Integrated Alkali Gradient pH Control Purification of Acidic Copper-Containing Etching Waste Solution and Cu2(OH)3Cl Conversion-Calcination Process for High-Purity CuO
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification of AEWS
2.3. Fabrication of CuO
2.4. Characterization
2.5. Measurement of CuO and Impurity Contents
2.5.1. Measurement of CuO Content
2.5.2. Measurement of Chloride Content
2.5.3. Measurement of Metal Impurities Contents
3. Results and Discussions
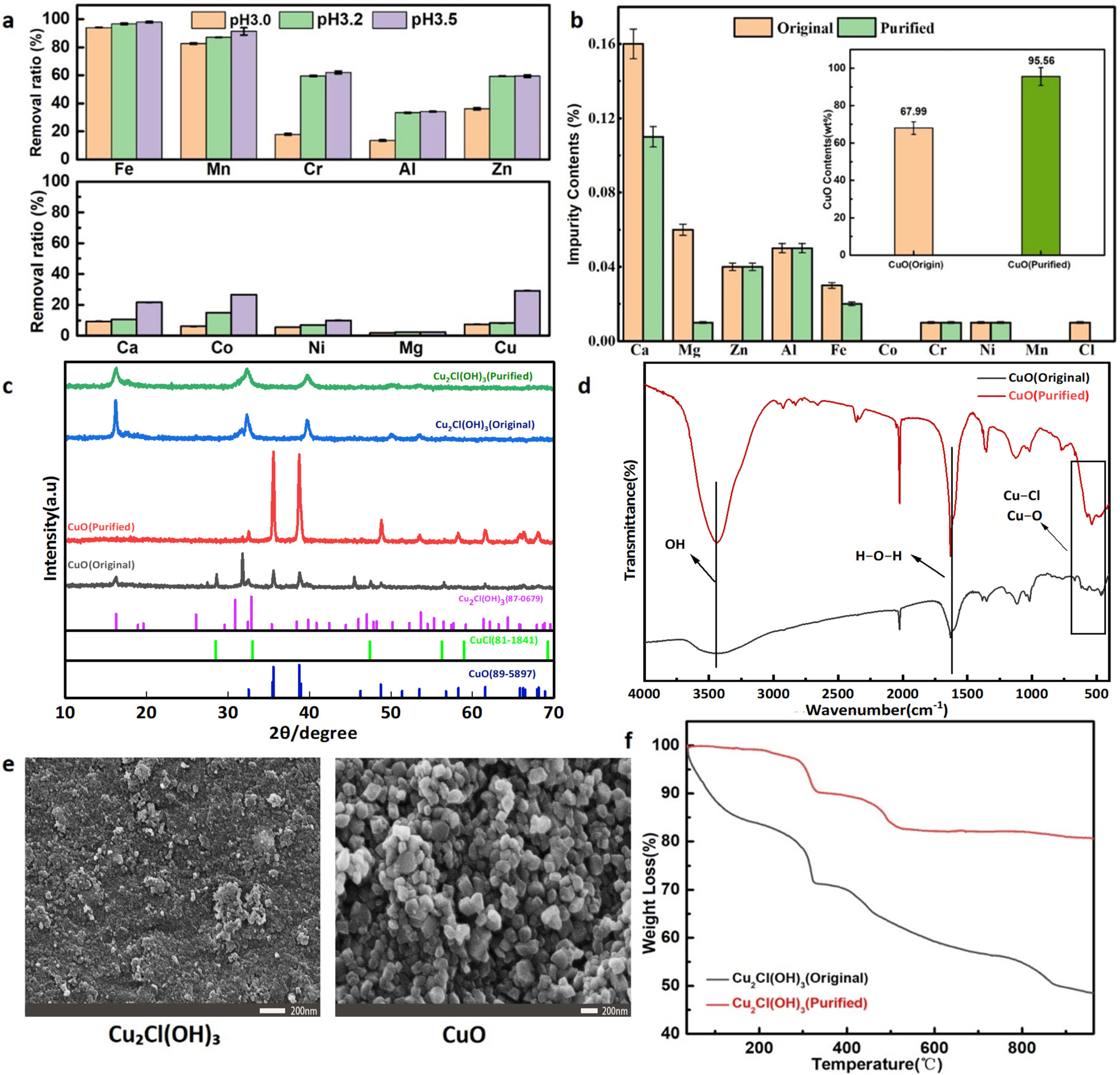
3.1. Removal of Metallic Impurities from AEWS
3.2. Preparation of CuO with AEWS
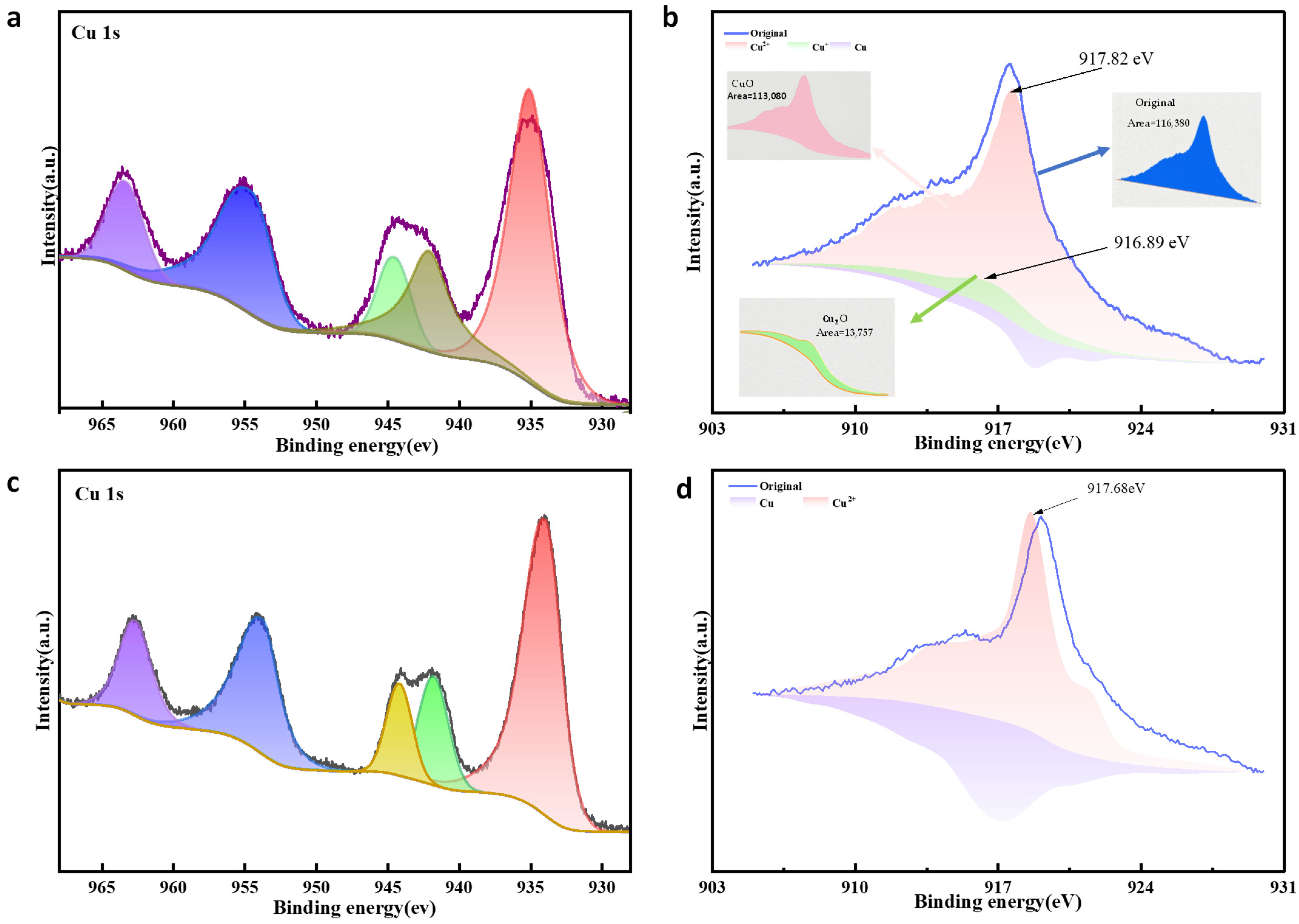
3.3. Refinement of High Purity CuO
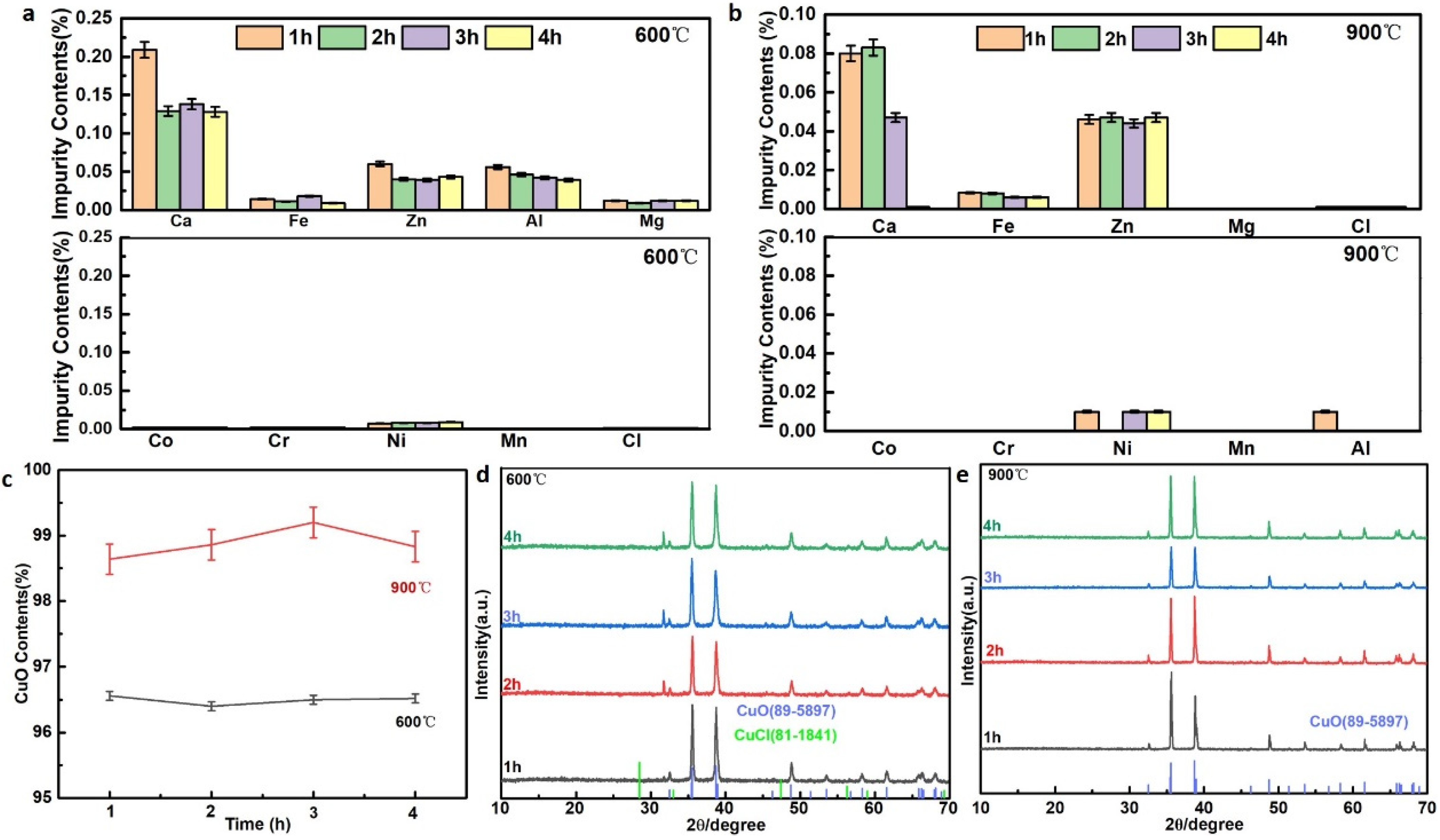
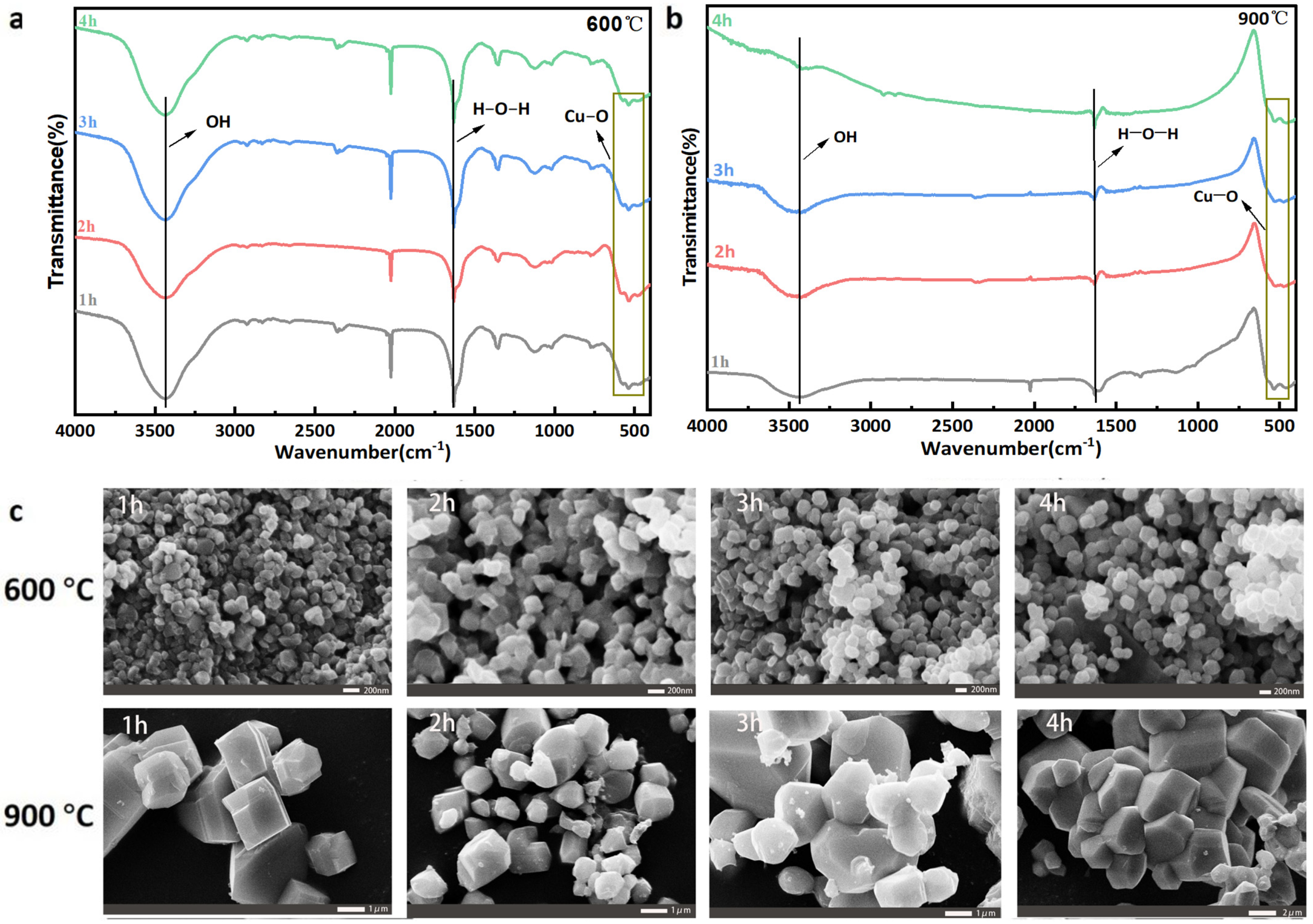
3.3.1. Calcination Technology
3.3.2. Optimizing the Precursor of CuO
3.4. Comparative Analysis
3.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives–A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Chang, Y.; Deng, L.; Meng, X.; Zhang, W.; Wang, C.; Wang, Y.; Zhao, S.; Li, L.; Crittenden, J.C. Closed-loop electrochemical recycling of spent copper (II) from etchant wastewater using a carbon nanotube modified graphite felt anode. Environ. Sci. Technol. 2018, 52, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zeng, X.; Song, Q.; Liu, L.; Li, J.H.; Li, J. Examining regeneration technologies for etching solutions: A critical analysis of the characteristics and potentials. J. Clean. Prod. 2016, 113, 973–980. [Google Scholar] [CrossRef]
- Ullah, A.A.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef]
- Binesh, A.; Venkatachalam, K. Copper in Human Health and Disease: A Comprehensive Review. J. Biochem. Mol. Toxic. 2024, 38, e70052. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. R. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Teschke, R. Copper, iron, cadmium, and arsenic, all generated in the universe: Elucidating their environmental impact risk on human health including clinical liver injury. Int. J. Mol. Sci. 2024, 25, 6662. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Sahu, S.K.; Pramanik, S. Recovery of high value copper and zinc oxide powder from waste brass pickle liquor by solvent extraction. Hydrometallurgy 2016, 165, 182–190. [Google Scholar] [CrossRef]
- Che, J.; Zhang, W.; Ma, B.; Wang, C. An efficient process for recovering copper as CuO nanoparticles from acidic waste etchant via chemical precipitation and thermal decomposition: Turning waste into value-added product. J. Clean. Prod. 2022, 369, 133404. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Qin, Y.; Zhou, M.; Sun, J. Iron-carbon microelectrolysis for wastewater remediation: Preparation, performance and interaction mechanisms. Chemosphere 2021, 278, 30483. [Google Scholar] [CrossRef]
- Ramírez Zamora, R.M.; Schouwenaars, R.; Durán Moreno, A.; Buitron, G. Production of activated carbon from petroleum coke and its application in water treatment for the removal of metals and phenol. Water Sci. Technol. 2000, 42, 119–126. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kano, K.; Suzuki, T.; Kobayashi, A. A novel technology for on-site cupric oxide recovery from cupric chloride etchant waste. Water Sci. Technol. 2011, 64, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Riu, D.H.; Kim, S.R.; Kim, B.I. Preparation of shape-controlled copper oxide powders from copper-containing solution. Mater. Leet 2002, 54, 229–237. [Google Scholar] [CrossRef]
- Qi, W.X.; Zhang, H.; Liu, K.; Li, J.; Zhang, X.X.; Xie, Y.Y.; Qi, T. Clean recovery of copper from waste printed circuit boards using ceric ammonium nitrate. Sep. Purif. Technol. 2023, 324, 124474. [Google Scholar] [CrossRef]
- Wang, L.P.; Chen, Y.J. Sequential precipitation of iron, copper, and zinc from wastewater for metal recovery. J. Environ. Eng. 2023, 145, 04018130. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, F.; Ma, Y. Ultrasonic recovery of copper and iron through the simultaneous utilization of Printed Circuit Boards (PCB) spent acid etching solution and PCB waste sludge. J. Hazard. Mater. 2011, 185, 155–161. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, J.; Yan, X.; Liu, X.; Xu, W.; Liu, W.; Lin, Z. Recovery of copper from printed circuit board (PCB) acidic etching wastewater: Ammonia regulates the crystallization of high valued copper salt. Surf. Interfaces 2022, 31, 969. [Google Scholar] [CrossRef]
- Wen, Z.J.; Eliot, R.; Hui, S.; Baikun, L.; Heng, Z.; Yu, L. Cu2Cl(OH)3 to nanostructured sisal-like Cu(OH)2 and CuO: Synthesis and characterization. J. Appl. Phys. 2009, 105, 064917. [Google Scholar]
- Chia-Chang, L.; Min-Shan, W. Continuous production of CuO nanoparticles in a rotating packed bed. Ceram. Int. 2016, 42, 2133–2139. [Google Scholar] [CrossRef]
- HG/T 5354-2018; Industrial Activated Copper Oxide. Chemical Industry Press: Beijing, China, 2018.
- Stec, M.; Jagustyn, B.; Słowik, K.; Ściążko, M.; Iluk, T. Influence of high chloride concentration on pH control in hydroxide precipitation of heavy metals. J. Sustain. Met. 2020, 6, 239–249. [Google Scholar] [CrossRef]
- BrbootI, M.M.; AbiD, B.A.; Al-ShuwaikI, N.M. Removal of heavy metals using chemicals precipitation. Eng. Technol. J. 2011, 29, 595–612. [Google Scholar] [CrossRef]
- Seo, E.Y.; Cheong, Y.W.; Yim, G.J.; Min, K.W.; Geroni, J.N. Recovery of Fe, Al and Mn in acid coal mine drainage by sequential selective precipitation with control of pH. Catena 2017, 148, 11–16. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Jay Chithra, M.; Sathya, M.; Pushpanathan, K.J.A.M.S. Effect of pH on crystal size and photoluminescence property of ZnO nanoparticles prepared by chemical precipitation method. Acta Metall. Sin. 2015, 28, 394–404. [Google Scholar] [CrossRef]
- Baltpurvins, K.A.; Burns, R.C.; Lawrance, G.A. Heavy metals in wastewater: Modelling the hydroxide precipitation of copper (II) from wastewater using lime as the precipitant. Waste Manag. 1996, 16, 717–725. [Google Scholar] [CrossRef]
- Luna, I.Z.; Hilary, L.N.; Chowdhury, A.S.; Gafur, M.A.; Khan, N.; Khan, R.A. Preparation and characterization of copper oxide nanoparticles synthesized via chemical precipitation method. Open Access Libr. J. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Momenil, M.; Mirhosseini; Nazar, Z.; Kazempour, A.; Hakimiyan, M. Antibacterial and photocatalytic activity of CuO nanostructure films with different morphology. J. Mater. Sci. Mater. Electron. 2016, 27, 8131–8137. [Google Scholar] [CrossRef]
- Meshram, S.P.; Adhyapak, P.V.; Mulik, U.P.; Amalnerkar, D.P. Facile synthesis of CuO nanomorphs and their morphology dependent sunlight driven photocatalytic properties. Chem. Eng. J. 2012, 204–206, 158–168. [Google Scholar] [CrossRef]
- Koga, N.; Okada, S.; Nakamura, T.; Tanaka, H. A kinetic study of the thermal decomposition of iron (III) hydroxide-oxides Part 2. Preparation and thermal decomposition of γ-FeO (OH). Thermochim. Acta 1995, 267, 195–208. [Google Scholar] [CrossRef]
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 2014, 20, 113–117. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kodani, S.; Koga, N. Physico-geometrical kinetic modeling of the thermal decomposition of magnesium hydroxide. Phys. Chem. C 2020, 124, 2458–2471. [Google Scholar] [CrossRef]
- Moezzi, A.; Cortie, M.; McDonagh, A. Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide. Dalton T 2016, 45, 7385–7390. [Google Scholar] [CrossRef]
- Koga, N. A comparative study of the effects of decomposition rate control and mechanical grinding on the thermal decomposition of aluminum hydroxide. J. Therm. Anal. Calorim. 2005, 8, 595–601. [Google Scholar] [CrossRef]
- Haq, I.U.; Akhtar, K.; Malook, K. Synthesis and characterization of monodispersed copper oxide and their precursor powder. Mater. Res. Bull. 2014, 57, 121–126. [Google Scholar] [CrossRef]
- Filipič, G.; Cvelbar, U. Copper oxide nanowires: A review of growth. Nanotechnology 2012, 23, 194001. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Ren, S.; Liu, S.; Xue, S. Integrated Alkali Gradient pH Control Purification of Acidic Copper-Containing Etching Waste Solution and Cu2(OH)3Cl Conversion-Calcination Process for High-Purity CuO. Processes 2025, 13, 2807. https://doi.org/10.3390/pr13092807
He D, Ren S, Liu S, Xue S. Integrated Alkali Gradient pH Control Purification of Acidic Copper-Containing Etching Waste Solution and Cu2(OH)3Cl Conversion-Calcination Process for High-Purity CuO. Processes. 2025; 13(9):2807. https://doi.org/10.3390/pr13092807
Chicago/Turabian StyleHe, Dengliang, Song Ren, Shuxin Liu, and Shishan Xue. 2025. "Integrated Alkali Gradient pH Control Purification of Acidic Copper-Containing Etching Waste Solution and Cu2(OH)3Cl Conversion-Calcination Process for High-Purity CuO" Processes 13, no. 9: 2807. https://doi.org/10.3390/pr13092807
APA StyleHe, D., Ren, S., Liu, S., & Xue, S. (2025). Integrated Alkali Gradient pH Control Purification of Acidic Copper-Containing Etching Waste Solution and Cu2(OH)3Cl Conversion-Calcination Process for High-Purity CuO. Processes, 13(9), 2807. https://doi.org/10.3390/pr13092807




