Targeting PRMT5: Current Inhibitors and Emerging Strategies for Therapeutic Intervention
Abstract
1. Protein Arginine Methyltransferases 5 (PRMT5) Overview
1.1. PRMT Enzymes
1.2. PRMT5 Structure
1.2.1. PRMT54:MEP504 Hetero-Octamer Complex Structure
1.2.2. PRMT5:MEP50 Monomer Structure
1.3. PRMT5 Inhibition Mechanisms in Cancer Therapy
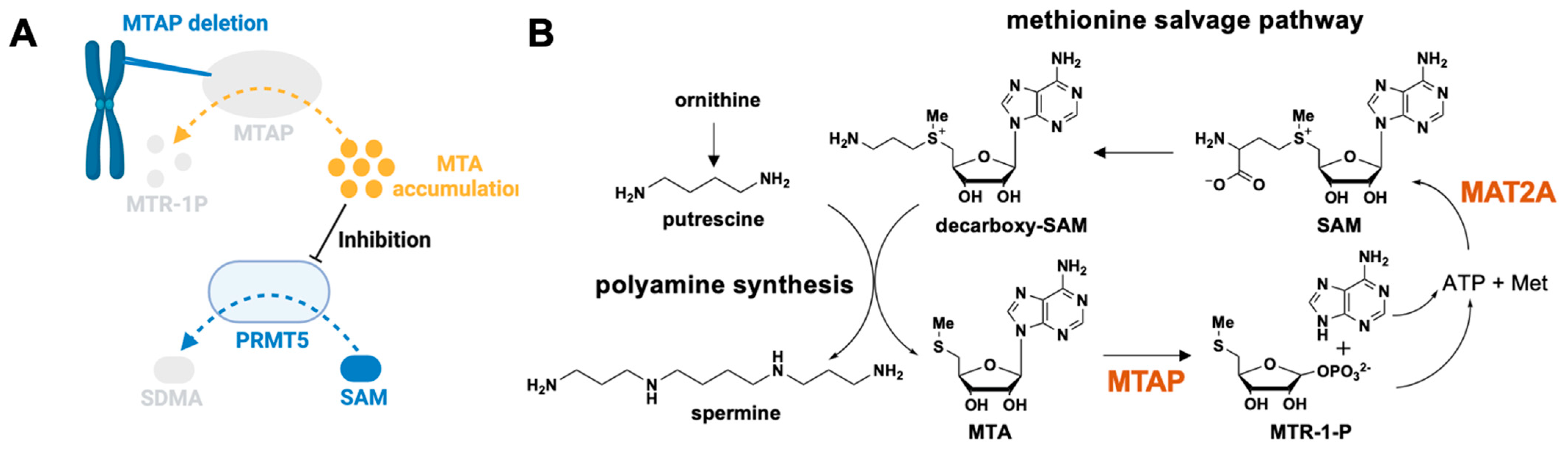
1.3.1. Regulation by Endogenous Ligand
1.3.2. Regulation by PTM
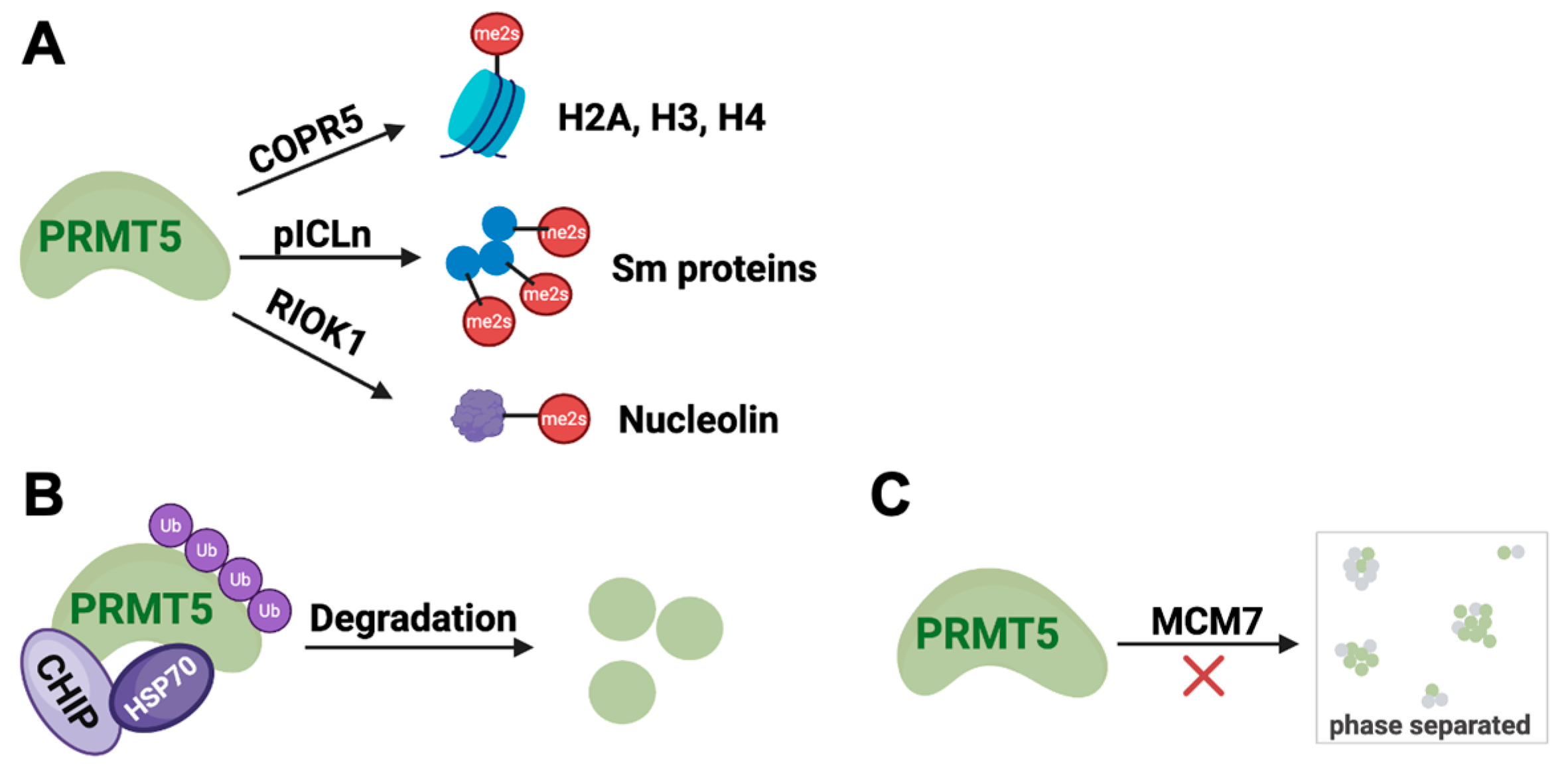
1.3.3. Regulation by Protein–Protein Interactions (PPI)
1.3.4. Regulation by Ubiquitin–Proteasome System (UPS)
1.3.5. Regulation by Liquid–Liquid Phase Separation (LLPS)
1.4. Current PRMT5 Inhibitors in Clinical Trials
2. Structure-Based PRMT5 Inhibitor Development
2.1. PRMT5 Inhibitors Targeting C-Terminal Catalytic Pocket
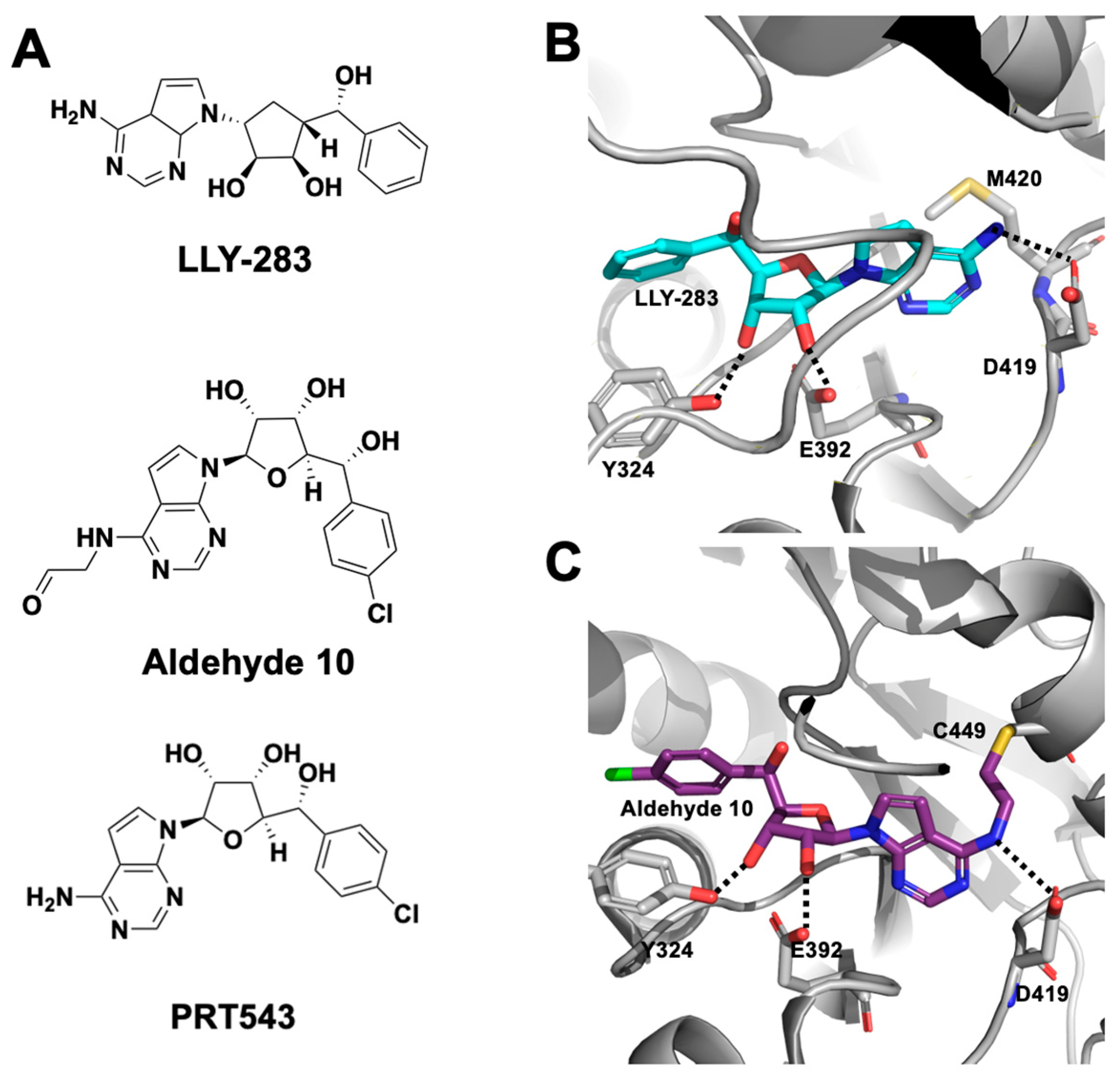
2.1.1. SAM-Cooperative Inhibitors
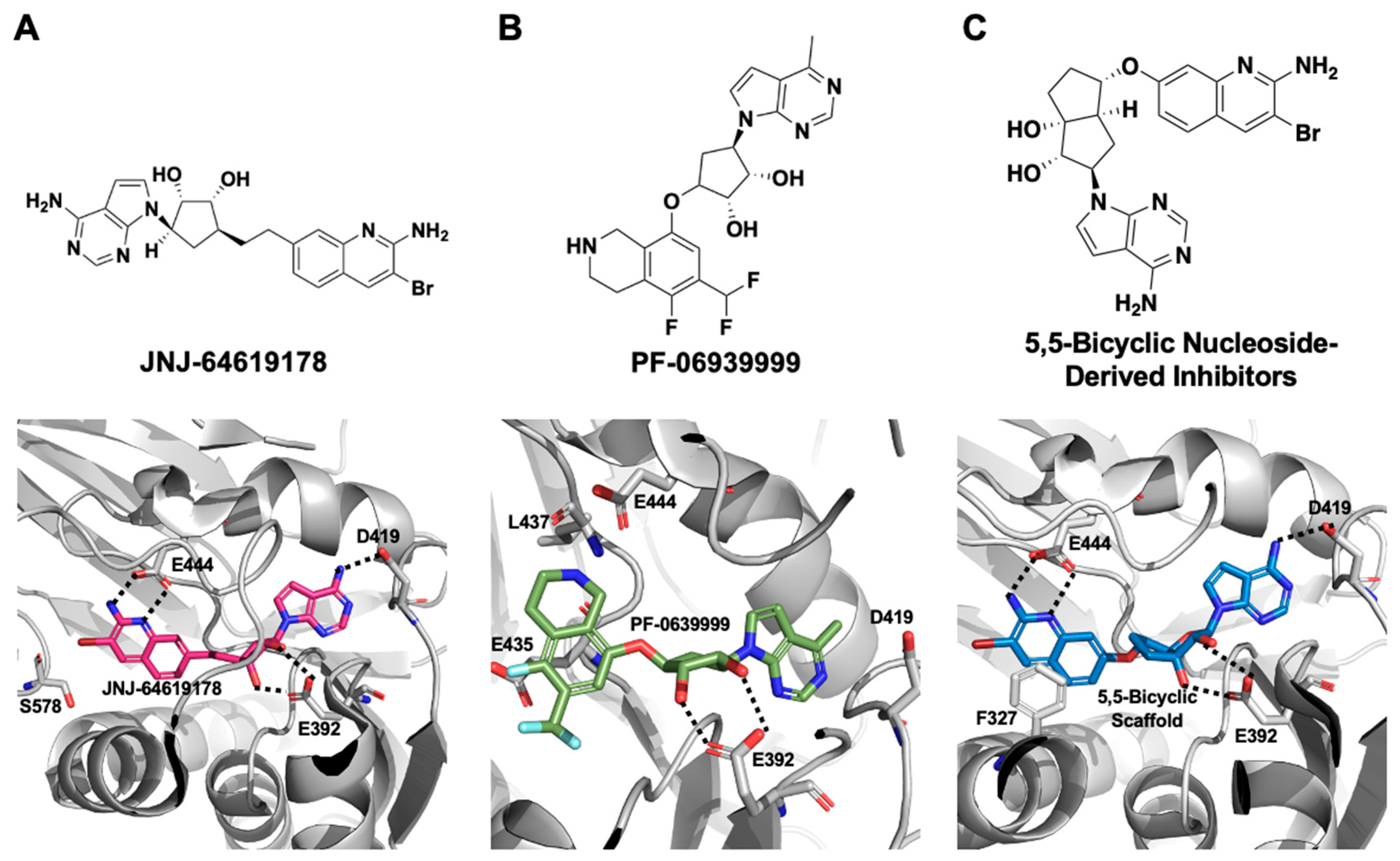
2.1.2. SAM-Competitive Inhibitors
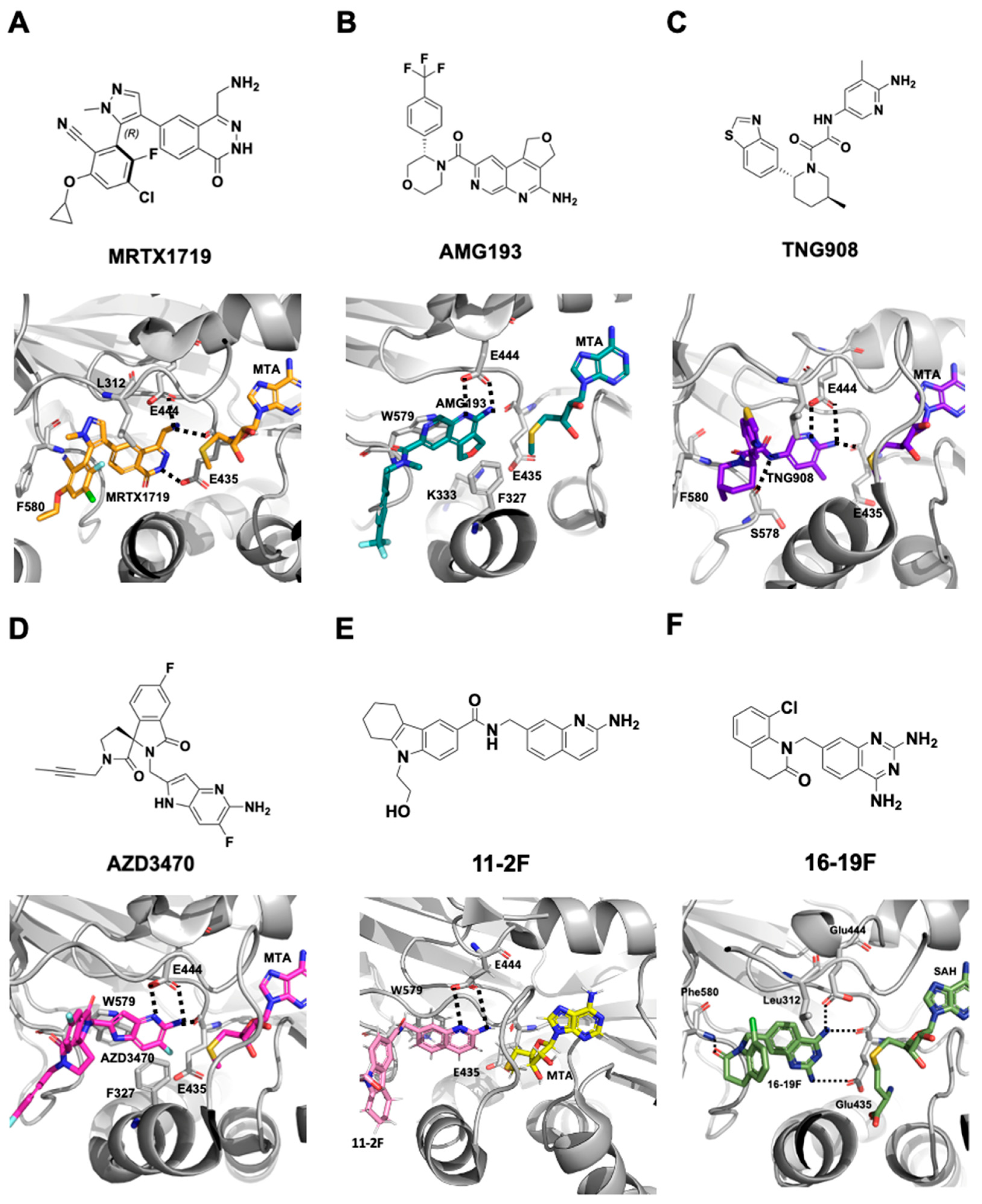
2.1.3. MTA-Cooperative PRMT5 Synthetic Lethal Inhibitors
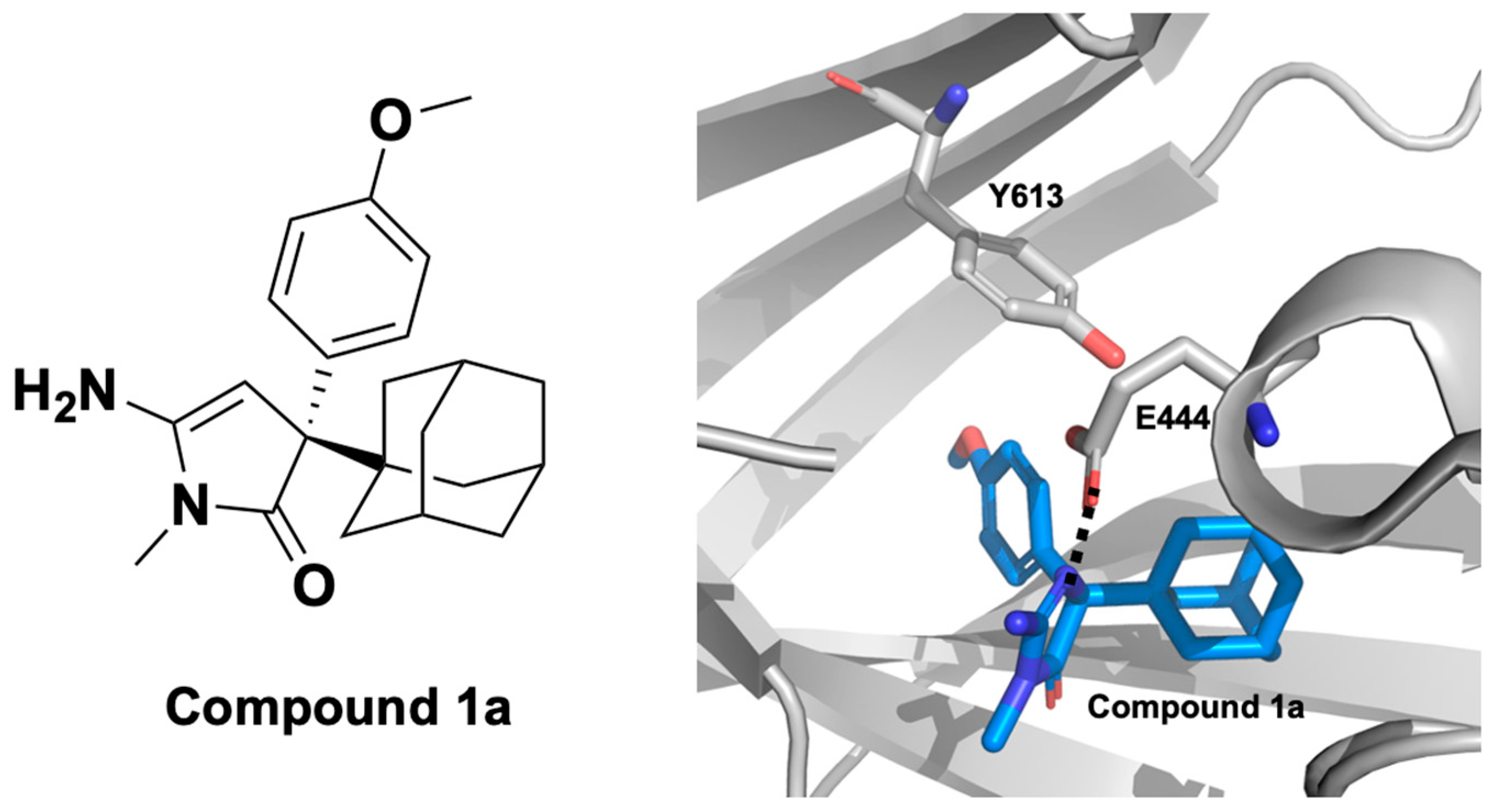
2.1.4. PRMT5 Allosteric Inhibitors
2.2. PRMT5 Inhibitors Targeting N-Terminal TIM Barrel Domain—Novel Inhibition Strategies
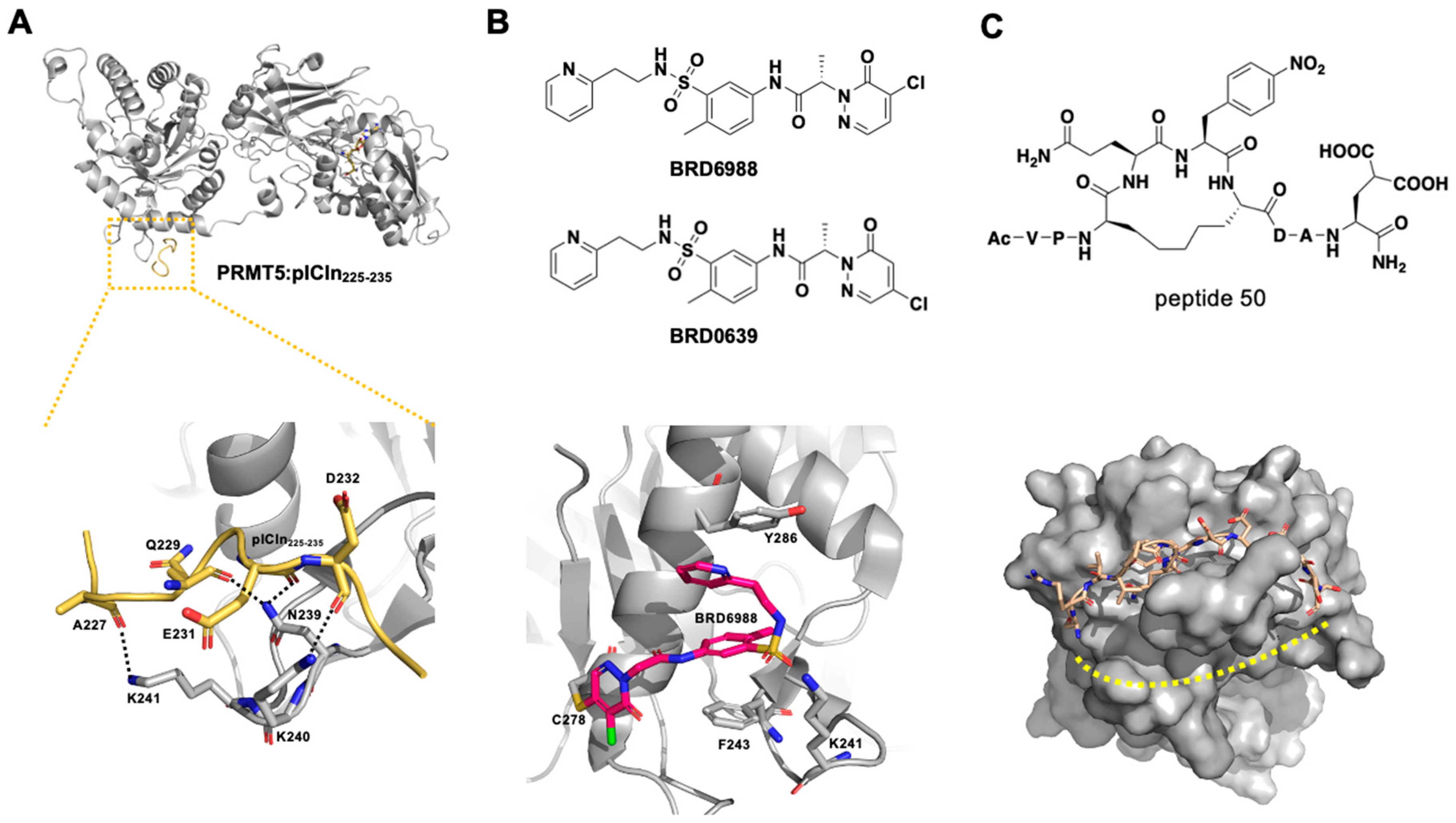
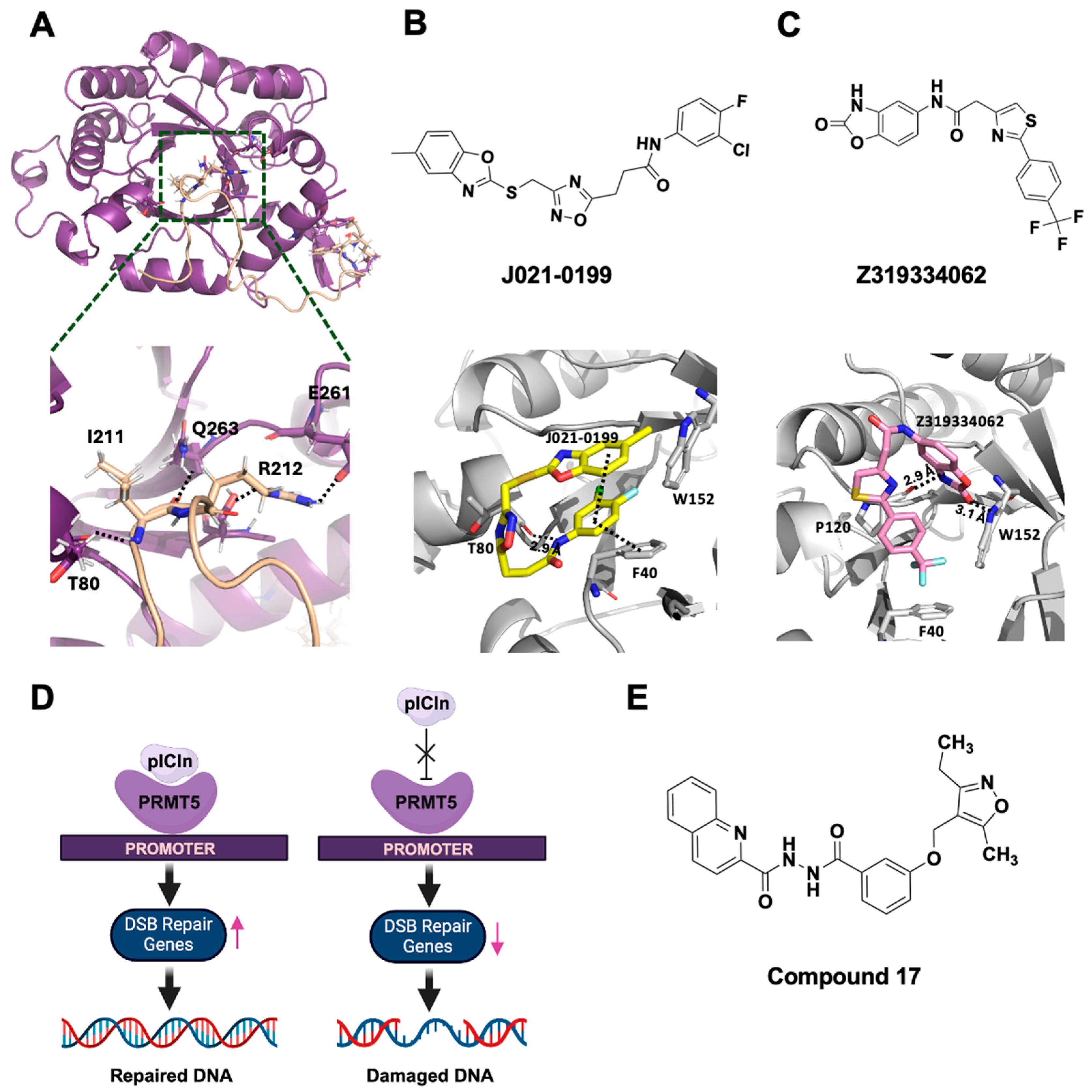
2.2.1. PRMT5–Substrate Adaptor Interaction Inhibitors
2.2.2. PRMT5–MEP50 Interaction Inhibitor
3. Discussion
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledges
Conflicts of Interest
References
- Liu, M.; Yao, B.; Gui, T.; Guo, C.; Wu, X.; Li, J.; Ma, L.; Deng, Y.; Xu, P.; Wang, Y.; et al. PRMT5-dependent transcriptional repression of c-Myc target genes promotes gastric cancer progression. Theranostics 2020, 10, 4437–4452. [Google Scholar] [CrossRef] [PubMed]
- Sachamitr, P.; Ho, J.C.; Ciamponi, F.E.; Ba-Alawi, W.; Coutinho, F.J.; Guilhamon, P.; Kushida, M.M.; Cavalli, F.M.G.; Lee, L.; Rastegar, N.; et al. PRMT5 inhibition disrupts splicing and stemness in glioblastoma. Nat. Commun. 2021, 12, 979. [Google Scholar] [CrossRef]
- Hoang, P.M.; Torre, D.; Jaynes, P.; Ho, J.; Mohammed, K.; Alvstad, E.; Lam, W.Y.; Khanchandani, V.; Lee, J.M.; Toh, C.M.C.; et al. A PRMT5-ZNF326 axis mediates innate immune activation upon replication stress. Sci. Adv. 2024, 10, eadm9589. [Google Scholar] [CrossRef]
- Hamard, P.J.; Santiago, G.E.; Liu, F.; Karl, D.L.; Martinez, C.; Man, N.; Mookhtiar, A.K.; Duffort, S.; Greenblatt, S.; Verdun, R.E.; et al. PRMT5 Regulates DNA Repair by Controlling the Alternative Splicing of Histone-Modifying Enzymes. Cell Rep. 2018, 24, 2643–2657. [Google Scholar] [CrossRef]
- Gillespie, M.S.; Chiang, K.; Regan-Mochrie, G.L.; Choi, S.Y.; Ward, C.M.; Sahay, D.; Garcia, P.; Arnold, R.; Davies, C.C. PRMT5-regulated splicing of DNA repair genes drives chemoresistance in breast cancer stem cells. Oncogene 2025, 44, 862–876. [Google Scholar] [CrossRef]
- Tan, L.; Xiao, K.; Ye, Y.; Liang, H.; Chen, M.; Luo, J.; Qin, Z. High PRMT5 expression is associated with poor overall survival and tumor progression in bladder cancer. Aging 2020, 12, 8728–8741. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shao, G.; Zhang, H.T.; Li, C.; Zhang, D.; Cheng, L.; Elzey, B.D.; Pili, R.; Ratliff, T.L.; Huang, J.; et al. Protein arginine methyltransferase 5 functions as an epigenetic activator of the androgen receptor to promote prostate cancer cell growth. Oncogene 2017, 36, 1223–1231. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Hu, X.; Zheng, Y.; Chen, X. Targeting PRMT5/Akt signalling axis prevents human lung cancer cell growth. J. Cell Mol. Med. 2019, 23, 1333–1342. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Feng, Y.; Li, Y.; Tamiya, H.; Tocci, S.; Ronai, Z.A. PRMT5 control of cGAS/STING and NLRC5 pathways defines melanoma response to antitumor immunity. Sci. Transl. Med. 2020, 12, eaaz5683. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, G.; Li, Y.; Wang, C.; Zhang, Y.; Luo, Y.; Xu, J.; Qiu, Y.; Ma, J.; Yang, J.; et al. Epigenetically targeting PRMT5 promotes antitumor immunity by inducing endogenous retroviruses expression and triggering viral mimicry response. Transl. Res. 2025, 281, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, H.; Xu, J.; Deng, C.; Qian, X.; Chu, S.; Zhu, W.G.; Zhu, J.; Yong, H.; Li, Z.; et al. PRMT5-Mediated ALKBH5 Methylation Promotes Colorectal Cancer Immune Evasion via Increasing CD276 Expression. Research 2025, 8, 0549. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yang, X.; Seabra, G.; Xu, X.; Dong, J.; Brant, J.O.; Zhou, W.; Guan, J.; Jiang, W.; Li, C. Discovery and Mechanism of 16–19F, a Novel Synthetic Lethal Inhibitor of the PRMT5•MTA Complex in MTAP-Deleted Cancer Cells. ACS Chem. Biol. 2025, 20, 1333–1346. [Google Scholar] [CrossRef]
- Krzyzanowski, A.; Gasper, R.; Adihou, H.; Hart, P.; Waldmann, H. Biochemical Investigation of the Interaction of pICln, RioK1 and COPR5 with the PRMT5-MEP50 Complex. Chembiochem 2021, 22, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, K.M.; Blomquist, C.; Acharya, N.; Li, R.; Ranaghan, M.J.; O’Keefe, M.; Rodriguez, D.J.; Young, M.J.; Kesar, D.; Pal, D.; et al. Molecular basis for substrate recruitment to the PRMT5 methylosome. Mol. Cell 2021, 81, 3481–3495 e7. [Google Scholar] [CrossRef] [PubMed]
- Antonysamy, S.; Bonday, Z.; Campbell, R.M.; Doyle, B.; Druzina, Z.; Gheyi, T.; Han, B.; Jungheim, L.N.; Qian, Y.; Rauch, C.; et al. Crystal structure of the human PRMT5:MEP50 complex. Proc. Natl. Acad. Sci. USA 2012, 109, 17960–17965. [Google Scholar] [CrossRef]
- Musiani, D.; Bok, J.; Massignani, E.; Wu, L.; Tabaglio, T.; Ippolito, M.R.; Cuomo, A.; Ozbek, U.; Zorgati, H.; Ghoshdastider, U.; et al. Proteomics profiling of arginine methylation defines PRMT5 substrate specificity. Sci. Signal 2019, 12, eaat8388. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Wilson, F.H.; Ruth, J.R.; Paulk, J.; Tsherniak, A.; Marlow, S.E.; Vazquez, F.; Weir, B.A.; Fitzgerald, M.E.; Tanaka, M.; et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 2016, 351, 1214–1218. [Google Scholar] [CrossRef]
- Mavrakis, K.J.; McDonald, E.R., 3rd; Schlabach, M.R.; Billy, E.; Hoffman, G.R.; deWeck, A.; Ruddy, D.A.; Venkatesan, K.; Yu, J.; McAllister, G.; et al. Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 2016, 351, 1208–1213. [Google Scholar] [CrossRef]
- Burgos, E.S.; Wilczek, C.; Onikubo, T.; Bonanno, J.B.; Jansong, J.; Reimer, U.; Shechter, D. Histone H2A and H4 N-terminal tails are positioned by the MEP50 WD repeat protein for efficient methylation by the PRMT5 arginine methyltransferase. J. Biol. Chem. 2015, 290, 9674–9689. [Google Scholar] [CrossRef]
- Majumder, S.; Alinari, L.; Roy, S.; Miller, T.; Datta, J.; Sif, S.; Baiocchi, R.; Jacob, S.T. Methylation of histone H3 and H4 by PRMT5 regulates ribosomal RNA gene transcription. J. Cell Biochem. 2010, 109, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lu, J.; Hu, X.; Hu, X.; Gao, X.; Zhou, J. PRMT5/FGFR3/AKT Signaling Axis Facilitates Lung Cancer Cell Metastasis. Technol. Cancer Res. Treat. 2023, 22, 15330338231161139. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, Y.M.; Lee, G.; Choi, Y.J.; Lim, B.O.; Kim, Y.J.; Choi, D.K.; Park, J.W. PRMT5 is essential for the eIF4E-mediated 5′-cap dependent translation. Biochem. Biophys. Res. Commun. 2014, 452, 1016–1021. [Google Scholar] [CrossRef]
- Qu, S.; Feng, B.; Xing, M.; Qiu, Y.; Ma, L.; Yang, Z.; Ji, Y.; Huang, F.; Wang, Y.; Zhou, J.; et al. PRMT5 K240lac confers ferroptosis resistance via ALKBH5/SLC7A11 axis in colorectal cancer. Oncogene 2025, 44, 2814–2830. [Google Scholar] [CrossRef]
- Du, C.; Hansen, L.J.; Singh, S.X.; Wang, F.; Sun, R.; Moure, C.J.; Roso, K.; Greer, P.K.; Yan, H.; He, Y. A PRMT5-RNF168-SMURF2 Axis Controls H2AX Proteostasis. Cell Rep. 2019, 28, 3199–3211 e5. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.; Zielinska, A.E.; Shaaban, A.M.; Sanchez-Bailon, M.P.; Jarrold, J.; Clarke, T.L.; Zhang, J.; Francis, A.; Jones, L.J.; Smith, S.; et al. PRMT5 Is a Critical Regulator of Breast Cancer Stem Cell Function via Histone Methylation and FOXP1 Expression. Cell Rep. 2017, 21, 3498–3513. [Google Scholar] [CrossRef] [PubMed]
- McKinney, D.C.; McMillan, B.J.; Ranaghan, M.J.; Moroco, J.A.; Brousseau, M.; Mullin-Bernstein, Z.; O’Keefe, M.; McCarren, P.; Mesleh, M.F.; Mulvaney, K.M.; et al. Discovery of a First-in-Class Inhibitor of the PRMT5-Substrate Adaptor Interaction. J. Med. Chem. 2021, 64, 11148–11168. [Google Scholar] [CrossRef] [PubMed]
- Pesiridis, G.S.; Diamond, E.; Van Duyne, G.D. Role of pICLn in methylation of Sm proteins by PRMT5. J. Biol. Chem. 2009, 284, 21347–21359. [Google Scholar] [CrossRef]
- Guderian, G.; Peter, C.; Wiesner, J.; Sickmann, A.; Schulze-Osthoff, K.; Fischer, U.; Grimmler, M. RioK1, a new interactor of protein arginine methyltransferase 5 (PRMT5), competes with pICln for binding and modulates PRMT5 complex composition and substrate specificity. J. Biol. Chem. 2011, 286, 1976–1986. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zeng, L.F.; He, Q.Y.; Tao, W.A.; Zha, Z.G.; Hu, C.D. The E3 ubiquitin ligase CHIP mediates ubiquitination and proteasomal degradation of PRMT5. Biochim. Biophys. Acta 2016, 1863, 335–346. [Google Scholar] [CrossRef]
- Liu, L.; Yin, S.; Gan, W. TRAF6 Promotes PRMT5 Activity in a Ubiquitination-Dependent Manner. Cancers 2023, 15, 2501. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kitajo, K. The effect of PRMT1-mediated arginine methylation on the subcellular localization, stress granules, and detergent-insoluble aggregates of FUS/TLS. PLoS ONE 2012, 7, e49267. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Dai, H.; Zhang, H.; Xie, M.; Zhang, H.; Chen, F.; Kang, X.; Bai, X.; Chen, Z. UBAP2L arginine methylation by PRMT1 modulates stress granule assembly. Cell Death Differ. 2020, 27, 227–241. [Google Scholar] [CrossRef]
- Wall, M.L.; Lewis, S.M. Methylarginines within the RGG-Motif Region of hnRNP A1 Affect Its IRES Trans-Acting Factor Activity and Are Required for hnRNP A1 Stress Granule Localization and Formation. J. Mol. Biol. 2017, 429, 295–307. [Google Scholar] [CrossRef]
- Tsai, W.C.; Gayatri, S.; Reineke, L.C.; Sbardella, G.; Bedford, M.T.; Lloyd, R.E. Arginine Demethylation of G3BP1 Promotes Stress Granule Assembly. J. Biol. Chem. 2016, 291, 22671–22685. [Google Scholar] [CrossRef]
- Hofweber, M.; Dormann, D. Friend or foe-Post-translational modifications as regulators of phase separation and RNP granule dynamics. J. Biol. Chem. 2019, 294, 7137–7150. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.H.; Hsu, K.W.; Wu, K.J. Liquid-liquid phase separation (LLPS) in cellular physiology and tumor biology. Am. J. Cancer Res. 2021, 11, 3766–3776. [Google Scholar] [PubMed]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Z.; Zhang, S.; Li, Y.; Wang, Y.; Fang, Z.; Ma, Y.; Liu, Z.; Zhang, W.; Li, D.; et al. Global profiling of arginine dimethylation in regulating protein phase separation by a steric effect-based chemical-enrichment method. Proc. Natl. Acad. Sci. USA 2022, 119, e2205255119. [Google Scholar] [CrossRef]
- Vinet, M.; Suresh, S.; Maire, V.; Monchecourt, C.; Nemati, F.; Lesage, L.; Pierre, F.; Ye, M.; Lescure, A.; Brisson, A.; et al. Protein arginine methyltransferase 5: A novel therapeutic target for triple-negative breast cancers. Cancer Med. 2019, 8, 2414–2428. [Google Scholar] [CrossRef]
- Haque, T.; Cadenas, F.L.; Xicoy, B.; Alfonso, A.; Platzbecker, U.; Avivi, I.; Brunner, A.M.; Chromik, J.; Morillo, D.; Patel, M.R.; et al. Phase 1 Study of JNJ-64619178, a Protein Arginine Methyltransferase 5 Inhibitor, in Patients with Lower-Risk Myelodysplastic Syndromes. Blood 2021, 138 (Suppl. S1), 2606. [Google Scholar] [CrossRef]
- A Phase 1, First-in-Human, Open-Label Study of the Safety, Pharmacokinetics, and Pharmacodynamics of JNJ-64619178, an Inhibitor of Protein Arginine Methyltransferase 5 (PRMT5) in Subjects with Advanced Cancers. 2018. Available online: https://clinicaltrials.gov/study/NCT03573310 (accessed on 18 January 2025).
- A Phase 1 Study to Evaluate The Safety, Pharmacokinetics, and Pharmacodynamics of Escalating Doses of PF-06939999 (PRMT5 INHIBITOR) in Participants with Advanced or Metastatic Non-Small Cell Lung Cancer, Head and Neck Squamous Cell Carcinoma, Esophageal Cancer, Endometrial Cancer, Cervical Cancer and Bladder Cancer. 2019. Available online: https://www.clinicaltrials.gov/study/NCT03854227 (accessed on 18 January 2025).
- Jensen-Pergakes, K.; Tatlock, J.; Maegley, K.A.; McAlpine, I.J.; McTigue, M.; Xie, T.; Dillon, C.P.; Wang, Y.; Yamazaki, S.; Spiegel, N.; et al. SAM-Competitive PRMT5 Inhibitor PF-06939999 Demonstrates Antitumor Activity in Splicing Dysregulated NSCLC with Decreased Liability of Drug Resistance. Mol. Cancer Ther. 2022, 21, 3–15. [Google Scholar] [CrossRef] [PubMed]
- A Phase 1, Open-Label, Multicenter, Dose Escalation and Expansion Study of PRT811 in Subjects with Advanced Solid Tumors, CNS Lymphoma, and Recurrent High-Grade Gliomas. 2019. Available online: https://www.clinicaltrials.gov/study/NCT02048488 (accessed on 18 January 2025).
- A Phase 1, Open-Label, Multicenter, Dose Escalation, Dose Expansion Study of PRT543 in Patients with Advanced Solid Tumors and Hematologic Malignancies. 2019. Available online: https://clinicaltrials.gov/study/NCT03886831?cond=myelofibrosis&intr=PRT543&rank=1 (accessed on 18 January 2025).
- A Phase I Study to Investigate the Safety, Tolerability, Preliminary Efficacy and Pharmacokinetics(PK) of SCR-6920 Capsule in Patients with Advanced Malignant Tumors. 2022. Available online: https://clinicaltrials.gov/study/NCT05528055?term=PRMT5%20inhibitor&viewType=Table&rank=2 (accessed on 18 January 2025).
- Yu, J.; Sun, Y.; Guo, S.; Wang, H.; Wu, J.; Jiang, X.; Chen, J.; Yang, G.; Yang, C. 675P A phase I study of safety, pharmacokinetics, and pharmacodynamics of SCR-6920, a protein arginine methyltransferase 5 (PRMT5) inhibitor, in patients with advanced malignant tumors. Ann. Oncol. 2023, 34, S474. [Google Scholar] [CrossRef]
- A Phase II Randomized Window of Opportunity Trial Evaluating Clinical and Biological Effects of PRMT5 Inhibitor, GSK3326595, in Early Stage Breast Cancer. Ontario Institute for Cancer Research; GlaxoSmithKline; London Regional Cancer Program; Canada, Hamilton Health Sciences Corporation (Eds.) 2020. Available online: https://clinicaltrials.gov/study/NCT04676516?intr=GSK3326595&rank=1 (accessed on 18 January 2025).
- Watts, J.M.; Bradley, T.J.; Thomassen, A.; Brunner, A.M.; Minden, M.D.; Papadantonakis, N.; Abedin, S.; Baines, A.J.; Barbash, O.; Gorman, S.; et al. A Phase I/II Study to Investigate the Safety and Clinical Activity of the Protein Arginine Methyltransferase 5 Inhibitor GSK3326595 in Subjects with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Blood 2019, 134 (Suppl. S1), 2656. [Google Scholar] [CrossRef]
- An Open Label, Phase 1, Treatment Study to Evaluate the Safety, Pharmacokinetics and Pharmacodynamics of IDE397 (MAT2A Inhibitor) in Adult Participants with Advanced Solid Tumors. 2021. Available online: https://www.clinicaltrials.gov/study/NCT04794699 (accessed on 18 January 2025).
- A Phase 1, Open-Label, Multicenter Clinical Trial of S095035 (MAT2A Inhibitor) in Adult Participants with Advanced or Metastatic Solid Tumors with Homozygous Deletion of MTAP. Institut de Recherches Internationales Servier (Ed.) 2023. Available online: https://clinicaltrials.gov/study/NCT06188702 (accessed on 18 January 2025).
- A Phase 1/2 Multiple Expansion Cohort Trial of MRTX1719 in Patients with Advanced Solid Tumors with Homozygous MTAP Deletion. 2022. Available online: https://www.clinicaltrials.gov/study/NCT05245500 (accessed on 18 January 2025).
- A Phase 1/1b/2 Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of AMG 193 Alone and in Combination with Docetaxel in Subjects with Advanced MTAP-Null Solid Tumors. 2021. Available online: https://clinicaltrials.gov/study/NCT05094336 (accessed on 18 January 2025).
- A Phase 1b Study Evaluating the Safety, Tolerability, Pharmacokinetics, and Efficacy of AMG 193 Alone or in Combination with Other Therapies in Subjects with Advanced Thoracic Tumors with Homozygous MTAP-Deletion (Master Protocol). 2024. Available online: https://clinicaltrials.gov/study/NCT06333951 (accessed on 18 January 2025).
- A Phase 1b Study Evaluating the Safety, Tolerability, Pharmacokinetics, and Efficacy of AMG 193 in Combination with Other Therapies in Subjects with Advanced Gastrointestinal, Biliary Tract, or Pancreatic Cancers with Homozygous MTAP-Deletion. 2024. Available online: https://clinicaltrials.gov/study/NCT06360354 (accessed on 18 January 2025).
- A Modular Phase I/II, Open-Label, Multicentre Study to Evaluate the Safety, Tolerability, and Efficacy of AZD3470, a PRMT5 Inhibitor, as Monotherapy and in Combination with Anticancer Agent(s) in Participants with Relapsed/Refractory Haematologic Malignancies. 2023. Available online: https://clinicaltrials.gov/study/NCT06130553 (accessed on 18 January 2025).
- PRIMROSE: A Modular Phase I/IIa, Multi-Centre, Dose Escalation, and Expansion Study of AZD3470, a MTA Cooperative PRMT5 Inhibitor, as Monotherapy and in Combination with Anticancer Agents in Patients with Advanced/Metastatic Solid Tumours That Are MTAP Deficient. 2023. Available online: https://clinicaltrials.gov/study/NCT06130553 (accessed on 18 January 2025).
- A Phase 1/2, Multi-Center, Open-Label Study to Evaluate the Safety, Tolerability, and Preliminary Anti-Tumor Activity of TNG462 in Patients with MTAP-Deleted Advanced or Metastatic Solid Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT05732831 (accessed on 18 January 2025).
- A Phase 1/2, Multi-Center, Open-Label Study to Evaluate the Safety, Tolerability, and Preliminary Anti-Tumor Activity of TNG908 in Patients with MTAP-Deleted Advanced or Metastatic Solid Tumors. 2022. Available online: https://clinicaltrials.gov/study/NCT05275478 (accessed on 18 January 2025).
- A Phase 1a/b Study Investigating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Preliminary Antitumor Activity of BGB-58067, an MTA-Cooperative PRMT5 Inhibitor in Patients with Advanced Solid Tumors. 2024. Available online: https://clinicaltrials.gov/study/NCT06589596 (accessed on 18 January 2025).
- A Multi-Center, Open-Label, Phase I/II Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Preliminary Anti-Tumor Activity of CTS3497 in Patients with MTAP Deficient Advanced Solid Tumors and Lymphomas. 2025. Available online: https://clinicaltrials.gov/study/NCT06971523 (accessed on 18 January 2025).
- A Phase 1a/1b Study Evaluating the Clinical Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Preliminary Anti-Tumor Efficacy of PEP08 as Monotherapy and Combination Therapy in MTAP-Del Advanced or Metastatic Solid Tumors. 2025. Available online: https://clinicaltrials.gov/study/NCT06973863 (accessed on 18 January 2025).
- A First-in-Human Study to Evaluate the Safety, Tolerability and Pharmacokinetics, Pharmacodynamics and Preliminary Clinical Activity of BAY 3713372, a Novel 2nd Generation PRMT5 Inhibitor, in Participants with MTAP-Deleted Solid Tumors. 2025. Available online: https://clinicaltrials.gov/study/NCT06914128 (accessed on 18 January 2025).
- Chan-Penebre, E.; Kuplast, K.G.; Majer, C.R.; Boriack-Sjodin, P.A.; Wigle, T.J.; Johnston, L.D.; Rioux, N.; Munchhof, M.J.; Jin, L.; Jacques, S.L.; et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat. Chem. Biol. 2015, 11, 432–437. [Google Scholar] [CrossRef]
- Siu, L.L.; Rasco, D.W.; Vinay, S.P.; Romano, P.M.; Menis, J.; Opdam, F.L.; Heinhuis, K.M.; Egger, J.L.; Gorman, S.A.; Parasrampuria, R. METEOR-1: A phase I study of GSK3326595, a first-in-class protein arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumours. Ann. Oncol. 2019, 30, v159. [Google Scholar] [CrossRef]
- Brehmer, D.; Beke, L.; Wu, T.; Millar, H.J.; Moy, C.; Sun, W.; Mannens, G.; Pande, V.; Boeckx, A.; van Heerde, E.; et al. Discovery and Pharmacological Characterization of JNJ-64619178, a Novel Small-Molecule Inhibitor of PRMT5 with Potent Antitumor Activity. Mol. Cancer Ther. 2021, 20, 2317–2328. [Google Scholar] [CrossRef]
- A Study of PRT543 in Participants with Advanced Solid Tumors and Hematologic Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03886831 (accessed on 18 January 2025).
- Smith, C.R.; Aranda, R.; Bobinski, T.P.; Briere, D.M.; Burns, A.C.; Christensen, J.G.; Clarine, J.; Engstrom, L.D.; Gunn, R.J.; Ivetac, A.; et al. Fragment-Based Discovery of MRTX1719, a Synthetic Lethal Inhibitor of the PRMT5*MTA Complex for the Treatment of MTAP-Deleted Cancers. J. Med. Chem. 2022, 65, 1749–1766. [Google Scholar] [CrossRef]
- Smith, C.R.; Aranda, R.; Christensen, J.G.; Engstrom, L.D.; Gunn, R.J.; Ivetac, A.; Ketcham, J.M.; Kuehler, J.; David Lawson, J.; Marx, M.A.; et al. Design and evaluation of achiral, non-atropisomeric 4-(aminomethyl)phthalazin-1(2H)-one derivatives as novel PRMT5/MTA inhibitors. Bioorg Med. Chem. 2022, 71, 116947. [Google Scholar] [CrossRef]
- Smith, C.R.; Kulyk, S.; Ahmad, M.U.D.; Arkhipova, V.; Christensen, J.G.; Gunn, R.J.; Ivetac, A.; Ketcham, J.M.; Kuehler, J.; Lawson, J.D.; et al. Fragment optimization and elaboration strategies—The discovery of two lead series of PRMT5/MTA inhibitors from five fragment hits. RSC Med. Chem. 2022, 13, 1549–1564. [Google Scholar] [CrossRef]
- Villalona-Calero, M.A.; Patnaik, A.; Maki, R.G.; O’Neil, B.; Abbruzzese, J.L.; Dagogo-Jack, I.; Devarakonda, S.; Wahlroos, S.; Lin, C.C.; Fujiwara, Y.; et al. Design and rationale of a phase 1 dose-escalation study of AMG 193, a methylthioadenosine (MTA)-cooperative PRMT5 inhibitor, in patients with advanced methylthioadenosine phosphorylase (MTAP)-null solid tumors. J. Clin. Oncol. 2022, 40, TPS3167. [Google Scholar] [CrossRef]
- Briggs, K.; Tsai, A.; Zhang, M.; Tonini, M.; Haines, B.; Huang, A.; Cottrell, K. TNG462 is a potential best-in-class MTA-cooperative PRMT5 inhibitor for the treatment of peripheral MTAP-deleted solid tumors. Eur. J. Cancer 2022, 174, S84. [Google Scholar] [CrossRef]
- Briggs, K.; Cottrell, K.; Tsai, A.; Zhang, M.; Tonini, M.; Yoda, S.; Lombardo, S.; Teng, T.; Davis, C.; Whittington, D.; et al. TNG908 is a brain-penetrant, MTA-cooperative PRMT5 inhibitor for the treatment of MTAP-deleted cancer. Eur. J. Cancer 2022, 174, S84. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, F. PRMT5:MEP50 WITH SCR-6920 AND SAM [PDB ID:8X6L]. 2025. Available online: https://www.rcsb.org/structure/8X6L (accessed on 4 September 2025).
- Shen, Y.; Gao, G.; Yu, X.; Kim, H.; Wang, L.; Xie, L.; Schwarz, M.; Chen, X.; Guccione, E.; Liu, J.; et al. Discovery of First-in-Class Protein Arginine Methyltransferase 5 (PRMT5) Degraders. J. Med. Chem. 2020, 63, 9977–9989. [Google Scholar] [CrossRef] [PubMed]
- Bonday, Z.Q.; Cortez, G.S.; Grogan, M.J.; Antonysamy, S.; Weichert, K.; Bocchinfuso, W.P.; Li, F.; Kennedy, S.; Li, B.; Mader, M.M.; et al. LLY-283, a Potent and Selective Inhibitor of Arginine Methyltransferase 5, PRMT5, with Antitumor Activity. ACS Med. Chem. Lett. 2018, 9, 612–617. [Google Scholar] [CrossRef]
- Lin, H.; Wang, M.; Zhang, Y.W.; Tong, S.; Leal, R.A.; Shetty, R.; Vaddi, K.; Luengo, J.I. Discovery of Potent and Selective Covalent Protein Arginine Methyltransferase 5 (PRMT5) Inhibitors. ACS Med. Chem. Lett. 2019, 10, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Sun, W.; Beke, L.; Berthelot, D.; Brehmer, D.; Brown, D.; Corbera, J.; Irving, S.; Meerpoel, L.; Nys, T.; et al. A Chemical Probe for the Methyl Transferase PRMT5 with a Novel Binding Mode. ACS Med. Chem. Lett. 2020, 11, 2227–2231. [Google Scholar] [CrossRef]
- Quiroz, R.V.; Reutershan, M.H.; Schneider, S.E.; Sloman, D.; Lacey, B.M.; Swalm, B.M.; Yeung, C.S.; Gibeau, C.; Spellman, D.S.; Rankic, D.A.; et al. The Discovery of Two Novel Classes of 5,5-Bicyclic Nucleoside-Derived PRMT5 Inhibitors for the Treatment of Cancer. J. Med. Chem. 2021, 64, 3911–3939. [Google Scholar] [CrossRef]
- Candito, D.A.; Ye, Y.; Quiroz, R.V.; Reutershan, M.H.; Witter, D.; Gadamsetty, S.B.; Li, H.; Sauri, J.; Schneider, S.E.; Lam, Y.H.; et al. Development of a Flexible and Robust Synthesis of Tetrahydrofuro[3,4-b]furan Nucleoside Analogues. J. Org. Chem. 2021, 86, 5142–5151. [Google Scholar] [CrossRef]
- Kalev, P.; Hyer, M.L.; Gross, S.; Konteatis, Z.; Chen, C.C.; Fletcher, M.; Lein, M.; Aguado-Fraile, E.; Frank, V.; Barnett, A.; et al. MAT2A Inhibition Blocks the Growth of MTAP-Deleted Cancer Cells by Reducing PRMT5-Dependent mRNA Splicing and Inducing DNA Damage. Cancer Cell 2021, 39, 209–224 e11. [Google Scholar] [CrossRef]
- Belmontes, B.; Slemmons, K.K.; Su, C.; Liu, S.; Policheni, A.N.; Moriguchi, J.; Tan, H.; Xie, F.; Aiello, D.A.; Yang, Y.; et al. AMG 193, a Clinical Stage MTA-Cooperative PRMT5 Inhibitor, Drives Antitumor Activity Preclinically and in Patients with MTAP-Deleted Cancers. Cancer Discov. 2024, 15, 139–161. [Google Scholar]
- Cottrell, K.M.; Briggs, K.J.; Whittington, D.A.; Jahic, H.; Ali, J.A.; Davis, C.B.; Gong, S.; Gotur, D.; Gu, L.; McCarren, P.; et al. Discovery of TNG908: A Selective, Brain Penetrant, MTA-Cooperative PRMT5 Inhibitor That Is Synthetically Lethal with MTAP-Deleted Cancers. J. Med. Chem. 2024, 67, 6064–6080. [Google Scholar] [CrossRef]
- Smith, J.M.; Barlaam, B.; Beattie, D.; Bradshaw, L.; Chan, H.M.; Chiarparin, E.; Collingwood, O.; Cooke, S.L.; Cronin, A.; Cumming, I.; et al. Discovery and In Vivo Efficacy of AZ-PRMT5i-1, a Novel PRMT5 Inhibitor with High MTA Cooperativity. J. Med. Chem. 2024, 67, 13604–13638. [Google Scholar] [CrossRef]
- Zhou, W.; Yadav, G.P.; Yang, X.; Qin, F.; Li, C.; Jiang, Q.X. Cryo-EM structure-based selection of computed ligand poses enables design of MTA-synergic PRMT5 inhibitors of better potency. Commun. Biol. 2022, 5, 1054. [Google Scholar] [CrossRef]
- Alinari, L.; Mahasenan, K.V.; Yan, F.; Karkhanis, V.; Chung, J.H.; Smith, E.M.; Quinion, C.; Smith, P.L.; Kim, L.; Patton, J.T.; et al. Selective inhibition of protein arginine methyltransferase 5 blocks initiation and maintenance of B-cell transformation. Blood 2015, 125, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Palte, R.L.; Schneider, S.E.; Altman, M.D.; Hayes, R.P.; Kawamura, S.; Lacey, B.M.; Mansueto, M.S.; Reutershan, M.; Siliphaivanh, P.; Sondey, C.; et al. Allosteric Modulation of Protein Arginine Methyltransferase 5 (PRMT5). ACS Med. Chem. Lett. 2020, 11, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Malamas, M.S.; Erdei, J.; Gunawan, I.; Turner, J.; Hu, Y.; Wagner, E.; Fan, K.; Chopra, R.; Olland, A.; Bard, J.; et al. Design and synthesis of 5,5′-disubstituted aminohydantoins as potent and selective human beta-secretase (BACE1) inhibitors. J. Med. Chem. 2010, 53, 1146–1158. [Google Scholar] [CrossRef]
- McDonald, E.R., 3rd; de Weck, A.; Schlabach, M.R.; Billy, E.; Mavrakis, K.J.; Hoffman, G.R.; Belur, D.; Castelletti, D.; Frias, E.; Gampa, K.; et al. Project DRIVE: A Compendium of Cancer Dependencies and Synthetic Lethal Relationships Uncovered by Large-Scale, Deep RNAi Screening. Cell 2017, 170, 577–592 e10. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowski, A.; Esser, L.M.; Willaume, A.; Prudent, R.; Peter, C.; ‘t Hart, P.; Waldmann, H. Development of Macrocyclic PRMT5-Adaptor Protein Interaction Inhibitors. J. Med. Chem. 2022, 65, 15300–15311. [Google Scholar] [CrossRef]
- Shen, Z.; Seabra, G.; Li, C. Virtual High-Throughput Screening of Ligands for Disrupting PRMT5/pICLn Interaction in Prostate Cancer Cells. ACS Med. Chem. Lett. 2025, 16, 875–879. [Google Scholar] [CrossRef]
- Shen, Z.; Seabra, G.; Brant, J.; Shirlekar, K.; Deleyrolle, L.; Lewis, B.; Li, C. Discovery of PRMT5 N-Terminal TIM Barrel Ligands from Machine-Learning-Based Virtual Screening. ACS Omega 2025, 10, 1156–1163. [Google Scholar] [CrossRef]
- Asberry, A.M.; Cai, X.; Deng, X.; Santiago, U.; Liu, S.; Sims, H.S.; Liang, W.; Xu, X.; Wan, J.; Jiang, W.; et al. Discovery and Biological Characterization of PRMT5:MEP50 Protein-Protein Interaction Inhibitors. J. Med. Chem. 2022, 65, 13793–13812. [Google Scholar] [CrossRef] [PubMed]



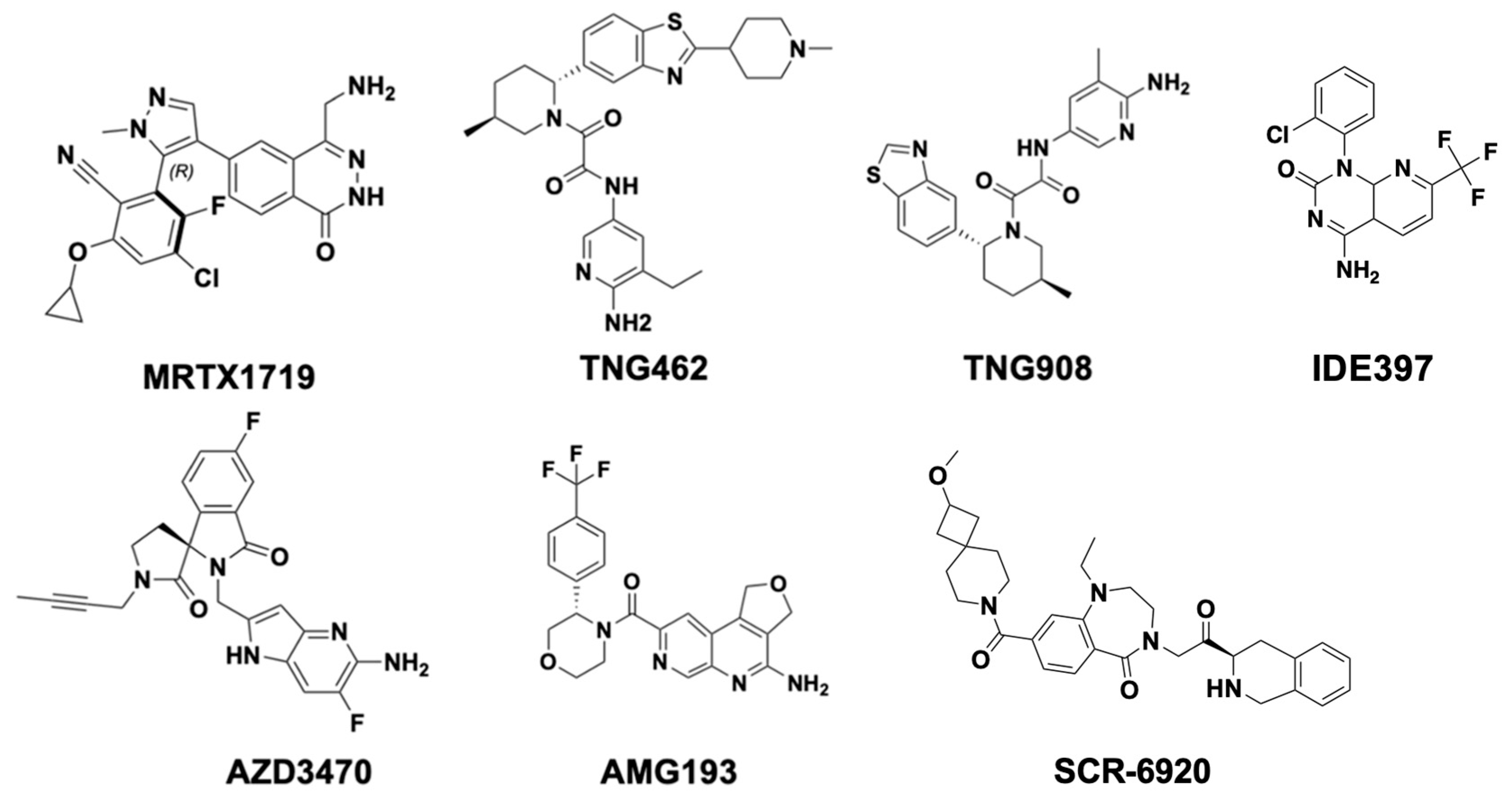

| Type | Compound | Company | Clinical Stage | First Identified (Year) | Discontinued (Year) | Indications |
|---|---|---|---|---|---|---|
| SAM-cooperative inhibitors | GSK3326595 | GlaxoSmithKline | Discontinued | 2017 | 2022 | Advanced solid tumors (AST) and non-Hodgkin’s lymphoma (NHL). |
| SCR-6920 | Simcere Pharmaceutical | Phase 2 | 2022 | N/A | AST and NHL | |
| SAM-competitive inhibitors | JNJ-64619178 | Janssen Pharmaceuticals | Discontinued | 2019 | 2022 | AST and NHL |
| PF-06939999 | Pfizer | Discontinued | 2019 | 2022 | Advanced or metastatic solid tumors, including endometrial cancer, HNSCC, NSCLC, urothelial cancer, cervical cancer, and esophageal cancer. | |
| PRT811 | Prelude Therapeutics | Discontinued | 2020 | 2022 | AST and high-grade gliomas. | |
| PRT543 | Prelude Therapeutics | Discontinued | 2020 | 2022 | AST and hematologic malignancies. | |
| MTA-cooperative inhibitors | TNG908 | Tango Therapeutics | Phase 1/2 | 2022 | N/A | MTAP-deleted solid tumors, including non-CNS solid tumors such as NSCLC and pancreatic cancer. |
| TNG462 | Tango Therapeutics | Phase 1/2 | 2023 | N/A | MTAP-deleted solid tumors, including NSCLC and pancreatic cancer. | |
| MRTX1719 | Mirati Therapeutics | Phase 1/2 | 2023 | N/A | Solid tumors harboring MTAP gene deletions. | |
| AMG 193 | Amgen | Phase 1/2 | 2024 | N/A | AST with MTAP deletions. | |
| BGB-58067 | BeiGene | Phase 1 | 2024 | N/A | Advanced malignant solid neoplasms. | |
| AZD3470 | AstraZeneca | Phase 1/2 | 2024 | N/A | MTAP-Deficient Advanced or Metastatic Solid Tumors | |
| BAY 3713372 | Bayer | Phase 1 | 2025 | N/A | MTAP-deleted Solid Tumors | |
| PEP08 | PharmaEngine | Phase 1 | 2025 | N/A | MTAP-Del Advanced or Metastatic Solid Tumors | |
| CTS3497 | CytosinLab Therapeutics | Phase 1/2 | 2025 | N/A | MTAP Deficient Advanced Solid Tumors and Lymphomas | |
| MAT2A inhibitors | IDE397 | IDEAYA Biosciences | Phase 1/2 | 2024 | N/A | MTAP-deleted solid tumors, including non-small cell lung cancer and urothelial cancer. |
| S095035 | Servier Pharmaceuticals | Phase 1 | 2019 | N/A | Advanced or metastatic solid tumors with homozygous deletion of MTAP. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Li, C. Targeting PRMT5: Current Inhibitors and Emerging Strategies for Therapeutic Intervention. Processes 2025, 13, 2878. https://doi.org/10.3390/pr13092878
Shen Z, Li C. Targeting PRMT5: Current Inhibitors and Emerging Strategies for Therapeutic Intervention. Processes. 2025; 13(9):2878. https://doi.org/10.3390/pr13092878
Chicago/Turabian StyleShen, Zhihang, and Chenglong Li. 2025. "Targeting PRMT5: Current Inhibitors and Emerging Strategies for Therapeutic Intervention" Processes 13, no. 9: 2878. https://doi.org/10.3390/pr13092878
APA StyleShen, Z., & Li, C. (2025). Targeting PRMT5: Current Inhibitors and Emerging Strategies for Therapeutic Intervention. Processes, 13(9), 2878. https://doi.org/10.3390/pr13092878










