Experimental Study on the Activation Energy of Coal Oxidation Under Different Oxygen Concentrations
Abstract
1. Introduction
2. Coal Sample Preparation and Experimental Setup
2.1. Coal Sample Preparation
2.2. Experimental Apparatus
3. Results and Discussion
3.1. Analysis of Stage Characteristics
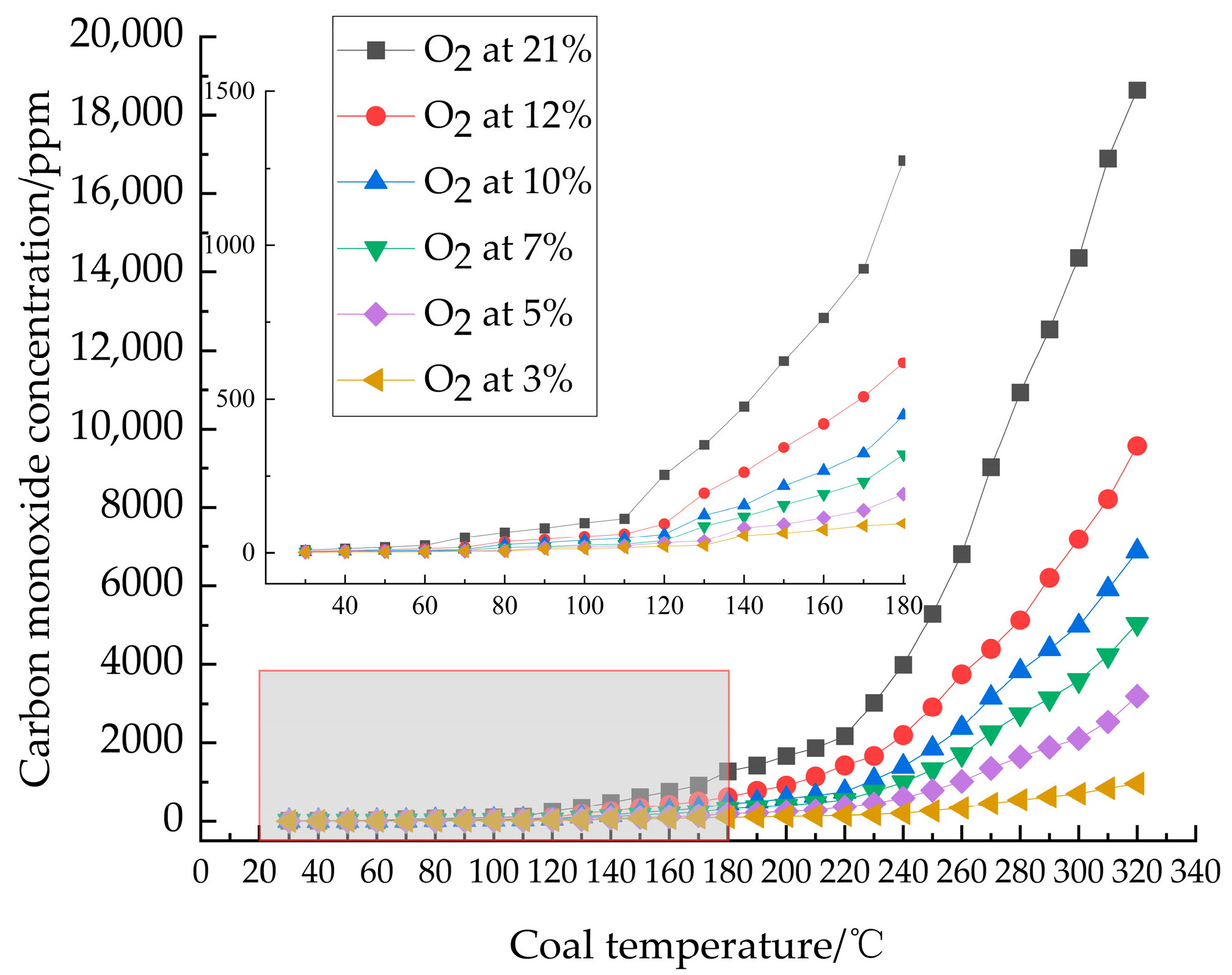
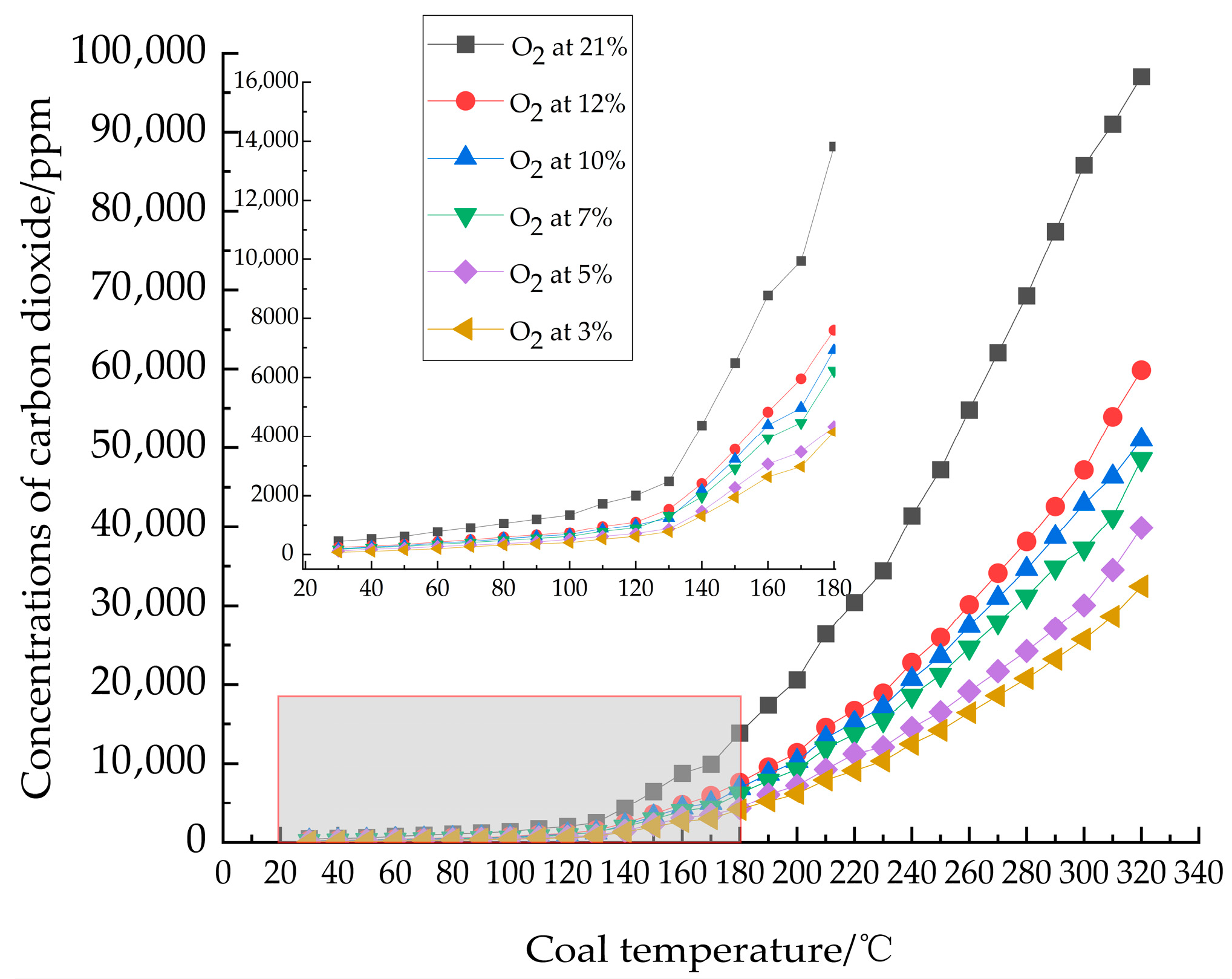
3.1.1. Analysis of Carbon Oxides
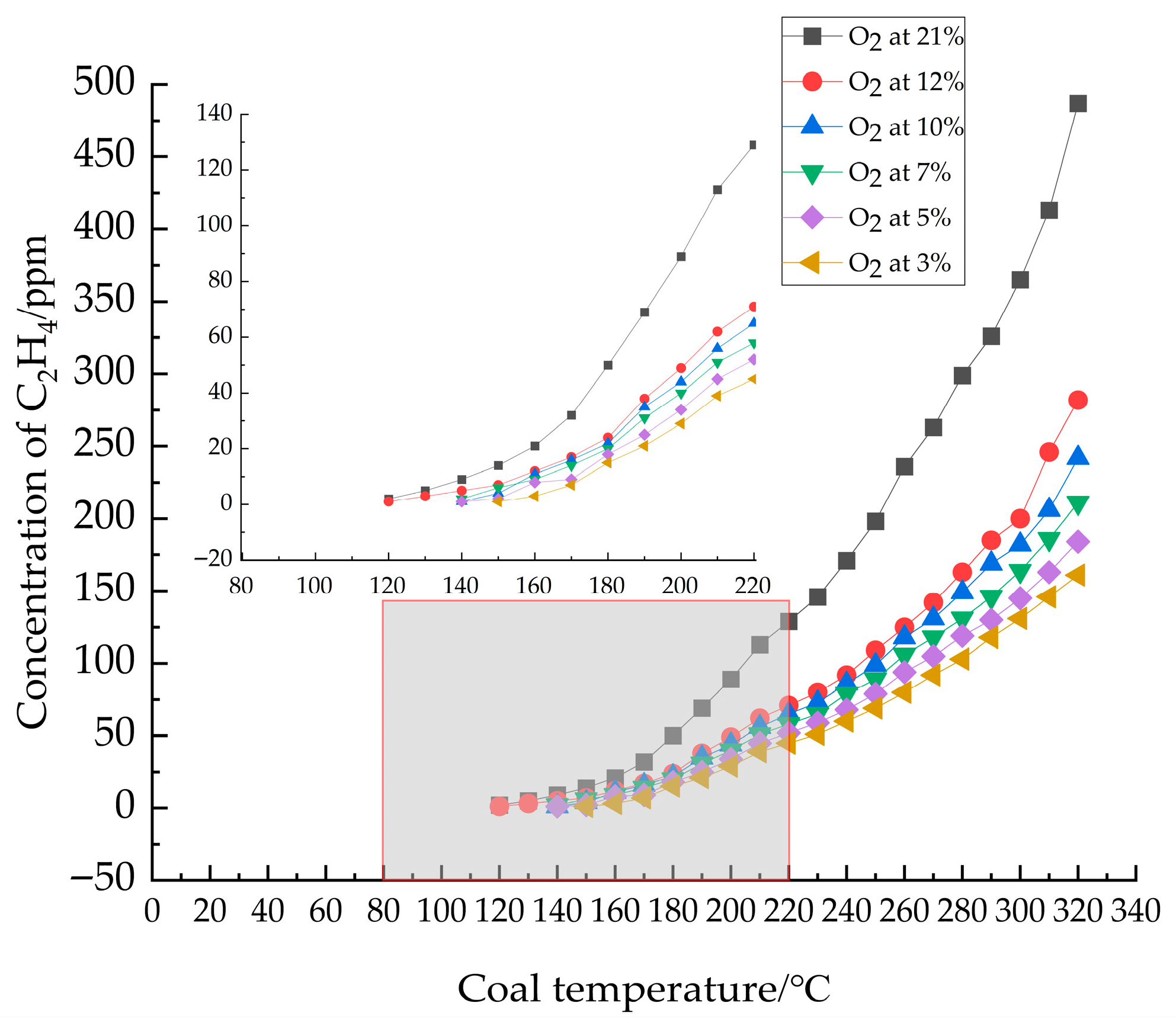
3.1.2. Analysis of C2H4
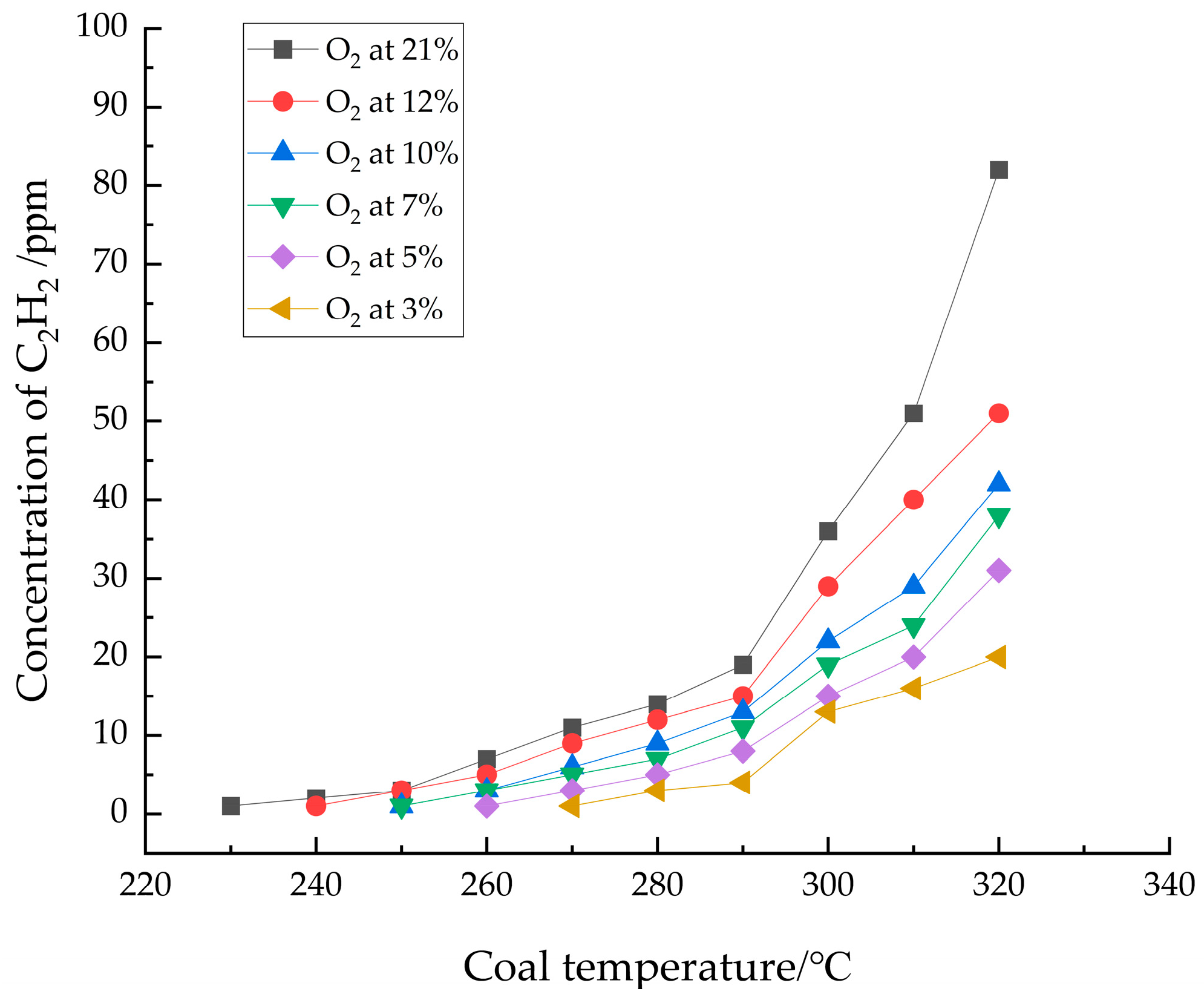
3.1.3. Analysis of C2H2
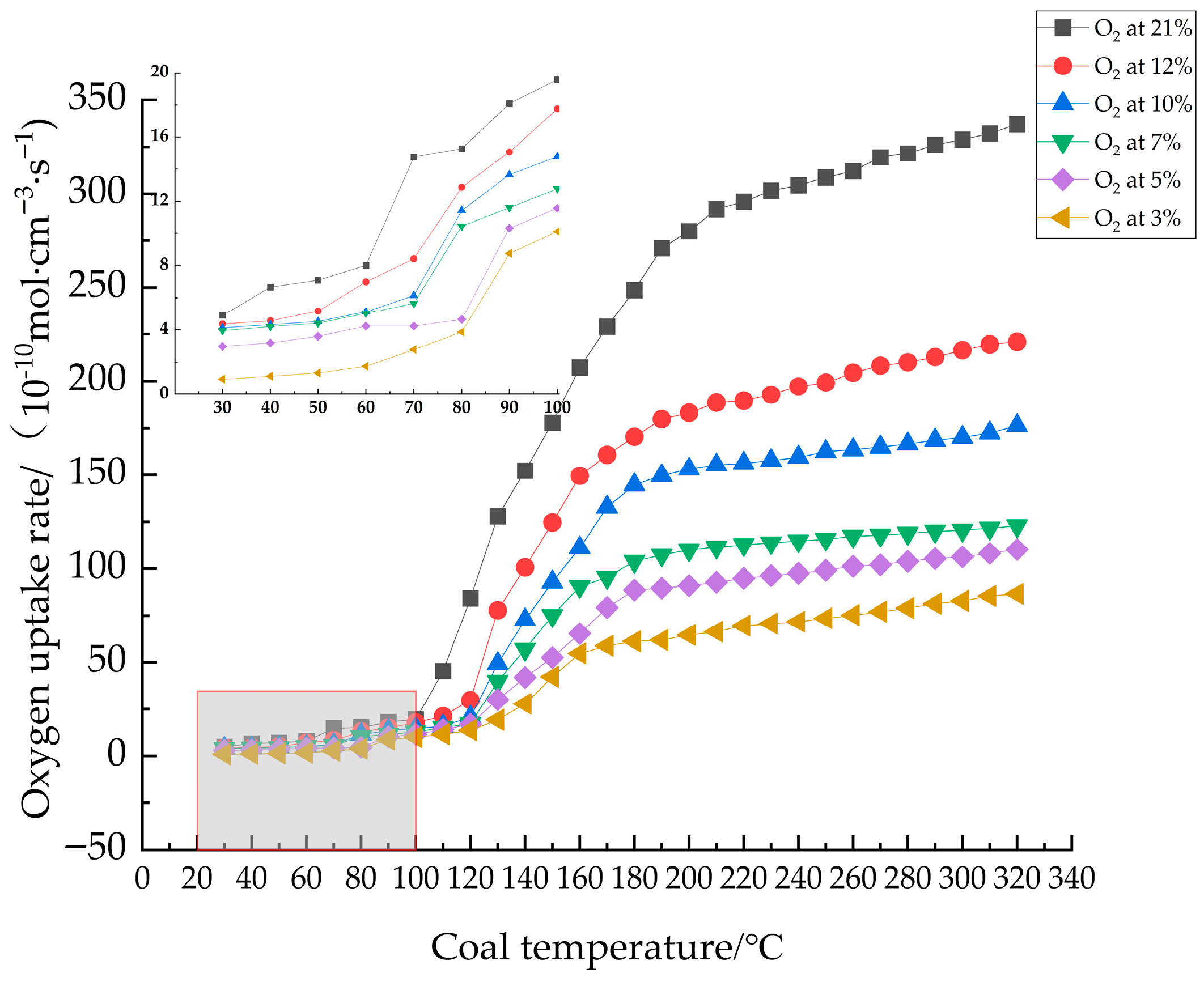
3.1.4. Oxygen Consumption Rate Analysis
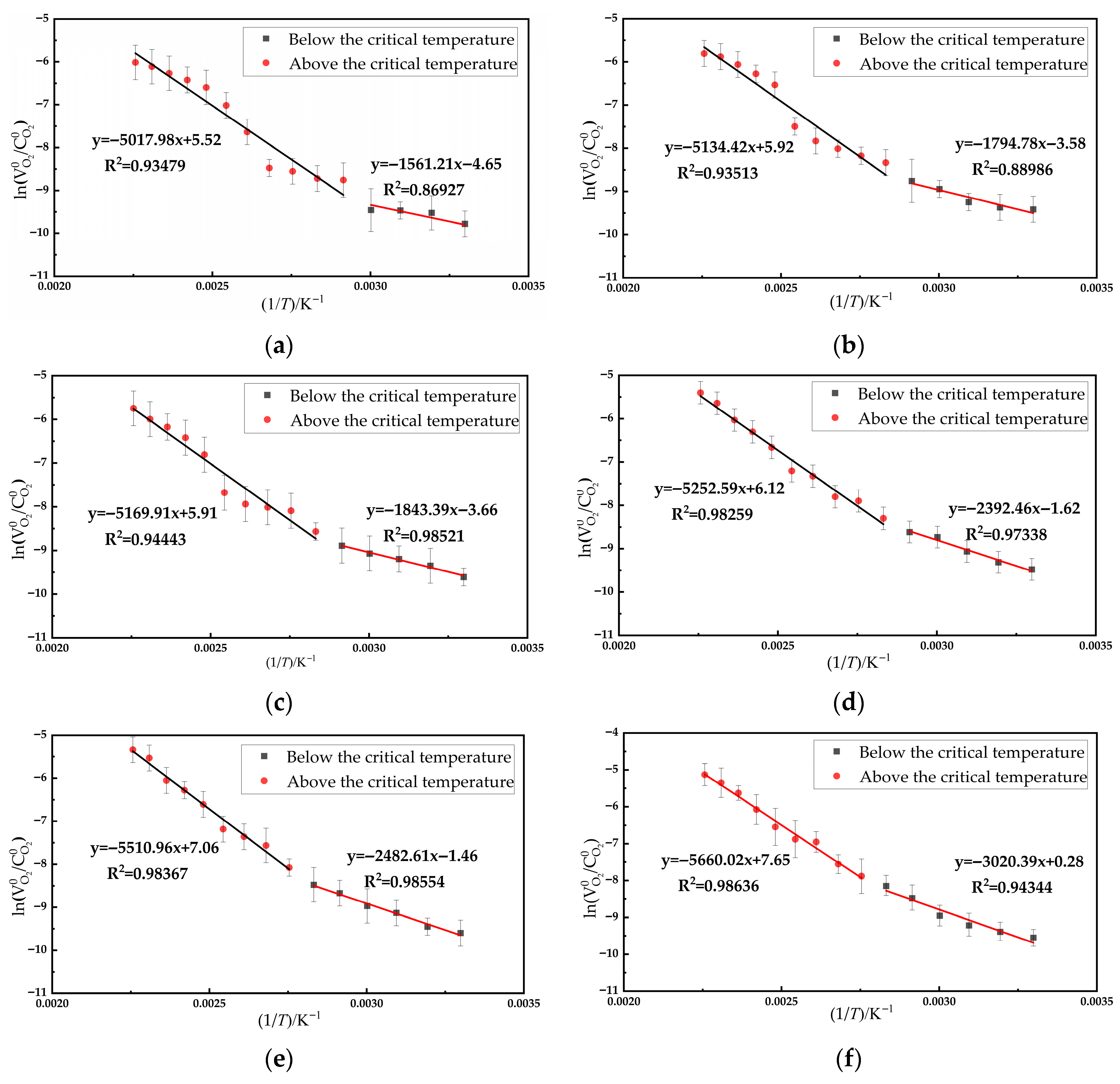
3.2. Activation Energy Calculation
4. Conclusions
- (1)
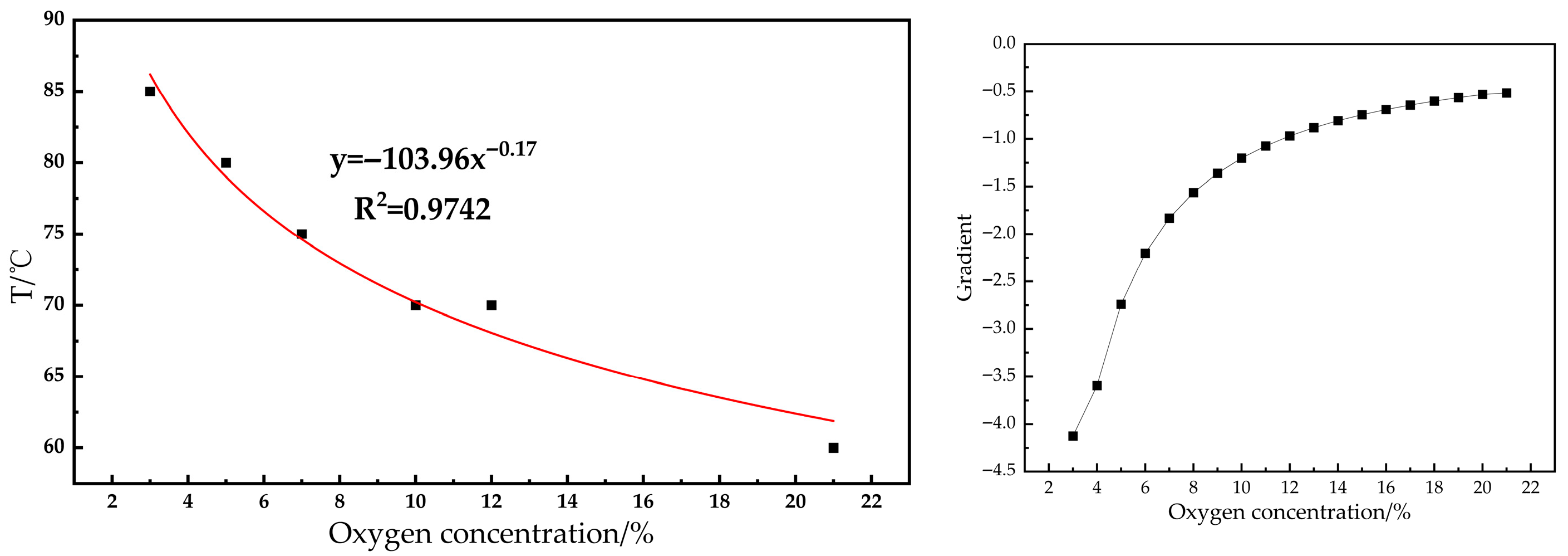
- Analysis of indicator gas release patterns revealed that CO and C2H4 release trends largely paralleled the increase in oxygen consumption rate, both escalating with temperature. Critical temperatures for coal samples under varying oxygen concentrations were determined: 60 °C at 21% O2, 70 °C at 12%, 10%, and 7% O2, and 80 °C at 5% and 3% O2. These critical temperatures served as the basis for delineating changes in activation energy.
- (2)
- Following the phase division, the kinetic analysis demonstrates a high degree of equation fitting, which effectively reflects the relationship between ln( /) and 1/T. Below the critical temperature, the coal samples exhibit an increase in activation energy with decreasing O2 concentration. Above the critical temperature, the coal samples also show an increase in activation energy with decreasing O2 concentration.
- (3)
- A significant disparity in activation energy of coal samples is observed across the critical temperature threshold, with the low-temperature phase consistently exhibiting lower activation energy than the high-temperature phase. In practical mining operations, preventive measures must be promptly implemented when oxygen concentration in goaf areas exceeds 6% to mitigate the risk of spontaneous coal combustion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, B.D.; Teng, X.Y.; Zhang, F.; Pan, Y.; Men, D.P.; Han, Y.J. Analysis and projections on China’s energy landscape in 2025 and the timing of peak coal consumption. China Coal 2025, 51, 33–41. [Google Scholar] [CrossRef]
- China Coal Research Institute (CCRI); Song, M. The Research Group on the Coal Industry Prosperity Index of China Minmetals (Beijing). Research report on the economic situation of China’s coal industry from 2024 to 2025. China Coal 2025, 51, 1–12. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wang, K.; Wang, W.F.; Yan, J.; Tang, Z.Q.; Kang, F.R.; Ren, S.J. Research progress and prospect of mine fire intelligent monitoring and early warning technology in recent 20 years. Coal Sci. Technol. 2024, 52, 154–177. [Google Scholar] [CrossRef]
- Moni, V.; Klouda, P.; Blata, J.; Helebrant, F. The application for a prediction of the coal spontaneous ignition-PREDISAM. Manag. Syst. Prod. Eng. 2017, 25, 81–87. [Google Scholar] [CrossRef]
- Qin, B.T.; Zhong, X.X.; Wang, D.M.; Xin, H.H.; Shi, Q.L. Research progress of coal spontaneous combustion process characteristics and prevention technology. Coal Sci. Technol. 2021, 49, 66–99. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Dai, F.; Zhang, X. Research on the Intrinsic correlation mechanism between stage oxidation characteristics and spontaneous combustion tendency of coals with different metamorphic degrees. Chem. Eng. Sci. 2025, 314, 121800. [Google Scholar] [CrossRef]
- Vikram, M.; Bhattacharjee, R.; Paul, P. Determination of spontaneous combustion propensity and ignition time of Indian coal using adiabatic oxidation method. Fuel 2025, 388, 134569. [Google Scholar] [CrossRef]
- Stuhlman, S.; Kumar, K. Activation energies and evolved gas analysis for Argonne premium coals. Int. J. Coal Prep. Util. 2024, 44, 51–66. [Google Scholar] [CrossRef]
- Wang, D.M.; Xin, H.H.; Qi, X.Y.; Dou, G.L.; Qi, G.S.; Ma, L.Y. Reaction pathway of coal oxidation at low temperatures: A model of cyclic chain reactions and kinetic characteristics. Combust. Flame 2016, 163, 447–460. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, H.; Wang, H.; Wang, G.; Bu, Y.; Liu, Y.; Zhou, C.; Sun, L. Study on the effect of the liquid nitrogen freeze-thaw cycle on the coal spontaneous combustion propensity. Appl. Therm. Eng. 2025, 264, 125386. [Google Scholar] [CrossRef]
- Liu, B.X.; Ma, S.Q.; Zhang, C.; Zhu, J.F. Study on the influence of particle size and heating rate on characteristic temperature and activation energy of coal oxidation. Saf. Coal Min. 2023, 54, 77–83. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, X.; Ma, C.; Luo, Z.; Li, Q.; Deng, J.; Sheng, Y.; Peng, B. Comparative study of the kinetic characteristics of coal spontaneous combustion. J. Therm. Anal. Calorim. 2023, 148, 4463–4476. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Zhang, Y.; Zhang, Y.; Ma, Q. Numerical Simulation Study on Coal Spontaneous Combustion: Effect of Porosity Distribution. Combust. Sci. Technol. 2023, 195, 472–493. [Google Scholar]
- Wang, H.; Li, J.; Dong, Z.; Fan, C.; Zhang, Y.; Chen, X. Effect of thermal damage on the pore–fracture system during coal spontaneous combustion. Fuel 2023, 339, 127439. [Google Scholar] [CrossRef]
- Li, J.L.; Xu, Z.; Zhao, Z.; Xu, S. Study on coal’s spontaneous combustion propensity based on the correlation between oxygen consumption and heat generation. Combust. Sci. Technol. 2023, 195, 2258–2273. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Wang, H.Y.; Song, Z.Y.; He, C.N. The relationship between oxidation kinetics characteristic parameters of coal adiabatic progress and metamorphic degree. J. China Coal Soc. 2014, 39, 498–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Li, Y.; Liu, Y. Characteristics of carbon monoxide production and oxidation kinetics during the decaying process of coal spontaneous combustion. Can. J. Chem. Eng. 2018, 96, 1752–1761. [Google Scholar] [CrossRef]
- Chen, X.; Ma, T.; Zhai, X.; Lei, C. Thermogravimetric and infrared spectroscopic study of bituminous coal spontaneous combustion to analyze combustion reaction kinetics. Thermochim. Acta 2019, 676, 84–93. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, Y.; Li, Q.; Ma, T. Research on spontaneous combustion tendency of bituminous coals in different metamorphic grades based on activation energy index. Min. Safety Environ. Prot. 2016, 43, 5–7. Available online: http://www.cnki.net/kcms/detail/50.1062.TD.20160202.1905.004.html (accessed on 5 September 2025). [CrossRef]
- Fetisova, O.Y.; Kuznetsov, P.; Purevsuren, B.; Avid, B. A kinetic study of the stepwise thermal decomposition of various coals from Mongolia. Solid. Fuel Chem. 2021, 55, 1–7. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.X.; Zhao, J.Y.; Song, J.J.; Zhang, Y.N. Experiment study on oxidation and activated energy of different partical size coal based on programmed temperature rising. Coal Sci. Technol. 2019, 47, 214–219. [Google Scholar] [CrossRef]
- Song, Y. Characteristics of generating carbon oxides at low-temperature oxidation stages of low-rank coal. Shock. Vib. 2022, 2022, 9380297. [Google Scholar] [CrossRef]
- Qu, L. A study on the prediction method of coal spontaneous combustion development period based on critical temperature. Environ. Sci. Pollut. Res. 2018, 25, 35748–35760. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Song, D.; Tan, B. Research on the critical temperature and stage characteristics for the spontaneous combustion of different metamorphic degrees of coal. Int. J. Coal Prep. Util. 2018, 38, 221–236. [Google Scholar] [CrossRef]
- Yan, H.; Nie, B.; Liu, P.; Chen, Z.; Yin, F.; Gong, J.; Lin, S.; Wang, X.; Kong, F.; Hou, Y. Experimental assessment of multi-parameter index gas correlation and prediction system for coal spontaneous combustion. Combust. Flame 2023, 247, 112485. [Google Scholar] [CrossRef]
- Deng, J.; Liu, L.; Lei, C.; Wang, C.; Xiao, Y. Spatiotemporal distributions of the temperature and index gases during the dynamic evolution of coal spontaneous combustion. Combust. Sci. Technol. 2021, 193, 1679–1695. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Xue, S.; Wu, J.; Tang, Y.; Chang, L. Assessment of spontaneous combustion status of coal based on relationships between oxygen consumption and gaseous product emissions. Fuel Process. Technol. 2018, 179, 60–71. [Google Scholar] [CrossRef]
- Qin, Y.; Chu, C.; Yan, L.; Xu, Y.; Guo, W.; Fan, Q.; He, J.; Li, F. Verification of the oxygen consumption rate of the remaining coal in the gob by research and experiment. Energy Sources Part A 2024, 46, 773–788. [Google Scholar] [CrossRef]
- Jia, X.; Wu, J.; Lian, C.; Rao, J. Assessment of coal spontaneous combustion index gas under different oxygen concentration environment: An experimental study. Environ. Sci. Pollut. Res. 2022, 29, 87257–87267. [Google Scholar] [CrossRef]
- Su, H.; Ji, H.; Chen, X. Model simplification of coal combustion kinetics: A case study of Weihuliang coal in Urumchi, China. Combust. Theor. Model. 2019, 23, 1071–1089. [Google Scholar] [CrossRef]
- Arisoy, A.; Beamish, B. Reaction kinetics of coal oxidation at low temperatures. Fuel 2015, 159, 412–417. [Google Scholar] [CrossRef]
- Deng, J.; Li, Q.; Xiao, Y.; Wen, H. The effect of oxygen concentration on the non-isothermal combustion of coal. Thermochim. Acta 2017, 653, 106–115. [Google Scholar] [CrossRef]
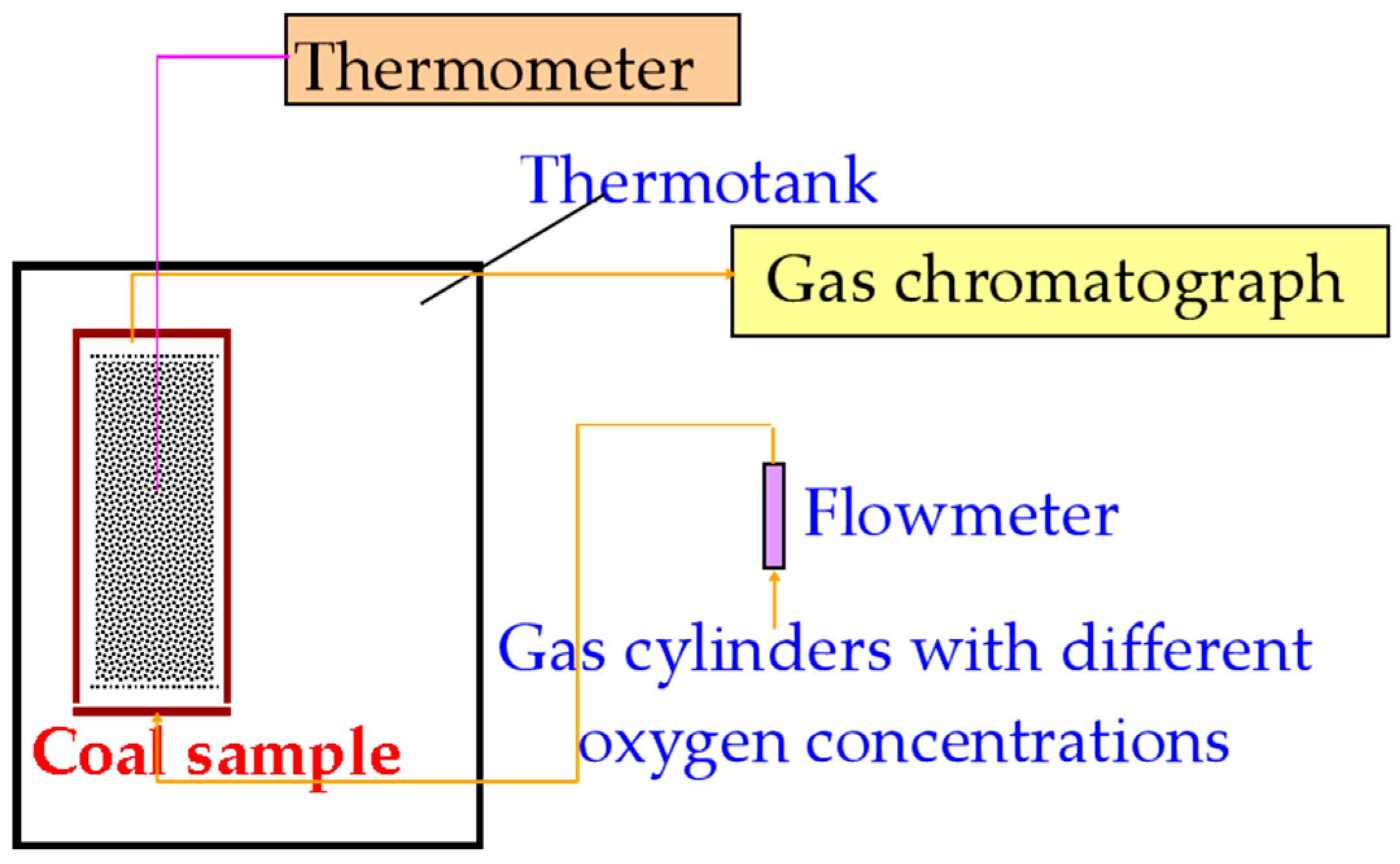
| Coal Sample | Aad (%) | Vad (%) | Mad (%) | FCad (%) | Oad (%) | St (%) | Q (MJ/kg) |
|---|---|---|---|---|---|---|---|
| Coal seam No. 1 | 9.8 | 32.5 | 6.2 | 51.5 | 9.3% | 0.85 | 27 |
| Serial Number | Granularity (mm) | Average Particle Size (mm) | High Test Tube Coal (cm) | Coal Weight (g) | Coal Volume (cm3) | Bulk Density (g/cm3) | Heating Rate (°C/min) |
|---|---|---|---|---|---|---|---|
| 1 | Mixed coal sample | 4.17 ± 0.01 | 13.35 | 500 | 1048 | 0.48 | 0.3 |
| 2 | Mixed coal sample | 4.17 ± 0.01 | 13.25 | 500 | 1039 | 0.48 | 0.3 |
| 3 | Mixed coal sample | 4.17 ± 0.01 | 13.34 | 500 | 1048 | 0.48 | 0.3 |
| 4 | Mixed coal sample | 4.17 ± 0.01 | 13.38 | 500 | 1050 | 0.48 | 0.3 |
| 5 | Mixed coal sample | 4.17 ± 0.01 | 13.25 | 500 | 1039 | 0.48 | 0.3 |
| 6 | Mixed coal sample | 4.17 ± 0.01 | 13.30 | 500 | 1044 | 0.48 | 0.3 |
| Oxygen Concentration/% | Critical Temperature/°C | E1/(kJ·mol−1) | E2/(kJ·mol−1) |
|---|---|---|---|
| 21 | 60 | 12.9799 | 41.7194 |
| 12 | 70 | 14.9218 | 42.6875 |
| 10 | 70 | 15.3259 | 42.9826 |
| 7 | 70 | 19.8909 | 43.6700 |
| 5 | 80 | 20.6404 | 45.8181 |
| 3 | 80 | 25.1115 | 47.0574 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Hui, J.; Cheng, X.; Zhang, L.; Li, Y.; Li, C.; Qi, C. Experimental Study on the Activation Energy of Coal Oxidation Under Different Oxygen Concentrations. Processes 2025, 13, 2889. https://doi.org/10.3390/pr13092889
Liu W, Hui J, Cheng X, Zhang L, Li Y, Li C, Qi C. Experimental Study on the Activation Energy of Coal Oxidation Under Different Oxygen Concentrations. Processes. 2025; 13(9):2889. https://doi.org/10.3390/pr13092889
Chicago/Turabian StyleLiu, Wenyong, Jing Hui, Xiaojiao Cheng, Lei Zhang, Yixin Li, Changsheng Li, and Chenyang Qi. 2025. "Experimental Study on the Activation Energy of Coal Oxidation Under Different Oxygen Concentrations" Processes 13, no. 9: 2889. https://doi.org/10.3390/pr13092889
APA StyleLiu, W., Hui, J., Cheng, X., Zhang, L., Li, Y., Li, C., & Qi, C. (2025). Experimental Study on the Activation Energy of Coal Oxidation Under Different Oxygen Concentrations. Processes, 13(9), 2889. https://doi.org/10.3390/pr13092889





