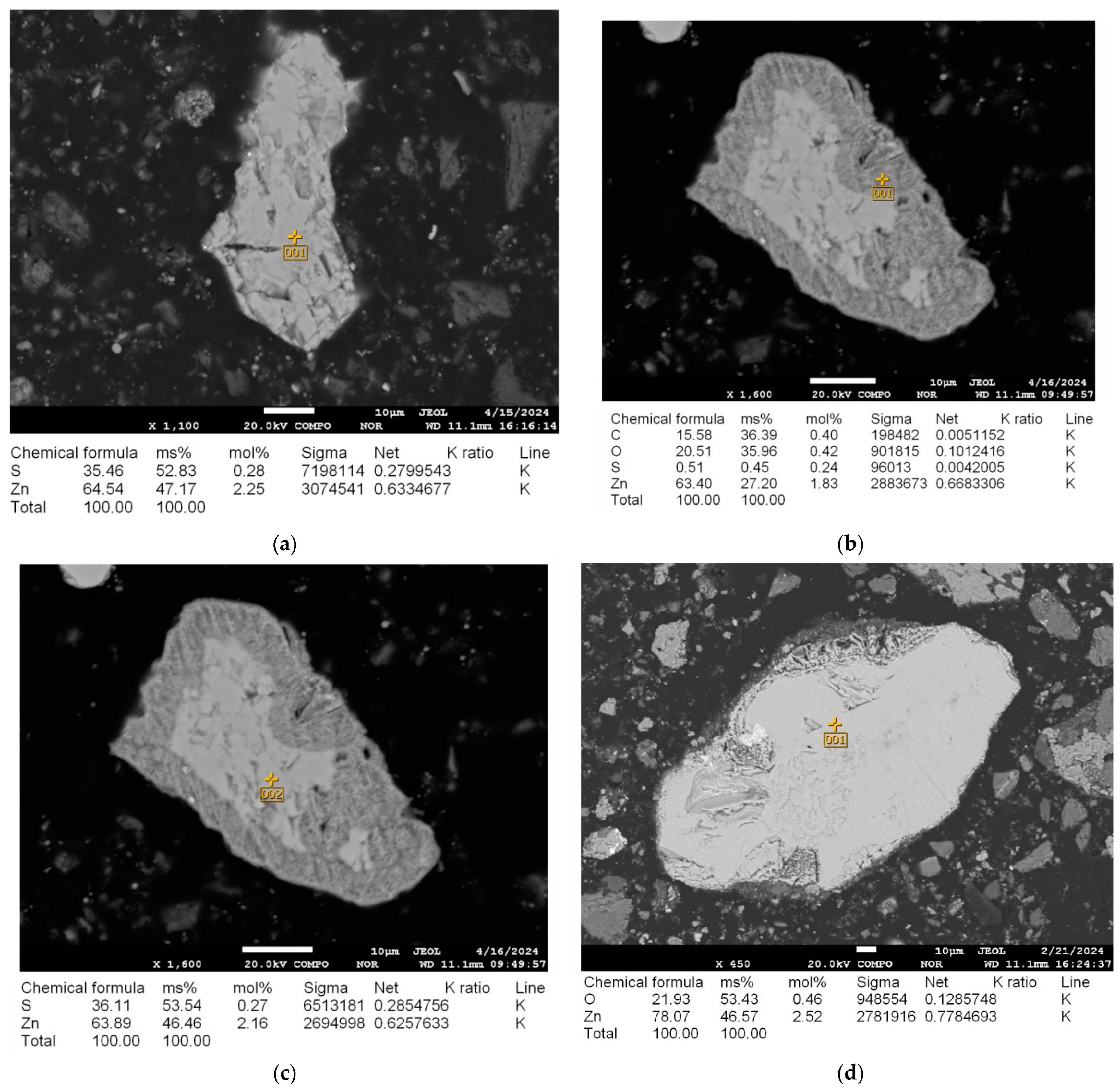
3.1. Phase Alteration Induced by Microwave Irradiation
3.2. Statistical Evaluation
- (1)
- Comparison of Predicted vs. Actual Values: The “Predicted vs. Actual Values” plot (see Figure 6d) shows that the predicted zinc recovery values closely correlate with the actual values, as the data points are positioned near the diagonal line. To quantify the model’s accuracy, the coefficient of determination (R2) was calculated as 0.803, indicating a high degree of fit between the model and experimental data. Additionally, the Mean Absolute Percentage Error (MAPE) was computed:
- (2)
- Cross-Validation: For further validation, the Leave-One-Out Cross-Validation (LOOCV) method was applied using Design Expert 7.0 software. In this method, each experimental result was sequentially excluded from the dataset, and the model was retrained on the remaining data to predict the excluded value. The average prediction error from LOOCV was 5.2%, which is consistent with the MAPE and supports the model’s reliability. Additionally, the adjusted was calculated, which accounts for the number of predictors in the model and confirms its generalizability.
Interaction Among Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current Technologies for Recovery of Metals from Industrial Wastes: An Overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Ultarakova, A.A.; Yessengaziyev, A.M.; Kuldeyev, E.I.; Kassymzhanov, K.K.; Uldakhanov, O.K. Processing of Titanium Production Sludge with the Extraction of Titanium Dioxide. Metalurgija 2021, 60, 411–414. [Google Scholar]
- Panichkin, A.; Wieleba, W.; Kenzhegulov, A.; Uskenbayeva, A.; Mamaeva, A.; Imbarova, A.; Kvyatkovskii, S.; Kasenova, B. Effect of thermal treatment of chromium iron melts on the structure and properties of castings. Mater. Res. Express 2023, 10, 086502. [Google Scholar] [CrossRef]
- Ospanov, K.; Smailov, K.; Nuruly, Y. Patterns of Non-Traditional Thermodynamic Functions ΔrG0/n and ΔfG0(Averaged) Changes for Cobalt Minerals. Chem. Bull. Kazakh Natl. Univ. 2020, 96, 22–30. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Tussupbayev, N.K.; Abdykirova, G.Z.; Koizhanova, A.K.; Fischer, D.Y.; Baltabekova, Z.A.; Samenova, N.O. Evaluation of the Efficiency of Using an Oxidizer in the Leaching Process of Gold-Containing Concentrate. Processes 2024, 12, 973. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Ma, R.; Li, Y.; Zhao, H.; Wang, H.; Jiang, S. An Innovative Method for the Efficient and Selective Extraction of Co, Ni, Cu, and Mn from Oceanic Cobalt-Rich Crusts by Ammonium Sulfate Roasting: Behavior, Roasting Kinetics and Mechanism. Miner. Eng. 2024, 207, 108543. [Google Scholar] [CrossRef]
- Zhao, F.; Jiang, X.; Wang, S.; Feng, L.; Li, D. The Recovery of Valuable Metals from Ocean Polymetallic Nodules Using Solid-State Metalized Reduction Technology. Minerals 2019, 10, 20. [Google Scholar] [CrossRef]
- Sakellariadou, F.; González, F.J.; Hein, J.R.; Rincón-Tomás, B.; Arvanitidis, N.; Kuhn, T. Seabed Mining and Blue Growth: Exploring the Potential of Marine Mineral Deposits as a Sustainable Source of Rare Earth Elements (MaREEs) (IUPAC Technical Report). Pure Appl. Chem. 2022, 94, 329–351. [Google Scholar] [CrossRef]
- Wegerer, S.; Semel, M.D.; Weixler, L. Vertical Exploration Approach for Seafloor Massive Sulfide Deposits. In Offshore Technology Conference; OTC: Columbus, OH, USA, 2023; ISBN 978-1-61399-974-5. [Google Scholar] [CrossRef]
- Balaram, V. Deep-Sea Mineral Deposits as a Future Source of Critical Metals, and Environmental Issues—A Brief Review. Miner. Miner. Mater. 2023, 2, 5. [Google Scholar] [CrossRef]
- Nath, S.; Singh, K.K.; Tangjang, S.; Das, S. Industrial Solid Wastes and Environment: An Overview on Global Generation, Implications, and Available Management Options. In Springer Water; Springer: Berlin/Heidelberg, Germany, 2024; pp. 221–246. [Google Scholar] [CrossRef]
- Varjani, S. Trends in Mitigation of Industrial Waste: Global Health Hazards, Environmental Implications and Waste Derived Economy for Environmental Sustainability. Sci. Total Environ. 2022, 811, 152357. [Google Scholar] [CrossRef]
- Bazarbayeva, S.M. Dataset on Industrial Waste Compositions in West Kazakhstan and Conditions for Processing Them into Construction Materials. Data Brief 2024, 54, 110265. [Google Scholar] [CrossRef] [PubMed]
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Amanzholova, L.; Mishra, B.; Abdikerim, B.; Yessimova, D. Modification of Natural Minerals with Technogenic Raw Materials. Metals 2022, 12, 1907. [Google Scholar] [CrossRef]
- Dosmukhamedov, N.; Zholdasbay, E.; Argyn, A. Integrated Chlorination Technology for Producing Alumina and Silica from Ash-Slag Waste of the TPP of Kazakhstan. J. Mater. Res. Technol. 2022, 23, 1435–1446. [Google Scholar] [CrossRef]
- Zhanikulov, N.N.; Sapargaliyeva, B.; Agabekova, A.B.; Alfereva, Y.O.; Syrlybekkyzy, S.; Nurshakhanova, L.K.; Nurbayeva, F.K.; Sabyrbaeva, G.S.; Kozlov, P.T.; Kolesnikova, O.G. Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement. J. Compos. Sci. 2023, 7, 226. [Google Scholar] [CrossRef]
- Jandieri, G. Increasing the Efficiency of Secondary Resources in the Mining and Metallurgical Industry. J. S. Afr. Inst. Min. Metall. 2023, 123, 1–8. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Fediuk, R.; Kolesnikova, O.; Zhanikulov, N.; Zhakipbayev, B.; Kuraev, R.; Akhmetova, E.; Shal, A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials 2022, 15, 324. [Google Scholar] [CrossRef]
- Rahmati, S.; Adavodi, R.; Hosseini, M.R.; Veglio’, F. Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals 2024, 14, 605. [Google Scholar] [CrossRef]
- Ultarakova, A.; Karshyga, Z.; Lokhova, N.; Yessengaziyev, A.; Kassymzhanov, K.; Mukangaliyeva, A. Studies of Niobium Sorption from Chloride Solutions with the Use of Anion-Exchange Resins. Processes 2023, 11, 1288. [Google Scholar] [CrossRef]
- Ultarakova, A.; Karshyga, Z.; Lokhova, N.; Yessengaziyev, A.; Kassymzhanov, K.; Mukangaliyeva, A. Studies on the Processing of Fine Dusts from the Electric Smelting of Ilmenite Concentrates to Obtain Titanium Dioxide. Materials 2022, 15, 8314. [Google Scholar] [CrossRef]
- Abdulvaliev, R.A.; Surkova, T.Y.; Baltabekova, Z.A.; Yessimova, D.M.; Stachowicz, M.; Smailov, K.M.; Dossymbayeva, Z.D.; Ainur, B. Effect of Amino Acids on the Extraction of Copper from Sub-Conditional Raw Materials. Kompleks. Ispolz. Miner. Syra Complex Use Miner. Resour. 2024, 335, 50–58. [Google Scholar] [CrossRef]
- Walke, S.; Mandake, M.B. Optimization of Chemical Engineering Processes in the Mining and Metal Industry: A Review. J. Mines Met. Fuels 2024, 2023, 240–251. [Google Scholar] [CrossRef]
- Kuandykova, A.; Taimasov, B.; Potapova, E.; Sarsenbaev, B.; Kolesnikov, A.; Begentayev, M.; Kuldeyev, E.; Dauletiyarov, M.; Zhanikulov, N.; Amiraliyev, B.; et al. Production of Composite Cement Clinker Based on Industrial Waste. J. Compos. Sci. 2024, 8, 257. [Google Scholar] [CrossRef]
- Toshkodirova, R.E.; Abdurakhmonov, S. Processing of Clinker—Technogenic Waste of Zinc Production. Univers. Tech. Sci. 2020, 11, 78–81. [Google Scholar]
- Trebukhov, S.; Volodin, V.; Nitsenko, A.; Burabaeva, N.; Ruzakhunova, G. Recovery of Zinc from the Concentrate of Domestic Waste Processing by Vacuum Distillation. Metals 2022, 12, 703. [Google Scholar] [CrossRef]
- Lobanov, V.G.; Kolmachikhina, O.B.; Polygalov, S.E.; Khabibulina, R.E.; Sokolov, L.V. Features of the Presence of Precious Metals in the Zinc Production Clinker. Russ. J. Non-Ferr. Met. 2022, 63, 594–598. [Google Scholar]
- Kiprono, N.R.; Kawalec, A.; Klis, B.; Smolinski, T.; Rogowski, M.; Kalbarczyk, P.; Samczynski, Z.; Norenberg, M.; Ostachowicz, B.; Adamowska, M.; et al. Radiation Techniques for Tracking the Progress of the Hydrometallurgical Leaching Process: A Case Study of Mn and Zn. Metals 2024, 14, 744. [Google Scholar] [CrossRef]
- Maltrana, V.; Morales, J. The Use of Acid Leaching to Recover Metals from Tailings: A Review. Metals 2023, 13, 1862. [Google Scholar] [CrossRef]
- Wang, S.; Gao, F.; Li, B.; Liu, Y.; Deng, T.; Zhang, Y.; Chen, W. Clinkerization of Carbonatable Belite–Melilite Clinker Using Solid Waste at Low Temperature. Constr. Build. Mater. 2024, 418, 135357. [Google Scholar] [CrossRef]
- Zhidebekkyzy, A.; Temerbulatova, Z.; Amangeldiyeva, B.; Sakhariyeva, A. Towards a Circular Economy: An Analysis of Kazakhstani Case. J. Econ. Res. Bus. Adm. 2023, 143, 16–32. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, G. Recent Status of Production, Administration Policies, and Low-Carbon Technology Development of China’s Steel Industry. Metals 2024, 14, 480. [Google Scholar] [CrossRef]
- Diaz, F.; Sommerfeld, M.; Hovestadt, G.; Latacz, D.; Friedrich, B. Lessons Learned from Attempts at Minimising CO2 Emissions in Process Metallurgy—Pyrolysed Secondary Raw Materials, Bio-Coke, and Hydrogen as Alternative Reducing Agents. Proceedings 2024. [Google Scholar] [CrossRef]
- Harvey, J.-P.; Courchesne, W.; Vo, M.D.; Oishi, K.; Robelin, C.; Mahue, U.; Leclerc, P.; Al-Haiek, A. Greener Reactants, Renewable Energies and Environmental Impact Mitigation Strategies in Pyrometallurgical Processes: A Review. MRS Energy Sustain. 2022, 9, 212–247. [Google Scholar] [CrossRef]
- Hamidi, A.; Nazari, P.; Shakibania, S.; Rashchi, F. Microwave Irradiation for the Recovery Enhancement of Fly Ash Components: Thermodynamic and Kinetic Aspects. Chem. Eng. Process. 2023, 191, 109472. [Google Scholar] [CrossRef]
- Lin, S.; Li, K.; Yang, Y.; Gao, L.; Omran, M.; Guo, S.; Chen, J.; Chen, G. Microwave-Assisted Method Investigation for the Selective and Enhanced Leaching of Manganese from Low-Grade Pyrolusite Using Pyrite as the Reducing Agent. Chem. Eng. Process. 2021, 159, 108209. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-Assisted Leaching—A Review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Stojković, M.; Ristić, M.; Đolić, M.; Perić Grujić, A.; Onjia, A. Recovery of Rare Earth Elements from Coal Fly and Bottom Ashes by Ultrasonic Roasting Followed by Microwave Leaching. Metals 2024, 14, 371. [Google Scholar] [CrossRef]
- Fang, X.; Peng, Z.; Yin, T.; Rao, M.; Li, G. Microwave Treatment of Copper–Nickel Sulfide Ore for Promotion of Grinding and Flotation. Metals 2024, 14, 565. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Baltabekova, Z.; Smailov, K. Harnessing Microwave Technology for Enhanced Recovery of Zinc from Industrial Clinker. Metals 2024, 14, 699. [Google Scholar] [CrossRef]
- Ye, L.; Peng, Z.; Tian, R.; Tang, H.; Zhang, J.; Rao, M.; Li, G. A novel process for highly efficient separation of boron and iron from ludwigite ore based on low-temperature microwave roasting. Powder Technol. 2022, 410, 117848. [Google Scholar] [CrossRef]
- Ma, A.; Zheng, X.; Gao, L.; Li, K.; Omran, M.; Chen, G. Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment. Metals 2021, 11, 1922. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, L.; Yang, K. Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore. Superalloys 2023, 13, 356. [Google Scholar]




| Elemental Composition, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | S | Mg | P | Si | Al | Na | Zr | Mn | Ca | Sb | Cr |
| 41.644 | 0.807 | 1.030 | 0.055 | 4.581 | 0.912 | 0.173 | 0.012 | 0.110 | 3.807 | 0.034 | 0.020 |
| K | Fe | Pb | Cu | As | Zn | Sr | Cl | Mo | Ti | Ba | Ni |
| 0.109 | 37.532 | 0.154 | 1.037 | 0.138 | 1.217 | 0.043 | 0.011 | 0.026 | 0.101 | 0.825 | 0.033 |
| Mineralogical Composition | Chemical Formula | Phase Abundance, % |
|---|---|---|
| Hematite | Fe2O3 | 24.2 |
| Magnesium iron oxide | Mg1.55Fe1.6O4 | 18.2 |
| Diopside, ferrian | Ca1.007(Mg0.805Fe0.214) ((Si1.75Fe0.241)O6) | 16.5 |
| Calcium Magnesium Iron Aluminum Silicate | Ca2(Mg,Fe+3,Al)6(Si,Al)6O20 | 15.1 |
| Gypsum | CaSO4·2H2O | 12.2 |
| Quartz | SiO2 | 8.1 |
| Albite, potassian | (K0.22Na0.78)(AlSi3O8) | 4.4 |
| Sphalerite | ZnS | 1.4 |
| Factors | Symbol | Coding Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Duration (min) | A | 30 | 195 | 360 |
| Concentration of H2SO4 (g/dm3) | B | 20 | 90 | 160 |
| Slurry Density (%) | C | 20 | 25 | 35 |
| Thermal Condition (°C) | D | 20 | 50 | 80 |
| Parameter | Values |
|---|---|
| Duration (min) | 30, 60 *, 120, 240, 300, 360 |
| Concentration of H2SO4 (g/dm3) | 20, 40, 60, 80, 100 *, 120, 140, 160 |
| Slurry Density (%) | 20 *, 25, 35 |
| Thermal Condition (°C) | 20, 40, 60 *, 80 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 5466.47 | 14 | 390.46 | 4.39 | 0.0037 | Significant |
| A- duration | 9.91 | 1 | 9.91 | 0.11 | 0.7432 | |
| B- concentration of H2SO4 | 63.10 | 1 | 63.10 | 0.71 | 0.4130 | |
| C- Slurry Density | 10.00 | 1 | 10.00 | 0.11 | 0.7421 | |
| D- thermal condition | 497.65 | 1 | 497.65 | 5.59 | 0.0319 | |
| AB | 23.38 | 1 | 23.38 | 0.26 | 0.6158 | |
| AC | 332.41 | 1 | 332.41 | 3.74 | 0.0724 | |
| AD | 1122.80 | 1 | 1122.80 | 12.62 | 0.0029 | |
| BC | 109.54 | 1 | 109.54 | 1.23 | 0.2847 | |
| BD | 460.63 | 1 | 460.63 | 5.18 | 0.0380 | |
| CD | 414.24 | 1 | 414.24 | 4.65 | 0.0476 | |
| A2 | 1389.91 | 1 | 1389.91 | 15.62 | 0.0013 | |
| B2 | 6.13 | 1 | 6.13 | 0.069 | 0.7965 | |
| C2 | 46.03 | 1 | 46.03 | 0.52 | 0.4831 | |
| D2 | 346.96 | 1 | 346.96 | 3.90 | 0.0670 | |
| Residual | 1334.90 | 15 | 88.99 | |||
| Lack of Fit | 1290.90 | 10 | 129.09 | 0.1661 | significant | |
| Pure Error | 44.00 | 5 | 8.80 | |||
| Cor Total | 6801.37 | 29 | 390.46 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenzhaliyev, B.; Berkinbayeva, A.; Baltabekova, Z.; Moldabayeva, G.; Smailov, K.; Saulebekkyzy, S.; Tolegenova, N.; Karim, D.; Omirbek, T. Investigation of Phase Transformations in Technogenic Raw Materials Under Microwave Treatment for Enhanced Zinc Leaching. Processes 2025, 13, 1099. https://doi.org/10.3390/pr13041099
Kenzhaliyev B, Berkinbayeva A, Baltabekova Z, Moldabayeva G, Smailov K, Saulebekkyzy S, Tolegenova N, Karim D, Omirbek T. Investigation of Phase Transformations in Technogenic Raw Materials Under Microwave Treatment for Enhanced Zinc Leaching. Processes. 2025; 13(4):1099. https://doi.org/10.3390/pr13041099
Chicago/Turabian StyleKenzhaliyev, Bagdaulet, Ainur Berkinbayeva, Zhazira Baltabekova, Gulnara Moldabayeva, Kenzhegali Smailov, Shynar Saulebekkyzy, Nazerke Tolegenova, Diana Karim, and Tursynkul Omirbek. 2025. "Investigation of Phase Transformations in Technogenic Raw Materials Under Microwave Treatment for Enhanced Zinc Leaching" Processes 13, no. 4: 1099. https://doi.org/10.3390/pr13041099
APA StyleKenzhaliyev, B., Berkinbayeva, A., Baltabekova, Z., Moldabayeva, G., Smailov, K., Saulebekkyzy, S., Tolegenova, N., Karim, D., & Omirbek, T. (2025). Investigation of Phase Transformations in Technogenic Raw Materials Under Microwave Treatment for Enhanced Zinc Leaching. Processes, 13(4), 1099. https://doi.org/10.3390/pr13041099






