Abstract
The valorization of agro-industrial byproducts like Habanero pepper (Capsicum chinense Jacq.) leaves has gained attention due to their high polyphenol content and bioactivity. In this study, phenolic-rich extracts were obtained using ultrasound-assisted extraction with natural deep eutectic solvents (NADES). Extracts were microencapsulated with maltodextrin, guar gum, and modified starch and incorporated into an isotonic beverage. The bioaccessibility of total and individual polyphenols were assessed through in vitro digestion by simulating fasting and postprandial conditions. Under fasting conditions, the enriched isotonic beverage showed significantly higher total phenolic content (6.98 ± 0.03 mg GAE/100 mL) compared to the control isotonic beverage (5.02 ± 0.22 mg GAE/100 mL), representing a 39% increase. Rutin and quercetin remained detectable throughout digestion, with final concentrations of 1.24 ± 0.05 and 1.10 ± 0.10 mg/100 mL, respectively, in the enriched beverage under postprandial conditions. These findings confirm the protective effect of the encapsulation matrix and highlight NADES as promising solvents for sustainable extraction. This work supports the integration of polyphenol microencapsulated into functional beverages as a novel strategy for delivering bioactive compounds from Capsicum chinense by-products.
1. Introduction
The Habanero pepper (Capsicum chinense Jacq.) is a pepper from the Yucatán Peninsula, internationally recognized for its unique sensory characteristics, phytochemical composition, and geographic designation of origin granted to the Jaguar and Mayapan varieties (IMPI, 2010) [1]. While the fruit is the main commercial product, the cultivation of Habanero pepper generates a large volume of agro-industrial byproducts such as stems, peduncles, and leaves, which are often discarded or burned as waste after harvest. These residues represent nearly 80% of the biomass produced in the region and are increasingly being explored as valuable sources of bioactive compounds [2,3,4].
Among the bioactives molecules found in Capsicum chinense leaves, phenolic compounds, especially flavonoids such as catechin, rutin, and quercetin, have attracted scientific interest due to their antioxidant, anti-inflammatory, hypoglycemic, and anticarcinogenic properties [5,6,7]. However, the successful recovery of these molecules for functional applications requires extraction techniques that comply with green chemistry principles to ensure both safety and efficiency [8].
NADES have emerged as an eco-friendly alternative to conventional organic solvents. They are composed of biodegradable, generally recognized as safe (GRAS) compounds such as sugars, amino acids, and organic acids. Hydrophilic NADES exhibit high extraction efficiency for phenolic compounds while being non-toxic and sustainable [9,10,11,12,13]. In particular, ultrasound-assisted extraction (UAE) with NADES has demonstrated enhanced polyphenol recovery from plant matrices, due to improved cavitation and solvent penetration [14,15].
Nonetheless, once extracted, polyphenols are vulnerable to degradation, particularly in aqueous environments and food matrices, due to their sensitivity to pH, temperature, and enzymatic activity. To overcome this limitation, microencapsulation technologies, such as spray drying using wall materials like maltodextrin, gum arabic, or modified starch, have been widely explored to protect sensitive bioactives, enhance their stability, and enable gradual and targeted release during gastrointestinal transit [16,17,18]. While these encapsulation systems have demonstrated efficacy in improving the digestive stability and bioaccessibility of polyphenols from various plant sources, their integration with NADES remains at an early stage [19,20]. Therefore, the combination of NADES-based extraction and microencapsulation represents a novel and promising approach, offering both efficient recovery of bioactives and functional protection throughout processing and digestion. This emerging strategy is gaining attention for its potential in the development of functional ingredients for food and nutraceutical applications.
Several studies have demonstrated the successful incorporation of microencapsulated phenolic-rich extracts into liquid food matrices. For example, Bernal-Millán et al. [21] microencapsulated oregano polyphenols and reported up to 85% stability during in vitro digestion of model beverages. Similarly, Wyspiańska et al. [22] demonstrated that isoflavone microcapsules added to isotonic drinks showed greater bioaccessibility and resistance to degradation during simulated digestion. In soymilk powder, microencapsulated isoflavones showed up to 1.7-fold higher release post-digestion when protected with maltodextrin–gum arabic blends [23].
Despite these advances, to the best of our knowledge, no studies have incorporated microencapsulated polyphenols extracted using NADES from Habanero pepper leaves into a functional beverage. Therefore, exploring the integration of such bioactive extracts into consumable formats offers a promising strategy for developing novel functional products derived from agro-industrial waste. Furthermore, evaluating their behavior under physiological conditions, such as in vitro gastrointestinal digestion, provides insight into their potential bioaccessibility and health benefits.
The present study investigates the behavior of individual polyphenols from microencapsulated Capsicum chinense leaf extract, obtained using NADES and integrated into an isotonic beverage. The results provide evidence for the potential of NADES-extracted, microencapsulated plant bioactives to serve as functional ingredients in beverages, contributing to sustainable innovation in the food and nutraceutical industries.
2. Materials and Methods
2.1. Plant Material
In this work, leaves from the Jaguar cultivar of Habanero pepper (Capsicum chinense Jacq.) were employed. The plants were grown in a greenhouse using black soil, referred locally in Mayan as Boox Lu’um, in Chablekal, Yucatán (21°06′02.3″ N, 89°33′40.5″ W). The leaves were obtained from the routine pruning practice performed at approximately 120 days post-transplantation, specifically from the area below the fork or stem division, a practice aimed at improving fruit yield and quality [24]. These pruned leaves are typically considered agro-industrial waste; therefore, their use represents a strategy for by-product valorization. For the purposes of this study, only leaves that were green, intact, and free from visible damage or disease were selected, while factors such as size or maturity stage were not considered relevant to the scope of this work.
2.2. Habanero Pepper Leaf Polyphenol Extraction by NADES
2.2.1. Habanero Pepper Leaf Pretreatment
This procedure was adapted with minor modifications from the method proposed by Chel-Guerrero et al. [3]. Initially, the Habanero pepper leaves were manually sorted and then dried in a stainless-steel tray dryer (JERSA®, HS60-AID model, Estado de Mexico, Mexico) at 44 °C for 48 h, to reach a moisture content below 5%. Once dried, the leaves were ground using a Braun® grinder (model KSM-2, Treviso, Italy), and the obtained powder was passed through a 500 µm mesh sieve (#35, Fisher Scientific, Boston, MA, USA) to ensure particle size uniformity, then was stored at room temperature (30 °C) in a resealable plastic bag lined with aluminum foil. This resulting powder was subsequently used for polyphenol extraction.
2.2.2. Polyphenol Extraction Using an Optimized NADES
Avilés-Betanzos et al. [25] reported that the optimal parameters for producing a polyphenol-rich extract from Habanero pepper leaves (HPRE) using a natural deep eutectic solvent (NADES) composed of choline chloride and glucose include a molar ratio of 1:0.8 (choline chloride:glucose) and 68% added water. This solvent was combined with the leaf powder at a 1:10 w/v ratio. The mixture was sonicated by ultrasound-assisted extraction using a Sonics Vibra-Cell® ultrasonic processor (Sonics®, New York, NY, USA; model CV 505), operated at fixed conditions of 5 min of extraction time, 750 W, 20 kHz frequency and 30% amplitude.
Following sonication, the extract was centrifuged at 4700 rpm for 30 min at 4 °C. The resulting supernatant was filtered through a 0.22 µm membrane and stored under refrigeration until further use. From this point forward, the recovered supernatant is referred to as NADES-68.
Polyphenol extraction was also carried out on the enriched and control isotonic beverage samples prior to in vitro digestion, to compare them with the digested samples. The extracts were obtained by sonicating 4 mL of each sample for 20 min using an ultrasonic cleaner (model SB 3200 DTDN, 180 W, 40 kHz, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China). The resulting extracts were centrifuged, filtered, and then stored under refrigeration at 8 °C in order to prevent the degradation of the bioactive compounds of interest.
2.2.3. Determination of Cytotoxicity and Oral Toxicity of Optimized NADES
For the determination of the cytotoxicity of NADES-68, different concentrations of the sample were prepared by diluting it in Dulbecco’s Modified Eagle Medium (DMEM) to reach the required final concentrations and subsequently sterilized by filtration through 0.22 µm membrane filters to avoid contamination of the culture medium. The cytotoxicity assay was conducted using the murine fibroblast cell line CCL-1 NCT clone L-929, obtained from the American Type Culture Collection (ATCC), in accordance with the guidelines established by the national pharmacopoeia, Farmacopea de los Estados Unidos Mexicanos [26].
Cell proliferation was performed using Dulbecco’s Modified Eagle Medium (DMEM) supplemented with fetal bovine serum, and cultures were maintained until they reached over 80% confluence. Once the medium was renewed, 10,000 cells were seeded into multiwell plates and exposed to the test conditions. The functional ingredient sample was applied at concentrations ranging from 6.3 to 0.78%. As controls, sodium dodecyl sulfate (SDS) at 100 µg/mL was used as the positive control, while the culture medium without any added sample served as the negative control. All treatments were performed in triplicate.
After 24 h of exposure, cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, a colorimetric method that evaluates cellular metabolic activity. Absorbance readings were measured at a wavelength of 570 nm.
For the evaluation of acute oral cytotoxicity of the NADES-68, the methodology established by the Organization for Economic Co-operation and Development (OECD) test guideline No. 425 was followed [27], nulliparous and non-pregnant female mice (Mus musculus) of the Bagg Albino strain (BALB/c) were used, with a body weight ranging from 20 to 22 g. The animals were 9 weeks old at the beginning of the study. Prior to the start of the experiment, they were acclimatized for 5 days and monitored daily for any signs of illness or abnormal behavior. Housing was provided in individual polycarbonate cages with stainless steel lids. Lighting was maintained on a 12 h light/dark cycle. The ambient temperature was controlled at 22–24 °C, while relative humidity was monitored and remained within the range of 46–59%, in accordance with the housing conditions established by NOM-062-ZOO-1999 and OECD guidelines. Animals were randomly assigned to treatment. A total of five healthy female mice were used in the study. Each animal was identified using indelible ink applied to the skin (neck area), and cage identification was performed using self-adhesive labels. Food was withdrawn 12 h prior to sample administration, while water was provided ad libitum. Body weight was recorded on the day of sample administration, as well as on days 7 and 14 post-administration.
The administered dose was based on the limit test, using a dose of 2000 mg/kg. Initially, the dose was administered to one animal, followed by four additional animals in accordance with the established methodology.
Observations were carried out continuously during the first 4 h following administration and subsequently monitored over a 14-day period. Throughout this time, various parameters were evaluated, including physical appearance (such as abnormal posture, hypothermia, piloerection, salivation, lacrimation, and nasal discharge), behavioral responses (such as food and water intake, spontaneous or reduced activity, exploratory behavior, aggressiveness, vocalizations, and signs of sedation), as well as physical examination indicators (including mortality-related signs, positioning of the head, limbs, and tail, muscle tone, convulsions, paralysis, and the presence of skin lesions).
All procedures involving animals were conducted in accordance with the ethical standards and regulations outlined in the Mexican Official Norm NOM-062-ZOO-1999 for the care and use of laboratory animals. The experimental protocol was approved by the Internal Committee for the Care and Use of Laboratory Animals of CIATEJ, A.C. (approval code: 2023-018B).
2.3. Microencapsulation of the Habanero Pepper Extract Obtained by NADES
Microencapsulated powder from HPRE was obtained by applying the optimal spray-drying conditions were applied to produce two distinct microencapsulates: one to obtain a high polyphenol content powder (89.4 °C inlet temperature, 7.8% guar gum) and another for a high antioxidant capacity powder (104.1 °C inlet temperature, 8.06% guar gum). The encapsulating agents used included maltodextrin (DE 17–20), modified starch, and guar gum [19].
The procedure to microencapsulate was performed as follows: the HPRE was mixed with the encapsulating agent at a weight ratio of 1:3 (HPRE:encapsulating agent), yielding a 5% solution. This homogeneous mixture was then spray-dried under the previously described conditions, using a MOBILE MINOR™ GEA® spray dryer (Model MM standard, Düsseldorf, Germany). The feeding was carried out at a fixed flow rate of 10 mL/min, achieved through a peristaltic pump (WATSON MARLOW®, model 520S, Düsseldorf, Germany), with an atomization pressure of 3.5 bar and an airflow rate of 80 kg/h.
The subsequent powder was collected via a cyclone separator, sealed in aluminum-lined plastic bags, weighed, and stored at room temperature (30 °C) for further analysis.
2.4. Enriched Isotonic Beverage with Microencapsulated Habanero Pepper Leaf Extract
2.4.1. Preparation of the Control and Enriched Isotonic Beverage
The control isotonic beverage was prepared according to the Mexican Official Standard NOM-218-SSA1-2011 [28], using 200 mL of purified water to which 12 g of sugar (6%), 1.168 g of table salt (3.98 meq sodium), and 400 µL of red fruit flavoring (Deiman®, TX, USA) were added. To prepare the enriched isotonic beverage, the same ingredients were used, with the addition of 4% w/v of the microencapsulated product. The solution was stirred until homogeneous consistency was achieved prior to in vitro digestion.
2.4.2. Determination of Total Polyphenol Content and Antioxidant Capacity of the Enriched Isotonic Beverage
According to the methodology of Johnson et al. [29], with some modifications, the determination of total polyphenols was carried out as described below: A volume of 50 µL of enriched isotonic beverage was transferred to a microplate well, followed by the addition of 400 µL of distilled water and 50 µL of Folin–Ciocalteu reagent (previously diluted 1:3 v/v with distilled water). After a reaction time of 5 min, 50 µL of 10% sodium carbonate solution was added. The plate was incubated at room temperature (30 °C) for 60 min, and the absorbance was measured at 764 nm using a microplate reader.
To determine TPC concentration a calibration curve was prepared using gallic acid as the standard (1 mg/mL stock solution). Serial dilutions were made to obtain the following concentrations: 5, 10, 25, 50, 75, 100, 125, 150, 175, and 200 µg/mL. A calibration curve showed an R2 of 0.9983 (Figure S1).
Results were expressed as mg gallic acid equivalent per 100 mL of isotonic beverage (mg GAE/100 mL).
For the determination of antioxidant capacity (Ax), the methodology of Hernandez-Moreno et al. [30] was followed with some modifications, 20 µL of the enriched isotonic beverage was added to 140 µL of an adjusted DPPH solution (4 mg of 2,2-diphenyl-1-picrylhydrazyl in 100 mL methanol) with an absorbance of 0.700 ± 0.002 at 517 nm (Aadj), previously calibrated using a microplate spectrophotometer (Thermo Scientific Multiskan FC). The absorbance of the sample (Asp), measured after 30 min of incubation, was used in Equation (1) to calculate the percentage of DPPH radical inhibition.
where Asp is the absorbance obtained from the sample and Aadj is the absorbance of the adjusted DPPH.
2.5. In Vitro Digestion of an Isotonic Beverage Enriched with Microencapsulated Habanero Pepper Leaf Extract
2.5.1. Experimental Design
To evaluate the effect of in vitro digestion on the total polyphenol content, polyphenol profile, and antioxidant capacity of an isotonic beverage enriched with microencapsulated Habanero pepper leaf extract, a 23 × 3 factorial design was applied. The first three factors—beverage type (−1: control, +1: enriched), digestion condition (−1: fasted, +1: postprandial), and digestive phase (−1: gastric, +1: intestinal) were assessed at two levels. The fourth factor, sampling time, was evaluated at three levels (−1: early, 0: intermediate, +1: final). The response variables included total polyphenol content (TPC), antioxidant capacity (% inhibition), and polyphenol profile (gallic acid, protocatechuic acid, coumaric acid, cinnamic acid, chlorogenic acid, rutin, quercetin + luteolin, kaempferol, and hesperidin). The experimental design is shown in Table 3.
2.5.2. Standard Food Preparation for Postprandial In Vitro Digestion of the Beverage Enriched with Microencapsulated Habanero Pepper Leaf Extract
Simulating human dietary intake through a standardized food model is a widely accepted strategy for evaluating the behavior of nutrients, bioactive compounds, or contaminants under controlled conditions. This approach enables reproducibility and facilitates the comparison between experimental treatments by minimizing the variability inherent in real diets [31].
To simulate the feeding conditions, a standard food formulation was developed based on a 2000 kcal diet with a macronutrient distribution of 60% carbohydrates, 25% lipids, and 15% proteins, in accordance with the typical dietary intake of the Mexican population [32]. This formulation was prepared by homogenizing 24.5 mL of pure corn cooking oil (Cristal®, Jalisco, Mexico), 155.4 g of nixtamalized corn flour (MASECA®, San Pedro Garza Garcia, Mexico), 16.1 g of white granulated sugar (Aurrera®, Ciudad de Mexico, Mexico), 36.4 g of bacteriological-grade meat peptone (Merck, Darmstadt, Germany), and 7.7 g of casein peptone (Becton Dickinson de México S.A. de C.V.) in 1000 mL of drinking water.
Once the mixture was obtained, it was heated to 75 °C while maintaining constant stirring for 15 min to ensure proper homogenization and pasteurization. Finally, the food was stored under refrigeration with a shelf life of 76 h.
2.5.3. In Vitro Fasted and Postprandial Digestion Simulation of the Isotonic Beverage Enriched with Microencapsulated Habanero Pepper Leaf Extract
The in vitro digestion simulation was performed following the procedure described by Torres-Martínez et al. [33], with slight modifications. In this study, two gastrointestinal conditions were simulated: fasting and postprandial states. The fasting state refers to the physiological condition in which no food has been consumed for an extended period, typically used as a baseline to assess compound stability without dietary interference [34]. In contrast, the postprandial state represents the period following food intake, during which digestive processes are actively engaged. Simulating both conditions allows for a more comprehensive evaluation of the behavior and bioaccessibility of phenolic compounds, since the presence of food components, such as proteins, lipids, and carbohydrates, can significantly influence the release, transformation, and absorption of bioactive molecules during digestion [35].
Under fasted conditions, the gastric phase was initiated by pre-equilibrating a 200 mL sample of isotonic beverage at 37 °C and adjusting the pH to 2.0–2.5 using 5 M HCl. Subsequently, 0.33 mg of pepsin was added, and the mixture was maintained under constant agitation (150 rpm) for 2 h.
At the end of the gastric phase, the pH was adjusted to 5.0–5.5 using 3 M NaOH to initiate the intestinal phase. Pancreatin (0.19 g, CREON®, IL, USA), lipase (1 mg), and bile salts (1 g, Difco®, NJ, USA) were then added, and the mixture was incubated for 4 h at 37 °C under constant agitation (150 rpm).
For the postprandial condition, 200 mL of the isotonic beverage was mixed with 200 mL of the standard food. After homogenization, a 200 mL aliquot was taken, adjusted to a pH of 2.0–2.5, and equilibrated at 37 °C. Pepsin (0.33 mg) was then added to initiate the gastric phase, followed by intestinal digestion as described above.
The in vitro digestion of standard food was also carried out under the previously described conditions.
All digestions were performed in triplicate, and samples were collected at beginning of the initial, intermediate, and final time of both the gastric and intestinal phases. Samples were centrifuged (8000 rpm, 4 °C, 30 min), and the supernatant was recovered and stored in chromatographic vials under refrigeration until further analysis.
2.5.4. Total Polyphenol Content and Antioxidant Capacity from In Vitro Digestion Stages of the Enriched Isotonic Beverage
During the gastric and intestinal digestion of the enriched isotonic beverage, samples were collected at time 0 (T0, beginning of digestion), intermediate time (Ti), and final time (Tf) to monitor changes in total polyphenol content (TPC) and antioxidant capacity. Sampling was carried out under both fasting and postprandial conditions.
The collected samples were centrifuged at 7000 rpm for 30 min at 4 °C. The recovered supernatant was filtered through a 0.22 µm membrane filter, and 50 µL aliquots were used for TPC determination according to Section 2.4.2.
Additionally, 20 µL of the supernatant from each digestion time point was used for the determination of antioxidant capacity, following the procedure described in Section 2.4.2.
2.5.5. Determination and Quantification of Individual Polyphenols in an Isotonic Beverage Enriched with Microencapsulated Habanero Pepper Leaf During In Vitro Digestion
The individual polyphenols were identified and quantified using a high-performance liquid chromatography (HPLC) Acquity H Class chromatograph (Waters, Milford, MA, USA) equipped with a reverse-phase Nova-Pak C18 column (Waters, Milford, MA, USA) and a diode-array detector (DAD). The chromatographic method used a constant flow rate of 0.8 mL/min. The mobile phases consisted of water with 0.2% formic acid (Phase A) and acetonitrile with 0.1% formic acid (Phase B), following the elution gradient below: 0–0.5 min 99% A; 0.5–9 min from 99% A to 70% A; 9–12 min 70% A; 12–12.10 min from 70% A to 99% A; 12.10–17 min 99% A. For quantification, a calibration curve was constructed using 10 polyphenol standards: gallic acid, protocatechuic acid, chlorogenic acid, p-coumaric acid, cinnamic acid, rutin, hesperidin, quercetin, luteolin, and kaempferol. Each compound was prepared at an initial concentration of 1 mg/mL, with a calibration range of 5 to 75 µg/mL, according to the protocol described by Chel-Guerrero et al. [3]. Identification of polyphenols in the microcapsules before and after in vitro digestion was carried out by comparing their retention times with those of the standards (Figure S2). Results were expressed as mg/100 mL of isotonic beverage.
2.6. Statistical Analysis
The experimental procedures were conducted in random order using a 23 × 3 factorial design. For each beverage sample derived from the experimental setup, triplicate measurements were recorded to determine total and individual polyphenols, antioxidant capacity by DPPH, color, moisture content, texture, and sampling point from in vitro digestion. Results are reported as mean values with their corresponding standard deviations. Data from analysis of variance (ANOVA), post hoc comparisons, as well as additional analyses, were processed using the Statgraphics Centurion XVII.II X64 software (Statgraphics Technologies Inc., Warrenton, VA, USA).
3. Results
3.1. Cytotoxicity and Acute Oral Toxicity of the Optimized NADES
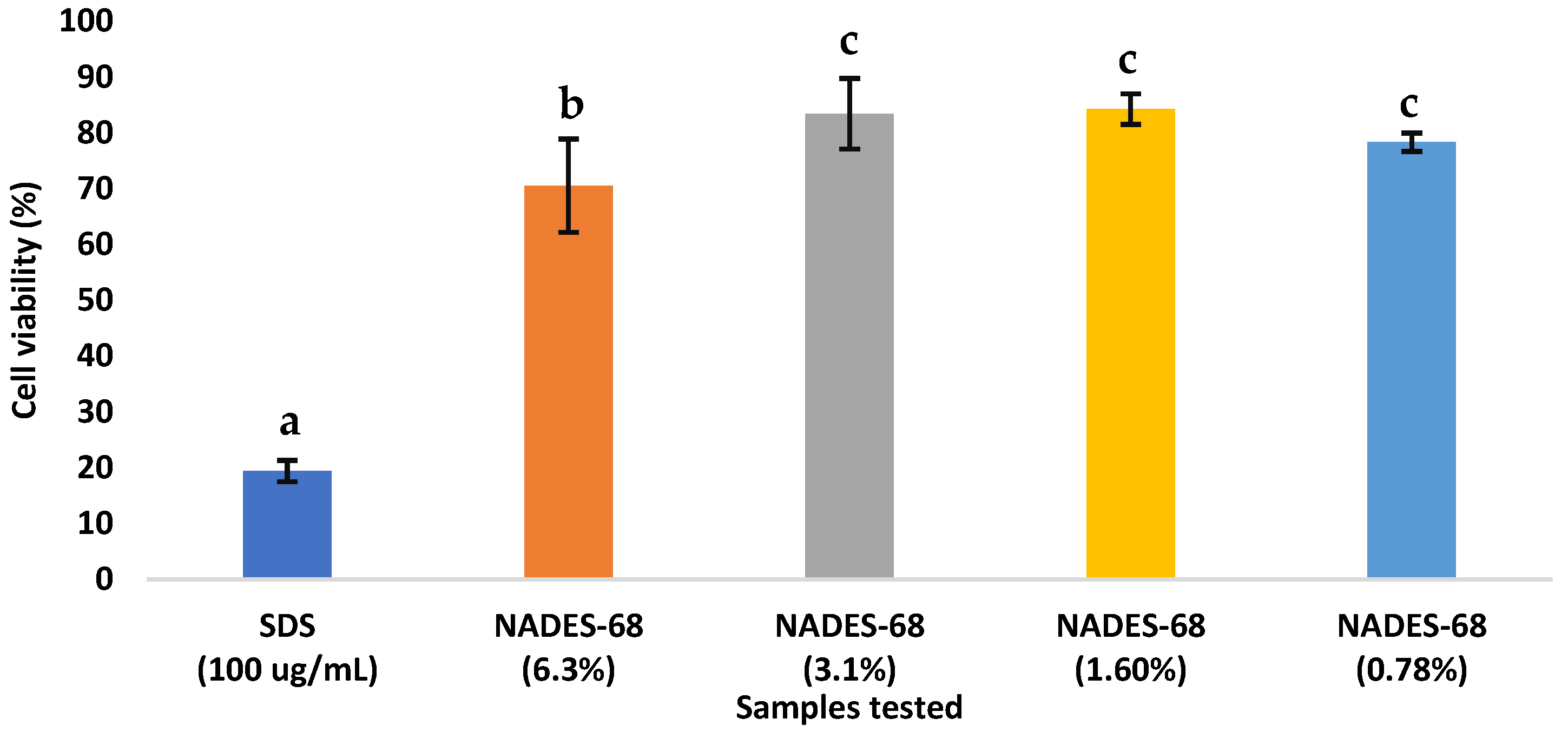
As shown in Figure 1, the cytotoxicity results indicate that cell viability was not affected by exposure to the different concentrations of the NADES-68 sample. In contrast, the positive control (SDS) reduced cell viability to approximately 20% and thus is considered cytotoxic.
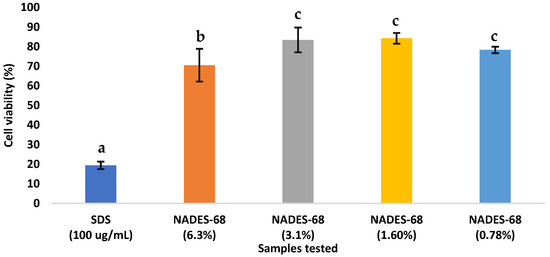
Figure 1.
Cell viability (%) of L-929 fibroblast cells after 24 h exposure to SDS (100 µg/mL, positive control) and different concentrations of NADES-68 (6.3%, 3.1%, 1.6%, and 0.78%) expressed as final concentrations in the culture medium. Bars represent mean ± SD (n = 3). Different letters indicate statistically significant differences (p < 0.05).
According to the Mexican Pharmacopeia, a reduction in cell viability below 70% of the control indicates potential cytotoxicity [26]. Therefore, it is concluded that the NADES-68 sample is not cytotoxic to the L-929 cell line. In the acute oral toxicity test, the experimental animals did not exhibit any signs of adverse effects from the test substance, and no mortality was observed at the administered dose.
All test subjects received a single oral dose of 2000 mg/kg of the NADES-68 formulation. Table 1 summarizes the body weight measurements recorded before treatment, and at 7 and 14 days post-treatment.

Table 1.
Body weight results during the treatment period.
No significant weight loss was observed in any of the animals throughout the observation period. In contrast, a slight increase in body weight was recorded in all individuals, suggesting normal growth and the absence of adverse effects associated with the administered dose. For instance, Animal 1 exhibited an increase from 20.7 g pre-treatment to 21.9 g at day 14, while Animal 4 showed the most pronounced gain, increasing from 22.3 g to 23.3 g; however, no statistically significant differences were observed before and after treatment (p > 0.05). The slight increase in body weight observed in all individuals suggests normal growth, which is considered a reliable indicator of the absence of systemic toxicity. This trend can be attributed to homeostatic regulation and normal metabolic activity, since no physiological stress or impaired nutrient absorption was induced by the tested dose. Following OECD guideline No. 425 have similarly reported stable or slightly increasing body weight as an indicator of health status in rodents exposed to non-toxic compounds [27].
The consistency of weight values across animals further supports the low toxicity profile of the tested formulation.
These results, combined with the lack of clinical signals or mortality, indicate that the NADES-68 formulation is well tolerated at the tested dose level.
Individual clinical observations of female test subjects after receiving a single oral dose of 2000 mg/kg of the NADES-68 formulation are shown in Table 2. Observations were recorded at multiple time points, including every hour for the first 4 h and daily for 14 days post-administration.

Table 2.
Individual clinical observations of test subjects following oral administration of NADES-68.
Throughout the entire observation period, all animals (1–1 to 1–5) consistently exhibited the code “Ø,” which, according to the observation criteria, indicates no signs of systemic toxicity. No adverse clinical signs such as piloerection (Pi), aggressiveness (Ag), convulsions (Co), hypothermia (Hi), or mortality (M) were reported at any of the evaluated time points.
These findings support preliminary evidence that NADES-68 does not induce acute systemic toxicity at a dose of 2000 mg/kg under the conditions of this study, aligning with the absence of mortality and stable body weight previously reported.
Necropsy evaluations indicated that all tissues and organs exhibited normal morphology, with no detectable lesions observed in any of the test animals. Due to the absence of visible pathological alterations, histopathological examination was not conducted. Based on the acute oral toxicity protocol employed, wherein no mortality or clinical signs of toxicity were observed at the limit dose of 2000 mg/kg, the substance identified as a “functional ingredient” can be classified as having no significant acute oral toxicity, with an estimated LD50 exceeding 2000 mg/kg.
3.2. Total Polyphenol Content, Polyphenol Profile and Antioxidant Capacity of an Isotonic Beverage Enriched with Microencapsulated Habanero Pepper Leaf Extract During In Vitro Digestion
Table 3 presents the results for total polyphenol content (TPC) and antioxidant capacity during the in vitro digestion of the enriched beverage, the control beverage, the standard food, and the extracts obtained from both beverages (control and enriched) through sonication.

Table 3.
Factorial Design 23 × 3 for the Evaluation of the Effect of in vitro Digestion on an Isotonic Beverage Enriched with Microencapsulated Habanero Pepper Leaf Extract.
Table 3.
Factorial Design 23 × 3 for the Evaluation of the Effect of in vitro Digestion on an Isotonic Beverage Enriched with Microencapsulated Habanero Pepper Leaf Extract.
| # Exp | Main Factors | Variables Response | ||||
|---|---|---|---|---|---|---|
| BT | Digestion Type | Digestive Phase | Sampling Time | TPC | Ax | |
| 1 | CT | FsT | GaT | ErL | 2.08 ± 0.34 a | 0.00 a |
| 2 | CT | FsT | GaT | MdL | 2.17 ± 0.13 a | 0.00 a |
| 3 | CT | FsT | GaT | FnL | 2.32 ± 0.14 ab | 0.00 a |
| 4 | CT | FsT | InT | ErL | 2.54 ± 0.13a bc | 0.00 a |
| 5 | CT | FsT | InT | MdL | 4.83 ± 0.05 e | 0.00 a |
| 6 | CT | FsT | InT | FnL | 5.02 ± 0.22 e | 0.00 a |
| 7 | CT | PtP | GaT | ErL | 13.77 ± 0.63 ghi | 63.58 ± 1.67 jk |
| 8 | CT | PtP | GaT | MdL | 14.40 ± 1.47 hij | 64.76 ± 1.93 k |
| 9 | CT | PtP | GaT | FnL | 15.33 ± 0.19 jklm | 56.16 ± 0.22 gh |
| 10 | CT | PtP | InT | ErL | 14.30 ± 1.67 hij | 72.94 ± 0.16 l |
| 11 | CT | PtP | InT | MdL | 16.18 ±0.38 klmn | 64.34 ± 3.94 k |
| 12 | CT | PtP | InT | FnL | 17.34 ± 0.70 n | 61.39 ± 0.36 ijk |
| 13 | ECh | FsT | GaT | ErL | 3.43 ± 0.86 bcd | 56.21 ± 1.78 gh |
| 14 | ECh | FsT | GaT | MdL | 5.06 ± 0.16 e | 60.08 ± 0.36 ij |
| 15 | ECh | FsT | GaT | FnL | 4.91 ±0.41 e | 50.91 ± 1.33 f |
| 16 | ECh | FsT | InT | ErL | 4.43 ± 0.71 de | 43.17 ± 3.32 e |
| 17 | ECh | FsT | InT | MdL | 7.08 ± 0.26 f | 34.19 ± 1.44 d |
| 18 | ECh | FsT | InT | FnL | 6.98 ± 0.03 f | 35.86 ± 4.23 d |
| 19 | ECh | PtP | GaT | ErL | 13.09 ±0.36 g | 63.09 ± 2.98 jk |
| 20 | ECh | PtP | GaT | MdL | 14.82 ±0.35 ij | 61.46 ± 3.37 ijk |
| 21 | ECh | PtP | GaT | FnL | 14.97 ±0.37 ijk | 59.07 ± 3.13 hi |
| 22 | ECh | PtP | InT | ErL | 13.55 ±2.76 gh | 54.63 ± 0.22 g |
| 23 | ECh | PtP | InT | MdL | 16.50 ± 0.94 mn | 11.75 ± 1.72 b |
| 24 | ECh | PtP | InT | FnL | 16.43 ± 0.11l mn | 35.29 ±3.32 d |
| 25 | StF | - | GaT | ErL | 14.02 ± 0.20 ghi | 53.79 ± 2.30 fg |
| 26 | StF | - | GaT | MdL | 15.23 ± 0.52 jkl | 62.80 ± 1.51 jk |
| 27 | StF | - | GaT | FnL | 15.50 ± 0.08 jklm | 58.85 ± 0.86 hi |
| 28 | StF | - | InT | ErL | 16.18 ± 0.09 klmn | 73.49 ± 2.31 l |
| 29 | StF | - | InT | MdL | 16.38 ±0.18 lmn | 76.16 ± 1.52 l |
| 30 | StF | - | InT | FnL | 16.32 ± 0.70 lmn | 73.92 ± 6.04 l |
| 31 | CTExT | - | - | - | 1.80 ± 0.03 a | 0.00 a |
| 32 | EChExT | - | - | - | 3.61 ± 0.10 cd | 21.19 ± 0.33 c |
Note: BT = Beverage type; CT = Control isotonic beverage; ECh = Enriched isotonic beverage; StF = Standard food; CTExT = Control isotonic beverage extraction by ultrasonic bath (20 min, 120 W, 40 kHz); EChExT = Enriched isotonic beverage extraction by ultrasonic bath (20 min, 180 W, 40 kHz); FsT = Fasting; PtP = Postprandial; GaT = Gastric phase; InT = Intestinal phase; ErL = Early time sampling; MdL = Middle time sampling; FnL = Final time sampling; TPC = Total polyphenol content (mg Gallic acid Equivalent/100 mL beverage); Ax = Antioxidant capacity (% Inhibition); Each treatment in the experimental design was performed in triplicate. Different letters in the same column indicate statistical difference (LSD, p < 0.05).
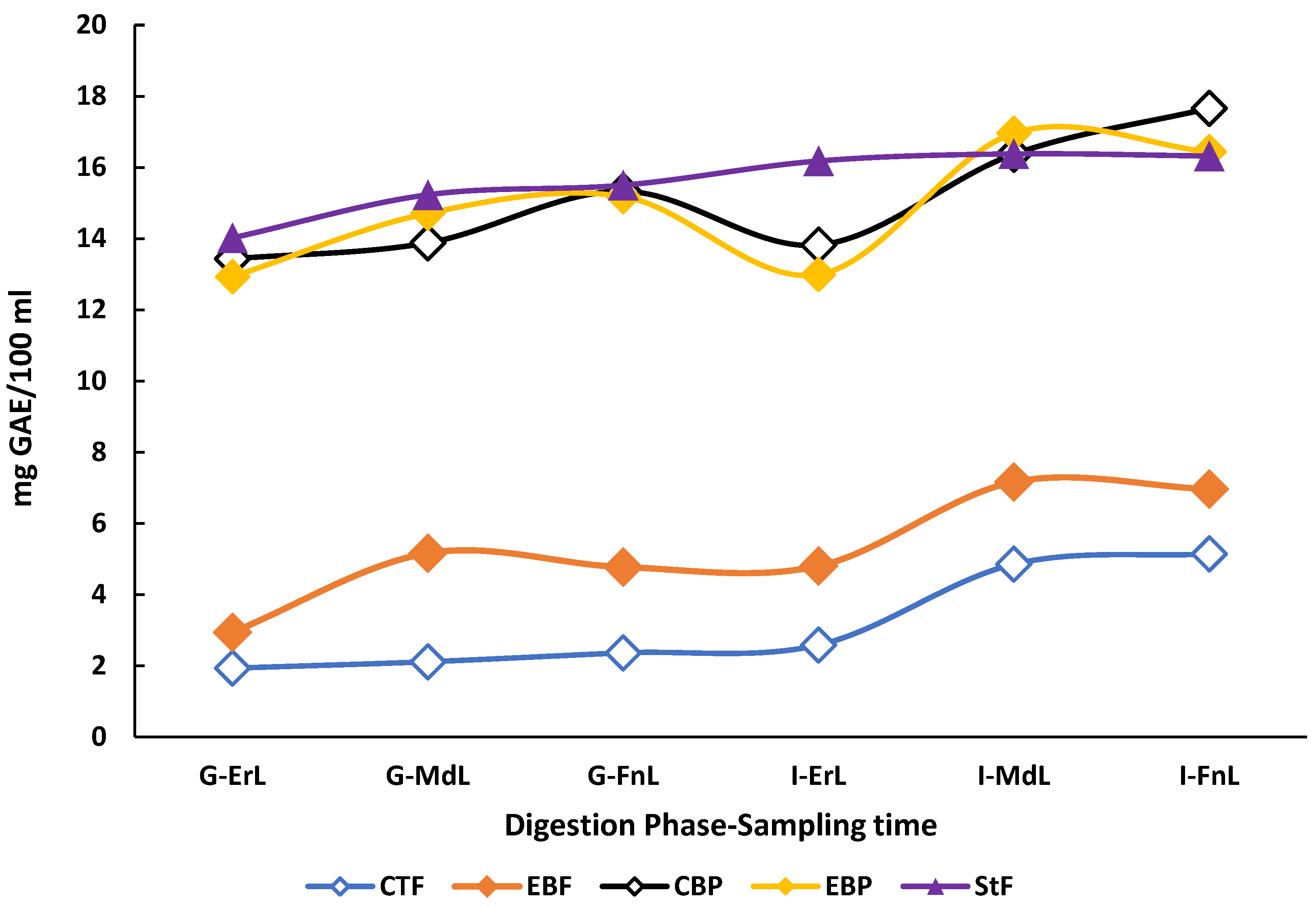
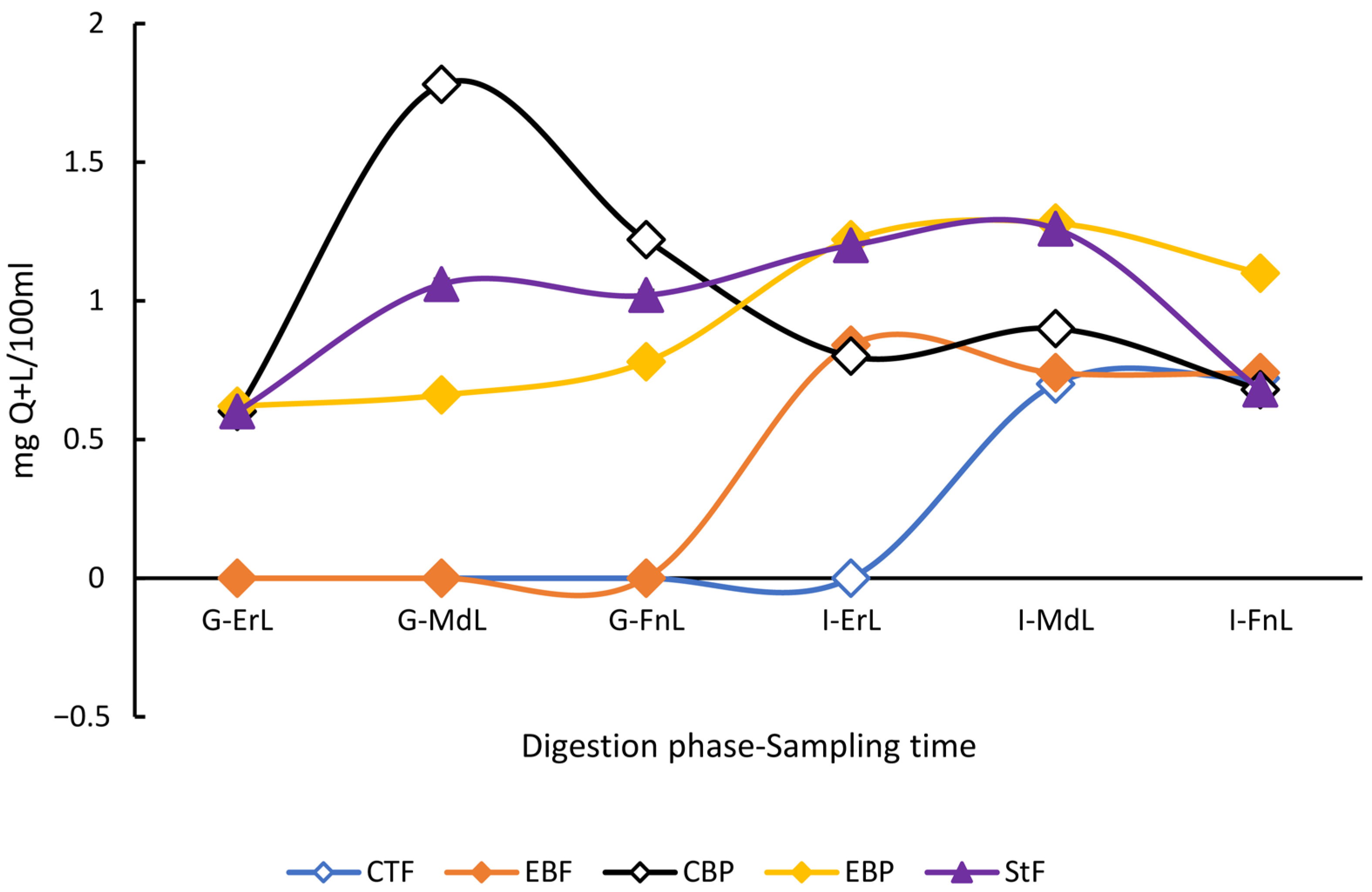
The TPC of the beverages throughout the digestion process exhibited distinct patterns depending on the digestion condition (fasted or postprandial). Beverages subjected to postprandial digestion conditions showed significantly higher TPC values in both gastric and intestinal phases compared to those digested under fasted conditions (p < 0.05). However, the standard food digested without the beverages did not show statistically significant differences in TPC when compared to either beverage under postprandial conditions (p < 0.05) (Figure 2).
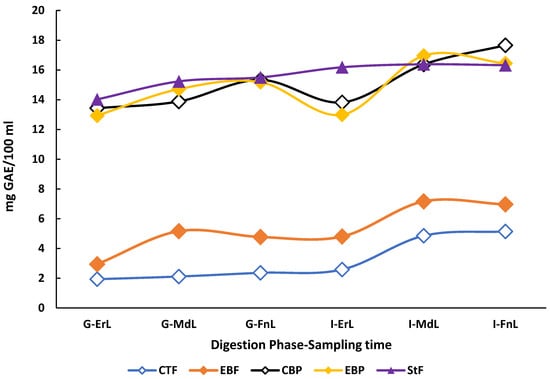
Figure 2.
Behavior of the total polyphenol content profile during the in vitro digestion of the enriched beverage with microencapsulated Habanero pepper leaf extract. GAE = Gallic acid equivalent; G-ErL = Gastric early time sampling; G-MdL = Gastric middle time sampling; G-FnL = Gastric final time sampling; I-ErL = Intestinal early time sampling; I-MdL = Intestinal middle time sampling; I-FnL = Intestinal final time sampling; CTF = Control beverage under fasting conditions; EBF = Enriched beverage under fasting conditions; CBP = Control beverage under postprandial conditions; EBP = Enriched beverage under postprandial conditions; StF = Standard food. All treatments in the experimental design were conducted in triplicate.
When comparing only the digestion of the control and enriched beverages, significant differences (p < 0.05) were observed in total polyphenol content (TPC). During the early sampling point of the gastric phase under fasting conditions, the enriched beverage exhibited a TPC of 3.43 ± 0.86 mg GAE/100 mL, while the control beverage showed a concentration of 2.08 ± 0.34 mg GAE/100 mL. These differences persisted throughout the gastric phase and became even more pronounced at the intermediate sampling point of the intestinal phase, where the enriched beverage reached 7.08 ± 0.26 mg GAE/100 mL compared to 4.83 ± 0.05 mg GAE/100 mL in the control beverage, representing a 46.5% increase.
The in vitro digestion (starting from the middle sampling time of the gastric phase) of the enriched beverage also showed a higher TPC compared to the sonicated enriched beverage (3.61 ± 0.10 mg GAE/100 mL).
Regarding the antioxidant capacity of both beverages during in vitro digestion, a similar behavior was observed between the enriched and control beverages under fasting conditions, when compared to the total polyphenol content profile.
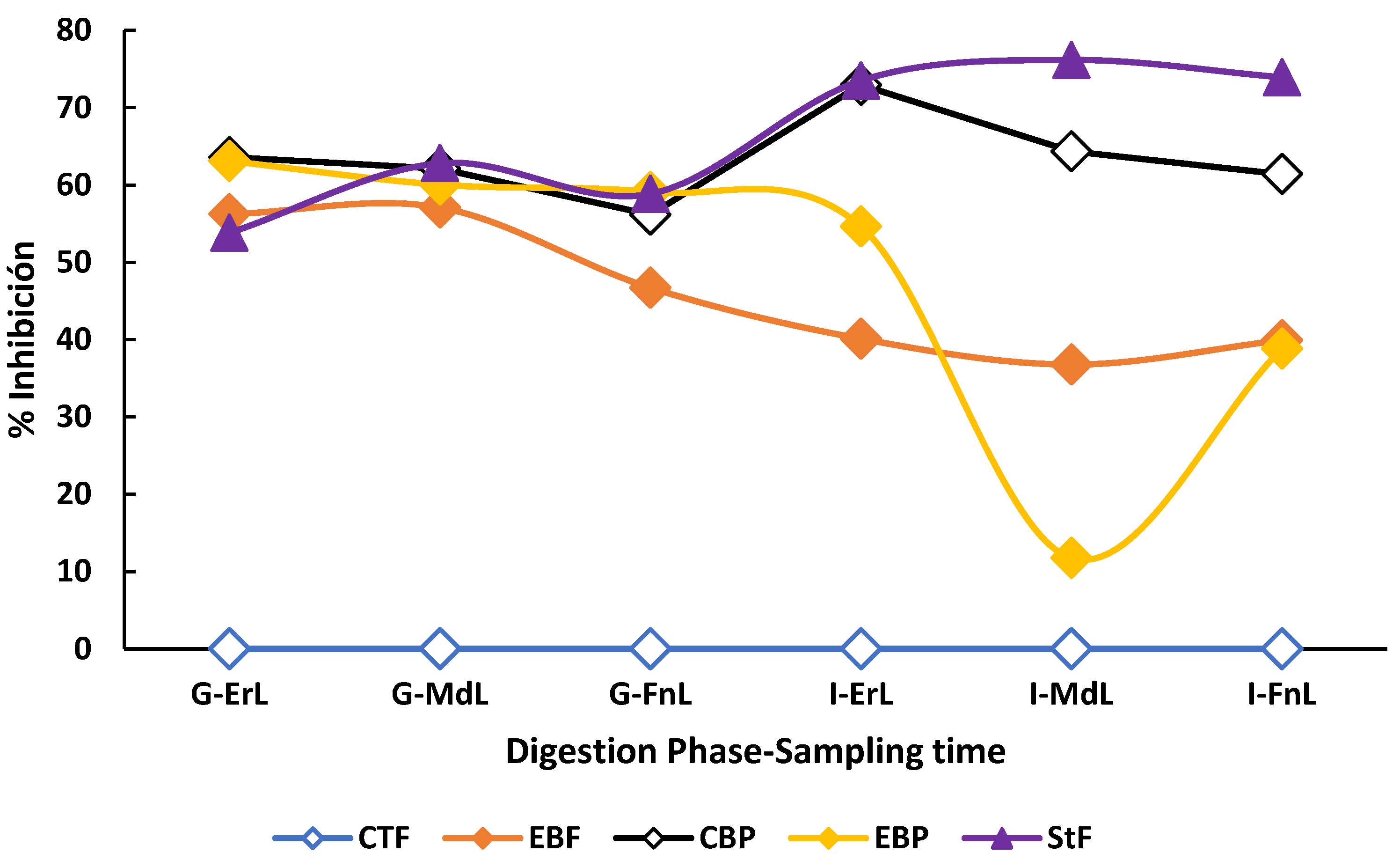
Throughout both the gastric and intestinal phases under fasting conditions, the enriched beverage exhibited significantly higher antioxidant capacity (p < 0.05) compared to its counterpart, which showed no detectable antioxidant activity at any sampling point—early, intermediate, or final—across the digestive phases (Figure 3).
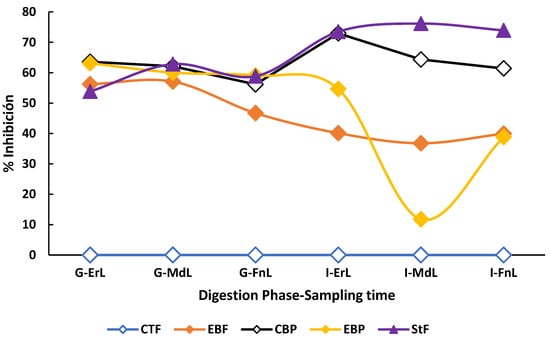
Figure 3.
Behavior of the antioxidant capacity during the in vitro digestion of the beverage enriched with microencapsulated Habanero pepper leaf extract. G-ErL = Gastric early time sampling; G-MdL = Gastric middle time sampling; G-FnL = Gastric final time sampling; I-ErL = Intestinal early time sampling; I-MdL = Intestinal middle time sampling; I-FnL = Intestinal final time sampling; CTF = Control beverage fasting condition; EBF = Enriched beverage fasting condition; CBP = Control beverage postprandial condition; EBP = Enriched beverage postprandial condition; StF = Standard Food. Each treatment in the experimental design was performed in triplicate.
On the other hand, during the gastric phase under postprandial conditions, no significant differences (p > 0.05) were observed among the enriched beverage (61.46 ± 3.37% inhibition), the control beverage (64.76 ± 1.93% inhibition), and the standard food (62.80 ± 1.51% inhibition). Likewise, at the final sampling time of the intestinal phase, no significant differences (p > 0.05) were observed in the enriched beverage under both fasting (35.86 ± 4.23% inhibition) and postprandial (35.29 ± 3.32% inhibition) conditions.
Regarding the standard food, it exhibited the highest antioxidant capacity (p < 0.05) at each sampling time, early (73.49 ± 2.31% inhibition), middle (76.16 ± 1.52% inhibition), and final (73.92 ± 6.04% inhibition) when compared to both beverages under fasting and postprandial conditions.
Finally, the extracts obtained by sonication from the enriched and control beverages showed an antioxidant capacity of 21.19 ± 0.33% inhibition and no detectable antioxidant activity, respectively.
The ANOVA from the 23 × 3 factorial design revealed that the main factors with a significant effect (p < 0.05) on total polyphenol content were the type of digestion (fasting or postprandial), type of beverage (enriched or control), digestive phase (gastric or intestinal), and the two-way interaction between digestion type and beverage type.
Regarding antioxidant capacity, the main factors influencing this dependent variable included digestion type (p < 0.0001), beverage type (p < 0.0001), and digestive phase (p < 0.0001), as well as two-way interactions between beverage type and digestion type (p < 0.0001), and beverage type and digestive phase (p < 0.0001). Additionally, a significant three-way interaction was identified among beverage type, digestion type, and digestive phase (p = 0.0324). Table 4 shows all the p-values from the 23 × 3 factorial design.

Table 4.
p-values for the effects and interactions of experimental design 23 × 3 factors on total polyphenol content and antioxidant capacity.
3.3. Polyphenol Profile of an Isotonic Beverage Enriched with Microencapsulated Habanero Pepper Leaf During In Vitro Digestion
Of the polyphenols analyzed (Section 2.5.4), only catechin, chlorogenic acid, coumaric acid, cinnamic acid, rutin, quercetin + luteolin, and hesperidin were detected in the samples (Table 5).

Table 5.
Polyphenol profile results from experimental design 23 × 3 of the enriched beverage with microencapsulated extract of Habanero pepper leaf during in vitro digestion.
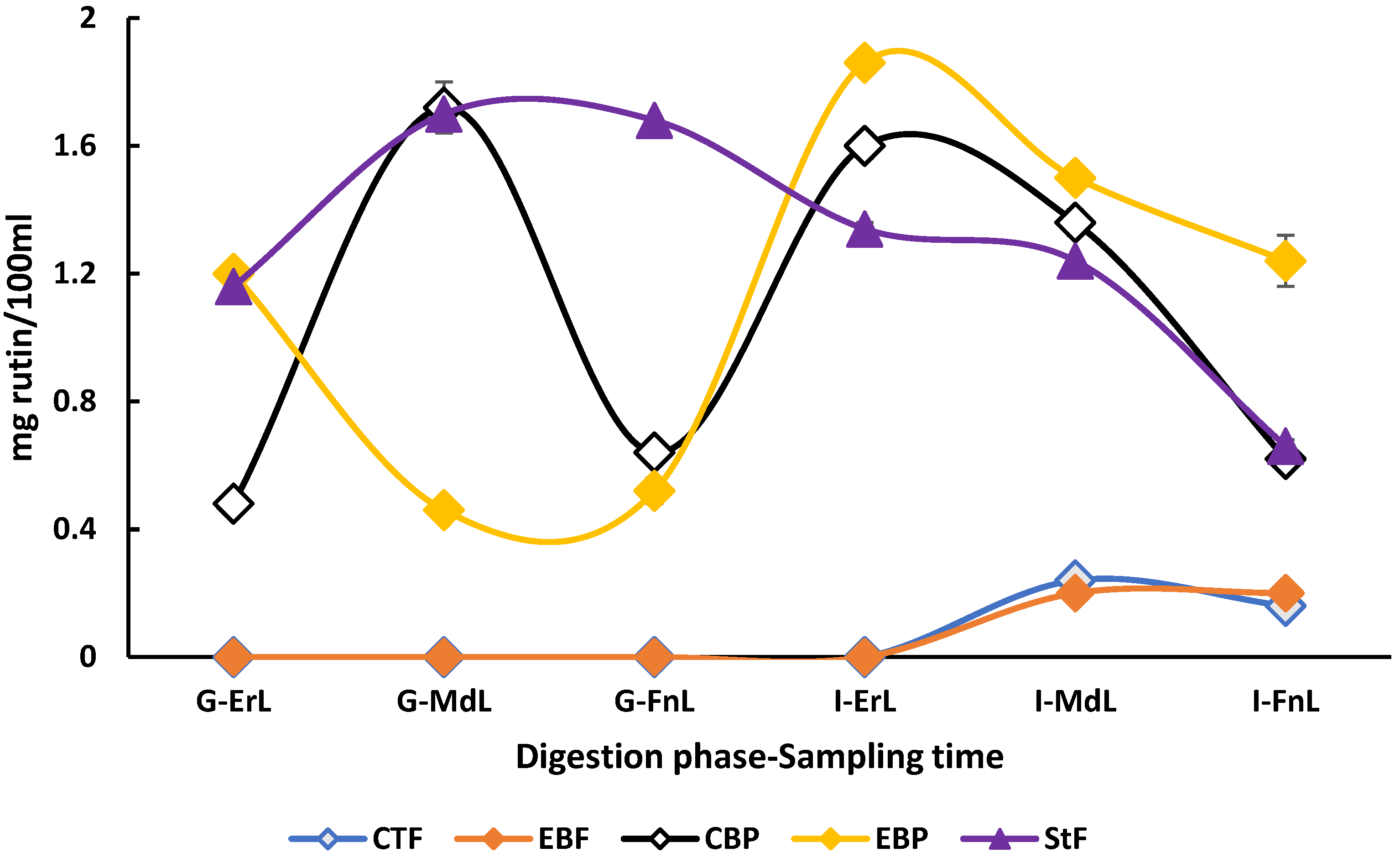
The highest concentrations (p < 0.05) of rutin (1.86 ± 0.02 mg/100 mL beverage) and hesperidin (0.70 ± 0.10 mg/100 mL beverage) were observed in the enriched beverage under postprandial conditions, rutin at the early sampling time of the intestinal phase, and hesperidin at the early sampling time of the gastric phase. As shown in Figure 4, the enriched beverage in the postprandial condition exhibited improved rutin bioaccessibility in the small intestine, despite starting with concentrations similar to those of the standard food.
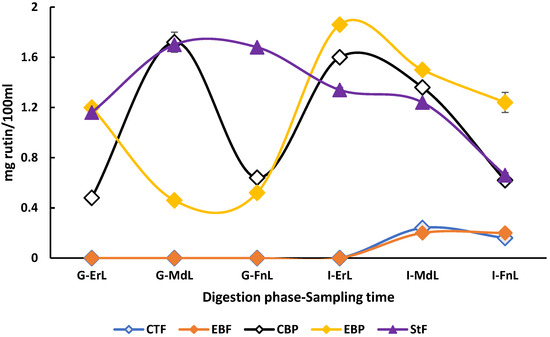
Figure 4.
Behavior of rutin the in vitro digestion of the beverage enriched with microencapsulated Habanero pepper leaf extract. G-ErL = Gastric early time sampling; G-MdL = Gastric middle time sampling; G-FnL = Gastric final time sampling; I-ErL = Intestinal early time sampling; I-MdL = Intestinal middle time sampling; I-FnL = Intestinal final time sampling; CTF = Control beverage fasting condition; EBF = Enriched beverage fasting condition; CBP = Control beverage postprandial condition; EBP = Enriched beverage postprandial condition; StF = Standard Food. Each treatment in the experimental design was performed in triplicate.
Although the individual concentrations of polyphenols such as catechin, chlorogenic acid, coumaric acid, cinnamic acid, and quercetin + luteolin (Q + L) were not the highest in the enriched beverage under either digestion condition (fasting or postprandial), it was observed that the levels of these compounds were maintained or even increased from the beginning of the gastric phase to the end of the intestinal phase. In contrast, the control isotonic beverages showed a decreasing behavior at both digestion conditions. For example, in the control beverage under postprandial conditions, Q + L was not detected (ND) in the gastric phase and detected with 0.74 ± 0.02 mg/100 mL by the end of intestinal digestion. In contrast, the enriched beverage under the same conditions began with a Q + L concentration of 0.62 ± 0.00 mg/100 mL and increased significantly to 1.10 ± 0.10 mg/100 mL by the end of the intestinal phase (Figure 5).
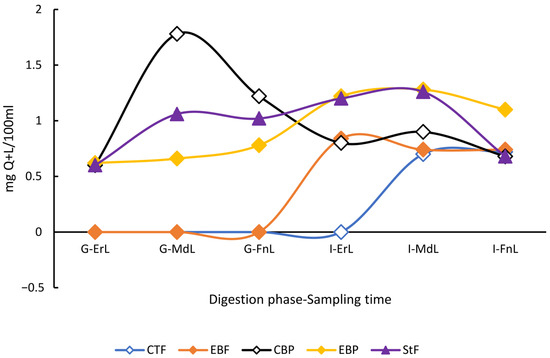
Figure 5.
Behavior of Quercetin + Luteolin the in vitro digestion of the beverage enriched with microencapsulated Habanero pepper leaf extract. G-ErL = Gastric early time sampling; G-MdL = Gastric middle time sampling; G-FnL = Gastric final time sampling; I-ErL = Intestinal early time sampling; I-MdL = Intestinal middle time sampling; I-FnL = Intestinal final time sampling; CTF = Control beverage fasting condition; EBF = Enriched beverage fasting condition; CBP = Control beverage postprandial condition; EBP = Enriched beverage postprandial condition; StF = Standard Food. Each treatment in the experimental design was performed in triplicate.
When comparing this behavior with the standard food and the digestions of the enriched beverage, it became evident that the concentrations of individual polyphenols showed no differences at the beginning of the gastric phase. However, by the end of the intestinal phase, standard food showed a decrease in the concentration of these phenolic compounds. For example, the concentration of rutin in the standard food decreased from 1.16 ± 0.00 mg/100 mL of beverage at the start of the gastric phase to 0.66 ± 0.00 mg/100 mL of beverage at the end of the intestinal phase of the in vitro digestion. In comparison, the enriched beverage under postprandial conditions started with a concentration of 1.20 ± 0.00 mg/100 mL of beverage in the gastric phase and ended with 1.24 ± 0.08 mg/100 mL of beverage in the intestinal phase.
Figure S3 shows the graphs illustrating the behavior of cinnamic acid during the in vitro digestion of the isotonic beverage enriched with microencapsulated Habanero pepper leaf extract, where the enriched beverage shows a higher concentration compared to the control beverage, both under postprandial conditions.
All results for the individual polyphenols were analyzed to determine which main factors and interactions had an effect (p < 0.05) on their concentration during in vitro digestion. Table 6 shows the p-values corresponding to the factors and their interactions for each individual polyphenol.

Table 6.
p-values for the effects and interactions of experimental design 23 × 3 factors on polyphenol profile from enriched beverage during in vitro digestion.
It was observed that the double interactions had a significant effect (p < 0.05) on the concentration of the identified individual polyphenols, except for the interaction between Beverage Type (A) × Digestion Type (B) on the concentration of cinnamic acid (CiA), and the interaction between Digestion Type (B) × Digestive Phase (C) on the concentration of catechin, with p-values > 0.05.
Triple interactions were also evaluated and showed a significant effect (p < 0.05) on the concentration of several individual polyphenols such as chlorogenic acid, cinnamic acid, quercetin + luteolin, kaempferol, and hesperidin. In the case of coumaric acid, the interaction A × B × Digestion Time (D) did not present a significant effect on its concentration during the in vitro digestion of the isotonic beverage (p > 0.05). On the other hand, the triple interaction A × C × Digestion Time (D) had no significant effect (p > 0.05) on catechin and coumaric acid.
4. Discussion
To the best of our knowledge, this is the first work evaluating the behavior of phenolic compounds during in vitro digestion in an isotonic beverage enriched with microencapsulated Habanero pepper leaf extract obtained by NADES. This study integrates green extraction technologies, underutilized plant material, and microencapsulation into a novel functional isotonic beverage (liquid matrix), allowing the assessment of polyphenol bioaccessibility and antioxidant performance across in vitro digestive phases (gastric and intestinal).
The choline chloride (ChCl) based-NADES extract (NADES-68) showed no cytotoxic effects on L-929 fibroblast cells at any of the tested concentrations (0.78–6.3%), with cell viability remaining above 70%, the safety threshold defined by the Mexican Pharmacopeia for cytotoxic classification. These results are consistent and comparable to those reported by Paiva et al. [12], where different ChCl -based NADES formulations were evaluated using various acids (citric, tartaric) and sugars (sucrose, glucose, xylose) as hydrogen bond donors (HBDs). Cell viability of L-292 cells exceeded 70% for NADES prepared with glucose and sucrose as HBDs in a 1:1 molar ratio with ChCl.
These results are consistent with the findings of Popović et al. [36], who systematically evaluated the cytotoxicity of twelve ChCl -based NADES on various cell lines, including HT-29 (human colon adenocarcinoma), Caco-2 (human intestinal epithelial cells), MCF-7 (human breast carcinoma), and MRC-5 (normal human lung fibroblasts), at concentrations ranging from 0% to 15% v/v. In their study, sugar-based NADES, such as ChCl:Glucose, ChCl:Fructose, and ChCl:Sucrose, demonstrated the highest cell viabilities across all tested lines. MRC-5 cells-maintained viabilities above 85% at concentrations up to 3.75% v/v, indicating low cytotoxicity. In contrast, acid-based NADES, especially those formulated with oxalic acid or lactic acid, resulted in significantly lower viability in cancer cell lines such as MCF-7, where cell viability dropped below 50% at the same 3.75% v/v concentration. These findings further emphasize the role of the HBD and NADES composition in modulating cytotoxic responses.
It has also been reported that sugar-based NADES exhibit lower cytotoxicity because they do not alter the cellular pH, unlike organic acid-based NADES presenting a pH < 6.5 [37,38]; consequently, the latter show higher cytotoxic effects on cancer cell lines such as MCF-7 [39,40], B16F10 [39], CaOV3 [39], and HeLaS3 [39,40].
In contrast to our findings, where the sugar-based NADES extract was successfully evaluated, Bezerra et al. [41] were unable to assess cytotoxicity for their sugar-based NADES formulation (lactic acid:glucose) due to its extremely acidic pH (<3), which likely destabilized the cell culture environment and interfered with the colorimetric assay conditions. This limitation highlights the strong influence of NADES acidity on assay feasibility. In our case, the extract obtained with a sugar-based and highly hydrated NADES (NADES-68) was diluted in DMEM to reach the final test concentrations, which maintained the medium close to neutrality and avoided assay artifacts, thus allowing reliable cytotoxicity evaluation. This outcome is consistent with literature showing that increasing water content raises NADES pH and improves biocompatibility, while choline- or sugar-based systems are generally less cytotoxic than organic acid-based NADES. However, Bezerra et al. [41] did evaluate other NADES formulations, including ChCl:glycerol (ChCl:GL) and urea:glycerol (U:GL), on cancer cell lines MCF-7 and MDA-MB-231. These NADES, with neutral to basic pH, showed significant cytotoxic effects: for instance, ChCl:GL reduced MCF-7 cell viability by up to 78.4% at 6.25% concentration, while U:GL and its extract reduced viability to 24.8% and 70.7%, respectively, at concentrations between 12.5% and 25%, supporting the relevance of pH and NADES composition in modulating biological effects. Similarly, the acute oral toxicity test confirmed the extract’s safety at the limit dose of 2000 mg/kg, with no signs of systemic toxicity, behavioral alterations, or weight loss in BALB/c mice. These findings place the extract within the non-toxic classification according to the Organization for Economic Co-operation and Development (OECD) guidelines [27], aligning with other NADES formulations intended for oral use [42,43]. Based on these safety results, the incorporation of polyphenols into functional beverages has gained increasing interest. While the safety profile of NADES and their microencapsulated forms supports their potential use in isotonic drinks, most current studies have focused on the addition of polyphenol extracts or microencapsulated extracts obtained using conventional solvents, and these have primarily been incorporated into non-isotonic beverages [44,45,46,47]. In this context, the present work specifically focuses on the incorporation of NADES-obtained microencapsulated phenolic extracts into isotonic beverages, aiming to address this gap in existing literature.
In the present study, the behavior of total microencapsulated phenolic compounds added to an isotonic beverage during in vitro digestion was similar to that reported by Wyspiańska et al. [22], who incorporated a microencapsulated soybean extract (spray drying, maltodextrin DE8) at 0.025% into an isotonic beverage.
The microencapsulated phenolic compounds from soybeans started with a concentration of 71 µg/100 mL in gastric phase, increasing their concentration during the intestinal phase up to 145 µg/100 mL. A similar behavior was observed for the microencapsulated phenolic compounds evaluated in the present study, which began with a concentration of 3.43 ± 0.86 mg GAE/100 mL at the beginning of the gastric phase and reached 6.98 ± 0.03 mg GAE/100 mL at the end of the intestinal phase under fasting conditions.
According to Jakobek, Silva et al., and Yeasmen & Orsat, [18,39,48,49], this behavior is attributed to the encapsulating agents effectively protecting the phenolic compounds from gastric conditions, allowing their release under intestinal conditions such as pH and enzymatic activity. This represents an advantage in terms of the bioaccessibility of the bioactive compounds of interest and their potential absorption in the small intestine.
On the other hand, the concentrations of phenolic compounds under postprandial conditions showed no differences between the gastric and intestinal phases, both in the enriched beverage and in the control beverage. This could be the result of the formation of complexes through covalent and non-covalent bonds, the latter occurring between the hydroxyl groups of the phenolic compounds and the amino groups of proteins, mainly involving β-sheet secondary structures due to the arrangement of their hydroxyl groups. These complexes lead to a decrease in polyphenol bioaccessibility [50].
The behavior differences observed between fasting and postprandial conditions can be explained by well-described molecular interactions of polyphenols with dietary macronutrients [51]. Phenolics such as catechin, rutin, and quercetin interact with proteins (e.g., pepsin, trypsin, β-lactoglobulin) through hydrogen bonding with carbonyl groups, hydrophobic contacts with aromatic residues (Trp, Tyr, Phe), and, in some cases, covalent binding via quinone intermediates with lysine or cysteine side chains [51,52,53]. In lipid-rich postprandial environments, polyphenols are incorporated into bile salt–phospholipid micelles (e.g., sodium taurocholate, phosphatidylcholine), which significantly enhances their solubility and stability [54]. Additionally, interactions with starch fractions (amylose, amylopectin) through hydrogen bonds and π-π stacking reduce enzymatic hydrolysis, linking polyphenol stabilization with hypoglycemic effects [55,56,57]. During digestion, low gastric pH promotes hydrolysis of unstable compounds such as anthocyanins, while neutral intestinal conditions favor ionization and oxidation of hydroxyl groups unless protective lipid micelles or protein complexes are present [52,54,58,59]. These molecular events collectively provide a mechanistic rationale for the higher stability and bioaccessibility of polyphenols observed under postprandial conditions compared to fasting states.
When analyzing the behavior of the antioxidant capacity, a higher percentage of DPPH radical inhibition is observed in the samples of the enriched beverage under fasting conditions, demonstrating that microencapsulation provides protection for the antioxidant compounds from Habanero pepper leaf extract against both gastric and intestinal digestive conditions. A similar behavior has been previously reported in different microencapsulated extracts obtained by spray drying when evaluated through in vitro digestion. For example, Bernal-Millán et al. [21] evaluated a microencapsulated oregano (Lippia graveolens) extract using DE10 maltodextrin through in vitro digestion. The results showed that during the gastric phase (50.08 μmol Trolox Equivalents (TE)/g), the antioxidant capacity of the microencapsulated extracts was lower compared to the intestinal phase (60.43 μmol TE/g).
Ramírez-Damián et al. [60] evaluated the bioaccessibility index (BI) of the antioxidant capacity, using the DPPH method, of red banana peel extracts microencapsulated with soy protein during in vitro digestion in both the gastric and intestinal phases. At the end of the gastric phase, a decrease in BI to 63.79% was observed; however, by the end of the intestinal phase, the BI increased to 77.32%.
Regarding the microencapsulates added to isotonic beverages, a different behavior was observed compared to that reported by Wyspiańska et al. [22]. In their study, an isotonic beverage enriched with polyphenols microencapsulated using maltodextrin as the sole wall material exhibited higher antioxidant capacity during the gastric phase (570.84 nmol TE/mL) compared to the intestinal phase (90.54 nmol TE/mL). In contrast, in the present study, the combination of maltodextrin, guar gum, and modified starch as encapsulating agents resulted in a more controlled and sustained release of phenolic compounds, leading to greater antioxidant capacity during the intestinal phase. Similar protective behavior has been reported by Martinović et al. [61], Cegledi et al. [62], and Ramírez Damián et al. [60], where matrices combining polysaccharides and gums demonstrated enhanced stability and controlled release of encapsulated phenolics under simulated gastrointestinal conditions.
The observed trend for a decrease in the antioxidant capacity of polyphenol-rich microencapsulates during the gastric phase, followed by an increase during the intestinal phase in an in vitro digestion, can be attributed to several factors. During the gastric phase, both hydrophilic and hydrophobic compounds may undergo degradation due to pH changes, as well as interactions with other metabolites or proteins present in the medium. In contrast, during the intestinal phase, the encapsulating agents are gradually decomposing by the action of pH and digestive enzymes [60,62]. This degradation process facilitates the protection and controlled release of the target compounds contained in the extract, which then contributes to scavenging reactive oxygen species and other oxidizing agents, thereby enhancing the antioxidant capacity observed in this phase.
However, polar phenolic compounds may exhibit lower radical-scavenging efficiency in emulsified systems because of their preferential solubilization within bile salt micelles, a phenomenon aligned with the “polar paradox” [63,64]. This partitioning effect favors the standard food matrix, which provides a more suitable environment for phenolic stabilization. In addition, structural transformations of polyphenols during gastrointestinal digestion, including hydrolysis or oxidation, can yield metabolites with lower antioxidant activity [48,49]. Finally, the DPPH assay itself has known limitations in complex digesta, since peptides, reducing sugars, and micellar components can interfere with radical quenching [65].
The presence of other compounds in Habanero pepper leaf extracts has been reported, which may contribute to antioxidant capacity, such as vitamin C, carotenoids [66], alkaloids, tannins, terpenoids, and volatile oils [3]. It should be considered that hydrophilic compounds, such as polyphenols, may exhibit underestimated antioxidant capacity in radical scavenging assays like DPPH due to steric and diffusional hindrance caused by micellar or emulsified structures.
Antioxidant efficiency of chlorogenate esters in emulsions depends not only on their polarity but also on chain length and interfacial localization, suggesting that structural factors can restrict access to reactive sites. In their broader theoretical analysis, they further proposed that the presence of association colloids, such as reverse micelles, may trap antioxidants away from oxidation interfaces, impairing their effectiveness [51,63,67,68]. Complementarily, Chat et al. [69] experimentally confirmed that micellar environments significantly influence the radical scavenging activity of polyphenols such as rutin, by altering the solubilization and mobility of both the antioxidant and the DPPH radical. Their results highlight how the type of surfactant (cationic, anionic, or non-ionic) and the micelle structure modulate antioxidant performance, thus reinforcing the idea that micellar entrapment can lead to inaccurate estimations of antioxidant activity in hydrophilic systems [70]. This behavior was observed in our study with the enriched beverage subjected to digestion under postprandial conditions.
Among the compounds reported in Habanero pepper leaves, a wide range of characteristic individual polyphenols, such as catechin, quercetin, and rutin, have been successfully extracted using NADES and encapsulated, demonstrating potential as functional ingredients for the development of functional foods and beverages [19,25,66]. These bioactive compounds of interest were identified and quantified during the in vitro digestion of the isotonic beverage enriched with microencapsulated Habanero pepper leaf extract.
One of the polyphenols identified in the enriched beverage during in vitro digestion was rutin, a glycosylated flavonoid (quercetin-3-rhamnosyl glucoside) widely reported in plant leaves. This compound is associated with anti-inflammatory, antidiabetic, and cardioprotective properties, and its therapeutic potential has been highlighted in the management of venous disorders and hemorrhoids [71,72]. Reflecting these benefits, rutin is already included in various nutraceutical formulations marketed for vascular support and antioxidant protection. In our study, rutin reached concentrations of up to 1.24 mg/100 mL at the end of intestinal digestion under postprandial conditions, demonstrating that microencapsulation enhanced its bioaccessibility. These levels highlight the potential of the enriched beverage to deliver bioaccessible rutin at physiologically relevant concentrations, reinforcing its applicability as a competitive candidate in the functional food and nutraceutical market. This finding is especially relevant considering that previous in vivo and in vitro studies have shown that rutin is absorbed in the small intestine, though less efficiently than its aglycone, quercetin [73]. Specifically, approximately 45% of rutin was retained by the rat small intestinal wall, indicating partial absorption. Additionally, the glycosidic nature of rutin and its interactions with intestinal proteins have been cited as barriers to efficient uptake. According to more recent findings, rutin’s low water solubility, limited permeability, and rapid metabolism in the gut also contribute to its poor bioavailability [74]. Ahmad et al. [75], who investigated the formulation and digestive behavior of dry casein–rutin composites intended for application in functional dairy beverages, using a standardized static in vitro digestion model, demonstrated that the encapsulation of rutin within micellar casein matrices significantly enhanced its gastrointestinal stability and micellar incorporation, achieving a bioaccessibility to 71.6%. This improvement was attributed to the protective effect of the protein matrix, which helped to solubilize rutin during the intestinal phase while preventing its early degradation under gastric conditions. Furthermore, bio-compatibility assays using Caco-2 intestinal epithelial cells revealed that these composites were non-cytotoxic and did not compromise the integrity of the epithelial barrier.
Similarly to rutin, quercetin was identified at high concentrations at the end of intestinal digestion when analyzing the enriched beverage under postprandial conditions, in comparison with the standard food and the control beverage under both fasting and postprandial conditions.
Quercetin is a flavonol abundant in plant-based foods, valued for its antioxidant, anti-inflammatory, and cardioprotective effects, attributed to its polyhydroxylated chemical structure. However, its clinical potential is limited by low water solubility and poor intestinal absorption, as most of the compound is metabolized during digestion, significantly reducing the availability of its active form in systemic circulation [76]. Despite these limitations, quercetin has gained interest in the nutraceutical and pharmaceutical markets due to its therapeutic applications. For example, QG5®, a commercial formulation containing Psidium guajava leaf extract standardized to deliver quercetin, is widely used as an adjuvant in the management of colitis and intestinal inflammation [77]. Its reported mechanisms of action include modulation of the immune response, reduction in pro-inflammatory signaling pathways, and support of intestinal barrier integrity, as supported by pre-clinical studies [74,78,79]. Additionally, quercetin is marketed in supplements such as NOW® Quercetin with Bromelain and Life Extension® Optimized Quercetin, primarily for vascular protection and immune support.
In vivo studies in rats by Carbonaro & Grant [74] confirmed the limited absorption of quercetin, reporting that only about 1.5% of the compound crosses the intestinal barrier, while a significant proportion remains bound to intestinal tissues. Furthermore, the authors suggested that interactions with intestinal proteins, such as albumin, may facilitate transport but limit the availability of the free and bioactive form in systemic circulation. However, in our study, quercetin was consistently detected throughout the in vitro digestion process of the enriched beverage under postprandial conditions. Specifically, concentrations were maintained from the onset of the gastric phase (1.22 ± 0.02 mg/100 mL), through the intermediate stage (1.28 ± 0.00 mg/100 mL), and even at the end of the intestinal phase (1.10 ± 0.10 mg/100 mL).
The consistent detection of rutin and quercetin at the end of the intestinal phase can be explained by their chemical structures and physicochemical interactions with the encapsulating matrix. Rutin, a glycosylated flavonol composed of quercetin and disaccharide rutinose, exhibits increased water solubility and enhanced stability against acidic hydrolysis and enzymatic degradation compared to its aglycone counterpart. Its bulky sugar moiety introduces steric hindrance, which limits its inhibitory interaction with digestive enzymes in the free form but favors stabilization when bound to starch or encapsulating agents [34]. In contrast, quercetin aglycone, although more susceptible to oxidative degradation, demonstrates moderate polarity that improves its dispersion and incorporation into micellar systems during digestion. Both flavonoids are known to form non-covalent interactions, such as hydrogen bonds and van der Waals forces, with dietary polysaccharides or encapsulants (e.g., maltodextrin, guar gum, and modified starch), resulting in the formation of protective complexes that slow their enzymatic degradation [80,81]. It should also be emphasized that digestion, through pH changes and enzymatic action, enhances the bioaccessibility of the metabolites of interest, as the concentrations detected in the ultrasound-assisted extracts were significantly lower (EChExT = 0.16 ± 0.00 mg rutin/100 mL isotonic beverage) when compared to those observed after digestion.
This enriched isotonic beverage formulation (NADES extract, spray drying microencapsulation, enriched isotonic beverage) appears to promote the gradual release of polyphenols, potentially mitigating their known limitations in solubility and intestinal absorption. By preserving their structural integrity and enhancing bioaccessibility, this delivery strategy may increase the likelihood of systemic uptake and thereby improve the expression of their well-documented biological activities.
5. Conclusions
This study demonstrates that the use of NADES is a promising green extraction strategy for obtaining phenolic-rich extracts from Habanero pepper leaves. The phenolic profile obtained, including compounds such as rutin and quercetin, confirms that NADES are effective for extracting valuable bioactives and are compatible with subsequent encapsulation processes. The microencapsulation of these extracts using a matrix of maltodextrin, guar gum, and modified starch provided clear protection during in vitro digestion, as evidenced by the sustained presence of individual phenolic compounds and superior antioxidant capacity. Under postprandial conditions, the enriched beverage exhibited higher total phenolic content and antioxidant activity than the control and fasting conditions, supporting its enhanced functionality in a physiologically relevant model. The maintained concentration of key polyphenols in the intestinal phase, particularly rutin and quercetin, highlights the successful controlled release and protection offered by the encapsulating agents. Overall, this work supports the integration of NADES-based extracts into functional beverage development, showing that when combined with an appropriate microencapsulation strategy they can improve the stability, bioaccessibility, and potential health benefits of polyphenols.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13092826/s1, Figure S1: Calibration curve for the determination of total polyphenol content in the isotonic beverage samples enriched with Habanero pepper leaf extract during in vitro digestion; Figure S2: Behavior of cinnamic acid during the in vitro digestion of the beverage enriched with microencapsulated Habanero pepper leaf extract. G-ErL = Gastric early time sampling; G-MdL = Gastric middle time sampling; G-FnL = Gastric final time sampling; I-ErL = Intestinal early time sampling; I-MdL = Intestinal middle time sampling; I-FnL = Intestinal final time sampling; CTF = Control beverage fasting condition; EBF = Enriched beverage fasting condition; CBP = Control beverage postprandial condition; EBP = Enriched beverage postprandial condition; StF = Standard Food. Each treatment in the experimental design was performed in triplicate. Figure S3: Figure S3. Calibration curve of individual polyphenols. 1 = Protocatechuic acid (3.122 min); 2 = Catechin (5.020 min); 3 = Chlorogenic acid (6.468 min); 4 = Coumaric acid (8.211 min); 5 = Cinnamic acid (8.492 min); 6 = Rutin (9.822 min); 7 = Quercetin + Luteolin (11.537 min); 8 = Kaempferol (13.487 min). Injections were performed in triplicate.
Author Contributions
Conceptualization, I.M.R.-B. and K.A.A.-B.; methodology, I.M.R.-B., M.G.-Á., E.P.-C. and K.A.A.-B.; software, K.A.A.-B.; validation, I.M.R.-B., M.G.-Á., J.V.C.-R., M.O.R.-S. and E.P.-C.; formal analysis I.M.R.-B., M.G.-Á. and E.P.-C.; investigation, I.M.R.-B., M.G.-Á. and K.A.A.-B.; resources, I.M.R.-B., E.P.-C. and M.G.-Á.; data curation, I.M.R.-B., M.G.-Á., J.V.C.-R., M.O.R.-S. and E.P.-C.; writing—original draft preparation, I.M.R.-B. and K.A.A.-B.; writing—review and editing, I.M.R.-B. and M.O.R.-S.; visualization, I.M.R.-B. and M.G.-Á.; supervision, I.M.R.-B.; project administration, I.M.R.-B.; funding acquisition, I.M.R.-B. and M.G.-Á. All authors have read and agreed to the published version of the manuscript.
Funding
Kevin Alejandro Avilés-Betanzos was supported by a SECIHTI scholarship (No. 661099).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Ex vivo Digestion Laboratory at CIATEJ and the colleagues who supported the in vitro digestion procedures with their knowledge and guidance, including nutritionist Juan Ramon Palomera Hernandez and agro-industrial engineering Cesar Femat Castañeda.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Instituto Mexicano de la Propiedad Industrial (IMPI). Declaración de Protección de la Denominación de Origen “Chile Habanero de la Península de Yucatán”. Diario Oficial de la Federación. 4 October 2010. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5159400&fecha=04/10/2010 (accessed on 15 March 2025).
- Olguín-Rojas, J.A.; Vázquez-León, L.A.; Palma, M.; Fernández-Ponce, M.T.; Casas, L.; Fernández Barbero, G.; Rodríguez-Jimenes, G.C. Re-Valorization of Red Habanero Chili Pepper (Capsicum chinense Jacq.) Waste by Recovery of Bioactive Compounds: Effects of Different Extraction Processes. Agronomy 2024, 14, 660. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.D.; Oney-Montalvo, J.E.; Rodríguez-Buenfil, I.M. Phytochemical Characterization of By-Products of Habanero Pepper Grown in Two Different Types of Soils from Yucatán, Mexico. Plants 2021, 10, 779. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of Solvent Polarity on the Ultrasound-Assisted Extraction and Antioxidant Activity of Phenolic Compounds from Habanero Pepper Leaves (Capsicum chinense) and Its Identification by UPLC-PDA-ESI-MS/MS. Ultrason. Sonochem. 2021, 76, 105658. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Villegas, R.; González-Amaro, R.M.; Figueroa-Hernández, C.Y.; Rodríguez-Buenfil, I.M. The Genus Capsicum: A Review of Bioactive Properties of Its Polyphenolic and Capsaicinoid Composition. Molecules 2023, 28, 4239. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Commun. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Ivanković, A. Review of 12 Principles of Green Chemistry in Practice. Int. J. Sustain. Green Energy 2017, 6, 39–45. [Google Scholar] [CrossRef]
- Huang, M.M.; Yiin, C.L.; Mun Lock, S.S.; Fui Chin, B.L.; Othman, I.; Binti Ahmad Zauzi, N.S.; Chan, Y.H. Natural Deep Eutectic Solvents (NADES) for Sustainable Extraction of Bioactive Compounds from Medicinal Plants: Recent Advances, Challenges, and Future Directions. J. Mol. Liq. 2025, 425, 127202. [Google Scholar] [CrossRef]
- Puma-Isuiza, G.; García-Chacón, J.M.; Osorio, C.; Betalleluz-Pallardel, I.; Chue, J.; Inga, M. Extraction of Phenolic Compounds from Lucuma (Pouteria lucuma) Seeds with Natural Deep Eutectic Solvents: Modelling Using Response Surface Methodology and Artificial Neural Networks. Front. Sustain. Food Syst. 2024, 8, 1401825. [Google Scholar] [CrossRef]
- Cabrera, L.; Xavier, L.; Zecchi, B. Extraction of Phenolic Compounds with Antioxidant Activity from Olive Pomace Using Natural Deep Eutectic Solvents: Modelling and Optimization by Response Surface Methodology. Discov. Food 2024, 4, 29. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Aznar-Ramos, M.J.; Razola-Díaz, M.d.C.; Verardo, V.; Gómez-Caravaca, A.M. Comparison between Ultrasonic Bath and Sonotrode Extraction of Phenolic Compounds from Mango Peel By-Products. Horticulturae 2022, 8, 1014. [Google Scholar] [CrossRef]
- Duarte, H.; Gomes, V.; Aliaño-González, M.J.; Faleiro, L.; Romano, A.; Medronho, B. Ultrasound-Assisted Extraction of Polyphenols from Maritime Pine Residues with Deep Eutectic Solvents. Foods 2022, 11, 3754. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Gad, A.A.M.; Barakat, A.Z.; Mohamed, S.A. Microencapsulation of Antioxidant Phenolics from Tamarind Seed Peels Using Chia Gum and Maltodextrin. Sci. Rep. 2025, 15, 89792. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Microencapsulation of Ultrasound-Assisted Phenolic Extracts of Sugar Maple Leaves: Characterization, in vitro Gastrointestinal Digestion, and Storage Stability. Food Res. Int. 2024, 182, 114133. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Optimization of Spray Drying Conditions for a Capsicum chinense Leaf Extract Rich in Polyphenols Obtained by Ultrasonic Probe/NADES. ChemEngineering 2024, 8, 131. [Google Scholar] [CrossRef]
- Fitri, R.; Lestari, T.; Sari, Y.; Sutriyo, S.; Mun’Im, A. Freeze Drying of Natural Deep Eutectic Solvent (NADES) Extract of Green Coffee Bean Coffea canephora Pierre ex A. Froehner. J. Res. Pharm. 2020, 24, 225–232. [Google Scholar] [CrossRef]
- Bernal-Millán, M.D.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-Dried Microencapsulation of Oregano (Lippia graveolens) Polyphenols with Maltodextrin Enhances Their Stability during in vitro Digestion. J. Chem. 2022, 2022, 8740141. [Google Scholar] [CrossRef]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Effect of Microencapsulation on Concentration of Isoflavones during Simulated in vitro Digestion of Isotonic Drink. Food Sci. Nutr. 2019, 7, 805–816. [Google Scholar] [CrossRef]
- Detchewa, P.; Aphibanthammakit, C.; Moongngarm, A.; Avallone, S.; Prasajak, P.; Boonpan, C.; Ruangdath, V.; Sriwichai, W. Microencapsulation Techniques and Encapsulating Materials Influenced the Shelf Life and Digestion Release of Vitamin E and Isoflavones in Soymilk Powder. Sci. Rep. 2025, 15, 95284. [Google Scholar] [CrossRef]
- Vásquez-Velázquez, M.V.; López-Vázquez, J.S.; Ruiz-Sánchez, E.; Medina-Dzul, K.B.; Latournerie-Moreno, L. Agronomic Behavior and Fruit Quality in Habanero Peppers (Capsicum chinense Jacq.) as a Response to Formative Pruning. Agro Prod. 2021, 14, 107–112. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; González-Ávila, M.; Scampicchio, M.; Morozova, K.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Natural Deep Eutectic Solvent Optimization to Obtain an Extract Rich in Polyphenols from Capsicum chinense Leaves Using an Ultrasonic Probe. Processes 2023, 11, 1729. [Google Scholar] [CrossRef]
- Farmacopea de los Estados Unidos Mexicanos. MGA-DM 10993-1: Pruebas de Biocompatibilidad. Pruebas para Citotoxicidad In Vitro; Suplemento 2020; Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS): Ciudad de México, Mexico, 2020. [Google Scholar]
- OECD. Test No. 425: Acute Oral Toxicity—Up-and-Down Procedure. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2008. [Google Scholar] [CrossRef]
- Secretaría de Salud. NOM-218-SSA1-2011. Productos y Servicios: Bebidas Saborizadas No Alcohólicas, Sus Congelados, Productos Concentrados para Prepararlas y Bebidas Adicionadas con Cafeína. Especificaciones y Disposiciones Sanitarias. Métodos de Prueba. Diario Oficial de la Federación, 10 February 2012. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5223556&fecha=10/02/2012 (accessed on 15 February 2025).
- Johnson, J.B.; Mani, J.S.; Naiker, M. Microplate Methods for Measuring Phenolic Content and Antioxidant Capacity in Chickpea: Impact of Shaking. Eng. Proc. 2023, 48, 15167. [Google Scholar] [CrossRef]
- Hernández-Moreno, L.V.; Salazar, J.R.; Pabón, L.C.; Hernández-Rodríguez, P. Antioxidant Activity and Quantification of Phenols and Flavonoids of Colombian Plants Used in Urinary Tract Infections. Rev. UDCA Actual. Divulg. Cient. 2022, 25, 1690. [Google Scholar] [CrossRef]
- Santana, M.; Freitas-Silva, O.; Mariutti, L.R.B.; Teodoro, A.J. A Review of in vitro Methods to Evaluate the Bioaccessibility of Phenolic Compounds in Tropical Fruits. Crit. Rev. Food Sci. Nutr. 2024, 64, 1780–1790. [Google Scholar] [CrossRef]
- Pérez-Tepayo, S.; Rodríguez-Ramírez, S.; Unar-Munguía, M.; Shamah-Levy, T. Trends in the Dietary Patterns of Mexican Adults by Sociodemographic Characteristics. Nutr. J. 2020, 19, 68. [Google Scholar] [CrossRef]
- Torres-Martínez, B.M.; Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; González-Ávila, M.; Rodríguez-Carpena, J.G.; Huerta-Leidenz, N.; Pérez-Álvarez, J.A.; Fernández-López, J.; Sánchez-Escalante, A. Use of Pleurotus ostreatus to Enhance the Oxidative Stability of Pork Patties during Storage and in vitro Gastrointestinal Digestion. Foods 2022, 11, 4075. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S. Postprandial Effects of Wine Consumption Along with a Meal on the Main Pathophysiological Systems. In Wine and Health; Moreno-Arribas, M.V., Bartolomé Suáldea, B., Eds.; Academic Press: London, UK, 2018; Chapter 6; pp. 125–146. [Google Scholar]
- Popović, B.M.; Gligorijević, N.; Aranđelović, S.; Macedo, A.C.; Jurić, T.; Uka, D.; Mocko-Blažek, K.; Serra, A.T. Cytotoxicity Profiling of Choline Chloride-Based Natural Deep Eutectic Solvents. RSC Adv. 2023, 13, 3520–3527. [Google Scholar] [CrossRef]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Koh, Q.Q.; Kua, Y.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Sugar-Based Natural Deep Eutectic Solvent (NADES): Physicochemical Properties, Antimicrobial Activity, Toxicity, Biodegradability and Potential Use as Green Extraction Media for Phytonutrients. Sustain. Chem. Pharm. 2023, 35, 101218. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural Deep Eutectic Solvents: Cytotoxic Profile. SpringerPlus 2016, 5, 913. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, Cytotoxic and Antioxidative Evaluation of Natural Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef]
- Bezerra, F.S.; Ramos, G.M.S.; Carvalho, M.G.O.; Carvalho, H.S.; de Souza, J.P.; de Carvalho Neto, S.L.; de Souza, S.M.A.G.U.; Ferraz, D.C.C.; Koblitz, M.G.B. Cytotoxic Potential of Sunflower Meal NaDES and Liquid–Liquid Extracts. Food Chem. 2025, 474, 143148. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, M.; Zhang, L. The Effect of Deep Eutectic Solvent on the Pharmacokinetics of Salvianolic Acid B in Rats and Its Acute Toxicity Test. J. Chromatogr. B 2017, 1063, 60–66. [Google Scholar] [CrossRef]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef]
- Radhouane, M.F.; da Silveira, T.F.F.; Ribeiro, J.; Rodrigues, P.; Guimarães, R.; Calhelha, R.; Mandim, F.; Charfi, I.; Ferreira, I.C.F.R.; Alves, M.J.; et al. Development, Characterization and Stability of a Novel Sport Drink Based on Thermal Water, Apple Juice and Hibiscus. Food Chem. Adv. 2024, 5, 100823. [Google Scholar] [CrossRef]
- Bendaali, Y.; Vaquero, C.; González, C.; Morata, A. Contribution of Grape Juice to Develop New Isotonic Drinks with Antioxidant Capacity and Interesting Sensory Properties. Front. Nutr. 2022, 9, 890640. [Google Scholar] [CrossRef]
- Antonio-Gómez, M.V.; Salinas-Moreno, Y.; Hernández-Rosas, F.; Herrera-Corredor, J.A.; Contreras-Oliva, A. Color and Stability of Anthocyanins of Chagalapoli (Ardisia compressa K.) Fruit Added to an Isotonic Beverage as Microcapsules and as Free Extract. Foods 2023, 12, 2009. [Google Scholar] [CrossRef]
- Tomczyk, M.; Zaguła, G.; Dżugan, M. A Simple Method of Enrichment of Honey Powder with Phytochemicals and Its Potential Application in Isotonic Drink Industry. LWT 2020, 125, 109204. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.S.; Gomes, M.H.G.; de Carvalho, L.M.; Abreu, T.L.; dos Santos Lima, M.; Madruga, M.S.; Kurozawa, L.E.; Bezerra, T.K.A. Microencapsulation of Organic Coffee Husk Polyphenols: Effects on Release, Bioaccessibility, and Antioxidant Capacity of Phenolics in a Simulated Gastrointestinal Tract. Food Chem. 2024, 434, 137435. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Gultekin Subasi, B.; Celebioglu, H.U.; Ozdal, T.; Capanoglu, E. Chemistry of Protein-Phenolic Interactions Toward the Microbiota and Microbial Infections. Front. Nutr. 2022, 9, 914118. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, L.; Liu, Y.; Li, J. Effects of Interface Generation, Droplet Size and Antioxidant Partitioning on the Oxidation Rate and Oxidative Stability of Water–in–Oil Emulsions: A Comparison of Coarse Emulsions and Nanoemulsions. Food Hydrocoll. 2023, 136, 108227. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic–Protein Interactions: Insight from In-Silico Analyses—A Review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Rehman, M.T.; AlAjmi, M.F.; Husain, F.M.; Hisamuddin, M.; Altwaijry, N. Molecular interaction of tea catechin with bovine β-lactoglobulin: A spectroscopic and in silico studies. Saudi Pharm. J. 2020, 28, 238–245. [Google Scholar] [CrossRef]
- Buchweitz, M.; Kroon, P.A.; Rich, G.T.; Wilde, P.J. Quercetin solubilisation in bile salts: A comparison with sodium dodecyl sulphate. Food Chem. 2016, 211, 356–364. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Kwaśny, D.; Borczak, B.; Kapusta-Duch, J.; Kron, I. The Influence of Different Polyphenols on the Digestibility of Various Kinds of Starch and the Value of the Estimated Glycemic Index. Appl. Sci. 2024, 14, 8065. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Xue, H.; Feng, J.; Tang, Y.; Wang, X.; Tang, J.; Cai, X.; Zhong, H. Research progress on the interaction of the polyphenol–protein–polysaccharide ternary systems. Chem. Biol. Technol. Agric. 2024, 11, 95. [Google Scholar] [CrossRef]
- Ramírez-Damián, M.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Téllez-Medina, D.I.; Ramos-Monroy, O.A. Microencapsulation of Red Banana Peel Extract and Bioaccessibility Assessment by in vitro Digestion. Processes 2022, 10, 768. [Google Scholar] [CrossRef]
- Martinović, J.; Ambrus, R.; Planinić, M.; Perković, G.; Šelo, G.; Klarić, A.M.; Bucić-Kojić, A. Spray-Drying Microencapsulation of Grape Pomace Extracts with Alginate-Based Coatings and Bioaccessibility of Phenolic Compounds. Gels 2025, 11, 130. [Google Scholar] [CrossRef]
- Cegledi, E.; Garofulić, I.E.; Zorić, Z.; Roje, M.; Dragović-Uzelac, V. Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods 2022, 11, 2852. [Google Scholar] [CrossRef]
- Farooq, M.; Hou, X.; Zheng, W.; Li, C.; Wang, B.; Ullah, A.; Li, J.; Tang, Z. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil–water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4283. [Google Scholar] [CrossRef]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What Makes Good Antioxidants in Lipid-Based Systems? The Next Theories Beyond the Polar Paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef]
- Zheng, L.; Lin, L.; Su, G.; Zhao, Q.; Zhao, M. Pitfalls of using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay to assess the radical scavenging activity of peptides: Its susceptibility to interference and low reactivity towards peptides. Food Res. Int. 2015, 76 Pt 3, 359–365. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Oney-Montalvo, J.E.; Cauich-Rodríguez, J.V.; González-Ávila, M.; Scampicchio, M.; Morozova, K.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Antioxidant Capacity, Vitamin C and Polyphenol Profile Evaluation of a Capsicum chinense By-Product Extract Obtained by Ultrasound Using Eutectic Solvent. Plants 2022, 11, 2060. [Google Scholar] [CrossRef]
- Berton-Carabin, C.; Villeneuve, P. Targeting Interfacial Location of Phenolic Antioxidants in Emulsions: Strategies and Benefits. Annu. Rev. Food Sci. Technol. 2023, 14, 63–83. [Google Scholar] [CrossRef]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain length affects antioxidant properties of chlorogenate esters in emulsion: The cutoff theory behind the polar paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef] [PubMed]
- Chat, O.A.; Najar, M.H.; Mir, M.A.; Rather, G.M.; Dar, A.A. Effects of surfactant micelles on solubilization and DPPH radical scavenging activity of Rutin. J. Colloid Interface Sci. 2011, 355, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Troncho, T.; Villeneuve, P. Evaluation of Antioxidant–Surfactant Interactions Using Size Distribution Taylor Dispersion Analysis: Measuring Antioxidant Partitioning and Size of Native-State Micelles. Curr. Res. Food Sci. 2025, 11, 101142. [Google Scholar] [CrossRef]
- Corsale, I.; Carrieri, P.; Martellucci, J.; Piccolomini, A.; Verre, L.; Rigutini, M.; Panicucci, S. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I–III degree hemorrhoidal disease: A double-blind multicenter prospective comparative study. Int. J. Colorectal Dis. 2018, 33, 1595–1600. [Google Scholar] [CrossRef]
- Mel, M.M.R.D.; Gunathilake, K.D.P.P.; Fernando, C.A.N. Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. Int. J. Biol. Macromol. 2020, 159, 316–323. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G. Absorption of quercetin and rutin in rat small intestine. Ann. Nutr. Metab. 2005, 49, 178–182. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.Y.; Li, H.-B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, A.; Purba, A.; Rashidinejad, A. Novel Rutin-Casein Composites as Functional Dry Ingredients for the Delivery of High Concentration of Rutin in Dairy Beverages: In Vitro Bioaccessibility, Cytotoxicity, Absorption, and Intestinal Barrier Integrity. Food Hydrocoll. 2025, 170, 111735. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, F.; Alonso-Castro, A.J.; Anaya, M.; González-Trujano, M.E.; Salgado-Ceballos, H.; Orozco-Suárez, S. Mexican Traditional Medicine: Traditions of Yesterday and Phytomedicines of Tomorrow. In Therapeutic Medicinal Plants: From Lab to the Market; Tiwari, A.K., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–34. [Google Scholar]
- Frenț, O.D.; Stefan, L.; Morgovan, C.M.; Duteanu, N.; Dejeu, I.L.; Marian, E.; Vicaș, L.; Manole, F. A Systematic Review: Quercetin—Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability. Int. J. Mol. Sci. 2024, 25, 12091. [Google Scholar] [CrossRef]
- Yuan, M.; Sun, T.; Zhang, Y.; Guo, C.; Wang, F.; Yao, Z.; Yu, L. Quercetin Alleviates Insulin Resistance and Repairs Intestinal Barrier in db/db Mice by Modulating Gut Microbiota. Nutrients 2024, 16, 1870. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, T.; Li, Z.; Gao, Y.; Cui, S.W.; Qiu, J. Comparison of Quercetin and Rutin Inhibitory Influence on Tartary Buckwheat Starch Digestion In Vitro and Their Differences in Binding Sites with the Digestive Enzyme. Food Chem. 2022, 367, 130762. [Google Scholar] [CrossRef]
- Zieliński, H.; Wiczkowski, W.; Honke, J.; Piskuła, M.K. In Vitro Expanded Bioaccessibility of Quercetin-3-Rutinoside and Quercetin Aglycone from Buckwheat Biscuits Formulated from Flours Fermented by Lactic Acid Bacteria. Antioxidants 2021, 10, 571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).