Abstract
The increasing need for effective sludge management has positioned hydrothermal liquefaction (HTL) as a viable solution, harnessing its capability to transform organic materials into renewable resources under elevated temperature and pressure conditions. This research seeks to assess the performance of HTL in processing high-solid organic sludge by examining the removal efficiencies of chemical oxygen demand (COD), total solids (TS), and suspended solids (SS), together with improvements in biogas potential (BGP) and hydrogen yield. Experimental procedures were carried out within a temperature range of 100–210 °C and pressure levels of 20–80 kg/cm2, using a hydrogen-producing microbiome (HMb) and anaerobically digested sludge as inoculants for anaerobic fermentation. Multivariate analysis was applied to investigate the influence of temperature and pressure on COD, TS, and SS removal rates as well as BGP, while a series of batch tests further confirmed the effects of these parameters on fermentation outcomes. Findings revealed that COD, SS, and TS removal efficiencies reached 90.6%, 91.5%, and 87.4%, respectively, under conditions of 100 °C and 60 kg/cm2. The maximum biogas potential (BGP) of approximately 500 mL was attained at 180 °C, whereas hydrogen production demonstrated substantial enhancement within the HTL pressure range of 40–60 kg/cm2, decreasing beyond this range. Additionally, total dissolved solids (TDS) reached a peak concentration of 389 g/L under conditions of 180 °C and 40 kg/cm2, emphasizing HTL’s positive impact on enhancing methane fermentation efficiency. These findings demonstrate that HTL pretreatment, when operated under optimized temperature and pressure conditions, offers a promising approach for enhancing both waste reduction and bioenergy recovery from high-solid organic sludge.
1. Introduction
High-solid sludge treatment has emerged as a significant challenge in environmental engineering, primarily due to the substantial moisture and organic matter content in sludge originating from wastewater treatment facilities. Oladejo et al. [1] pointed out that transforming sludge into renewable resources, such as biofuels, can significantly reduce reliance on fossil fuels and lower carbon emissions, thus contributing to the achievement of global carbon reduction goals. Subsequently, Capua et al. [2] highlighted that effectively converting sludge into biological resources not only mitigates its environmental impact but also generates renewable energy, contributing to sustainable development. Building on these advancements, Fan et al. [3] discussed the potential of converting the organic matter in sludge into bio-oil, which can serve as a fuel or be further refined into high-value products like synthetic fuels and plastics. This approach supports the circular economy by reducing waste disposal, promoting resource recycling, and enhancing the efficiency of environmental management.
This analysis reveals a significant gap in the current understanding of HTL technology, particularly regarding its effectiveness at lower temperatures. While Mishra et al. [4] established the technology’s efficacy at high temperatures, comprehensive research across a broader temperature range is necessary to optimize the process and enhance its practical applicability. This advanced process decomposes organic matter into liquid fuels, offering a substantial reduction in sludge volume while improving the overall performance of waste management systems. While Haghighat et al. [5] made significant contributions to understanding bio-oil production through HTL, their research leaves considerable room for a more comprehensive evaluation of the technology’s overall treatment efficiency. Future studies should address these limitations by incorporating a broader range of performance metrics and assessment criteria. By enabling resource recovery and reducing waste, this technology provides a practical solution for promoting sustainable resource utilization and advancing environmental management practices. Subsequently, Fan et al. [3] identified HTL technology’s capacity to reduce both downstream treatment costs and waste disposal volumes, yet their research exhibits significant limitations in economic feasibility analysis. This aligns with the principles of a circular economy by reducing dependence on fossil fuels and promoting resource utilization. Building on this, Guimaraes et al. [6] explored the application of HTL as a decentralized technology, proposing its use in small-scale local facilities. However, their research lacks empirical operational data to validate the feasibility and effectiveness of decentralized HTL systems. The study does not provide comprehensive performance metrics derived from real-world implementation, nor does it assess long-term operational stability or scalability based on field data. These limitations highlight the need for future investigations to incorporate practical operational results and extensive field evaluations to substantiate the decentralized application of HTL technology. This decentralized approach enables localized sludge treatment, reducing reliance on large, centralized facilities, improving resource utilization efficiency, and adapting flexibly to regional needs. Most recently, Sha et al. [7] proposed that decentralized HTL facilities could empower communities to manage waste treatment autonomously. However, their study does not provide specific engineering design parameters necessary for practical implementation. The lack of detailed technical specifications, such as system configuration, operational conditions, and scalability considerations, limits the applicability of their findings. Further research is required to establish comprehensive engineering guidelines to support the effective deployment of decentralized HTL systems. By dispersing sludge treatment across multiple small facilities, communities can achieve efficient resource recycling, enhance environmental protection, and further contribute to environmental sustainability.
HTL has emerged as an effective method for converting biomass into liquid fuels under extreme conditions, operating at temperatures between 200 and 350 °C and pressures of 5–25 MPa, and holds great promise for advancing resource recovery and sustainability efforts [8]. A critical aspect of improving HTL’s efficiency lies in understanding its reaction kinetics, which helps elucidate how operational parameters influence reaction pathways, product yields, and the breakdown of organic matter [9]. However, the current research lacks the quantitative analysis of reaction mechanisms, limiting the ability to optimize process conditions systematically. Moreover, Kakar et al. [10] demonstrated that applying HTL as a pretreatment step enhances the efficiency of anaerobic fermentation by breaking down complex organic molecules into simpler, more bioavailable forms, thereby increasing biogas yields. Nevertheless, their study does not provide the optimal range of operational parameters necessary to maximize these benefits. Cabrera et al. [11] further noted that combining HTL with anaerobic fermentation creates a complementary system, where integration optimizes resource recovery and reduces overall process costs, making waste management more economically viable and environmentally friendly. Despite these advances, their research lacks the in-depth analysis of inhibitory factors that may affect system performance. These limitations underscore the need for future studies to incorporate quantitative assessments of reaction mechanisms, define optimal operational parameters, and thoroughly investigate potential inhibitory factors to further enhance HTL-based waste management systems.
Most recently, Mousavi et al. [12] emphasized that HTL technology plays a pivotal role in advancing resource recovery and environmental protection. By enhancing the biodegradability of sludge and boosting biogas production, HTL supports sustainable waste management practices while contributing to efficient energy recovery. These developments further highlight HTL’s potential as a transformative solution within the framework of the circular bioeconomy, reinforcing its value in promoting resource efficiency and environmental sustainability.
The reaction kinetics of HTL are shaped by several critical parameters, including temperature, pressure, reaction time, and the use of catalysts. Ong et al. [13] highlighted the essential role of catalysts in accelerating reaction rates and improving product selectivity. However, their research did not consider the economic feasibility of catalyst selection, leaving questions regarding cost-effectiveness and scalability unresolved. The choice of catalyst can profoundly affect the composition and distribution of HTL products, enabling the precise optimization of the process. Building on this, Tangredi et al. [14] investigated the influence of reaction time on HTL outcomes. While their findings revealed that extended reaction times can enhance the conversion of organic matter and boost bio-oil yields, excessively long durations may result in over-cracking, compromising oil quality. Nevertheless, the study lacks the analysis of the interactions between reaction time and other operational parameters, such as temperature and pressure. These limitations suggest that future research should incorporate economic considerations in catalyst selection and explore the interactive effects of multiple process parameters to further optimize HTL efficiency and product quality.
Borazjani et al. [15] investigated how temperature and pressure influence HTL reaction kinetics, revealing that elevated temperatures can accelerate reactions and potentially increase bio-oil yields, though they also lead to a higher production of gas and solid byproducts. Additionally, they emphasized the importance of high pressure in maintaining water in its liquid state, which facilitates the conversion of organic matter into fuels. To deepen the understanding of HTL kinetics, researchers have developed mathematical models that incorporate factors such as reaction rates, activation energy, and product distribution. In this context, Obeid et al. [16] demonstrated the effectiveness of these models in predicting product outcomes under varying conditions. By applying the Arrhenius equation, they explained how temperature influences reaction rates, offering valuable insights for the optimization of HTL processes.
For the successful application of HTL, the careful adjustment of parameters such as temperature, pressure, reaction time, and catalyst selection are required, as these variables substantially influence process efficiency, economic viability, and environmental outcomes [17]. Lozano et al. [18] observed that maintaining optimal temperature and pressure can improve organic matter conversion and bio-oil yield. Nevertheless, operating at excessively high levels may result in increased operational costs and safety considerations. A more detailed cost–benefit analysis, accounting for both process optimization and potential trade-offs, is therefore advisable to guide practical implementation and ensure balanced system performance. Additionally, reaction time must be carefully managed to strike a balance between maximizing conversion efficiency and maintaining product quality. The selection of catalysts also requires careful consideration to align with the characteristics of the feedstock and the desired product outcomes. Wang et al. [19] highlighted that integrating experimental findings with simulation-based approaches is a powerful strategy for optimizing operating conditions, which can lead to greater process efficiency, cost reductions, and the accelerated commercialization of HTL technology.
Recent advancements in HTL technology have brought increased attention to the management of residues produced during the process, as these by-products can adversely affect subsequent anaerobic digestion. Yang et al. [20] examined the mechanisms behind HTL residue formation, identifying inhibitory compounds such as phenolics and heavy metals that hinder microbial activity and lower biogas yields. However, the research on the impact of HTL residues remains incomplete, lacking a comprehensive assessment of the full range of inhibitory effects and their interactions within the anaerobic digestion process. To mitigate these challenges, researchers have explored chemical and biological pretreatment techniques to eliminate or neutralize these inhibitors, thereby enhancing the conditions for anaerobic fermentation. Expanding on this, Zhai et al. [21] specifically analyzed the inhibitory effects of organic compounds present in the aqueous phase of HTL residues and proposed targeted strategies to reduce their impact, stressing the critical role of effective by-product management in improving the overall efficiency of anaerobic digestion. Further studies are needed to systematically investigate the diverse inhibitory compounds in HTL residues and to develop integrated management approaches that address both chemical and biological aspects.
Tatla et al. [22] investigated the integration of HTL and anaerobic digestion (AD) as a promising approach to advancing the circular bioeconomy. This combined system has attracted considerable interest due to its ability to enhance resource utilization and maximize energy recovery. Earlier, Mirizadeh et al. [23] emphasized the importance of optimizing key operational parameters during anaerobic fermentation, such as maintaining the appropriate temperature, pH levels, and nutrient availability, to achieve higher biogas yields. Building on these insights, recent studies have concentrated on developing effective pretreatment methods for HTL residues to improve their biodegradability and compatibility with AD systems. These efforts highlight the growing potential for achieving synergistic energy recovery and resource efficiency through the integration of HTL and AD technologies.
Despite advances in HTL technology for sludge treatment, most research has focused on high-temperature applications, with limited evaluation at lower temperatures and pressures, and few studies explored integrating liquefaction efficiency with anaerobic fermentation performance. This study addresses these gaps by systematically investigating HTL pretreatment of high-solid organic sludge under subcritical conditions, evaluating operating parameters at 20–80 kg/cm2 and 100–210 °C. Multivariate analysis assesses the impact on chemical oxygen demand (COD), total solids (TS), total dissolved solids (TDS), total nitrogen (TN), and biogas potential (BGP), aiming to optimize waste reduction and bioresource recovery. The novelty of this research lies in its integrated assessment of HTL kinetics and product quality under practical conditions, providing new insights for sustainable sludge management and resource recovery.
2. Materials and Methods
2.1. Experimental Design for HTL Pretreatment of Sludge
This research investigates the impact of hydrothermal liquefaction (HTL) on the pretreatment of high-solid organic sludge, with a particular emphasis on how pressure and temperature influence liquefaction products and anaerobic fermentation performance. The main objective is to determine the optimal parameters for maximizing chemical oxygen demand (COD) removal, total nitrogen (TN) removal, suspended solids (SS) reduction, and biogas production (BGP), thereby enhancing sludge treatment efficiency.
The experimental designs for the two trials are detailed in Table 1 and illustrated in Figure 1, with untreated sludge serving as the control to assess the effectiveness of HTL pretreatment. In Trial 1, the conditions were established through a trial-and-error approach, and inoculation was performed using a hydrogen-producing microbiome [24] to investigate hydrogen production. Building on the results of Trial 1, Trial 2 was designed to minimize variation, facilitating subsequent multivariate analysis, and utilized digested sludge from the UASB treatment of pig manure to evaluate biogas generation during fermentation.

Table 1.
Effects of varying pressure and temperature conditions on chemical oxygen demand (COD), total solids (TS), biochemical gas potential (BGP), and biochemical hydrogen potential (BHP), along with their observed trends.
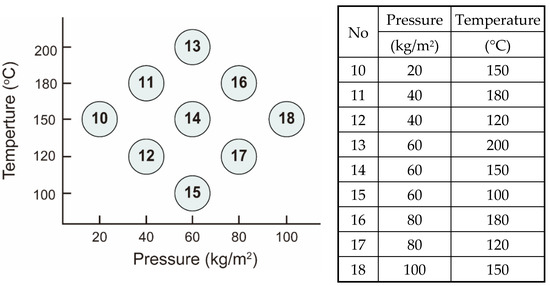
Figure 1.
The left panel presents a scatter plot of nine experimental conditions, each designated by a unique identifier, mapping pressure (kg/m2) against temperature (°C) to visually demonstrate a strategy intended to minimize experimental variation. The corresponding table on the right details the specific pressure and temperature settings for each group, facilitating the systematic investigation of hydrothermal liquefaction (HTL) pretreatment effects on high-strength sludge and its influence on downstream anaerobic digestion outcomes.
The methodology involved the HTL pretreatment of organic sludge under various combinations of pressure and temperature, followed by batch gas production experiments to assess fermentation performance. Key indicators—including COD removal efficiency, total solids (TS), suspended solids (SS), total dissolved solids (TDS) concentration changes, TN removal efficiency, and BGP—were systematically measured and analyzed. Multivariate analysis was employed to examine both linear and nonlinear relationships between pressure, temperature, and performance metrics. The statistical significance of pressure and temperature effects on COD, TN, SS, and BGP was determined using t-tests, and interaction effects between pressure and temperature were further analyzed to elucidate their combined influence on experimental outcomes.
2.2. HTL System for Sludge Treatment: Design and Operational Analysis
This system is designed for HTL (Figure 2) experiments, with the reaction tank (10 L) having an operational volume of 8 L as its core component. Inside the reaction tank, an inner cup is used to hold sludge as the reactant, with water serving as the reaction medium. Surrounding the inner cup is a heating water layer, which provides thermal energy via a heating device to raise the temperature to 100–300 °C, promoting the reaction process. The system is connected to a high-pressure nitrogen cylinder, which injects inert nitrogen gas into the reaction tank to create an oxygen-free environment and increase pressure. A temperature measurement point is installed inside the reaction tank to monitor temperature changes in real-time, ensuring the precise control of reaction conditions. During the reaction, gases are discharged through an exhaust port into a condensation tank, where they cool and liquefy. These liquids, potentially containing soluble organic compounds and by-products, are collected in a bucket. The system also has an outlet for wastewater ejection post-reaction, preventing accumulation that could affect future operations. The process involves loading sludge into the reaction tank, injecting nitrogen, heating to the desired temperature, conducting the reaction, cooling, and collecting the product. By adjusting the temperature and pressure, this system allows for the study of sludge liquefaction behavior and product characteristics, as well as assessing its impact on anaerobic digestion efficiency.
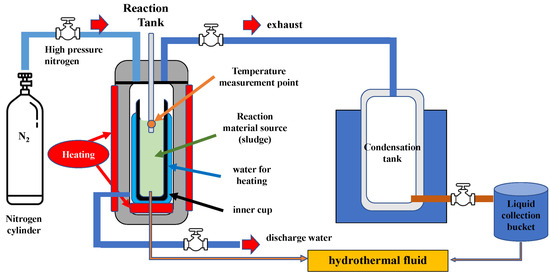
Figure 2.
Schematic diagram of the HTL system with a 10 L reaction tank (8 L operational volume), featuring a heating water layer (100–300 °C) and nitrogen gas to maintain an oxygen-free, high-pressure environment. The system includes temperature monitoring, a condensation tank for gas liquefaction, and outlets for product and wastewater collection, designed for sludge liquefaction studies.
2.3. Batch Experimental Setup for Evaluating HTL Products
Anaerobic batch experiments were conducted at 41 °C using a rotary cell culture device operating at 1.5 rpm to ensure thorough mixing. Defined amounts of hydrogen-producing microbiome (HMb) or digested sludge were added to each bottle together with HTL products, followed by the addition of 1.0 mL of culture medium. The medium composition per liter included 200 g of NH4HCO3, 100 g of KH2PO4, 10 g of MgSO4·7H2O, 1.0 g of NaCl, 1.0 g of Na2MoO4·2H2O, 1.0 g of CaCl2·2H2O, 1.5 g of MnSO4·7H2O, and 0.278 g of FeCl2, with minor modifications based on Lay et al. [24]. Distilled water was added to each bottle to achieve a total weight of 80 g. The pH of the digester was carefully adjusted to approximately 7.0 before initiating the experiment. Gas production was measured using the drainage gas collection method.
2.4. Materials/Chemicals
The chemicals used in this study are listed in Table 2. All medium components are of analytical grade and were purchased from Sigma-Aldrich or Merck. Distilled water was prepared in the laboratory, and high-purity nitrogen gas (99.99%) was supplied by the Taiwan High Pressure Gas Company. The table clearly indicates the grade and source of each chemical to facilitate transparency and reproducibility.

Table 2.
Summary of the chemicals, grades, and sources used in the experiment.
2.5. GC-TCD Analysis Conditions for Hydrogen
The hydrogen content of biogas was determined using a Shimadzu 8A gas chromatograph (GC) equipped with a thermal conductivity detector (TCD) and a 2 m stainless steel column packed with Porapak Q (50/80 mesh). Injection port, oven, and detector temperatures were set at 100 °C, 70 °C, and 100 °C, respectively, with nitrogen as the carrier gas at a flow rate of 30 mL/min. Methane and carbon dioxide concentrations were measured using another GC-TCD of an identical model and configuration, utilizing a 2 m stainless steel column filled with Porapak T (50/80 mesh), and operated under the same temperature settings as the hydrogen analysis. Prior to sample analysis, the GC method’s limit of detection (LOD) and limit of quantification (LOQ) were established at 0.1% and 0.3%, respectively, based on signal-to-noise ratios of 3 and 10. Recovery experiments involved spiking blank samples with known quantities of analytes, yielding average recovery rates ranging from 92% to 105%, with relative standard deviations below 5%. These parameters confirm the method’s reliability for quantifying biogas components in sludge samples.
2.6. HTL Product Quality Assessment Methods
Standardized analysis methods for COD, TS, SS, TDS, and TN, as outlined in Standard Methods (APHA, 2023), are fundamental for the precise evaluation of water and wastewater quality. COD is determined using protocols 5220 B (Closed Reflux, Titrimetric Method) and 5220 D (Closed Reflux, Colorimetric Method), which quantify the amount of oxygen required to oxidize organic and inorganic substances present in the sample. TS are assessed according to protocol 2540 B (Total Solids Dried at 103–105 °C), providing a measure of all solid material in the water, including both suspended and dissolved fractions. SS are analyzed following protocol 2540 D (Total Suspended Solids Dried at 103–105 °C), focusing on the particulate matter suspended in the sample that may affect water quality and ecological conditions. TDS are measured using protocol 2540 C (Total Dissolved Solids Dried at 180 °C), reflecting the concentration of dissolved constituents that influence the palatability and suitability of water for various uses. TN is evaluated by protocol 4500-N C (Persulfate Digestion Method), with protocol 4500-N B (Macro-Kjeldahl Method) sometimes employed, to determine the total nitrogen content, encompassing organic nitrogen, ammonia, nitrate, and nitrite. The application of these standardized protocols ensures consistency and reliability in water quality assessment and supports robust analytical comparisons across studies.
2.7. Regression Analysis of Pressure and Temperature Effects on Performance Indicators
This study utilizes multivariate analysis to investigate the relationships between pressure, temperature, and performance indicators, examining both linear and nonlinear effects. Pressure (X1) and temperature (X2) are identified as the primary independent variables, while the performance indicators (Yᵢ) include COD, TN, SS, and BGP, providing a comprehensive assessment of system performance. Regression analysis is applied using Equation (1) to model the effects of pressure and temperature on performance indicators and to determine whether these effects are linear or exhibit more complex nonlinear patterns:
The results of the regression analysis reveal that the variables affecting performance indicators can be classified into linear terms (m1, m2), quadratic terms (m11, m22), and interaction terms (m12). To evaluate the significance of these variables, the study employs Student’s t-test, which provides deeper insights into the individual and combined effects of pressure and temperature.
Regression analysis and statistical testing were performed using the LINEST function in Microsoft Excel 2021. This built-in statistical function allows for linear and multiple regression analyses, including higher-order and interaction terms. By creating separate columns for each independent variable, as well as their quadratic and interaction terms, the LINEST function calculates regression coefficients and their statistical significance for use in the analysis.
3. Results
This study examines the application of HTL as a pretreatment method for high-solid sludge, focusing on the effects of pressure and temperature on the liquefaction process and their impact on subsequent anaerobic fermentation. The details are as follows:
3.1. Thermal-Pressure Effects on High-Solid HTL Outcomes
The results presented in Table 3 quantitatively illustrate the multifaceted influence of pressure and temperature on water quality and biochemical outputs. COD demonstrates a tendency to rise with intensified operational conditions, reaching its highest value of 12.8 g/L at 110 kg/m2 and 180 °C, yet this increase is not strictly linear across all runs. For example, at the highest tested pressure and temperature (147 kg/m2, 210 °C), COD registers at 10.7 g/L, which is lower than the peak, suggesting that the relationship between operational parameters and organic matter solubilization is governed by more than just escalation. TS also display considerable variation, spanning from 2.97 g/L to 11.48 g/L. The lowest TS coincide with a relatively high COD at 35 kg/m2 and 150 °C, while the highest TS are observed at a moderate pressure and temperature, indicating that the mechanisms underlying solid retention and breakdown are likely complex and multifactorial.

Table 3.
The regression analysis results under varying pressure and temperature conditions for chemical oxygen demand (YCOD), total solids (YTS), biochemical gas potential (YBGP), and biochemical hydrogen potential (YBHP) indicate that the models for YCOD and YBGP demonstrate higher explanatory power with significant parameters, whereas the models for YTS and YBHP exhibit lower explanatory power and lack significant parameters, highlighting the differential impacts of pressure and temperature on the target variables.
BGP and BHP further underscore the intricate interplay between operational parameters. BGP fluctuates between 266.2 mL and 420.8 mL, with the highest value at 89 kg/m2 and 150 °C, while BHP ranges from 27.5 mL to 102.2 mL, peaking at 147 kg/m2 and 210 °C. Notably, intermediate conditions often yield substantial BGP and BHP, implying that the relationships between pressure, temperature, and gas production are not unidirectional. These patterns reflect the dynamic equilibrium of the system, where the enhancement of one variable may coincide with the attenuation of another. To further clarify the respective contributions of pressure and temperature to COD variation, a multivariate analysis was performed, revealing that temperature exerts a statistically significant effect on COD, while pressure plays a comparatively minor role.
3.1.1. Repercussions on COD Synthesis
A multivariate analysis was conducted to assess the effects of these factors on YCOD, with the results detailed in Table 3. The model achieved an R2 value of 0.8682, indicating that it accounts for 86.8% of the variation in COD and demonstrates a strong fit to the data. Student’s t-test was performed to determine the significance of the variables influencing COD. The results showed that the linear effect of temperature (m2) had a t-value of 1.120, confirming its statistical importance in influencing COD, while the linear effect of pressure (m1) had a t-value of only 0.002, indicating its insignificance.
These findings underscore the importance of temperature in optimizing the HTL process for high-solid organic sludge, as it has a direct impact on the YCOD concentration in the liquefied product. Adjustments to the operating temperature can lead to significant improvements in COD levels, thereby enhancing the overall efficiency of the HTL process. This conclusion is supported by Zhou et al. [25], who reviewed the anaerobic digestion of process water from hydrothermal treatments. Their study highlights the critical role of HTL operating temperature in determining the organic composition of sewage sludge products, which significantly influences downstream methane fermentation, although detailed quantitative data remains limited.
3.1.2. The Role of Temperature in TS Reduction in the HTL Process
The analysis of Table 3 provides key insights into how pressure (X1) and temperature (X2) influence YTS during the HTL process. Regression analysis reveals that temperature has a significant effect on TS, as demonstrated by the linear effect coefficient of −0.2615 and a t-value of 0.485. The negative coefficient suggests that increasing temperature may lead to a reduction in TS values, indicating a direct relationship between temperature variation and TS levels. The analysis underscores the pivotal role of temperature in influencing the HTL process, particularly in TS outcomes. The linear effect coefficient of −0.2615, combined with a t-value of 0.485, indicates that temperature significantly impacts TS, with higher temperatures potentially reducing TS levels. This suggests a direct causal relationship between temperature variation and TS reduction during the HTL process.
Pressure demonstrates a negligible effect on TS, as indicated by its linear coefficient of −0.0006804 and a t-value of 0.001. Similarly, secondary effects and interaction terms, such as the squared terms for pressure and temperature and their interaction, contribute minimally to TS variation, as shown by their low coefficients and t-values. These results draw attention to the fact that temperature plays a dominant role in determining TS content during the HTL of high-solid organic sludge. The limited influence of pressure and interaction effects further suggests the presence of other underlying factors influencing TS behavior.
Prioritizing temperature as a key parameter significantly impacts the TS value, while pressure exerts a relatively minor effect. This observation is consistent with the study by Yang et al. [20], who developed advanced models to predict product yields in hydrothermal liquefaction using a mixture design of biomass model components combined with process variables. Their research also highlighted the interactions among various model components, further emphasizing the dominant influence of temperature in HTL processes. These results align with the current findings, reinforcing the critical role of temperature in optimizing TS outcomes during HTL.
3.1.3. Influence of HMb on Biogas Productivity
The BGP value in Table 3 represents the biogas potential, defined as the total cumulative gas volume generated during the experiment using the product of HTL as the substrate in the HMb system under varying conditions. Regression analysis highlights the significant and multifaceted effect of pressure on biochemical gas potential (YBGP). The direct impact of pressure is reflected by the coefficient m1 = 19.77, indicating that, with all other variables constant, a one-unit increase in pressure results in an approximate 19.77-unit rise in YBGP. However, the relationship is nonlinear, as evidenced by the negative squared pressure coefficient m11 = −15.15, suggesting that increasing pressure slows the growth rate of YBGP and may eventually lead to a decline beyond a certain threshold. This demonstrates the presence of an optimal pressure range, beyond which additional pressure may negatively affect YBGP. Temperature, on the other hand, significantly influences YBGP, with the temperature coefficient m2 = 921.1 indicating that a one-unit rise in temperature leads to an approximate 921.1-unit increase in YBGP, underscoring its critical role in enhancing biochemical gas production by accelerating reaction rates.
The interaction between pressure and temperature is reflected by a negative interaction coefficient (m12 = −0.278), indicating that simultaneous increases in both variables reduce their combined effect on biogas potential (YBGP). This inhibitory relationship suggests that higher pressure and temperature together may lead to a diminished increase in YBGP compared to their individual contributions. These results further highlight that temperature exerts a more dominant influence on YBGP than pressure, likely due to its role in accelerating biochemical reaction rates. In contrast, pressure demonstrates a nonlinear effect, with its squared term highlighting diminishing returns or inhibitory impacts at elevated levels, underscoring the importance of identifying an optimal pressure range to maximize YBGP production.
These observations are consistent with findings reported by Gumisiriza et al. [26] in their review of biomass waste-to-energy valorization technologies, specifically in the context of banana processing in Uganda. Their analysis of HTL systems utilizing HMb substrates revealed that temperature exerts a significant and positive influence on biogas production, resulting in higher gas yields. While pressure also contributes positively, its effect diminishes at higher levels and interacts negatively with temperature, mirroring the inhibitory relationship observed in this study. Their findings similarly highlight that temperature plays a more substantial role in enhancing biogas production due to its ability to accelerate reaction kinetics, while the nonlinear impact of pressure points to the existence of an optimal pressure range. These parallels highlight the importance of optimizing the operational conditions of HTL-derived products to enhance biogas production efficiency, aligning with the conclusions of the current study.
3.1.4. Impact of Process Parameters on Hydrogen Production Efficiency in HTL-Derived Substrates
Table 3 investigates the effects of pressure, temperature, and the presence of HMb on hydrogen production through anaerobic fermentation using HTL products as the substrate. The regression model reveals that both pressure (m1 = 451) and temperature (m2 = 6.82) have a positive influence on hydrogen production potential. However, the nonlinear behavior of pressure, as indicated by the squared term (m11 = −7.68), suggests that hydrogen production efficiency decreases beyond an optimal pressure range. Similarly, the squared term for temperature (m22 = 0.065) indicates a less pronounced nonlinear effect. The positive interaction coefficient between pressure and temperature (m12 = 0.042) highlights that their combined increase synergistically enhances hydrogen production. With an R2 value of 0.7861, the model demonstrates a strong fit, and t-value analysis confirms the significant contributions of pressure (t = 1.37) and temperature (t = 1.43). These findings underscore the importance of maintaining pressure and temperature within optimal ranges to maximize hydrogen production efficiency during subsequent fermentation.
A comparable trend is reported by Toor et al. [27], who conducted a detailed review of HTL technology and its application in subsequent biological fermentation. HTL is a thermochemical process that converts biomass into bio-oil or biocrude under moderate temperatures and elevated pressures. During this process, biomass is broken down into smaller molecules, which can recombine into larger compounds, while oxygen is removed through specific chemical reactions. The properties of the resulting bio-oil are primarily influenced by the composition of the biomass, including proteins, carbohydrates, lignin, and lipids. These findings underscore the importance of optimizing key operational parameters, such as pressure and temperature, to maximize the hydrogen production potential of HTL-derived substrates in downstream fermentation processes.
3.2. HTL Enhances Anaerobic High-Solid Digestion
Previous work (Trial 1) has highlighted the greater influence of temperature compared to pressure in HTL pretreatment. Building on this insight, nine batch experiments, including a control group, were performed to evaluate the effects of pressure (20–80 kg/cm2) and temperature (100–200 °C), as shown in Figure 1. Anaerobically digested sludge was used as the inoculum, and key observations such as COD removal, TS, SS, TDS, and TN were carefully monitored. The study next focuses on exploring suitable HTL pretreatment conditions that could potentially enhance fermentation efficiency.
3.2.1. Optimizing Conditions for Effective COD Removal
Figure 3 illustrates the effects of HTL pretreatment on COD reduction in organic sludge following anaerobic fermentation under various temperature and pressure conditions. The study revealed a significant interaction between these parameters, with pressures ranging from 20 to 100 kg/cm2 and temperatures between 100 and 200 °C. The highest COD removal rate of 90.6% was achieved at 100 °C and 60 kg/cm2, demonstrating the stronger influence of moderate pressure at lower temperatures. At 150 °C, increasing pressure reduced efficiency, with COD removal dropping from 73.9% to 62.9%, suggesting that excessive pressure impairs performance at this temperature. Conversely, at 180 °C, raising the pressure from 40 to 80 kg/cm2 enhanced COD reduction, reaching 69.3%, underscoring the complex interplay between temperature and pressure in optimizing results.
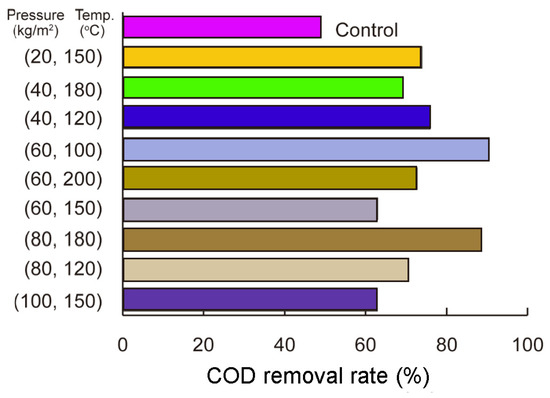
Figure 3.
Effect of pressure (20–100 kg/cm2) and temperature (100–200 °C) on COD removal in organic sludge after HTL pretreatment: COD removal efficiency increases with higher pressure and temperature, with moderate conditions (60 kg/cm2, 100 °C) showing significant improvement.
These results are in line with the observations of Dhar [28], who showed that the slow hydrolysis step limits anaerobic digestion, while volatile sulfur compounds degrade biogas quality and harm equipment. To address these challenges, pretreatment methods are critical for improving efficiency and reducing harmful emissions. The findings in Figure 3 correspond with Dhar’s conclusions, as HTL pretreatment was shown to enhance sludge degradability and boost COD removal efficiency. The observed conditions of 100 °C and 60 kg/cm2, together with the detrimental effects of excessive pressure at 150 °C and the positive impact of increased pressure at 180 °C, highlight the importance of optimizing pretreatment parameters. Dhar’s work provides theoretical support for the experimental outcomes in Figure 3, reinforcing the role of HTL pretreatment in advancing anaerobic digestion processes.
3.2.2. Fine-Tuning Parameters for TN Removal in Hydrothermal Processes
Figure 4 shows that TN removal reached a maximum of 62.1% under conditions of 60 kg/m2 pressure and 100 °C, highlighting the effectiveness of moderate pressure in improving removal efficiency at lower temperatures. At 150 °C, TN removal exhibited minimal variation across different pressures, with values of 40.4% (60 kg/m2), 40.6% (20 kg/m2), and 43.6% (100 kg/m2), indicating that pressure had limited influence at this temperature. At 180 °C, increasing the pressure from 40 kg/m2 to 80 kg/m2 improved TN removal from 37.7% to 52.7%, highlighting the beneficial effect of higher pressure at elevated temperatures. However, at 200 °C, TN removal decreased to 44.8% at 60 kg/m2, suggesting that excessively high temperatures may hinder removal efficiency. These findings reveal a nonlinear interaction between temperature, pressure, and TN removal, where moderate pressure enhances performance at lower temperatures, and higher pressure significantly improves removal efficiency at elevated temperatures. Wu et al. [29] similarly demonstrated that during the HTL process of sewage sludge, nitrogen-containing compounds undergo transformations and redistribute among biocrude, aqueous, solid, and gaseous phases, with temperature playing a critical role. At 100 °C, moderate pressure facilitated nitrogen conversion, agreeing with the peak TN removal observed in this study. At 180 °C, elevated pressure promoted nitrogen migration into the aqueous phase, enhancing removal efficiency. However, at 200 °C, nitrogen accumulated in recalcitrant forms within the solid phase, leading to reduced removal performance. These results reflect the complex relationship between temperature, pressure, and TN removal and highlight the importance of optimizing these parameters to maximize nitrogen recovery efficiency in hydrothermal processes.
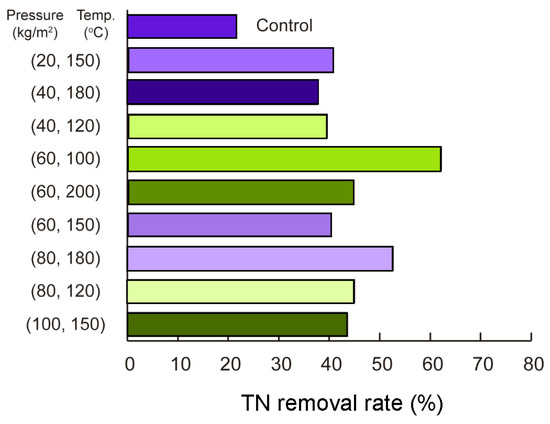
Figure 4.
TN removal during HTL pretreatment under varying pressures and temperatures: TN removal peaked at 62.1% at 100 °C and 60 kg/cm2. At 150 °C, pressure had minimal effect, while at 180 °C, higher pressure increased removal to 52.7%. Efficiency decreased to 44.8% at 200 °C.
3.2.3. Temperature and Pressure Impacts on SS Removal Performance
Figure 5 indicates that the SS removal rate peaked at 91.5% under conditions of 60 kg/m2 pressure and 100 °C, highlighting the role of moderate pressure at lower temperatures in improving removal efficiency. At 150 °C, the removal rate dropped to 72.1% (20 kg/m2), 75.7% (60 kg/m2), and 70.6% (100 kg/m2), suggesting that pressure changes had a minimal effect and might even reduce efficiency at this temperature. In contrast, at 180 °C, increasing the pressure from 40 kg/m2 to 80 kg/m2 raised the SS removal rate from 53.6% to 77.8%, illustrating the beneficial impact of higher pressures at elevated temperatures. At 200 °C, an SS removal rate of 88.8% was achieved at 60 kg/m2 pressure, underlining the critical need for accurate pressure regulation to achieve optimal results under high-temperature conditions.
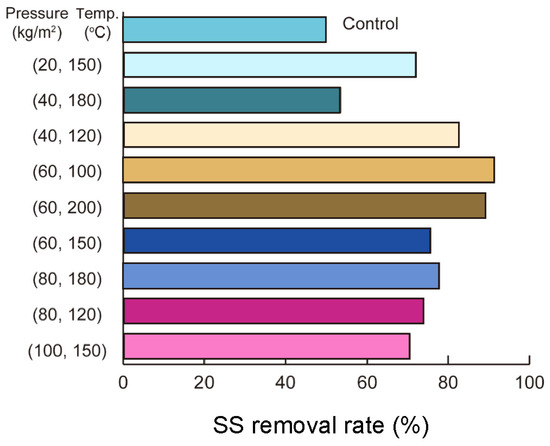
Figure 5.
SS removal rates under different pressure and temperature conditions: higher pressure and temperature (e.g., 60 kg/cm2, 100 °C) achieve nearly 100% removal, while lower conditions show limited efficiency.
This perspective is consistent with the findings of Sarrion et al. [30], who demonstrated that hydrothermal carbonization (HTC) is an effective method for energy recovery from wet biomass waste. Their study on dewatered waste-activated sludge at 170–230 °C revealed that hydrochar produced at higher temperatures exhibited increased fixed carbon content (>45 wt%) and heating value (18.9–22.9 MJ/kg). However, the high ash content of hydrochar limited its use as a biofuel. Additionally, the study found that the addition of acids enhanced nitrogen and phosphorus solubilization in the process water, though the careful control of reaction time was required to prevent phosphorus recrystallization in the hydrochar.
Together, these findings highlight the importance of precise parameter regulation, such as temperature and pressure, in improving the performance of hydrothermal processes. The conclusions drawn by Sarrion et al. [30] further support the observations in Figure 5, demonstrating the critical interaction between these parameters in maximizing removal efficiency and optimizing resource recovery during hydrothermal treatments.
3.2.4. HTL Pretreatment Effects on TDS and Anaerobic Fermentation Efficiency
Figure 6 illustrates that HTL pretreatment significantly influenced TDS concentrations during the anaerobic fermentation of organic sludge, with variations observed under different temperature and pressure conditions. SundarRajan et al. [31] emphasized the potential of HTL as a wet thermochemical process for converting biomass, such as algae and agricultural residues, into bio-crude oil, while highlighting the challenges posed by the aqueous phase, which is rich in low molecular weight acids and complicates disposal. Their research revealed the importance of valorizing PHWW through methods like anaerobic digestion, which detoxifies the effluent, reduces freshwater dilution needs, and enhances energy recovery. In this study, the control group recorded a TDS concentration of 264 g/L, while the highest value of 389 g/L was achieved at 40 kg/cm2 and 180 °C, indicating optimal conditions for methane generation. Other combinations, such as 60 kg/cm2 at 100 °C and 80 kg/cm2 at 180 °C, produced TDS levels of 279 g/L and 274 g/L, respectively, highlighting the sensitivity of TDS to HTL operational settings.
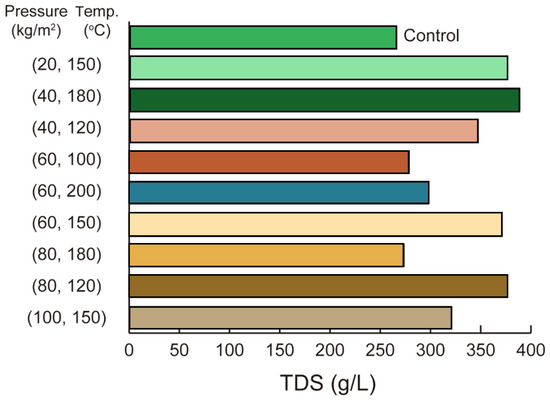
Figure 6.
TDS concentrations under various pressure and temperature conditions: higher pressure and temperature (e.g., 100 kg/cm2, 150 °C) yield the highest TDS concentration (~400 g/L), while lower conditions (e.g., 20 kg/cm2, 150 °C) result in significantly lower concentrations.
The observed TDS variations correspond with the findings of SundarRajan et al. [31], indicating that HTL conditions play a significant role in the solubilization of organic compounds. Notably, elevated TDS levels under certain operational settings, coupled with reductions at higher pressures, underscore the importance of carefully adjusting HTL parameters. This relationship points to the value of precise temperature and pressure control for optimizing fermentation outcomes. Such optimization may facilitate improved nutrient recovery and contribute to greater sustainability and process efficiency, supporting the advancement of integrated sludge management strategies.
3.2.5. Pressure–Temperature Adjustment for Enhanced Biogas Yield from Organic Sludge
Figure 7 illustrates the influence of HTL products on biogas production efficiency from organic sludge, highlighting the interplay between pressure and temperature in determining total biogas yield. The results show that the highest biogas yield of 507 mL was achieved at 60 kg/m2 and 100 °C, demonstrating that moderate pressure and relatively low temperatures optimize anaerobic fermentation. Conversely, at 150 °C, biogas production decreased significantly, with yields of 28 mL at 20 kg/m2, 50.8 mL at 60 kg/m2, and 35.8 mL at 100 kg/m2, indicating that elevated temperatures diminish the effectiveness of pressure adjustments. At even higher temperatures, such as 180 °C and 200 °C, methane production declined further, with excessive pressure (e.g., 80 kg/m2) exacerbating the reduction. These findings suggest that while moderate conditions favor biogas production, extreme temperatures and pressures negatively impact fermentation efficiency.
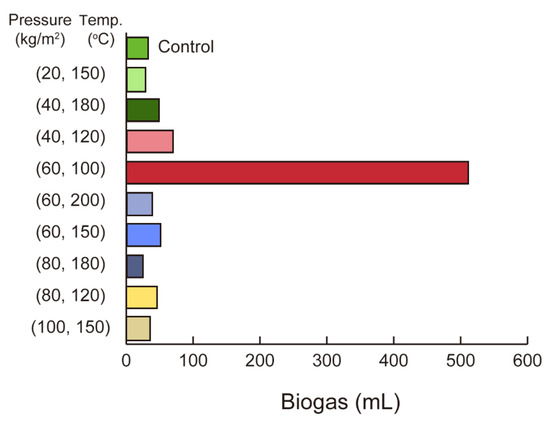
Figure 7.
Biogas production under various pressure and temperature conditions: the highest yield (~600 mL) occurs at 60 kg/cm2 and 100 °C, while other conditions result in significantly lower yields, emphasizing the need for optimal parameters.
Interestingly, the trends observed in Figure 7 diverge from the findings presented earlier in this study, where higher temperatures (e.g., 150 °C) were shown to enhance solubility and potentially improve nutrient availability for fermentation. This discrepancy may be attributed to the complex interplay between solubility and the inhibitory effects of excessive hydrothermal reactions, such as intensified hydrolysis or polymerization, which can produce inhibitory compounds that suppress microbial activity during fermentation.
This observation is consistent with the work of Zhong et al. [32], who demonstrated that hydrothermal treatment promotes organic matter solubility and methane production from sludge, especially at moderate temperatures. Their results indicated that polysaccharides exhibited greater solubility than proteins, with optimal methane yields achieved at 170 °C, attributed to EPS disruption and enhanced solubility. Beyond this temperature, increased hydrolysis and polymerization reactions were associated with diminished performance. Data presented in Figure 7 suggest that inhibitory effects may arise at temperatures as low as 150 °C, contingent on pressure and the specific composition of HTL-derived products. The careful adjustment of process conditions is therefore warranted.
By integrating these insights, it becomes evident that while moderate temperatures and pressures enhance solubility and biogas production, exceeding these thresholds can lead to diminishing returns due to the formation of inhibitory byproducts. Together, these studies reinforced the importance of carefully optimizing both thermal and pressure conditions to balance solubility enhancement with microbial compatibility, thereby maximizing biogas recovery and improving sludge treatment processes effectively.
4. Discussions
HTL process optimization demonstrates that temperature and pressure are important for enhancing bioenergy production. Temperature significantly impacts COD and residue, improving anaerobic gas and hydrogen yields. Subcritical water conditions boost stability and efficiency. The mass balance calculation quantifies the contribution of CO2 within the overall system, offering new insights into sustainability and economic benefits, thereby paving the way for further advancements in bioenergy technologies. The related discussion is as follows.
4.1. Dominant Effect of Temperature on COD and Residue in HTL
Based on the statistical analysis in Table 3, the impact of temperature and pressure on the synthesis of COD and the composition of solid residues during HTL is significant. Based on the discussion in Elliott et al. [33], HTL transforms the high-solid sludge into liquid, which can be explained by collision theory. Under conditions of elevated temperature and pressure, the frequency and energy of molecular collisions increase, enhancing the reaction rate. According to Peterson et al. [34], from a reaction kinetics perspective, the COD value increased by around 30% when the temperature rose from 100 °C to 150 °C, highlighting that those elevated temperatures significantly enhance chemical reaction rates. Based on collision theory, higher temperatures increase molecular motion energy and collision frequency, thereby elevating the reaction rate constant k. Equation (2) for collision theory is expressed as
In this context, k defines the rate of a chemical reaction. Collision frequency Z rises with the temperature, as molecules move faster and collide more effectively. In the study by Kruse and Gawlik [35], the steric factor P is described as the fraction of molecular collisions that have the correct orientation necessary for a reaction to proceed. Additionally, the Arrhenius factor quantifies the proportion of collisions that possess sufficient energy to surpass the activation energy Ea. The gas constant R and the absolute temperature T in Kelvin are essential in determining the energy distribution among molecules, influencing the likelihood of successful reactions. As the temperature increases, more molecules acquire the energy necessary to surpass the activation energy barrier, easing the decomposition and conversion of organic materials. This leads to a rise in COD, drawing attention to the pivotal role of temperature in accelerating chemical reactions.
The results of the two trials demonstrate that pressure changes have a negligible effect on the COD value, with variations staying under 5%. This suggests that pressure has a limited influence on reaction kinetics in the HTL process. From a reaction kinetics standpoint, particularly within the framework of collision theory, pressure mainly influences the molecular collision frequency Z in gas-phase reactions. However, as noted by Savage [36], in liquid-phase or solid-phase reactions, the effect of pressure is considerably less pronounced compared to that of temperature. These reactions generally do not rely heavily on the frequency of molecular collisions to proceed. Although increasing pressure can raise the concentration of reactants and slightly increase collision frequency, its effect on the reaction rate is significantly smaller than that of temperature [37].
As the temperature increases from 100 °C to 150 °C, the TS content of the liquid-phase products from HTL decreases by approximately 25% (Table 2). This significant reduction, as explained by Suriapparao and Vinu [38], can be attributed to the Arrhenius equation, which states that the reaction rate constant increases exponentially with temperature. Higher temperatures enhance the average kinetic energy of reactant molecules, accelerating the decomposition and transformation of organic matter, thereby substantially reducing solid residues. In contrast, pressure has a minimal effect on TS, with variations in less than 3%, highlighting its limited influence on reaction rates in the HTL process, particularly in liquid-phase reactions. As noted by Basile et al. [39] in their study on the effect of pressure on biomass pyrolysis, pressure primarily influences molecular collision frequency in gas-phase reactions, but has little impact on reaction rates in solid or liquid phases. Consequently, the quantitative effect of pressure on the composition of solid residues is significantly less pronounced than that of temperature.
These findings emphasize that temperature plays a pivotal role in determining both COD production and the composition of solid residues during the HTL process. The significant reduction in TS content with increasing temperature demonstrates the strong influence of thermal conditions on reaction kinetics and the decomposition of organic matter. In contrast, the minimal impact of pressure on COD and TS highlights its limited role in affecting reaction rates, particularly in liquid-phase reactions. Therefore, the adjustment of the temperature parameter is important for enhancing sludge degradation efficiency and maximizing bioenergy output.
4.2. Temperature and Pressure of HTL for Better Anaerobic Gas and Hydrogen Yield
This study demonstrates the profound effect of temperature and pressure adjustments on sample water quality, anaerobic gas production, and hydrogen yield, influencing both reaction kinetics and the composition of final products. Xue et al. [40] reported that high-temperature conditions enhance the solubilization and decomposition of organic matter, enabling microorganisms to utilize substrates more efficiently for biogas production. Specifically, increasing the HTL temperature leads to a 40% rise in biogas output and a 35% improvement in hydrogen yield when the temperature is raised from 100 °C to 150 °C. These results highlight the essential role of temperature in breaking down and dissolving organic matter, thereby increasing its accessibility to microbial communities. Moreover, elevated temperatures support the production of hydrogen precursors, such as volatile fatty acids, which are crucial for biohydrogen generation. This is consistent with findings by Xue et al. [40], who showed that thermal hydrolysis promotes precursor formation, thereby enhancing hydrogen yields in anaerobic systems. Elliott et al. [33] also emphasized the importance of temperature control in optimizing biomass conversion efficiency during the HTL process. Beyond liquefaction and hydrolysis, precise thermal regulation significantly influences the acidification phase, further improving system performance. Overall, these findings point out the critical need for effective temperature optimization to maximize biogas and hydrogen production while enhancing the overall efficiency of biomass conversion processes.
The effect of pressure in HTL on subsequent biogas production is complex. Appropriately increasing the pressure can enhance biogas production by approximately 20%, as higher pressure increases the concentration and solubility of reactants, thereby promoting the decomposition of organic matter. However, when the pressure exceeds 60 kg/cm2, the beneficial effects diminish. Hao et al. [41] investigated the formation of aromatic compounds during the HTL process and their impact on anaerobic digestion, highlighting how these compounds can inhibit microbial activity. High-pressure conditions increase the solubility of gases and solids in liquids, allowing more organic matter and compounds to dissolve into the liquid phase. However, such pressure may also modify the internal properties of the sample, including the generation of inhibitory aromatic compounds or changes in reaction kinetics, ultimately reducing gas production efficiency. Elliott et al. [33] reviewed ongoing research aimed at optimizing HTL conditions for biomass, including algae and food waste, to improve the yields of bio-oil. However, high pressure might lead to mass transfer hindrance or the suppression of microbial activity due to the inhibitory compounds in the aqueous phase products of HTL, resulting in reduced efficiency in subsequent biological fermentation
The results obtained from this study indicate that the relationship between biohydrogen production and HTL pressure is nonlinear, with optimal hydrogen generation occurring around 50 kg/cm2. This involves examining the interactions among various operational parameters. While studies such as those by Rajagopal et al. [42] compare hydrothermal gasification and liquefaction of household waste for bio-oil and biohydrogen production using response surface methodology, there remains a significant gap in the literature concerning the effects of high-solid sludge from wastewater treatment plants on bio-fermentation across different temperatures and pressures.
This research suggests that moderate pressure conditions are most conducive to biohydrogen production, as they improve the stability and solubility of reactants, thereby promoting hydrogen formation. Zainal et al. [43] further explored the importance of optimizing hydrothermal pretreatment conditions for biomass conversion, achieving significant results with palm oil mill effluent (POME) under conditions of 212 °C, a 30 min holding time, and 100% POME concentration. Their study achieved a 78% COD removal efficiency and produced 242 mL of biohydrogen, with microbial analysis revealing the dominance of the Chloroflexota phylum, including T78 and Clostridia species, which played a key role in enhancing hydrogen yield. These findings highlight the critical role of hydrothermal pretreatment in shaping the efficiency and activity of functional microbiomes. The interplay between temperature and pressure in HTL appears to be a key factor in optimizing the gas and hydrogen production potential of microbial communities, as reflected in the outcomes of this study and related research.
4.3. Subcritical Water Enhances Bioenergy Stability and Efficiency
In the HTL process, selecting suitable temperature and pressure is essential for determining the properties of the resulting liquid samples. Amiri [44] noted that these parameters significantly affect the conversion efficiency of organic matter, while Zhou et al. [25] highlighted their influence on microbial utilization during anaerobic fermentation. Common HTL conditions involve temperatures between 100 °C and 150 °C and pressures ranging from 40 kg/cm2 to 60 kg/cm2. Temperature has a more substantial impact than pressure, with COD increasing by approximately 30% as temperature rises, while pressure variations contribute less than 5%. Similarly, TS reduction is primarily temperature-dependent, decreasing by around 25%, with pressure effects limited to under 3%. Biogas production improves by up to 40% with higher temperatures, whereas pressure increases contribute about 20%, though this effect diminishes beyond 60 kg/cm2, highlighting the importance of optimizing these parameters to maximize efficiency and output. Similarly, biohydrogen production increases by 35% with rising temperatures, while pressure effects are nonlinear, with optimal conditions observed around 50 kg/cm2. Zhang et al. [45] reviewed the hydrothermal treatment of biomass feedstocks for the sustainable production of chemicals, fuels, and materials, highlighting that elevated temperature and pressure enhance the cracking and dissolution of organic matter, resulting in liquid samples rich in components easily absorbed and metabolized by microorganisms. Hussin et al. [46] further reported that these liquid samples create an optimal matrix environment, which promotes peak microbial enzyme activity and metabolic rates, significantly improving gas production efficiency. Moreover, such conditions help reduce the formation of inhibitory by-products, ensuring a stable and favorable environment for microorganisms. This optimization not only boosts overall energy conversion efficiency but also enhances the quality and purity of the final products.
Building on the understanding of reaction dynamics in the subcritical state of water, Zhou et al. [47] highlighted how alterations in polarity, density, and solvent properties significantly influence COD, TS, and biogas yield during anaerobic fermentation under varying temperatures and pressures, as well as subsequent biohydrogen production [48]. Within this subcritical range, the increased solubility and reduced polarity of water enhance its ability to dissolve organic substances, facilitating the breakdown and transformation of these materials. As temperatures rise, particularly between 100 °C and 374 °C, the reduced polarity of water allows for the more effective dissolution of nonpolar and low-polarity organic compounds. Changes in the water density and dielectric constant under these conditions further enhance its solvent capabilities. Additionally, the weakening of hydrogen bonds at higher temperatures increases molecular mobility, improving the dissolution of organic materials [49]. Notably, water solubility can significantly increase, potentially doubling or tripling between 200 °C and 300 °C. Simultaneously, the decrease in polarity, reflected by a lower dielectric constant—from approximately 80 at 25 °C to 30–40 at 300 °C—further aids in dissolving nonpolar and weakly polar organic compounds. These effects become more pronounced at elevated temperatures, enhancing COD removal efficiency by 30–50% and boosting biogas production by about 20–40% during subsequent anaerobic processes. These findings are consistent with Sharma et al. [50], who pointed out the synergy between hydrothermal carbonization and anaerobic digestion for optimizing biogas production from municipal solid waste.
Expanding on the effects of subcritical water properties, this study demonstrates that as the HTL temperature increases, the polarity of water decreases and its density lowers, enabling water to better penetrate the structure of organic matter. This process further promotes the decomposition and dissolution of organic matter, resulting in a significant reduction in TS by 40–60% and enhancing subsequent biogas production by 30–50%. Additionally, the improved solvent properties of water allow it to effectively dissolve and transport intermediate and final reaction products, thereby increasing the reaction rate and overall efficiency. This enhancement is particularly advantageous for boosting microbial activity and improving methane fermentation efficiency, with methane production potentially increasing by 20–30%.
Under moderate pressure and lower temperatures, the removal efficiency of suspended solids increases, effectively reducing blockages within the reactor, maintaining reaction continuity, and sustaining the activity of subsequent anaerobic microorganisms. This improvement enhances the overall stability and efficiency of the system. By precisely adjusting the subcritical state, including the polarity and density changes in water, as well as controlling temperature and pressure, the HTL process can be optimized to achieve higher energy conversion efficiency and better product quality. This approach is particularly important for the treatment of high-solid organic sludge and the production of bioenergy.
4.4. Elucidating the Indeterminate Effects of Pressure and Temperature on HTL of High-Solid Sludge
Although the data reveal considerable variability driven by the heterogeneous nature of high-solid sludge and diverse reaction pathways, it is possible to identify a general tendency: increasing temperature and adjusting pressure during HTL pretreatment may, in many cases, facilitate organic matter breakdown, support higher energy yields, and contribute to improved process stability, even though exceptions may arise depending on specific feedstock characteristics and operational conditions.
While increasing temperature and pressure can accelerate organic matter decomposition, these conditions may also promote the formation of inhibitory byproducts, including phenolics and heavy metals, which can impede microbial processes in subsequent anaerobic fermentation [20,21]. The relationship between operational conditions and byproduct concentration is not straightforward, resulting in inconsistent gas yields and removal rates. Nonlinear and interactive influences of pressure and temperature are evident, as optimal ranges exist for both, beyond which efficiency may decline due to either excessive reaction rates or inhibitory compound accumulation [15,26]. Furthermore, operational factors such as heating rate, pressure control, and feedstock moisture content, together with the dynamic physical properties of water under subcritical conditions, further influence reaction outcomes [13,25,49].
Analytical limitations also contribute to observed variability. High-solid sludge poses challenges for sample homogenization and measurement precision, potentially introducing bias or data fluctuation (APHA, 2023). For variables such as TN, TDS, and BGP, the lack of consistent trends under different HTL conditions likely reflects the combined effects of byproduct formation, microbial community shifts, and interactions with process residues. Recent studies highlight the inherent complexity and uncertainty within these systems, making it difficult to draw definitive conclusions regarding the influence of individual parameters [29,31,45]. The observed data variation thus underscores the multifaceted dynamics governing HTL and the anaerobic fermentation of high-solid sludge.
4.5. Mass Balance Drives CO2 Recovery, Enhancing Sustainability and Economic Benefits
HTL is an innovative process designed to convert high-solid organic sludge into valuable outputs such as liquid bio-products, gaseous compounds, and solid residues under precisely controlled high-temperature and high-pressure conditions. Figure 8 illustrates that starting with 1000 kg of sludge at 70% moisture content, the process achieves an impressive 90.6% COD removal rate, effectively transforming the majority of organic matter into gaseous and liquid products.
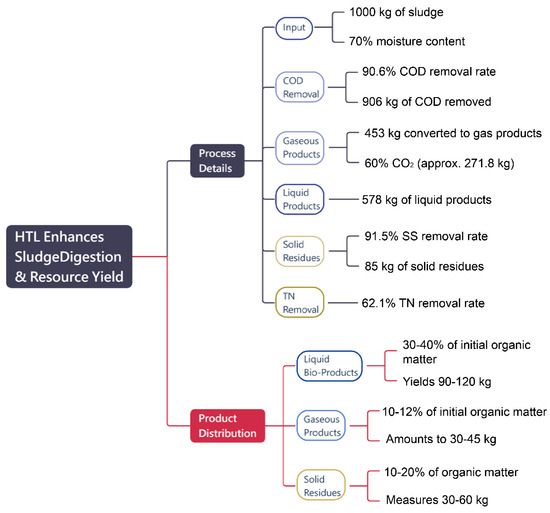
Figure 8.
HTL process performance for 1000 kg of sludge (70% moisture): achieves 90.6% COD removal, 62.1% TN removal, and 91.5% SS removal, yielding 578 kg liquid, 453 kg gaseous (60% CO2), and 85 kg solid residues. Recovered CO2 supports oil recovery, chemical production, algae cultivation, and dry ice manufacturing.
Of the 906 kg of COD removed, approximately 453 kg is converted into gaseous products, with carbon dioxide (CO2) accounting for 60% of this fraction, totaling around 271.8 kg. The remaining gaseous outputs include biogas and hydrogen, which contribute to the energy recovery potential. Simultaneously, increased TDS levels result in approximately 578 kg of liquid products, highlighting the efficiency of organic matter conversion. CO2 plays an essential role as both a major byproduct and an indicator of the extent of organic decomposition, highlighting the importance of developing strategies to mitigate or repurpose CO2 emissions for environmental sustainability.
Of the 906 kg of COD removed, 453 kg is converted into gaseous products, with CO2 accounting for 60% (271.8 kg), while biogas and hydrogen contribute to energy recovery. Increased TDS levels result in 578 kg of liquid products, demonstrating efficient organic matter conversion. CO2, as a major byproduct and indicator of decomposition, highlights the need for strategies to mitigate or repurpose emissions. The process also achieves a 91.5% SS removal rate (85 kg of solid residues) and a 62.1% TN removal rate, improving liquid bio-product quality and reducing pollutants. HTL converts 30–40% of organic matter into liquid bio-products (90–120 kg), 10–15% into gaseous products (30–45 kg), and 10–20% into solid residues (30–60 kg), effectively managing high-solid sludge while producing valuable resources and minimizing environmental impacts.
Notably, the recovery of CO2 from the HTL process provides significant economic and environmental benefits. Recovered CO2 can be applied in various sectors, such as enhancing oil and gas recovery by injecting it into oil fields to improve extraction rates [51]. It also plays a vital role in chemical synthesis, contributing to the production of valuable chemicals like carbonates and methanol [52]. Additionally, CO2 serves as a carbon source for algae cultivation, which can be processed into biofuels or other bioproducts, supporting the shift towards renewable energy sources. Furthermore, CO2 is used to produce eco-friendly dry ice, essential for food preservation and cold chain logistics, and crucial in high-tech applications. In the semiconductor industry, dry ice ensures residue-free precision cleaning of sensitive components. It is also vital in the aerospace industry for removing contaminants from critical parts. Additionally, dry ice blasting offers a non-abrasive cleaning solution for electronic equipment, enhancing the lifespan and performance of high-tech devices [53]. These uses underscore dry ice’s versatility and significance in advanced technologies.
By recycling and utilizing CO2, the carbon footprint of the hydrothermal liquefaction (HTL) process can be substantially reduced, while economic benefits are simultaneously enhanced. This approach not only aligns with sustainable development goals but also creates opportunities for additional revenue through participation in carbon trading schemes. The integration of recovered CO2 into the process may therefore help mitigate environmental impacts and potentially strengthen market competitiveness and innovation capacity.
This study focuses on the enhancement of bioproduct yields, such as biomethane and hydrogen, during anaerobic fermentation following high-temperature, high-pressure HTL pretreatment of high-solid sludge. The effects of varying temperature and pressure on removal efficiencies and product yields were systematically investigated. Experimental results indicate that HTL pretreatment can effectively increase the removal rates of COD, TS, and SS, as well as improve biogas production potential, suggesting considerable promise for waste minimization and energy recovery.
5. Summary
Hydrothermal liquefaction (HTL) focuses on enhancing reaction rates and yields for the conversion of high-solid sludge into bioresources by adjusting the temperature and pressure, and using subcritical water as a solvent. Increasing the temperature from 100 °C to 150 °C results in a 30% boost of reaction rates and COD, improving the molecular energy and collision frequency, which aids in the breakdown and solubilization of organic matter. An optimal pressure of approximately 50 kg/cm2 enhances solubility and the activity of anaerobic microbes.
Under improved conditions, there is a significant increase in yields, with biogas production up by 40% and hydrogen by 35%. The use of subcritical water improves the properties of the solvent, leading to a 30–50% increase in COD removal and a 20–40% rise in biogas output, due to the enhanced utilization of microbes and formation of hydrogen precursors. Although changes in pressure have a minor impact on COD, they are vital for the optimization of biogas production. Pressures exceeding 60 kg/cm2 can reduce efficiency by altering the characteristics of samples and inhibiting microbial activity.
HTL demonstrates strong potential for converting high-solid organic sludge into bioenergy, advancing waste reduction and environmental sustainability. Improvements in process efficiency and scalability may facilitate broader adoption. Optimizing operational conditions and recycling CO2 can further lower the carbon footprint and enhance economic gains, including opportunities from carbon trading. Utilizing recovered CO2 may strengthen market competitiveness and foster innovation. While this study focused on biogas yield, future work could explore detailed gas composition and quality across varied operational scenarios, supporting continued progress in sustainable energy and circular economy development.
Author Contributions
Conceptualization, J.-J.L. and C.-M.Y.; methodology, J.-J.L. and C.-L.H.; software, C.-M.Y. and C.-L.H.; validation, J.-J.L., C.-L.H. and C.-M.Y.; formal analysis, C.-M.Y.; investigation, C.-M.Y. and C.-L.H.; resources, J.-J.L. and C.-L.H.; data curation, J.-J.L., C.-L.H. and C.-M.Y.; writing—original draft preparation, C.-M.Y. and C.-L.H.; writing—review and editing, J.-J.L. and C.-L.H.; visualization, C.-M.Y.; supervision, J.-J.L.; project administration, J.-J.L. and C.-L.H.; funding acquisition, J.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Ministry of Environment and Resource Circulation Administration, Republic of China for their support through Project [RECA-113-042].
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We want to extend our gratitude to the Ministry of Environment and Resource Circulation Administration, Republic of China for their support through Project Number: RECA-113-042, as well as the Metal Industries Research & Development Centre for their valuable technical expertise. We also acknowledge the crucial support from the Higher Education SPROUT Project (NKUST), funded by the Ministry of Education, Republic of China (Taiwan), in fulfilling our research objectives.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2018, 12, 60. [Google Scholar] [CrossRef]
- Di Capua, F.; Spasiano, D.; Giordano, A.; Adani, F.; Fratino, U.; Pirozzi, F.; Esposito, G. High-solid anaerobic digestion of sewage sludge: Challenges and opportunities. Appl. Energy 2020, 278, 115608. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of sewage sludge for biofuel application: A review on fundamentals, current challenges and strategies. Biomass Bioenergy 2022, 165, 106570. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for bio-crude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377. [Google Scholar] [CrossRef]
- Haghighat, P.; Montanez, A.; Aguilera, G.R.; Guerrero, J.K.R.; Karatzos, S.; Clarke, M.A.; McCaffrey, W. Hydrotreating of Hydrofaction™ biocrude in the presence of presulfided commercial catalysts. Sustain. Energy Fuels 2019, 3, 744–759. [Google Scholar] [CrossRef]
- Real Guimarães, H.; Marcon Bressanin, J.; Lopes Motta, I.; Ferreira Chagas, M.; Colling Klein, B.; Bonomi, A.; Maciel Filho, R.; Djun Barbosa Watanabe, M. Decentralization of sustainable aviation fuel production in Brazil through Biomass-to-Liquids routes: A techno-economic and environmental evaluation. Energy Convers. Manag. 2023, 276, 116547. [Google Scholar] [CrossRef]
- Sha, C.; Shen, S.; Zhang, J.; Zhou, C.; Lu, X.; Zhang, H. A Review of Strategies and Technologies for Sustainable Decentralized Wastewater Treatment. Water 2024, 16, 3003. [Google Scholar] [CrossRef]
- Shahbeik, H.; Kazemi Shariat Panahi, H.; Dehhaghi, M.; Guillemin, G.J.; Fallahi, A.; Hosseinzadeh-Bandbafha, H.; Amiri, H.; Rehan, M.; Raikwar, D.; Latine, H.; et al. Biomass to biofuels using hydrothermal liquefaction: A comprehensive review. Renew. Sustain. Energy Rev. 2024, 189, 113976. [Google Scholar] [CrossRef]
- Kumar, R. A review on the modelling of hydrothermal liquefaction of biomass and waste feedstocks. Energy Nexus 2022, 5, 100042. [Google Scholar] [CrossRef]
- Kakar, F.L.; Tadesse, F.; Elbeshbishy, E. Comprehensive Review of Hydrothermal Pretreatment Parameters Affecting Fermentation and Anaerobic Digestion of Municipal Sludge. Processes 2022, 10, 2518. [Google Scholar] [CrossRef]
- Cabrera, D.V.; Barria, D.A.; Camu, E.; Celis, C.; Tester, J.W.; Labatut, R.A. Enhancing energy recovery of wastewater treatment plants through hydrothermal liquefaction. Environ. Sci. Water Res. Technol. 2023, 9, 474–488. [Google Scholar] [CrossRef]
- Mousavi, S.; Damizia, M.; Hamidi, R.; De Filippis, P.; de Caprariis, B. Techno-economic assessment of gasoline production from Fe-assisted lignocellulosic biomass hydrothermal liquefaction process with minimized waste stream. Energy Convers. Manag. 2024, 320, 118982. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Tangredi, A.; Barca, C.; Ferrasse, J.-H.; Boutin, O. Effect of process parameters on phosphorus conversion pathways during hydrothermal treatment of sewage sludge: A review. Chem. Eng. J. 2023, 463, 142342. [Google Scholar] [CrossRef]
- Borazjani, Z.; Azin, R.; Osfouri, S. Kinetics studies and performance analysis of algae hydrothermal liquefaction process. Biomass Convers. Biorefinery 2024, 14, 19257–19284. [Google Scholar] [CrossRef]
- Obeid, R.; Lewis, D.M.; Smith, N.; Hall, T.; van Eyk, P. Reaction kinetics and characterisation of species in renewable crude from hydrothermal liquefaction of monomers to represent organic fractions of biomass feedstocks. Chem. Eng. J. 2020, 389, 124397. [Google Scholar] [CrossRef]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M. Optimization of process parameters for bio-crude and value-added chemical recovery through hydrothermal liquefaction of microalgae. Biomass Convers. Biorefinery 2024, 14, 13445–13456. [Google Scholar] [CrossRef]
- Lozano, E.M.; Løkke, S.; Rosendahl, L.A.; Pedersen, T.H. Production of marine biofuels from hydrothermal liquefaction of sewage sludge. Preliminary techno-economic analysis and life-cycle GHG emissions assessment of Dutch case study. Energy Convers. Manag. X 2022, 14, 100178. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Zeng, Y.; Xu, C.C. Development of a global kinetic model based on chemical compositions of lignocellulosic biomass for predicting product yields from hydrothermal liquefaction. Renew. Energy 2023, 215, 118956. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Yang, L. A review on hydrothermal co-liquefaction of biomass. Appl. Energy 2019, 250, 926–945. [Google Scholar] [CrossRef]
- Zhai, Y.; Chen, H.; Xu, B.; Xiang, B.; Chen, Z.; Li, C.; Zeng, G. Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: Yield and composition of bio-oil, immobilization and risk assessment of heavy metals. Bioresour. Technol. 2014, 159, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tatla, H.K.; Ismail, S.; Khan, M.A.; Dhar, B.R.; Gupta, R. Coupling hydrothermal liquefaction and anaerobic digestion for waste biomass valorization: A review in context of circular economy. Chemosphere 2024, 361, 142419. [Google Scholar] [CrossRef]
- Mirizadeh, S.; Nosrati, M.; Shojaosadati, S.A. Synergistic effect of nutrient and salt stress on lipid productivity of Chlorella vulgaris through two-stage cultivation. BioEnergy Res. 2020, 13, 507–517. [Google Scholar] [CrossRef]
- Lay, J.J.; Li, S.H.; Han, C.L.; Zhong, W.Z. Nitrogen and sulfur metabolism in anaerobic hydrogen-producing microbiome as a challenge for 2, 4-DCP dechlorination. J. Environ. Chem. Eng. 2024, 12, 114488. [Google Scholar] [CrossRef]
- Zhou, Y.; Remon, J.; Pang, X.; Jiang, Z.; Liu, H.; Ding, W. Hydrothermal conversion of biomass to fuels, chemicals and materials: A review holistically connecting product properties and marketable applications. Sci. Total Environ. 2023, 886, 163920. [Google Scholar] [CrossRef]
- Gumisiriza, R.; Hawumba, J.F.; Okure, M.; Hensel, O. Biomass waste-to-energy valorisation technologies: A review case for banana processing in Uganda. Biotechnol. Biofuels 2017, 10, 11. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Dhar, B.R. Pretreatment of Municipal Waste Activated Sludge for Enhanced Anaerobic Digestion and Volatile Sulfur Compounds Control; The University of Western Ontario: London, ON, Canada, 2011. [Google Scholar]
- Wu, B.; Berg, S.M.; Remucal, C.K.; Strathmann, T.J. Evolution of N-Containing Compounds during Hydrothermal Liquefaction of Sewage Sludge. ACS Sustain. Chem. Eng. 2020, 8, 18303–18313. [Google Scholar] [CrossRef]
- Sarrion, A.; de la Rubia, A.; Coronella, C.; Mohedano, A.F.; Diaz, E. Acid-mediated hydrothermal treatment of sewage sludge for nutrient recovery. Sci. Total Environ. 2022, 838 Pt 4, 156494. [Google Scholar] [CrossRef]
- SundarRajan, P.; Gopinath, K.P.; Arun, J.; GracePavithra, K.; Adithya Joseph, A.; Manasa, S. Insights into valuing the aqueous phase derived from hydrothermal liquefaction. Renew. Sustain. Energy Rev. 2021, 144, 111019. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, D.; Liu, R.; Ding, Y.; Dai, X. Effects of hydrothermal treatment on organic compositions, structural properties, dewatering and biogas production of raw and digested sludge. Sci. Total Environ. 2022, 848, 157618. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, J.M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Kruse, A.; Gawlik, A. Biomass Conversion in Water at 330−410 °C and 30−50 MPa. Identification of Key Compounds for Indicating Different Chemical Reaction Pathways. Ind. Eng. Chem. Res. 2003, 42, 267–279. [Google Scholar] [CrossRef]
- Savage, P.E. Organic chemical reactions in supercritical water. Chem. Rev. 1999, 99. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, M.; Majidian, N.; Hallajisani, A.; Samipourgiri, M. Production of bio-oil from sewage sludge: A review on the thermal and catalytic conversion by pyrolysis. Sustain. Energy Technol. Assess. 2020, 42, 100870. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Effects of Biomass Particle Size on Slow Pyrolysis Kinetics and Fast Pyrolysis Product Distribution. Waste Biomass Valorization 2017, 9, 465–477. [Google Scholar] [CrossRef]
- Basile, L.; Tugnoli, A.; Stramigioli, C.; Cozzani, V. Influence of pressure on the heat of biomass pyrolysis. Fuel 2014, 137, 277–284. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Hao, S.; Ren, S.; Zhou, N.; Chen, H.; Usman, M.; He, C.; Shi, Q.; Luo, G.; Zhang, S. Molecular composition of hydrothermal liquefaction wastewater from sewage sludge and its transformation during anaerobic digestion. J. Hazard. Mater. 2020, 383, 121163. [Google Scholar] [CrossRef]
- Rajagopal, J.; Gopinath, K.P.; Neha, R.; Aakriti, K.; Jayaraman, R.S.; Arun, J.; Pugazhendhi, A. Processing of household waste via hydrothermal gasification and hydrothermal liquefaction for bio-oil and bio-hydrogen production: Comparison with RSM studies. J. Environ. Chem. Eng. 2022, 10, 107218. [Google Scholar] [CrossRef]
- Zainal, B.S.; Yu, K.L.; Mohamed, H.; Ong, H.C.; Mahlia, T.M.I. Hydrothermal pretreatment: A sustainable approach to biohydrogen production from palm oil mill effluent. Sustain. Energy Technol. Assess. 2024, 72, 104062. [Google Scholar] [CrossRef]
- Amiri, H. ADVANCING SUSTAINABLE RESOURCE UTILISATION: A REVIEW Of AQUATIC BIOREFINERIES. Planet. Sustain. 2024, 2, 26–35. [Google Scholar] [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal Treatment of Biomass Feedstocks for Sustainable Production of Chemicals, Fuels, and Materials: Progress and Perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef]
- Hussin, F.; Hazani, N.N.; Khalil, M.; Aroua, M.K. Environmental life cycle assessment of biomass conversion using hydrothermal technology: A review. Fuel Process. Technol. 2023, 246, 107747. [Google Scholar] [CrossRef]
- Zhou, F.; Hearne, Z.; Li, C.-J. Water—The greenest solvent overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. [Google Scholar] [CrossRef]
- Tushar, M.; DiMaria, P.C.; Al-Salem, S.M.; Dutta, A.; Xu, C.C. Biohydrogen Production by Catalytic Supercritical Water Gasification: A Comparative Study. ACS Omega 2020, 5, 15390–15401. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Sarmah, A.K.; Dubey, B.K. Downstream augmentation of hydrothermal carbonization with anaerobic digestion for integrated biogas and hydrochar production from the organic fraction of municipal solid waste: A circular economy concept. Sci. Total Env. 2020, 706, 135907. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Al-Marri, M.J.; Mahmoud, M.; Shawabkeh, R.; Aparicio, S. CO2 enhanced gas recovery and sequestration in depleted gas reservoirs: A review. J. Pet. Sci. Eng. 2021, 196, 107685. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; He, N.; Zhao, W.; Xiang, H.; Gupta, M.K.; Iqbal, A.; Khan, A.M. Assessment of energy consumption, carbon emissions and cost metrics under hybrid MQL-Dry ice blasting system: A novel cleaner production technology for manufacturing sectors. J. Clean. Prod. 2022, 360, 132111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).