Microwave-Assisted Synthesis of Antimicrobial Silver Nanoparticles Using Propolis Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis Material and Preparation of Non-Ethanolic Propolis Extracts
2.2. Preparation of AgNPs
2.3. Reagents
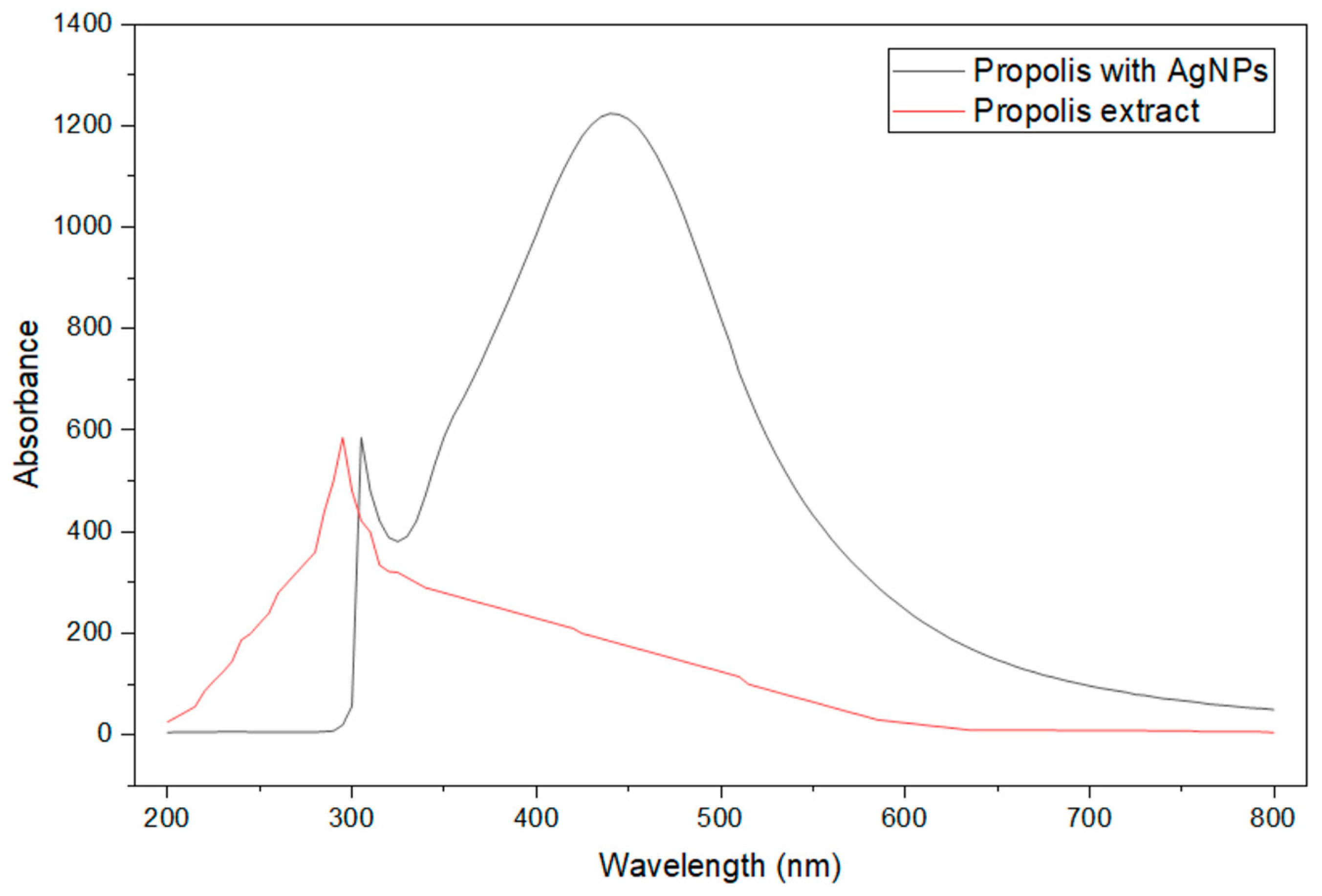
2.4. Spectrophotometric Studies
Determination of the Total Content of Phenolic Compounds, Flavonoids, Proanthocyanidins and Hydroxycinnamic Acid Derivatives
2.5. Evaluation of Antioxidant Activity
2.6. Microscopy
2.7. Antimicrobial Activity
2.8. Color Measurement of Propolis/AgNPs
2.9. Statistical Analysis
3. Results
3.1. Color Change of Propolis and Propolis/AgNPs
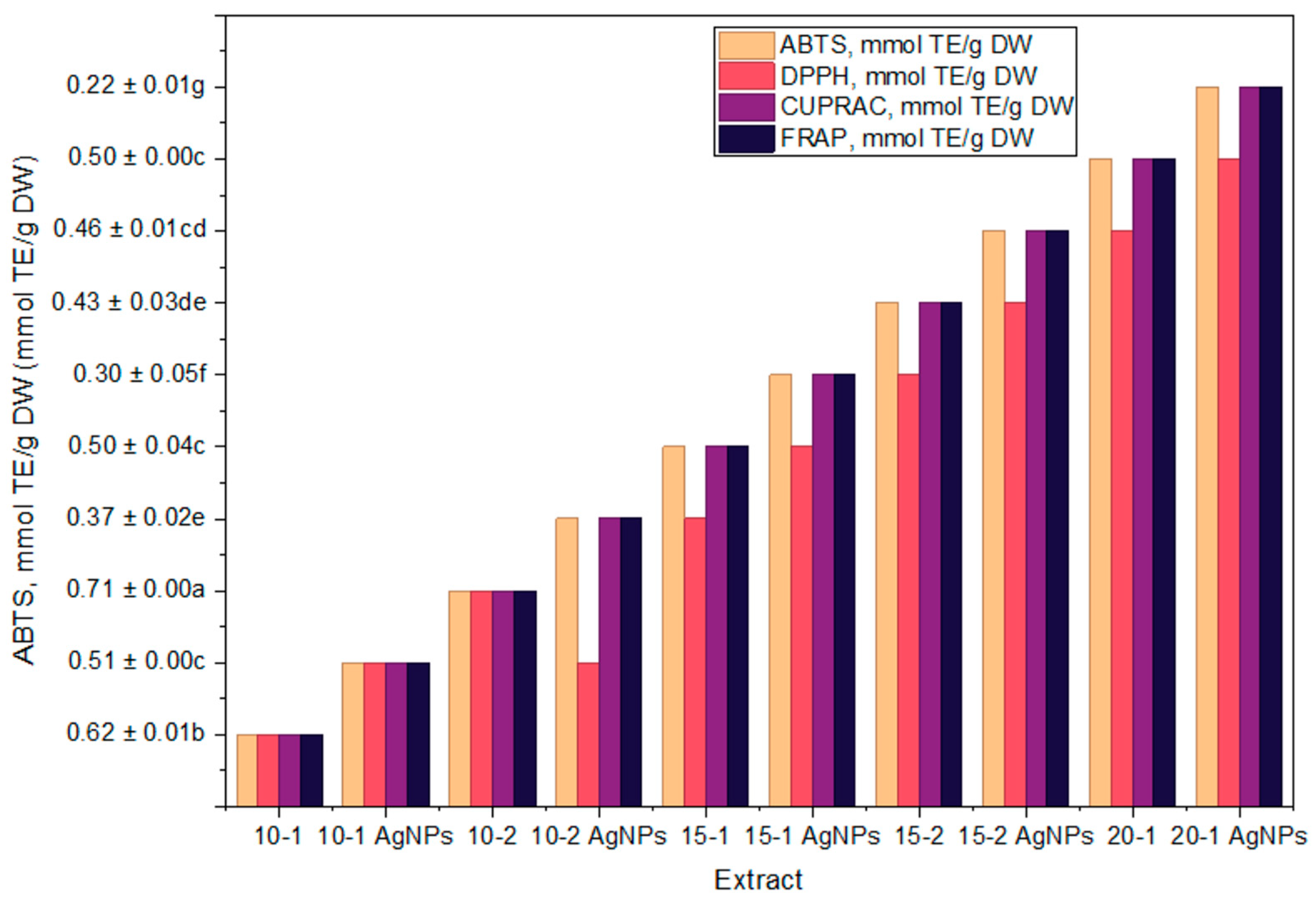
3.2. Determination of Antioxidant Properties of Propolis and Propolis/AgNPs
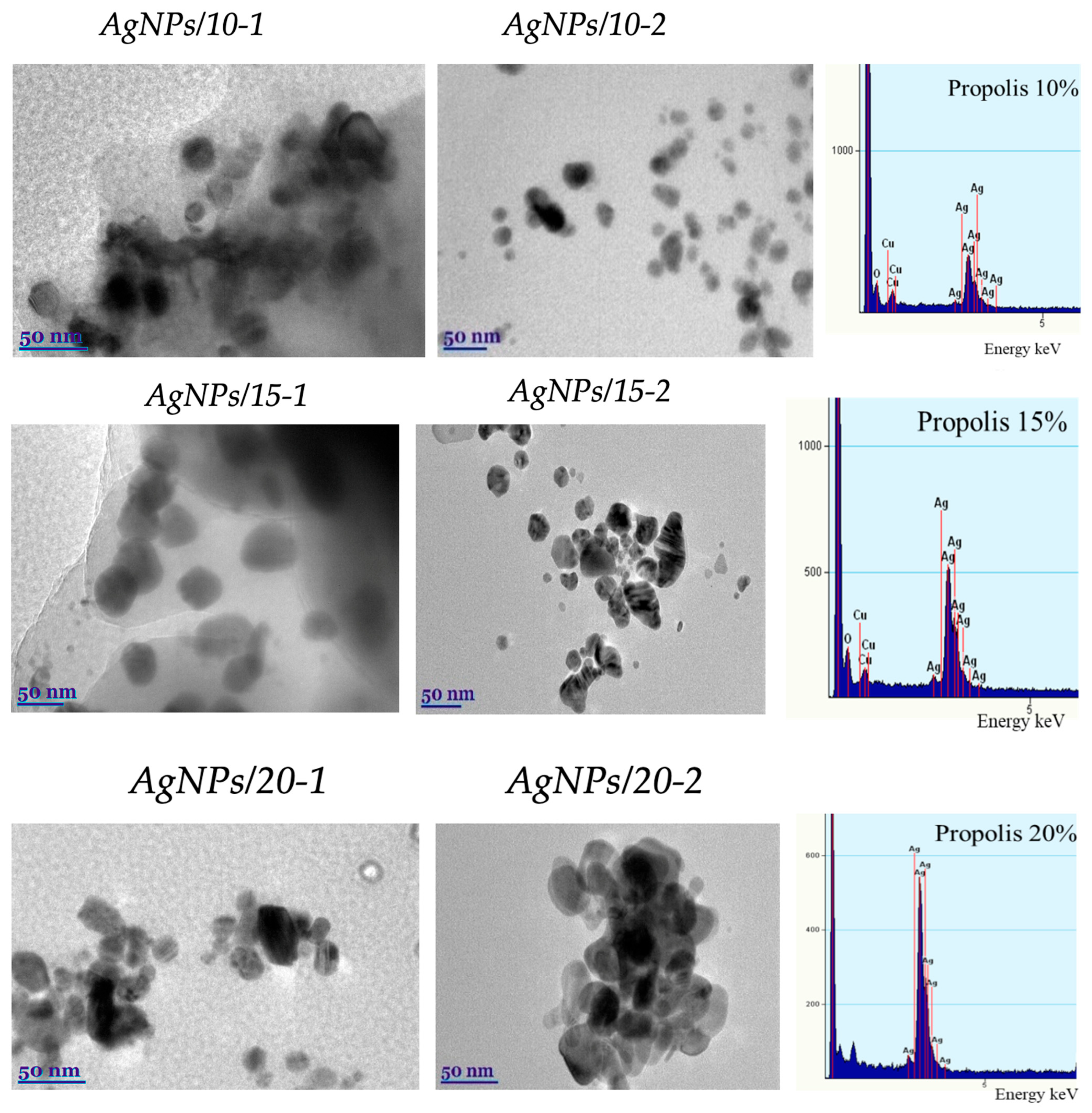
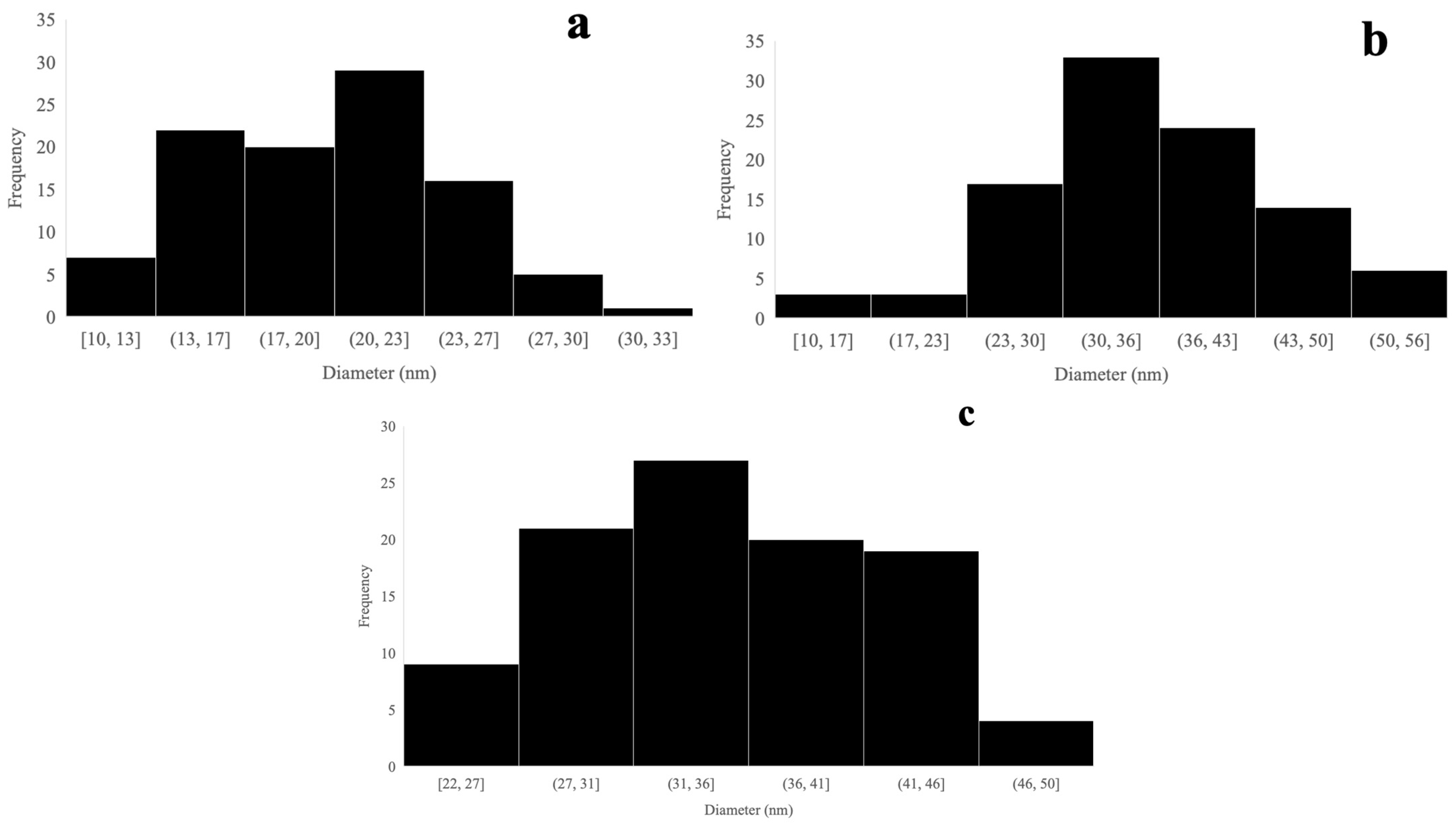
3.3. Transmission Electron Microscopy (TEM) of Propolis/AgNPs
3.4. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mançano, R.R.; Matheus, L.R.; Castro, L.E.N.; Barroso, T.L.C.T.; Da Rosa, R.G.; Ferreira, V.C.; Forster-Carneiro, T.; Colpini, L.M.S. Extraction Techniques for Propolis and Its Utilization in Silver Nanoparticle Synthesis: A Comprehensive Review. Eur. Food Res. Technol. 2025, 251, 1397–1433. [Google Scholar] [CrossRef]
- Zehra, S.H.; Ramzan, K.; Viskelis, J.; Viskelis, P.; Balciunaitiene, A. Advancements in Green Synthesis of Silver-Based Nanoparticles: Antimicrobial and Antifungal Properties in Various Films. Nanomaterials 2025, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Balciunaitiene, A.; Zehra, S.H.; Liaudanskas, M.; Zvikas, V.; Viskelis, J.; Nuapia, Y.B.; Siukscius, A.; Singh, P.K.; Janulis, V.; Viskelis, P. Biosynthesis of Silver Nanoparticles via Medusomyces Gisevii Fermentation with Origanum Vulgare L. Extract: Antimicrobial Properties, Antioxidant Properties, and Phytochemical Analysis. Molecules 2025, 30, 1706. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Recent Advances in Antibacterial Applications of Metal Nanoparticles (MNPs) and Metal Nanocomposites (MNCs) against Multidrug-Resistant (MDR) Bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 419–428. [Google Scholar] [CrossRef]
- Dantas, K.N.M.; Andrade, L.R.; Lisboa, E.; Santana, V.L.; Santos, A.L.S.; Mello, T.P.; Sangenito, L.S.; Lima, Á.S.; Fricks, A.T.; Begnami, A.F.; et al. Antimycotic Nail Polish Based on Humic Acid-coated Silver Nanoparticles for Onychomycosis. J. Chem. Tech. Biotech. 2021, 96, 2208–2218. [Google Scholar] [CrossRef]
- Santos, T.S.; Passos, E.M.D.; Seabra, M.G.D.J.; Souto, E.B.; Severino, P.; Mendonça, M.D.C. Entomopathogenic Fungi Biomass Production and Extracellular Biosynthesis of Silver Nanoparticles for Bioinsecticide Action. Appl. Sci. 2021, 11, 2465. [Google Scholar] [CrossRef]
- Bankova, V. Chemical Diversity of Propolis and the Problem of Standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Umsza-Guez, M.A.; Ramos Rodrigues, D.M.; Gálvez-Ruiz, J.C.; De Paula Castro, T.L.; Balderrama-Carmona, A.P. Comparison of the Biological Potential and Chemical Composition of Brazilian and Mexican Propolis. Appl. Sci. 2021, 11, 11417. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Propolis: A Review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Duarte Silveira, M.A.; De Jong, D.; Dos Santos Galvão, E.B.; Ribeiro, J.C.; Silva, T.C.; Berretta, A.A.; Amorim, T.C.; San Martin, R.L.A.; Rebelo Da Conceição, L.F.M.; Dantas Gomes, M.M.; et al. Efficacy of Propolis as an Adjunct Treatment for Hospitalized COVID-19 Patients: A Randomized, Controlled Clinical Trial. MedRxiv 2021, 138, 111526. [Google Scholar] [CrossRef]
- Sokolonski, A.R.; Fonseca, M.S.; Machado, B.A.S.; Deegan, K.R.; Araújo, R.P.C.; Umsza-Guez, M.A.; Meyer, R.; Portela, R.W. Activity of Antifungal Drugs and Brazilian Red and Green Propolis Extracted with Different Methodologies against Oral Isolates of Candida spp. BMC Complement. Med. Ther. 2021, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Galvéz-Ruíz, J.C.; Ikner, L.A.; Umsza-Guez, M.A.; De Paula Castro, T.L.; Gerba, C.P. In Vitro Antiviral Effect of Mexican and Brazilian Propolis and Phenolic Compounds against Human Coronavirus 229E. Int. J. Environ. Health Res. 2023, 33, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, H.K. Assessment of Propolis Treated by Different Extraction Methods. Braz. Arch. Biol. Technol. 2022, 65, e22210251. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, Z.; Yuan, J.; Zhang, C.; Cao, W.; Qin, X. Collagen Peptides Derived from Sipunculus Nudus Accelerate Wound Healing. Molecules 2021, 26, 1385. [Google Scholar] [CrossRef]
- Contieri, L.S.; De Souza Mesquita, L.M.; Sanches, V.L.; Chaves, J.; Pizani, R.S.; Da Silva, L.C.; Viganó, J.; Ventura, S.P.M.; Rostagno, M.A. Recent Progress on the Recovery of Bioactive Compounds Obtained from Propolis as a Natural Resource: Processes, and Applications. Sep. Purif. Technol. 2022, 298, 121640. [Google Scholar] [CrossRef]
- Teixeira, T.D.; Machado, B.A.S.; Barreto, G.D.A.; Dos Anjos, J.P.; Leal, I.L.; Nascimento, R.Q.; Hodel, K.V.S.; Umsza-Guez, M.A. Extraction of Antioxidant Compounds from Brazilian Green Propolis Using Ultrasound-Assisted Associated with Low- and High-Pressure Extraction Methods. Molecules 2023, 28, 2338. [Google Scholar] [CrossRef] [PubMed]
- Madkour, L.H. Ecofriendly Green Biosynthesized of Metallic Nanoparticles: Bio-Reduction Mechanism, Characterization and Pharmaceutical Applications in Biotechnology Industry. Glob. Drugs Ther. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Kustov, L.; Vikanova, K. Synthesis of Metal Nanoparticles under Microwave Irradiation: Get Much with Less Energy. Metals 2023, 13, 1714. [Google Scholar] [CrossRef]
- Badrillah, N.; Susanti, D.; Kamil, T.K.T.M.; Swandiny, G.F.; Widyastuti, Y.; Zaini, E.; Taher, M. Silver Nanoparticles Biogenically Synthesised Using Maclurodendron Porteri Extract and Their Bioactivities. Heliyon 2024, 10, e25454. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Szymczak, M.; Maciejewska, M.; Laskowski, Ł.; Laskowska, M.; Ostaszewski, R.; Skiba, G.; Franiak-Pietryga, I. All That Glitters Is Not Silver—A New Look at Microbiological and Medical Applications of Silver Nanoparticles. Int. J. Mol. Sci. 2021, 22, 854. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Minaeian, S.; Shahverdi, H.R.; Jamalifar, H.; Nohi, A.-A. Rapid Synthesis of Silver Nanoparticles Using Culture Supernatants of Enterobacteria: A Novel Biological Approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Satpathy, S.; Patra, A.; Ahirwar, B.; Delwar Hussain, M. Antioxidant and Anticancer Activities of Green Synthesized Silver Nanoparticles Using Aqueous Extract of Tubers of Pueraria tuberosa. Artif. Cells Nanomed. Biotechnol. 2018, 46, 71–85. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Viskelis, P.; Viskelis, J.; Streimikyte, P.; Liaudanskas, M.; Bartkiene, E.; Zavistanaviciute, P.; Zokaityte, E.; Starkute, V.; Ruzauskas, M.; et al. Green Synthesis of Silver Nanoparticles Using Extract of Artemisia Absinthium L., Humulus Lupulus L. and Thymus Vulgaris L., Physico-Chemical Characterization, Antimicrobial and Antioxidant Activity. Processes 2021, 9, 1304. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Anticancer, Antimicrobial, Antioxidant, and Catalytic Activities of Green-Synthesized Silver and Gold Nanoparticles Using Bauhinia Purpurea Leaf Extract. Bioprocess Biosyst. Eng. 2019, 42, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Green Synthesis and Characterization of Gold and Silver Nanoparticles Using Mussaenda Glabrata Leaf Extract and Their Environmental Applications to Dye Degradation. Environ. Sci. Pollut. Res. 2017, 24, 17347–17357. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Fozia, F.; Gul, A.; Ahmad, I.; Ullah, R.; Bari, A.; Mothana, R.A.; Hussain, H. Phyto-Extract-Mediated Synthesis of Silver Nanoparticles Using Aqueous Extract of Sanvitalia Procumbens, and Characterization, Optimization and Photocatalytic Degradation of Azo Dyes Orange G and Direct Blue-15. Molecules 2021, 26, 6144. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Riaz, S.; Naseem, S. Microwave-Assisted Green Synthesis and Characterization of Silver Nanoparticles Using Melia Azedarach for the Management of Fusarium Wilt in Tomato. Front. Microbiol. 2020, 11, 238. [Google Scholar] [CrossRef]
- Abboud, Y.; Eddahbi, A.; El Bouari, A.; Aitenneite, H.; Brouzi, K.; Mouslim, J. Microwave-Assisted Approach for Rapid and Green Phytosynthesis of Silver Nanoparticles Using Aqueous Onion (Allium Cepa) Extract and Their Antibacterial Activity. J. Nanostruct. Chem. 2013, 3, 84. [Google Scholar] [CrossRef]
- Bano, S.; Nazir, S.; Nazir, A.; Munir, S.; Mahmood, T.; Afzal, M.; Ansari, F.L.; Mazhar, K. Microwave-Assisted Green Synthesis of Superparamagnetic Nanoparticles Using Fruit Peel Extracts: Surface Engineering, T2 Relaxometry, and Photodynamic Treatment Potential. Int. J. Nanomed. 2016, 11, 3833–3848. [Google Scholar] [CrossRef]
- Bhat, R.; Sharanabasava, V.G.; Deshpande, R.; Shetti, U.; Sanjeev, G.; Venkataraman, A. Photo-Bio-Synthesis of Irregular Shaped Functionalized Gold Nanoparticles Using Edible Mushroom Pleurotus Florida and Its Anticancer Evaluation. J. Photochem. Photobiol. B Biol. 2013, 125, 63–69. [Google Scholar] [CrossRef]
- Petruzzi, L.; Rosaria Corbo, M.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef]
- Oršolić, N.; Landeka Jurčević, I.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Perak Junaković, E.; Terzić, S.; Jutrić, D. Effect of Propolis on Diet-Induced Hyperlipidemia and Atherogenic Indices in Mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef]
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The Use of Propolis in Dentistry, Oral Health, and Medicine: A Review. J. Oral Biosci. 2021, 63, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential Role of Propolis in Wound Healing: Biological Properties and Therapeutic Activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing Veterinary Drug Residues in Animal Products: A Review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA, 2019.
- Keum, G.B.; Kim, E.S.; Cho, J.; Song, M.; Oh, K.K.; Cho, J.H.; Kim, S.; Kim, H.; Kwak, J.; Doo, H.; et al. Analysis of Antibiotic Resistance Genes in Pig Feces during the Weaning Transition Using Whole Metagenome Shotgun Sequencing. J. Anim. Sci. Technol. 2023, 65, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tosi, E.A.; Ré, E.; Ortega, M.E.; Cazzoli, A.F. Food Preservative Based on Propolis: Bacteriostatic Activity of Propolis Polyphenols and Flavonoids upon Escherichia Coli. Food Chem. 2007, 104, 1025–1029. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent Progress in Pharmacological Research of Propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef]
- Salatino, A. Perspectives for Uses of Propolis in Therapy against Infectious Diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Kubilienė, L.; Žvikas, V.; Trumbeckaitė, S. Comparison of Ethanolic and Aqueous-Polyethylenglycolic Propolis Extracts: Chemical Composition and Antioxidant Properties. Evid.-Based Complement. Altern. Med. 2021, 2021, 5557667. [Google Scholar] [CrossRef]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative Preparation of Propolis Extracts: Comparison of Their Composition and Biological Activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is There a Potential for the Development of New Drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.M.; DeCastelli, L.; Nobile, M.; Martucci, F.; Mosconi, G.; Fontana, M.; Castrica, M.; Arioli, F.; Panseri, S. Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT 2020, 131, 1097831. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, D.Y.; Kang, H.J.; Yun, S.H.; Mariano, E., Jr.; Lee, J.; Kim, J.H.; Hur, S.J. Analysis of changes in antibiotic resistance in the human body using an in vitro digestion model incorporating human gut microbiota. Heliyon 2023, 9, e16128. [Google Scholar] [CrossRef] [PubMed]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi. Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.; Varma, R.S. Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Let. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademi˙ r, S.E.N.; Altun, M. Total antioxidant capacity assay of human serum using copper (II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Revilla, I.; Vivar-Quintana, A.M.; González-Martín, I.; Escuredo, O.; Seijo, C. The potential of near infrared spectroscopy for determining the phenolic, antioxidant, color and bactericide characteristics of raw propolis. Michrochem. J. 2017, 134, 211–217. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Bhatia, R.S.; Nandre, V.S.; Gundu, S.S.; Jagtap, S.D.; Kulkani, M.V. HPLC, NMR based chemical profiling and biological characterisation of Indian propolis. Fitoterapia 2017, 122, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Gałkowska, D.; Bugaj, R.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2014, 29, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Salleh, S.N.A.S.; Hanapiah, N.A.M.; Johari, W.L.W.; Ahmad, H.; Osman, N.H. Analysis of bioactive compounds and chemical composition of Malaysian stingless bee propolis water extracts. Saudi J. Biol. Sci. 2021, 28, 6705–6710. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic Constituents, Antioxidant and Antimicrobial Activity and Clustering Analysis of Propolis Samples Based on PCA from Different Regions of Anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef]
- Ibrahim, M.E.E.; Alqurashi, R.M. Anti-fungal and antioxidant properties of propolis (bee glue) extracts. Int. J. Food Microbiol. 2022, 361, 109463. [Google Scholar] [CrossRef]
- Fikri, A.M.; Sulaeman, A.; Marliyati, S.A.; Fahrudin, M. Antioxidant Activity and Total Phenolic Content of Stingless Bee Propolis from Indonesia. J. Api. Sci. 2019, 63, 139–147. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Giménez, A.; Schmeda-Hirschmann, G. Chemical profiling and antioxidant activity of Bolivian propolis. J. Sci. Food Agric. 2016, 96, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, X.; Wang, K.; Cao, X.; Zhang, C.; Zheng, H.; Hu, F. Antioxidant activities and molecular mechanisms of the ethanol extracts of Baccharis propolis and Eucalyptus propolis in RAW64. 7 cells. Pharm. Biol. 2016, 54, 2220–2235. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Puzeryte, V.; Radenkovs, V.; Krasnova, I.; Memvanga, P.B.; Viskelis, P.; Streimikyte, P.; Viskelis, J. Sustainable–green synthesis of silver nanoparticles using aqueous Hyssopus Officinalis and Calendula Officinalis extracts and their antioxidant and antibacterial activities. Molecules 2022, 27, 7700. [Google Scholar] [CrossRef]
- Luzala, M.M.; Muanga, C.K.; Kyana, J.; Safari, J.B.; Zola, E.N.; Mbusa, G.V.; Nuapia, Y.B.; Liesse, J.I.; Nkanga, C.I.; Krause, R.W.M.; et al. A critical review of the antimicrobial and antibiofilm activities of green-synthesized plant-based metallic nanoparticles. Nanomaterials 2022, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Bertotto, C.; Bilck, A.P.; Yamashita, F.; Anjos, O.; Siddique, M.A.B.; Harrison, S.; Brunton, N.P.; Carpes, S.T. Development of a biodegradable plastic film extruded with the addition of a Brazilian propolis by-product. LWT 2022, 157, 113124. [Google Scholar] [CrossRef]
- Şengül, M.; Seda, U.F.U.K. The effects of different extraction methods and solvents on antioxidant properties of propolis. BSJ Agri. 2023, 6, 386–393. [Google Scholar]
- Balčiūnaitienė, A.; Liaudanskas, M.; Puzerytė, V.; Viškelis, J.; Janulis, V.; Viškelis, P.; Griškonis, E.; Jankauskaitė, V. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Balčiūnaitienė, A.; Štreimikytė, P.; Puzerytė, V.; Viškelis, J.; Štreimikytė-Mockeliūnė, Ž.; Maželienė, Ž.; Sakalauskienė, V.; Viškelis, P. Antimicrobial activities against opportunistic pathogenic bacteria using green synthesized silver nanoparticles in plant and lichen enzyme-assisted extracts. Plants 2022, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Kędzia, B.; Hołderna-Kędzia, E. Aktywność antybiotyczna propolisu krajowego i europejskiego. The antibiotic activity of native and European propolis. Post. Fitoter. 2013, 2, 97–107. [Google Scholar]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Wahyuni, T.D.; Hatta, M.; Bukhari, A.; Santoso, A.; Massi, M.N. Increasing Natural Resistance Associated Macrophage Protein 1 serum level after Miana treatment in BALB/c induced Klebsiella pneumoniae experimental research. Ann. Med. Surg. 2021, 65, 102262. [Google Scholar] [CrossRef]
- Taufik, F.F.; Natzir, R.; Patellongi, I.; Santoso, A.; Hatta, M.; Junita, A.R.; Syukri, A.; Primaguna, M.R.; Dwiyanti, R.; Febrianti, A. In vivo and in vitro inhibition effect of propolis on Klebsiella pneumoniae: A review. Ann. Med. Surg. 2022, 81, 104388. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Hussain, E.A.; Shujaat, S.; Rasheed, M.A. Green Synthesis of Propolis Mediated Silver Nanoparticles with Antioxidant, Antibacterial, Anti-Inflammatory Properties and Their Burn Wound Healing Efficacy in Animal Model. Biomed. Phys. Eng. Express 2025, 11, 015050. [Google Scholar] [CrossRef] [PubMed]
- Karimitabar, Z.; Farmani, A.; Azimzadeh, M.; Alikhani, M.S.; Moghadam Shakib, M.; Alikhani, M.Y. The Antimicrobial Activity of Propolis Ethanolic Extract and Silver Nanoparticles Synthesized by Green Method on Gram-Positive and Negative Bacteria. Avicenna J. Clin. Microbiol. Infect. 2023, 10, 131–136. [Google Scholar] [CrossRef]
- Singh, S.; Nirala, S.K.; Kumar, D.; Shukla, S.; Shrivastava, S.; Bhadauria, M. Physicochemical Characterization and In Vitro Biological Activity of Silver Nanoparticles of Propolis Extract. BioNanoScience 2024, 14, 2181–2196. [Google Scholar] [CrossRef]


| Sample | L* | a* | b* |
|---|---|---|---|
| 10%-1 | 54.23 | 1.73 | 9.48 |
| 10%-1 AgNPs | 31.52 | 2.45 | 2.68 |
| 15%-1 | 55.54 | 1.72 | 17.12 |
| 15%-1 AgNPs | 29.77 | 0.96 | 1.28 |
| 15%-2 | 42.86 | 1.73 | 16.52 |
| 15%-2 AgNPs | 42.86 | 1.73 | 16.52 |
| 20%-1 | 54.24 | 1.34 | 20.71 |
| 20%-1 AgNPs | 33.93 | 1.51 | 6.31 |
| 20%-2 | 41.54 | 2.89 | 17.36 |
| 20%-2 AgNPs | 29.71 | 3.76 | 2.73 |
| Extract | The Total Proantocyanidin Content, mg EE/g DW | The Total Hydroxycinnamic Acid Derivatives Content, mg ChAE/g DW | The Total Phenolic Content, mg GAE/g DW | The Total Flavonoid Content, mg RE/g DW |
|---|---|---|---|---|
| 10-1 | 3.46 ± 0.69 c | 4.47 ± 0.45 d | 92.93 ± 3.72 d | 15.73 ± 0.63 c |
| 10-1 AgNPs | 1.17 ± 0.23 e | 0.68 ± 0.14 g | 6.66 ± 0.67 f | 3.58 ± 0.36 fg |
| 10-2 | 3.28 ± 0.66 c | 4.27 ± 0.43 d | 93.30 ± 3.73 d | 16.02 ± 0.64 c |
| 10-2 AgNPs | 1.05 ± 0.21 e | 0.78 ± 0.16 fg | 7.21 ± 0.72 f | 3.11 ± 0.31 g |
| 15-1 | 3.27 ± 0.56 c | 5.95 ± 0.45 bc | 122.42 ± 3.67 b | 20.16 ± 0.60 b |
| 15-1 AgNPs | 1.71 ± 0.26 de | 0.87 ± 0.13 efg | 12.63 ± 0.95 f | 6.18 ± 0.46 e |
| 15-2 | 3.84 ± 0.58 bc | 5.79 ± 0.43 c | 111.58 ± 3.35 c | 21.07 ± 0.63 b |
| 15-2 AgNPs | 1.68 ± 0.25 de | 0.98 ± 0.15 efg | 12.96 ± 0.97 f | 5.01 ± 0.38 ef |
| 20-1 | 4.98 ± 0.50 ab | 7.28 ± 0.36 a | 114.39 ± 2.29 c | 31.02 ± 0.62 a |
| 20-1 AgNPs | 2.63 ± 0.26 cd | 1.76 ± 0.18 e | 21.71 ± 1.09 e | 10.30 ± 0.52 d |
| 20-2 | 5.23 ± 0.52 a | 6.81 ± 0.34 ab | 145.69 ± 2.91 a | 31.89 ± 0.64 a |
| 20-2 AgNPs | 2.91 ± 0.29 cd | 1.62 ± 0.16 ef | 22.20 ± 1.11 e | 9.56 ± 0.48 d |
| Extract | ABTS, mmol TE/g DW | DPPH, mmol TE/g DW | CUPRAC, mmol TE/g DW | FRAP, mmol TE/g DW |
|---|---|---|---|---|
| 10-1 | 0.62 ± 0.01 b | 0.19 ± 0.00 cd | 0.29 ± 0.00 c | 1.93 ± 0.01 b |
| 10-1 AgNPs | 0.51 ± 0.00 c | 0.14 ± 0.00 ef | 0.16 ± 0.00 fg | 1.78 ± 0.01 c |
| 10-2 | 0.71 ± 0.00 a | 0.26 ± 0.00 a | 0.35 ± 0.00 b | 2.04 ± 0.04 a |
| 10-2 AgNPs | 0.37 ± 0.02 e | 0.14 ± 0.00 ef | 0.24 ± 0.01 d | 1.70 ± 0.02 d |
| 15-1 | 0.50 ± 0.04 c | 0.18 ± 0.01 cde | 0.22 ± 0.03 de | 1.23 ± 0.02 f |
| 15-1 AgNPs | 0.30 ± 0.05 f | 0.09 ± 0.00 g | 0.19 ± 0.00 ef | 1.16 ± 0.01 g |
| 15-2 | 0.43 ± 0.03 de | 0.20 ± 0.00 b | 0.17 ± 0.00 f | 1.29 ± 0.01 e |
| 15-2 AgNPs | 0.46 ± 0.01 cd | 0.14 ± 0.03 ef | 0.19 ± 0.01 ef | 1.05 ± 0.00 h |
| 20-1 | 0.50 ± 0.00 c | 0.17 ± 0.03 cde | 0.39 ± 0.01 a | 1.20 ± 0.00 fg |
| 20-1 AgNPs | 0.22 ± 0.01 g | 0.10 ± 0.02 fg | 0.13 ± 0.02 g | 0.69 ± 0.01 i |
| 20-2 | 0.43 ± 0.01 de | 0.15 ± 0.02 de | 0.33 ± 0.02 b | 1.17± 0.04 g |
| 20-2 AgNPs | 0.27 ± 0.00 fg | 0.09 ± 0.01 g | 0.09 ± 0.01 h | 0.68 ± 0.00 i |
| Samples | Reference (Standard) Cultures of Microorganisms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | ß-streptococcus | Staphylococcus epidermidis | Escherichia coli | Klebsiella pneumoniae | Pseudomonas aeruginosa | Proteus vulgaris | Bacillus cereus | Enterococcus faecalis | Candida albicans | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 10-1 | 10.00 ± 0.10 de | 11.80 ± 0.10 d | 11.65 ± 0.55 e | 11.90 ± 0.10 d | 10.10 ± 0.55 d | 9.50 ± 0.10 de | 8.87 ± 0.00 e | 6.75 ± 0.05 e | 8.55 ± 0.01 c | 7.40 ± 0.45 e |
| 10-1 AgNPs | 19.10 ± 0.10 b | 17.9 ± 0.10 bc | 15.90 ± 0.10 c | 20.8 ± 0.35 ab | 22.20 ± 0.01 a | 20.00 ± 0.10 a | 15.55 ± 0.04 c | 12.55 ± 0.55 b | 16.25 ± 0.00 a | 12.00 ± 0.1 d |
| 10-2 | 10.40 ± 0.15 d | 9.70 ± 0.10 ef | 9.05 ± 0.25 f | 10.15 ± 0.10 e | 9.75 ± 0.45 d | 10.3 ± 0.25 d | 9.00 ± 0.44 e | 7.00 ± 0.00 de | 9.05 ± 0.15 c | 8.15 ± 0.39 e |
| 10-2 AgNPs | 20.50 ± 0.15 a | 20.40 ± 0.70 a | 19.70 ± 0.01 a | 21.70 ± 0.15 a | 20.35 ± 0.15 b | 20.00 ± 0.90 a | 19.04 ± 0.00 a | 11.55 ± 0.10 c | 14.00 ± 0.45 b | 16.00 ± 0.1 c |
| 15-1 | 9.80 ± 0.00 e | 10.00 ± 0.50 e | 8.55 ± 0.10 fg | 10.00 ± 0.01 e | 9.85 ± 0.40 d | 8.95 ± 0.65 ef | 8.50 ± 0.75 e | 7.85 ± 0.15 d | 9.45 ± 0.87 c | 8.00 ± 0.55 e |
| 15-1 AgNPs | 20.00 ± 0.20 a | 19.10 ± 0.55 ab | 14.65 ± 0.25 d | 17.25 ± 0.60 c | 21.10 ± 0.90 ab | 16.50 ± 0.10 bc | 17.44 ± 0.55 b | 12.50 ± 0.25 b | 13.85 ± 0.20 b | 18.40 ± 0.2 a |
| 20-1 | 8.50 ± 0.50 f | 8.50 ± 0.50 f | 7.80 ± 0.00 g | 10.60 ± 0.30 e | 9.73 ± 0.60 d | 8.55 ± 0.00 ef | 6.50 ± 0.01 f | 6.95 ± 0.00 e | 9.35 ± 0.85 c | 6.20 ± 0.01 f |
| 20-1 AgNPs | 17.50 ± 0.05 c | 18.00 ± 0.90 bc | 16.50 ± 0.45 c | 21.10 ± 0.09 ab | 20.00 ± 0.55 bc | 17.35 ± 0.50 b | 11.48 ± 0.00 d | 13.00 ± 0.0 b | 14.00 ± 0.95 b | 17.32 ± 0.6 b |
| 20-2 | 8.00 ± 0.10 f | 8.75 ± 0.02 ef | 7.87 ± 0.85 g | 10.85 ± 0.15 e | 8.80 ± 0.01 d | 8.02 ± 0.05 f | 6.74 ± 0.02 f | 7.01 ± 0.55 de | 8.55 ± 0.01 c | 7.65 ± 0.10 e |
| 20-2 AgNPs | 18.00 ± 0.25 c | 17.50 ± 0.50 c | 18.20 ± 0.10 b | 20.45 ± 0.75 b | 18.75 ± 0.00 c | 15.85 ± 0.05 c | 15.45 ± 0.25 c | 14.00 ± 0.45 a | 15.33 ± 0.54 ab | 16.95 ± 0.00 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balciunaitiene, A.; Zehra, S.H.; Liaudanskas, M.; Viskelis, J.; Trumbeckaite, S.; Kubiliene, L.; Saunoriute, S.; Memvanga, P.B.; Ive, D.K.; Kyana, J.; et al. Microwave-Assisted Synthesis of Antimicrobial Silver Nanoparticles Using Propolis Extracts. Processes 2025, 13, 2861. https://doi.org/10.3390/pr13092861
Balciunaitiene A, Zehra SH, Liaudanskas M, Viskelis J, Trumbeckaite S, Kubiliene L, Saunoriute S, Memvanga PB, Ive DK, Kyana J, et al. Microwave-Assisted Synthesis of Antimicrobial Silver Nanoparticles Using Propolis Extracts. Processes. 2025; 13(9):2861. https://doi.org/10.3390/pr13092861
Chicago/Turabian StyleBalciunaitiene, Aiste, Syeda Hijab Zehra, Mindaugas Liaudanskas, Jonas Viskelis, Sonata Trumbeckaite, Loreta Kubiliene, Sandra Saunoriute, Patrick B. Memvanga, Dadit K. Ive, Joseph Kyana, and et al. 2025. "Microwave-Assisted Synthesis of Antimicrobial Silver Nanoparticles Using Propolis Extracts" Processes 13, no. 9: 2861. https://doi.org/10.3390/pr13092861
APA StyleBalciunaitiene, A., Zehra, S. H., Liaudanskas, M., Viskelis, J., Trumbeckaite, S., Kubiliene, L., Saunoriute, S., Memvanga, P. B., Ive, D. K., Kyana, J., & Viskelis, P. (2025). Microwave-Assisted Synthesis of Antimicrobial Silver Nanoparticles Using Propolis Extracts. Processes, 13(9), 2861. https://doi.org/10.3390/pr13092861













