Ferrate-Modified Biochar Boosts Ryegrass Phytoremediation of Petroleum and Zinc Co-Contaminated Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Synthesis and Physicochemical Characterization
2.2. Pot Experiment Design
2.3. Soil and Plant Properties Analysis
2.4. Soil Enzyme Activity Assay
2.5. Soil Microbial Community Analysis
2.5.1. Soil Phospholipid Fatty Acids Determination
2.5.2. DNA Extraction and PCR Amplification
2.5.3. Illumina Novaseq Sequencing
2.5.4. Bioinformatic Analysis
2.6. Statistical Analysis
3. Results and Discussion
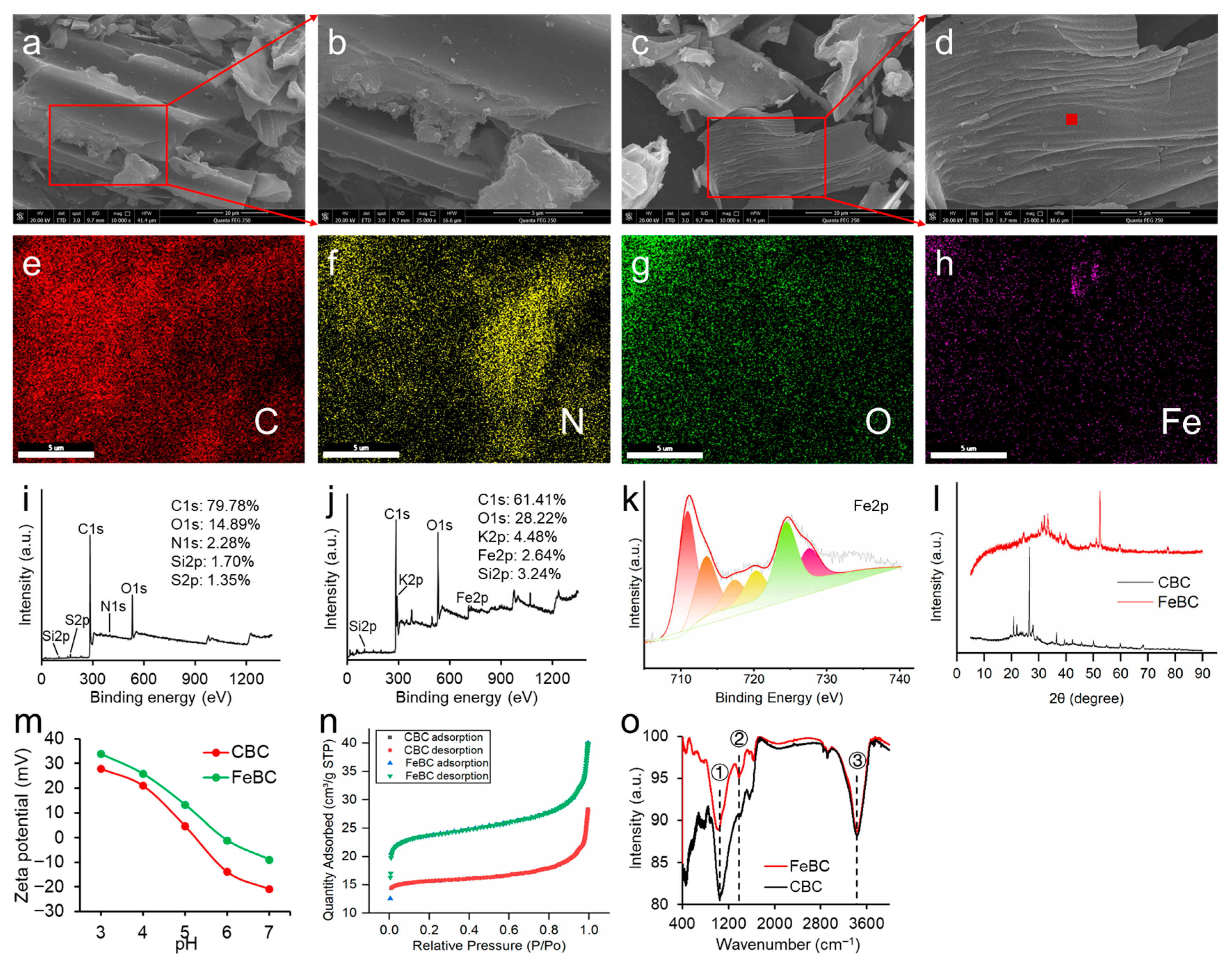
3.1. Physicochemical Characterizations and Adsorption Capacity of FeBC
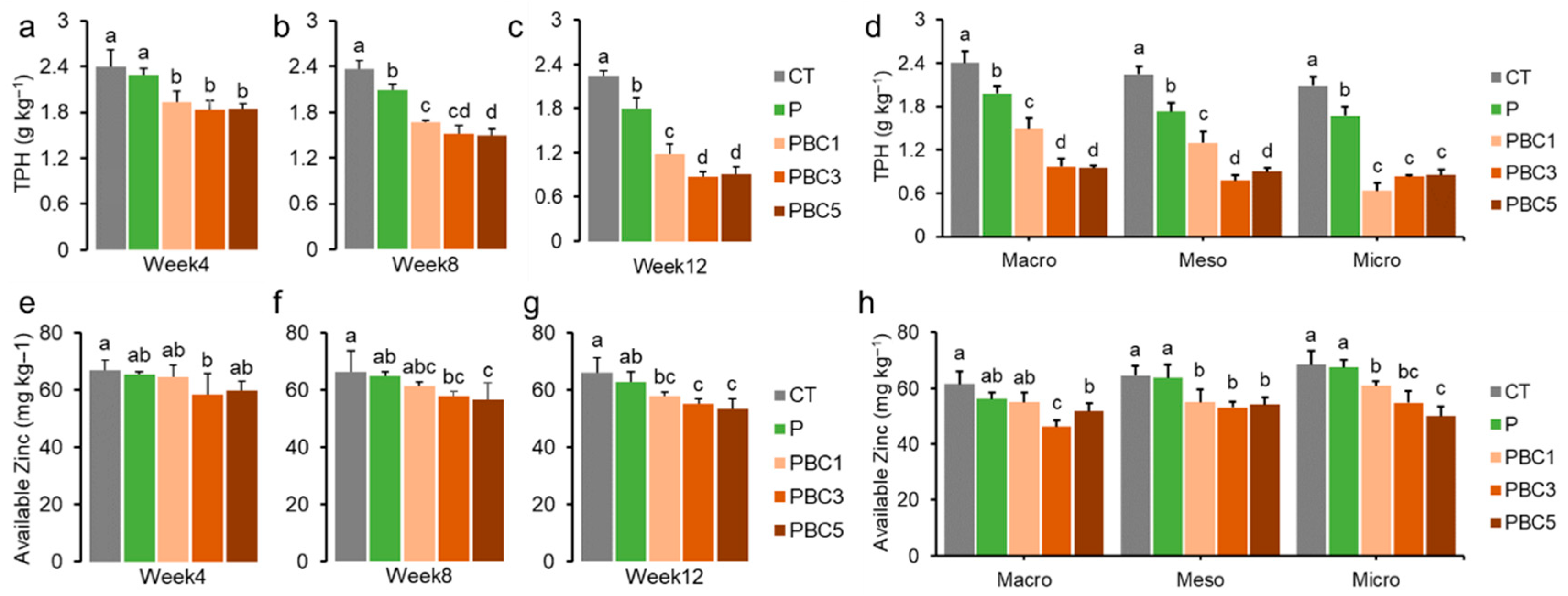
3.2. Soil and Plant Properties
3.3. Soil Enzyme Activities
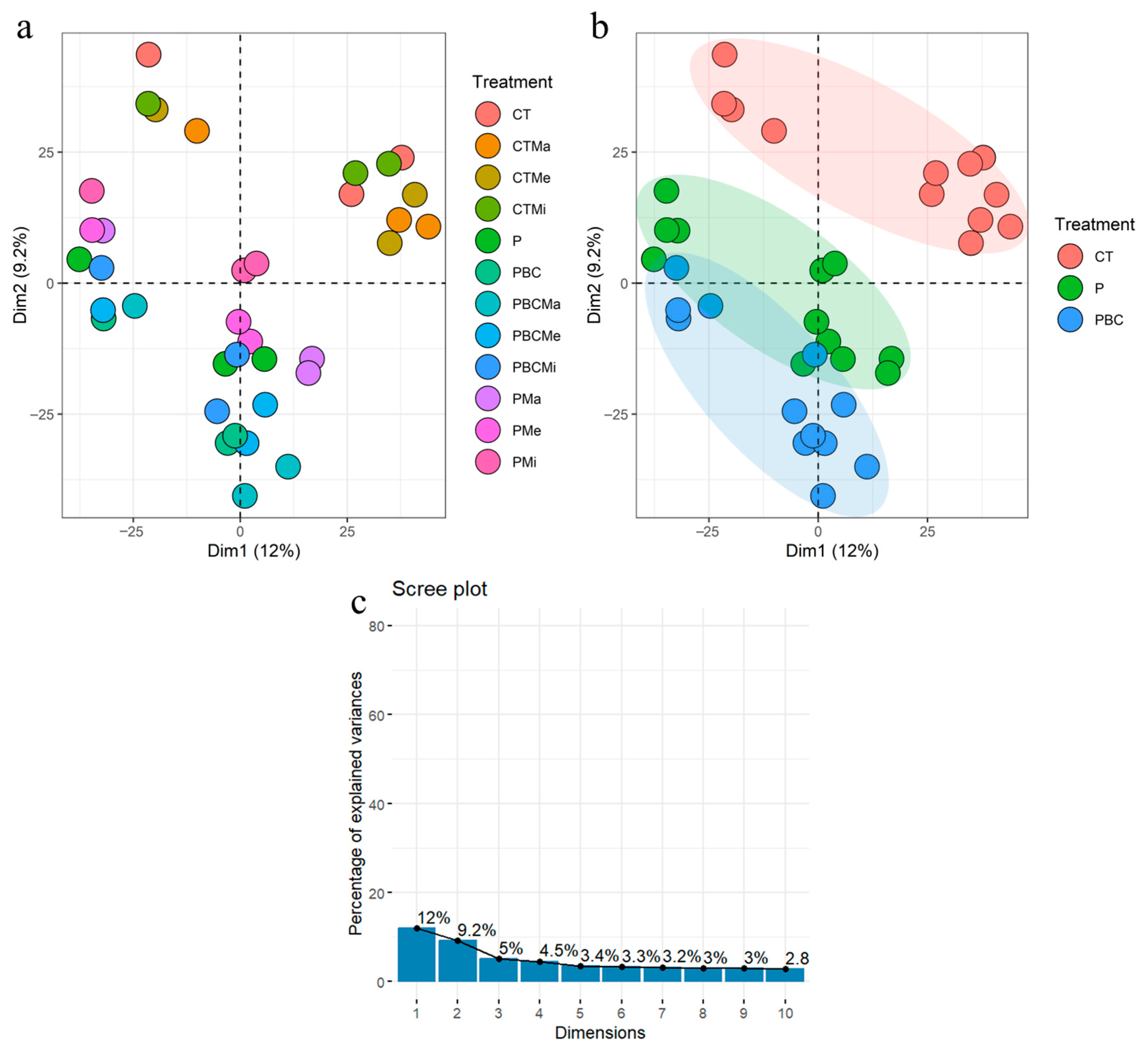
3.4. Soil Phospholipid Fatty Acids
3.5. Soil Microbial Communities
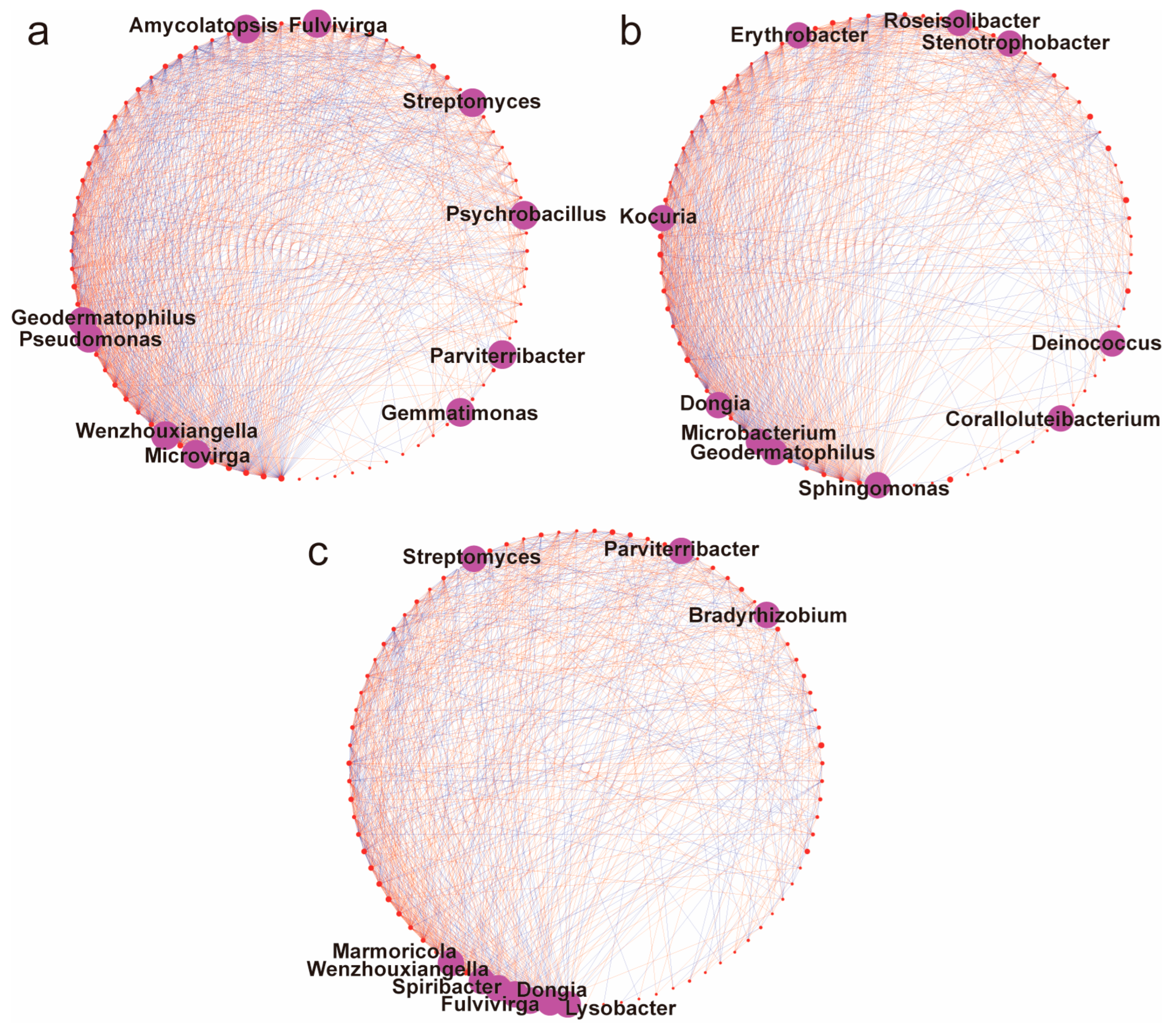
3.6. Soil Microbial Co-Occurrence Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Gu, M.X.; Zhu, W.; Zhang, M.R.; Zhou, W.Z. Petroleum-contamination drives the shift of microbiome through modifying soil metallome. Land Degrad. Dev. 2022, 33, 1718–1730. [Google Scholar] [CrossRef]
- Tang, J.C.; Wang, M.; Wang, F.; Sun, Q.; Zhou, Q.X. Eco-toxicity of petroleum hydrocarbon contaminated soil. J. Environ. Sci. 2011, 23, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wu, M.L.; Liu, H.; Xu, Y.R.; Liu, Z.L. Effect of petroleum hydrocarbon pollution levels on the soil microecosystem and ecological function. Environ. Pollut. 2022, 293, 118511. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Chen, S.G.; Zhou, W.Z. Enhanced phytoremediation of petroleum-contaminated soil by biochar and urea. J. Hazard. Mater. 2023, 453, 131404. [Google Scholar] [CrossRef]
- Kong, D.X.; Liu, Z.; Wu, J. Omics shed light on the mechanisms of petroleum-contaminated soil remediation: When biochar meets nitrogen. Land Degrad. Dev. 2023, 34, 5109–5121. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 21, 240–261. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Yan, J.R.; Zuo, X.X.; Yang, S.J.; Chen, R.Z.; Cai, T.M.; Ding, D.H. Evaluation of potassium ferrate activated biochar for the simultaneous adsorption of copper and sulfadiazine: Competitive versus synergistic. J Hazard. Mater. 2022, 424, 127435. [Google Scholar] [CrossRef]
- Tian, H.; Peng, S.; Zhao, L.; Chen, Y.; Cui, K. Simultaneous adsorption of Cd(II) and degradation of OTC by activated biochar with ferrate: Efficiency and mechanism. J. Hazard. Mater. 2023, 447, 130711. [Google Scholar] [CrossRef]
- Wilson, G.W.T.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Erktan, A.; Cécillon, L.; Graf, F. Increase in soil aggregate stability along a Mediterranean successional gradient in severely eroded gully bed ecosystems: Combined effects of soil, root traits and plant community characteristics. Plant Soil 2016, 398, 121–137. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Germida, J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 2015, 80, 3–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Shengzhe, E.; Wang, Y.; Su, S.; Bai, L.; Wu, C.; Zeng, X. Long-term manure application enhances the stability of aggregates and aggregate-associated carbon by regulating soil physicochemical characteristics. Catena 2021, 203, 105342. [Google Scholar] [CrossRef]
- Chen, L.J.; Wu, L.; Qi, S.; Yuqi, C.; CongYa, W.; Sheng, L. Long-term Camellia oleifera cultivation influences the assembly process of soil bacteria in different soil aggregate particles. Land Degrad. Dev. 2023, 34, 441–452. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Emerson, W.W.; Foster, R.C.; Oades, J.M. Organo-mineral complexes in relation to soil aggregation and structure. Interact. Soil Miner. Nat. Org. Microbes 1986, 17, 521–548. [Google Scholar] [CrossRef]
- Zheng, N.; Luo, M.; Meng, D.; Xu, D.; Liu, Z.; Shao, Y.; Ma, L. Effect of soil aggregate separation methods on the occurrence characteristics of typical pollutants. Processes 2022, 10, 216. [Google Scholar] [CrossRef]
- Luo, X.S.; Yu, S.; Li, X.D. Distribution, availability, and sources of trace metals in different particle size fractions of urban soils in Hong Kong: Implications for assessing the risk to human health. Environ. Pollut. 2011, 159, 1317–1326. [Google Scholar] [CrossRef]
- Qu, J.; Wu, Z.; Liu, Y.; Li, R.; Wang, D.; Wang, S.; Wei, S.; Zhang, J.; Tao, Y.; Jiang, Z.; et al. Ball milling potassium ferrate activated biochar for efficient chromium and tetracycline decontamination: Insights into activation and adsorption mechanisms. Bioresour. Technol. 2022, 360, 127407. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.Y.; Zheng, G.D.; Li, J.; Qin, J.X.; Wang, L.; Ouyang, X.D.; Wang, J.L.; Gu, W.B. Effects of petroleum contamination on soil metal(loid) s and microbial communities. J. Environ. Sci. 2025, 157, 662–673. [Google Scholar] [CrossRef]
- QIIME2. Available online: https://docs.qiime2.org/2019.1/ (accessed on 13 August 2025).
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. Dada2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zhang, Y.; Luo, J.; Chen, L.; Guan, Y. Novel co-polymerization of polypyrrole/polyaniline on ferrate modified biochar composites for the efficient adsorption of hexavalent chromium in water. Chemosphere 2022, 303, 135254. [Google Scholar] [CrossRef]
- Duckworth, O.W.; Polizzotto, M.L.; Thompson, A. Bringing soil chemistry to environmental health science to tackle soil contaminants. Front. Environ. Sci. 2022, 10, 981607. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Aparicio, J.D.; Lacalle, R.G.; Artetxe, U.; Urionabarrenetxea, E.; Becerril, J.M.; Polti, M.A.; Soto, M. Successful remediation of soils with mixed contamination of chromium and lindane: Integration of biological and physico-chemical strategies. Environ. Res. 2021, 194, 110666. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.P.; Abreu, C.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Joseph, S.; Tsechansky, L.; Privat, K.; Schreiter, I.J.; Schüth, C.; Graber, E.R. Biochar aging in contaminated soil promotes Zn immobilization due to changes in biochar surface structural and chemical properties. Sci. Total Environ. 2018, 626, 953–961. [Google Scholar] [CrossRef]
- Xu, P.D.; Zhu, J.; Wang, H.; Shi, L.; Zhuang, Y.; Fu, Q.L.; Chen, J.Z.; Hu, H.Q.; Huang, Q.Y. Regulation of soil aggregate size under different fertilizations on dissolved organic matter, cellobiose hydrolyzing microbial community and their roles in organic matter mineralization. Sci. Total Environ. 2021, 755, 142595. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Change 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhou, H.; Gu, J.F.; Huang, F.; Yang, W.J.; Wang, S.L.; Liao, B.H. Effects of nano-Fe3O4-modified biochar on iron plaque formation and Cd accumulation in rice (Oryza sativa L.). Environ. Pollut. 2020, 260, 113970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhou, H.; Zeng, P.; Wang, S.L.; Yang, W.J.; Huang, F.; Liao, B.H. Nano-Fe3O4-modified biochar promotes the formation of iron plaque and cadmium immobilization in rice root. Chemosphere 2021, 276, 130212. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Diplock, A.T. Glutathione peroxidase (EC 1.11. 1.9), glutathione-S-transferase (EC 2.5. 1.13), superoxide dismutase (EC 1.15. 1.1) and catalase (EC 1.11. 1.6) activities in tissues of ducklings deprived of vitamin E and selenium. Brit. J. Nutr. 1983, 50, 437–444. [Google Scholar] [CrossRef]
- Ashokan, K.V.; Mundaganur, D.S.; Mundaganur, Y.D. Catalase: Phylogenetic characterization to explore protein cluster. J. Res. Bioinform. 2010, 1, 1–8. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Takio, N.; Yadav, M.; Yadav, H.S. Catalase-mediated remediation of environmental pollutants and potential application—A review. Biocatal. Biotransformation 2021, 39, 389–407. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Kim, D.Y.; Kim, J.G. Degradation characteristics of waste lubricants under different nutrient conditions. J. Hazard. Mater. 2007, 143, 65–72. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of biochar application on soil organic carbon composition and enzyme activity in paddy soil under water-saving irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Application of biochar changed the status of nutrients and biological activity in a calcareous soil. J. Soil Sci. Plant Nutr. 2020, 20, 450–459. [Google Scholar] [CrossRef]
- Sadañoski, M.A.; Tatarin, A.S.; Barchuk, M.L.; Gonzalez, M.; Pegoraro, C.N.; Fonseca, M.I.; Villalba, L.L. Evaluation of bioremediation strategies for treating recalcitrant halo-organic pollutants in soil environments. Ecotoxicol. Environ. Saf. 2020, 202, 110929. [Google Scholar] [CrossRef] [PubMed]
- Aislabie, J.; Deslippe, J.R.; Dymond, J. Soil microbes and their contribution to soil services. In Ecosystem Services in New Zealand–Conditions and Trends; Manaaki Whenua Press: Lincoln, New Zealand, 2013; Volume 1, pp. 143–161. [Google Scholar]
- Saccá, M.L.; Barra Caracciolo, A.; Di Lenola, M.; Grenni, P. Ecosystem Services Provided by Soil Microorganisms. In Soil Biological Communities and Ecosystem Resilience; Lukac, M., Grenni, P., Gamboni, M., Eds.; Sustainability in Plant and Crop Protection; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Qi, X.; Xiao, S.; Chen, X.; Ali, I.; Gou, J.; Wang, D.; Han, M. Biochar-based microbial agent reduces U and Cd accumulation in vegetables and improves rhizosphere microecology. J. Hazard. Mater. 2022, 436, 129147. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Wang, F.; Bian, Y.; Jiang, X. Combined effects of maize straw biochar and oxalic acid on the dissipation of polycyclic aromatic hydrocarbons and microbial community structures in soil: A mechanistic study. J. Hazard. Mater. 2019, 364, 325–331. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Yu, Y.; Huang, J.; Zhou, Z.; Zeng, J.; Yan, Q. Ecological stability of microbial communities in Lake Donghu regulated by keystone taxa. Ecol. Indic. 2022, 136, 108695. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Y.; Ding, C.; Ren, X.; Yuan, J.; Sun, F.; Li, Y. Integrated metagenomics and molecular ecological network analysis of bacterial community composition during the phytoremediation of cadmium-contaminated soils by bioenergy crops. Ecotoxicol. Environ. Saf. 2017, 145, 111–118. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Q.; Liu, W.; Zhong, D.; Ge, Y.; Christie, P.; Luo, Y. Soil microbial community and association network shift induced by several tall fescue cultivars during the phytoremediation of a petroleum hydrocarbon-contaminated soil. Sci. Total Environ. 2021, 792, 148411. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Taylor, J.W. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef]
- Röttjers, L.; Faust, K. From hairballs to hypotheses–biological insights from microbial networks. FEMS Microbiol. Rev. 2018, 42, 761–780. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Hu, G.; Hu, C.; Xu, C.; Zhang, Z.; Ning, K. Comparative genomics analysis reveals genetic characteristics and nitrogen fixation profile of Bradyrhizobium. iScience 2024, 27, 108948. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, G.; Tomada, S.; Pertot, I. The impact of the omics era on the knowledge and use of Lysobacter species to control phytopathogenic micro-organisms. J. Appl. Microbiol. 2018, 124, 15–27. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zheng, G.; Liu, Z.; Li, J. Ferrate-Modified Biochar Boosts Ryegrass Phytoremediation of Petroleum and Zinc Co-Contaminated Soils. Processes 2025, 13, 2827. https://doi.org/10.3390/pr13092827
Wang X, Zheng G, Liu Z, Li J. Ferrate-Modified Biochar Boosts Ryegrass Phytoremediation of Petroleum and Zinc Co-Contaminated Soils. Processes. 2025; 13(9):2827. https://doi.org/10.3390/pr13092827
Chicago/Turabian StyleWang, Xinyu, Guodong Zheng, Zhe Liu, and Jie Li. 2025. "Ferrate-Modified Biochar Boosts Ryegrass Phytoremediation of Petroleum and Zinc Co-Contaminated Soils" Processes 13, no. 9: 2827. https://doi.org/10.3390/pr13092827
APA StyleWang, X., Zheng, G., Liu, Z., & Li, J. (2025). Ferrate-Modified Biochar Boosts Ryegrass Phytoremediation of Petroleum and Zinc Co-Contaminated Soils. Processes, 13(9), 2827. https://doi.org/10.3390/pr13092827





