Innovative Ultrasound-Assisted Extraction for Phenolic and Antioxidant Evaluation of Brazilian Green Propolis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of Ultrasound-Assisted Extraction (UAE)
2.3. Box–Behnken Experimental Design (BBD) and Response Surface Methodology (RSM)
2.4. UHPLC-DAD Analysis
2.5. Statistical Analysis
3. Results and Discussion
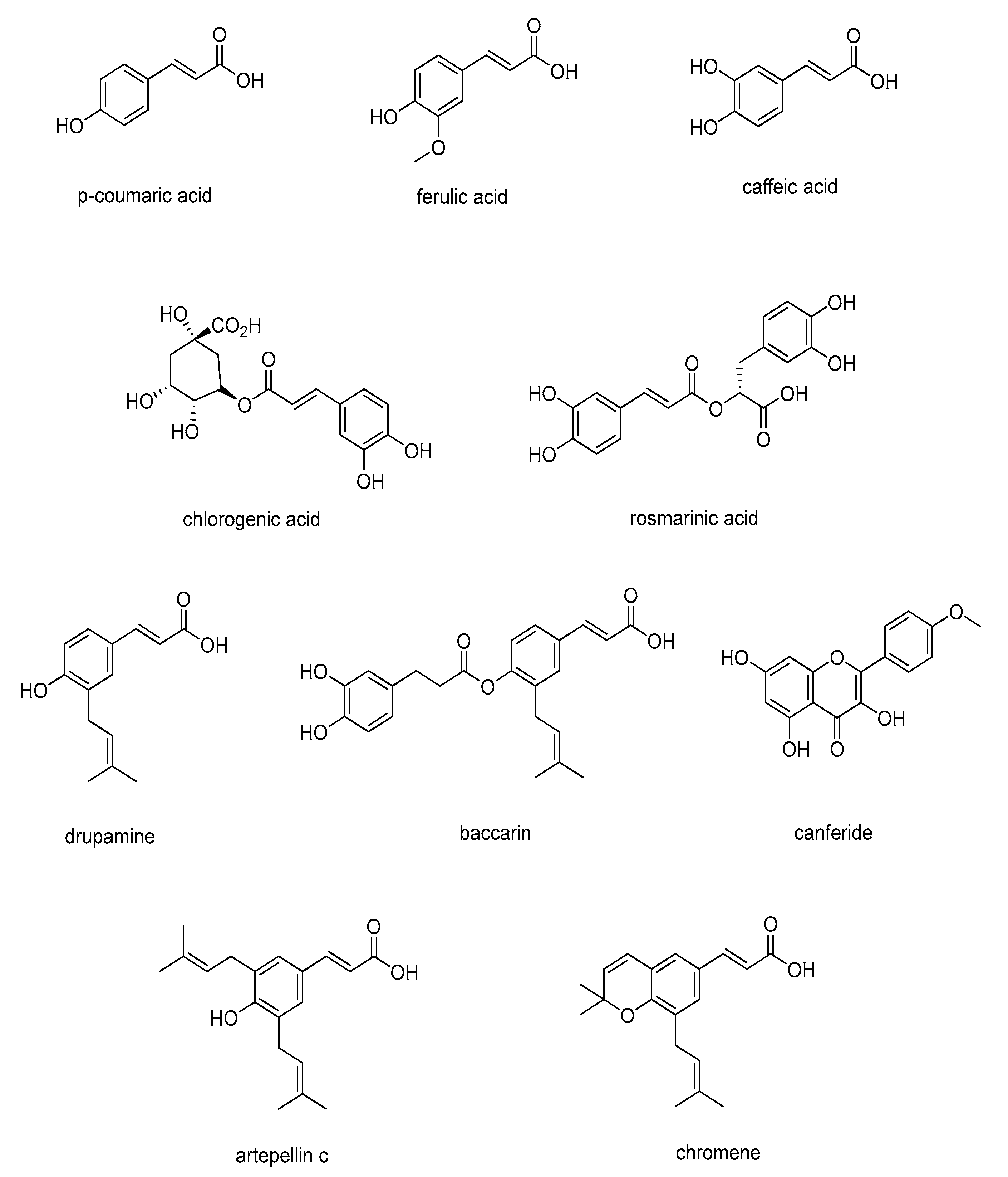
3.1. Identification of Compounds
3.2. Univariate Study of Optimal Range of Methanol/Ethanol Percentage in Water
3.3. Univariate Study of Optimal Range of Temperature
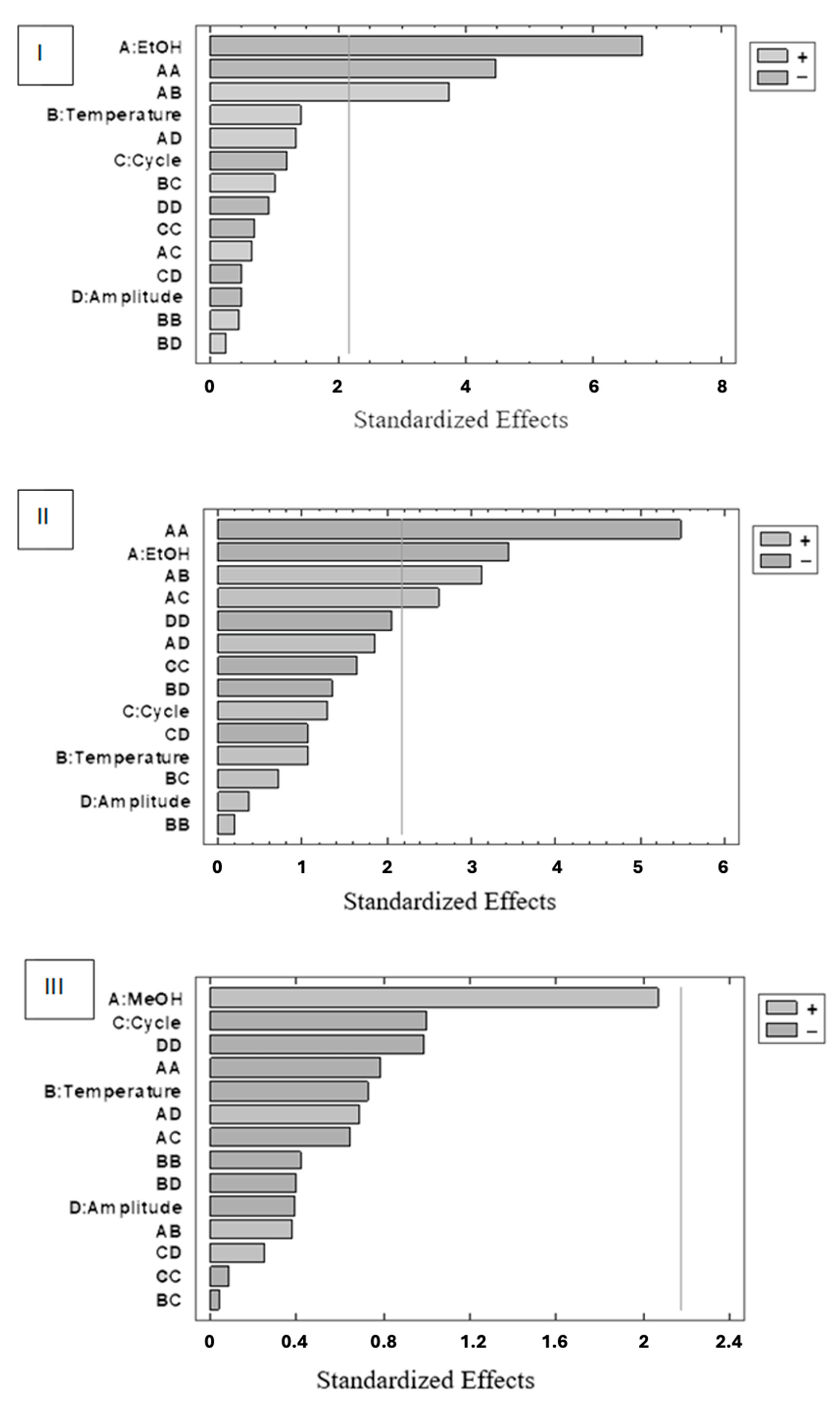
3.4. Optimizing Ultrasound-Assisted Extraction (UAE)
0.145865*AC + 0.298932*AD + 0.0853767*B^2 + 0.223795*BC + 0.0548125*BD − 0.133105*C^2 − 0.110412*CD
− 0.176655*D^2
0.754325*AC + 0.5327*AD + 0.0510083*B^2 + 0.205125*BC − 0.387025*BD − 0.412967*C^2 − 0.307325*CD −
0.513704*D^2
− 0.530667*AC + 0.567187*AD − 0.29868*B^2 − 0.0337332*BC − 0.325636*BD − 0.0611652*C^2 + 0.206203*CD
− 0.700847*D^2
3.4.1. Optimizing the Extraction Time
3.4.2. Precision Evaluation of the Optimized UAE Method
3.5. Chemical Compounds Identified and Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Armstrong, L.; Raeski, P.A.; Almeida, V.P.; Minteguiaga, M.; Raman, A.N.V.; Junior, A.G.; Naman, B.; Manfron, J. Baccharis dracunculifolia DC. A Review of Research Advances From 2004 to 2024, With New Micromorphology and Essential Oil Investigations. J. Herb. Med. 2024, 48, 100952. [Google Scholar] [CrossRef]
- Risoto, M.; Facanali, R.; Haber, L.L.; Vieira, M.A.R.; Isobe, M.T.C.; Cavallari, M.M.; Bajay, M.M.; Zucchi, M.I.; Pinheiro, J.B.; Marques, M.O.M. Chemical and genetic diversity of Baccharis dracunculifolia DC. (Asteraceae) from the Cerrado biome. Biochem. Syst. Ecol. 2023, 111, 104735. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, F.B.; Castro, R.N. Comparação entre a composição química e capacidade antioxidante de diferentes extratos de própolis verde. Quim. Nova 2016, 39, 1192–1199. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Balderrama-Carmona, A.P.; Umsza-Guez, M.A.; Aparecida, B. Antiviral effects of Brazilian green and red propolis extracts on Enterovirus surrogates. Environ. Sci. Pollut. Res. 2020, 27, 28510–28517. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; Portapilla, G.B.; Silva, G.M.; Duarte, A.; Rotta, C.G.; Henrique, C.; Albuquerque, S.; Bastos, J.K.; Campo, V.L. Synthesis, antitumor activity and in silico analyses of amino acid derivatives of artepillin C, drupanin and baccharin from green propolis. Bioorg. Med. Chem. 2021, 47, 116372. [Google Scholar] [CrossRef]
- Andriana, Y.; Abdurrahman, M.F.; Haryanti, P.; Pangestuti, R.; Kurnianto, D.; Sefrienda, A.R.; Apriyati, E.; Wungkana, J.; Indriati, A.; Litaay, C. Optimization of ultrasonic-assisted extraction (UAE) for phenolics and antioxidant activity from cocoa (Theobroma cacao) leaves and phytochemical profiling using GC-MS and LC-HRMS. Biocatal. Agric. Biotechnol. 2025, 65, 103557. [Google Scholar] [CrossRef]
- Setyani, W.; Murwanti, R.; Sulaiman, T.N.S.; Hertiani, T. Application of Response Surface Methodology (RSM) for the Optimization of Ultrasound-Assisted Extraction (UAE) of Moringa oleifera: Extraction Yield, Content of Bioactive Compounds, and Biological Effects In Vitro. Plants 2023, 12, 2455. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, I.T.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Optimization of microwave-assisted extraction of phenolic compounds from chestnut processing waste using response surface methodology. J. Clean. Prod. 2023, 395, 136452. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.V.D.; Freires, S.C.V.; Palheta, H.C.D.O.; Ferreira, P.H.d.M. Impact of Use of Ultrasound-Assisted Extraction on the Quality of Brazil Nut Oil (Bertholletia excelsa HBK). Separations 2025, 12, 182. [Google Scholar] [CrossRef]
- Cardoso, R.V.; da Silva, D.V.T.; Santos-Sodré, S.J.L.; Pereira, P.R.; Freitas, C.S.; Moterle, D.; Kanis, L.A.; Silva, L.H.M.D.; Rodrigues, A.M.D.C.; Paschoalin, V.M.F. Green Ultrasound-Assisted Extraction of Bioactive Compounds from Cumari-Do-Pará Peppers (Capsicum chinense Jacq.) Employing Vegetable Oils as Solvents. Foods 2024, 13, 2765. [Google Scholar] [CrossRef]
- Aiello, G.; Xu, R.; Pugliese, R.; Bartolomei, M.; Li, J.; Bollati, C.; Rueller, L.; Robert, J.; Arnoldi, A.; Lammi, C. Quality Assessment of the Protein Ingredients Recovered by Ultrasound-Assisted Extraction from the Press Cakes of Coconut and Almond Beverage Preparation. Foods 2022, 11, 3693. [Google Scholar] [CrossRef]
- Linares, G.; Rojas, M.L. Ultrasound-Assisted Extraction of Natural Pigments From Food Processing By-Products: A Review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef]
- Teixeira, B.V.; Vidigal, M.C.T.R.; Stringheta, P.C. Optimization of ultrasound-assisted extraction of anthocyanins from purple tomatoes. Cienc. Rur. 2024, 54, e20220604. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, D.-P.; Lin, S.-J.; Li, Y.; Zheng, J.; Zhou, Y.; Zhang, J.-J.; Li, H.-B. Ultrasound-Assisted Extraction and Identification of Natural Antioxidants from the Fruit of Melastoma sanguineum Sims. Molecules 2017, 22, 306. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Kingwascharapong, P.; Mazumder, A.R.; Rawdkuen, S. Optimization of extraction of phenolic compounds and antioxidants from passion fruit and rambutan seeds using response surface methodology. J. Agric. Food Res. 2023, 14, 100888. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; López-Castillo, J.G.; Palma, M.; Barbero, G.F.; Carrera, C. Ultrasound-Assisted Extraction of Total Phenolic Compounds and Antioxidant Activity in Mushrooms. Agronomy 2022, 12, 1812. [Google Scholar] [CrossRef]
- Gunalan, S.; Thangaiah, A.; Rathnasamy, V.K.; Janaki, J.G.; Thiyagarajan, A.; Kuppusamy, S.; Arunachalam, L. Microwave-assisted extraction of biomolecules from moringa (Moringa oleifera Lam.) leaves var. PKM 1: An optimization study by response surface methodology (RSM). Kuwait J. Sci. 2023, 50, 339–344. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M.; Barroso, C.G. Multiresponse optimization of a UPLC method for the simultaneous determination of tryptophan and 15 tryptophan-derived compounds using a Box-Behnken design with a desirability function. Food Chem. 2017, 225, 1–9. [Google Scholar] [CrossRef]
- Kumar, K.; Shivmurti, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Chacon, W.D.C.; Sorita, G.D.; Monteiro, A.R.; Verruck, S.; Montero, L.; Cifuentes, A.; Álvarez-Rivera, G.; Ayala Valencia, G. Brazilian green propolis-loaded starch nanoparticles: Influence of extraction conditions on bioactivity and kinetic stability under thermal and pH stress. Int. J. Biol. Macromol. 2025, 322, 146861. [Google Scholar] [CrossRef] [PubMed]
- Kuvendziev, S.; Dimitrievska, I.; Stojchevski, M.; Marinkovski, M. ANN modeling and RSM optimization of ultrasound-assisted extraction of protodioscin-rich extracts from Tribulus terrestris L. Ultrason. Sonochem. 2024, 111, 107141. [Google Scholar] [CrossRef]
- Loureiro, R.P.; Castro, T.L.A.; Cardoso, C.A.L. Optimization of the extraction of bioactive compounds from the leaves of Campomanesia sessiliflora (O. Berg) Mattos: Standardizing the utilization of a new resource. J. Appl. Res. Med. Aromat. Plants 2025, 44, 100615. [Google Scholar] [CrossRef]
- Cabral, I.S.R.; Oldoni, T.L.C.; Alencar, S.M.; Rosalen, P.L.; Ikegaki, M. The correlation between the phenolic composition and biological activities of two varieties of Brazilian propolis (G6 and G12). Braz. J. Pharm. Sci. 2012, 48, 557. [Google Scholar] [CrossRef]
- Sun, S.; Liu, M.; He, J.; Li, K.; Zhang, X.; Yin, G. Identification and Determination of Seven Phenolic Acids in Brazilian Green Propolis by UPLC-ESI-QTOF-MS and HPLC. Molecules 2019, 24, 1791. [Google Scholar] [CrossRef]
- Nakajima, Y.; Tsuruma, K.; Shimazawa, M.; Satoshi, M.; Hideaki, H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement. Altern. Med. 2009, 9, 4. [Google Scholar] [CrossRef]
- Messerli, S.M.; Ahn, M.R.; Kunimasa, K.; Yanagihara, M.; Tatefuji, T.; Hashimoto, K.; Mautner, V.; Uto, Y.; Hori, H.; Kumazawa, S.; et al. Artepillin C (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytother. Res. 2009, 23, 423. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Hoshi, M.; Matsunaga, T.; Inoue, T.; Ichihara, K.; Ikari, A. Autophagy inhibition enhances anticancer efficacy of artepillin C, a cinnamic acid derivative in Brazilian green propolis. Biochem. Biophys. Res. Commun. 2018, 26, 437. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Ribeiro, P.V.; Almeida, M.O.; Mejía, J.A.A.; Casoti, R.; Kenupp, J.B. Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green propolis. J. Pharm. Biomed. Anal. 2020, 30, 112922. [Google Scholar] [CrossRef] [PubMed]
| Factor | −1 | 0 | +1 | Units |
|---|---|---|---|---|
| A: % Ethanol or Methanol/water | 50 | 75 | 100 | % |
| B: Temperature | 20 | 40 | 60 | °C |
| C: Cycle | 0.2 | 0.6 | 1.0 | s−1 |
| D: Amplitude (W) | 20 | 40 | 60 | % |
| Experiment | A | B | C | D | MeOHTP | EtOHSP | EtOHCP |
|---|---|---|---|---|---|---|---|
| 1 | 0 | −1 | 1 | 0 | 21.65 | 4.99 | 16.19 |
| 2 | 0 | 1 | 0 | −1 | 22.25 | 5.44 | 16.22 |
| 3 | −1 | −1 | 0 | 0 | 21.56 | 5.86 | 15.79 |
| 4 | 1 | 0 | 0 | 1 | 22.31 | 3.88 | 14.91 |
| 5 | 0 | −1 | 0 | −1 | 22.38 | 5.84 | 15.57 |
| 6 | 0 | 1 | 1 | 0 | 22.23 | 5.83 | 16.80 |
| 7 | 0 | 0 | 1 | 1 | 22.26 | 5.13 | 15.58 |
| 8 | 1 | 1 | 0 | 0 | 21.33 | 5.99 | 16.73 |
| 9 | −1 | 0 | 0 | −1 | 21.11 | 5.93 | 15.89 |
| 10 | −1 | 0 | −1 | 0 | 22.59 | 6.19 | 16.37 |
| 11 | 1 | 0 | 0 | −1 | 21.12 | 3.31 | 13.84 |
| 12 | 0 | 1 | 0 | 1 | 20.81 | 5.56 | 15.87 |
| 13 | −1 | 1 | 0 | 0 | 18.99 | 5.18 | 14.97 |
| 14 | 0 | −1 | −1 | 0 | 21.78 | 5.82 | 16.39 |
| 15 | 0 | 0 | −1 | 1 | 19.81 | 5.63 | 15.86 |
| 16 | 1 | 0 | −1 | 0 | 26.70 | 3.55 | 12.44 |
| 17 | −1 | 0 | 0 | 1 | 20.02 | 5.30 | 14.83 |
| 18 | 1 | −1 | 0 | 0 | 22.65 | 3.33 | 13.95 |
| 19 | 0 | 1 | −1 | 0 | 22.50 | 5.77 | 16.18 |
| 20 | 1 | 0 | 1 | 0 | 20.96 | 3.58 | 14.71 |
| 21 | 0 | 0 | 1 | −1 | 22.24 | 5.72 | 16.26 |
| 22 | 0 | −1 | 0 | 1 | 22.25 | 5.75 | 16.76 |
| 23 | 0 | 0 | −1 | −1 | 20.61 | 5.77 | 15.31 |
| 24 | −1 | 0 | 1 | 0 | 18.96 | 5.63 | 15.62 |
| 25 | 0 | 0 | 0 | 0 | 22.39 | 5.69 | 16.56 |
| 26 | 0 | 0 | 0 | 0 | 22.37 | 5.91 | 16.68 |
| 27 | 0 | 0 | 0 | 0 | 22.56 | 5.66 | 16.76 |
| Samples | Solvent (%) | Temperature (°C) | Amplitude (W) | Cycle (s−1) |
|---|---|---|---|---|
| EtOHSP | 50 | 20 | 20 | 0.6 |
| EtOHCP | 80 | 60 | 43 | 0.8 |
| MeOHTP | 100 | 37 | 38 | 0.2 |
| Compound | EtOHSP (mg/g) | EtOHCP (mg/g) | MeOHTP (mg/g) |
|---|---|---|---|
| Chlorogenic acid | 1.24 | 0.91 | 3.31 |
| Coumaric acid | 0.88 | 0.62 | 2.45 |
| Caffeic acid | 1.12 | 0.76 | 3.01 |
| Ferulic acid | 0.79 | 0.55 | 2.65 |
| Artepillin C | 3.18 | 4.52 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.B.; Fernández Barbero, G.; Carrera, C.; Palma, M.; Epifânio, N.M.M.; Kawahito, T.C.; Júnior, V.V.; Chaves, D.S.A. Innovative Ultrasound-Assisted Extraction for Phenolic and Antioxidant Evaluation of Brazilian Green Propolis. Processes 2025, 13, 2880. https://doi.org/10.3390/pr13092880
Pereira DB, Fernández Barbero G, Carrera C, Palma M, Epifânio NMM, Kawahito TC, Júnior VV, Chaves DSA. Innovative Ultrasound-Assisted Extraction for Phenolic and Antioxidant Evaluation of Brazilian Green Propolis. Processes. 2025; 13(9):2880. https://doi.org/10.3390/pr13092880
Chicago/Turabian StylePereira, Debora B., Gerardo Fernández Barbero, Ceferino Carrera, Miguel Palma, Neide M. M. Epifânio, Taina C. Kawahito, Valdir V. Júnior, and Douglas S. A. Chaves. 2025. "Innovative Ultrasound-Assisted Extraction for Phenolic and Antioxidant Evaluation of Brazilian Green Propolis" Processes 13, no. 9: 2880. https://doi.org/10.3390/pr13092880
APA StylePereira, D. B., Fernández Barbero, G., Carrera, C., Palma, M., Epifânio, N. M. M., Kawahito, T. C., Júnior, V. V., & Chaves, D. S. A. (2025). Innovative Ultrasound-Assisted Extraction for Phenolic and Antioxidant Evaluation of Brazilian Green Propolis. Processes, 13(9), 2880. https://doi.org/10.3390/pr13092880










