Optimized Extraction and Component Identification of Physalis alkekengi L. Calyx Polyphenols and Antioxidant Dynamics During Thermal Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
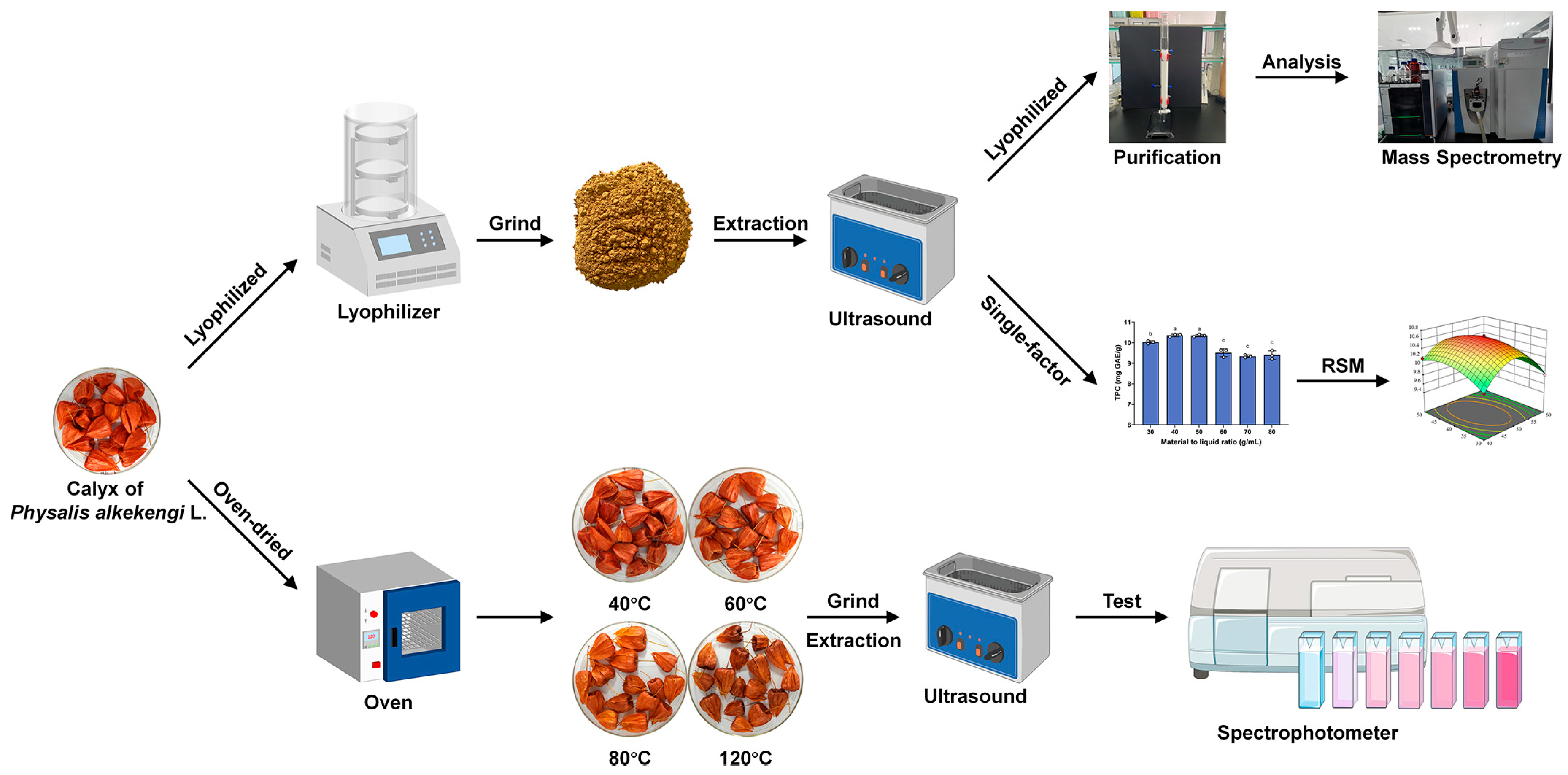
2.3. Extraction of Polyphenols from CPAL
2.3.1. Preparation of CPAL Powder
2.3.2. CPAL-Drying Experiment
2.3.3. Ultrasonic-Assisted Extraction
2.3.4. Quantification of the Total Phenolic Content
2.4. Extraction Optimization of CPAL Polyphenols
2.4.1. Design of the Single-Factor Experiment
2.4.2. RSM Experiment
2.5. Characterization of Polyphenolics in CPAL
2.5.1. Purification of the CPAL Ethanol Extract
2.5.2. Analysis by UPLC-Q Exactive HF Orbitrap-MS
2.6. Antioxidant Assays
2.6.1. DPPH Radical-Scavenging Assay
2.6.2. ABTS Radical-Scavenging Assay
2.6.3. Ferric-Reducing Antioxidant Power Assay
2.7. Statistical Analysis
3. Results and Discussion
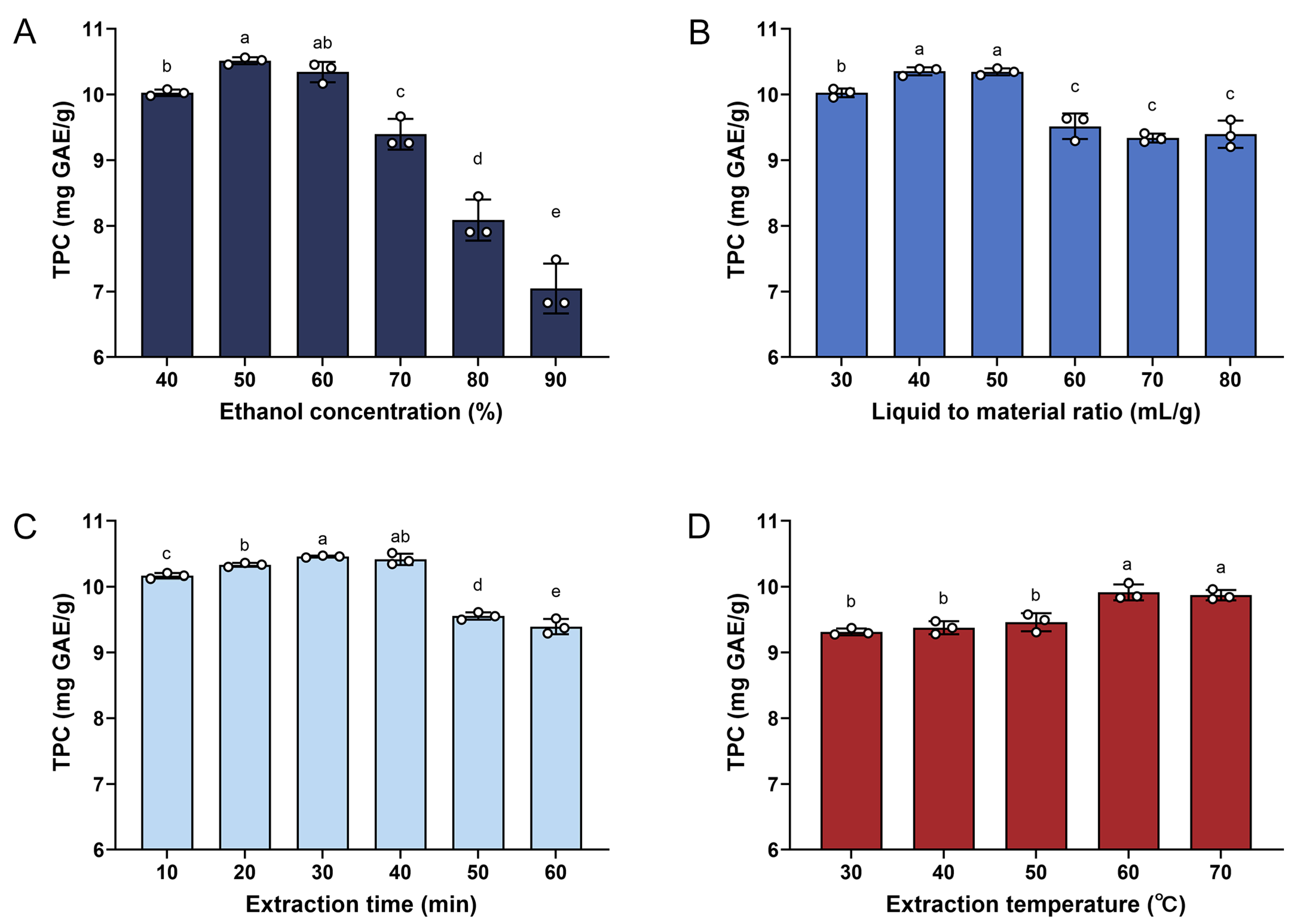
3.1. Single-Factor Experiment
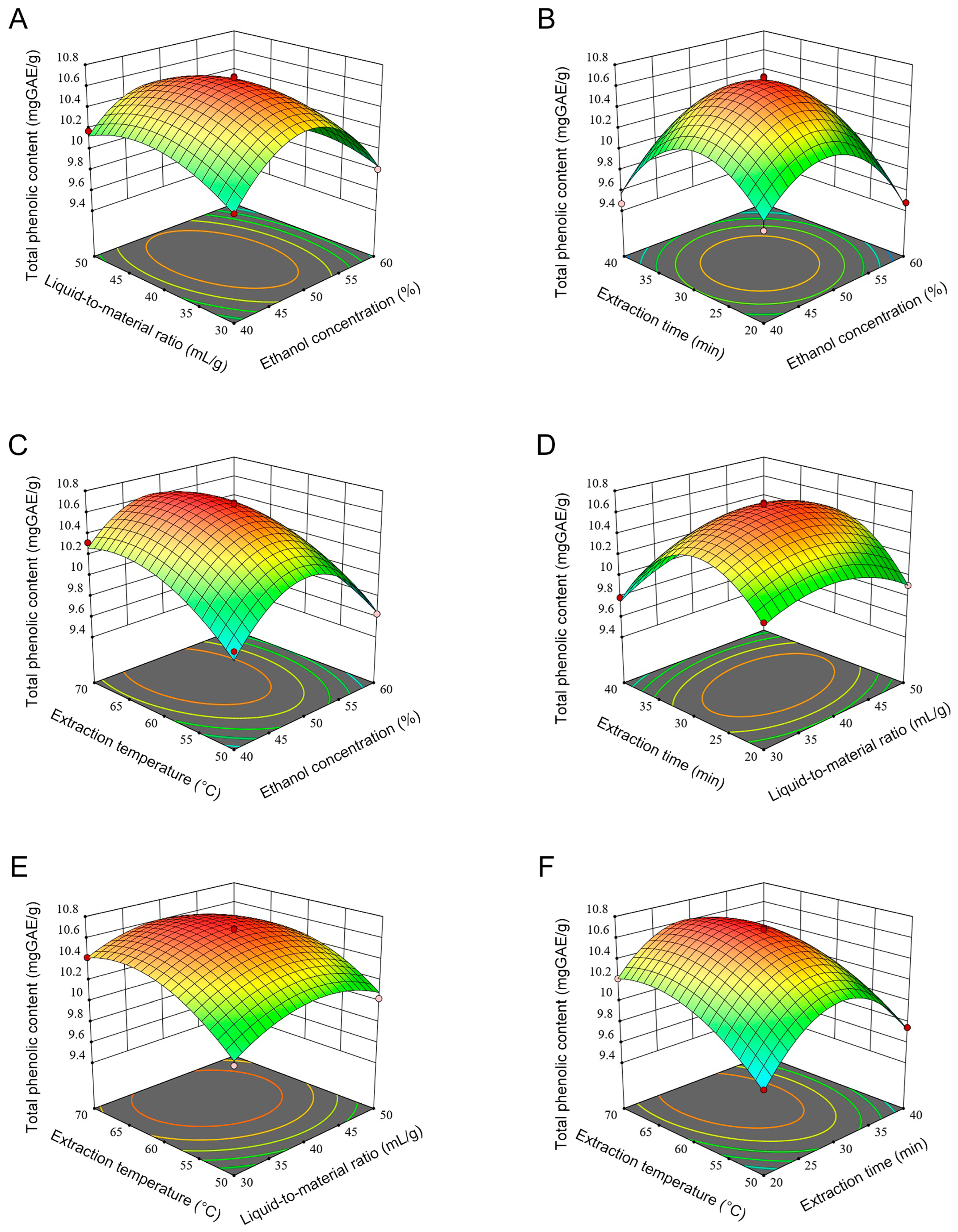
3.2. Analysis of RSM
3.2.1. Analysis of Variance and Regression Equation
− 0.0425X2X4 − 0.0775X3X4 − 0.5422X12 − 0.2110X22 − 0.5223X32 − 0.2310X42.
3.2.2. Verification Experiment
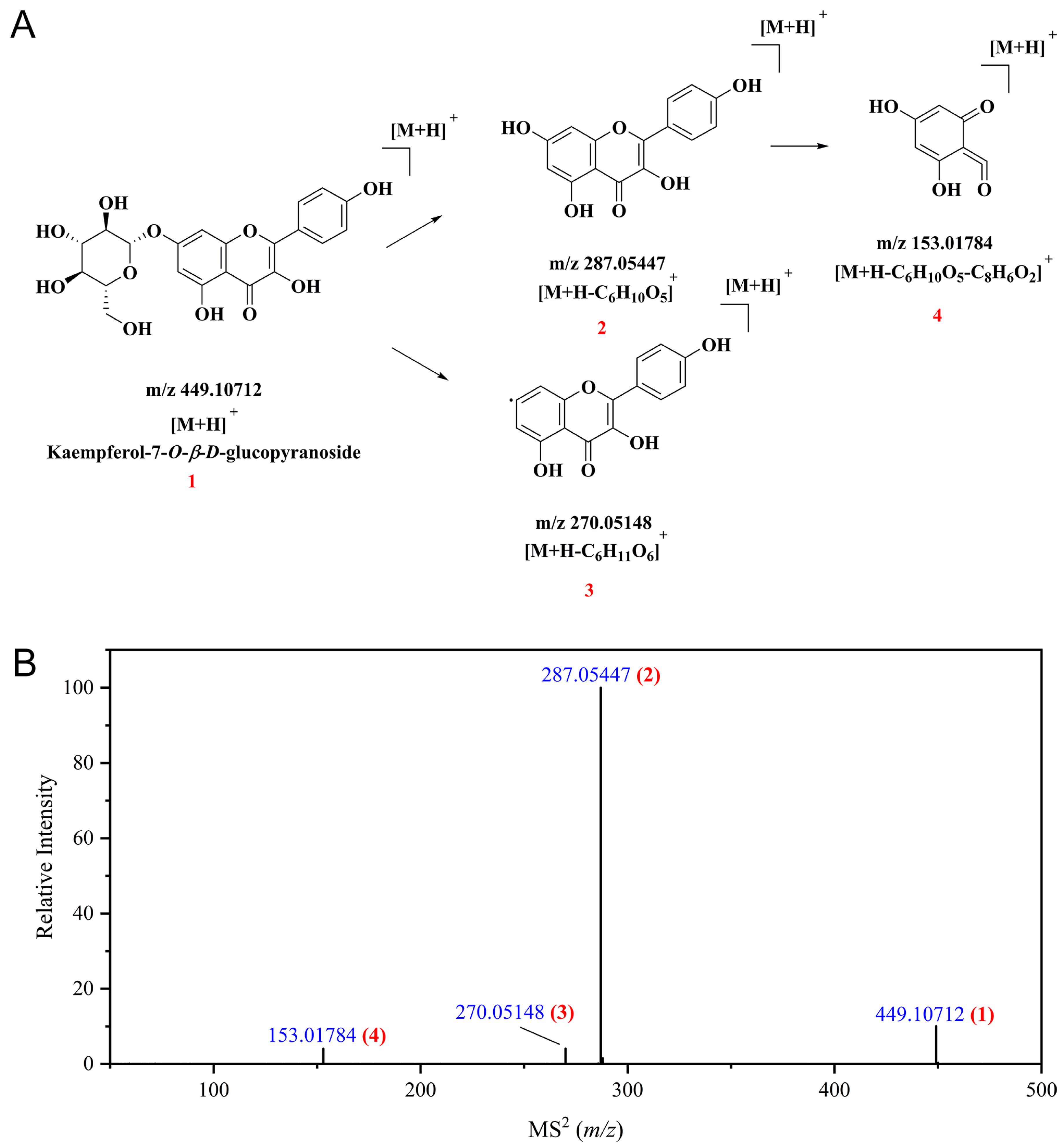
3.3. Profiles of Polyphenolic Compounds from CPAL
3.4. Antioxidant Activities
3.5. The Changes in the Polyphenol Content and Antioxidant Capacity of CPAL During Drying
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPAL | Calyx of Physalis alkekengi L. |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | Ferric-reducing antioxidant power |
| TPC | Total phenolic content |
| UPLC | Ultra-performance liquid chromatography |
References
- Liu, X.; Bian, J.; Li, D.; Liu, C.; Xu, S.; Zhang, G.; Zhang, L.; Gao, P. Structural features, antioxidant and acetylcholinesterase inhibitory activities of polysaccharides from stem of Physalis alkekengi L. Ind. Crops Prod. 2019, 129, 654–661. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, C.; Yu, D.; Tian, B.; Li, W.; Sun, Z. Research progress on the chemical components and pharmacological effects of Physalis alkekengi L. var. franchetii (Mast.) Makino. Heliyon 2023, 9, e20030. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, L.; Chen, Y.; Wang, C.-H.; ShenTu, J.-z.; Zheng, Y.-L. Physalin B induces G2/M cell cycle arrest and apoptosis in A549 human non-small-cell lung cancer cells by altering mitochondrial function. Anticancer Drugs 2019, 30, 128–137. [Google Scholar] [CrossRef]
- Popova, V.; Petkova, Z.; Mazova, N.; Ivanova, T.; Petkova, N.; Stoyanova, M.; Stoyanova, A.; Ercisli, S.; Okcu, Z.; Skrovankova, S.; et al. Chemical composition assessment of structural parts (seeds, peel, pulp) of Physalis alkekengi L. fruits. Molecules 2022, 27, 5787. [Google Scholar] [CrossRef]
- Teng, Y.; Yuan, Q.; Wu, Y.; Wu, S.; Su, J.; Zhang, P.; Zhang, Y. Research on the chemical constituents against Alzheimer’s disease of the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino. Chem. Biodivers. 2023, 20, e202301075. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Cao, F.; Yang, B.; Kuang, H. Natural products from Physalis alkekengi L. var. franchetii (Mast.) Makino: A review on their structural analysis, quality control, pharmacology, and pharmacokinetics. Molecules 2022, 27, 695. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, Y.; Fang, G.; Zhang, Y.; Wang, S. Polysaccharides from fruit calyx of Physalis alkekengi var. francheti: Isolation, purification, structural features and antioxidant activities. Carbohyd. Polym. 2009, 77, 188–193. [Google Scholar] [CrossRef]
- Chang, H.-T. Grand challenges in analytical science. Front. Anal. Sci. 2021, 1, 725070. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, Z.; Zhao, X.; Sun, L.; Wang, L.; Ren, X.; Deng, Y.; Mafra, I. Assessing the quality of calyx of Physalis alkekengi L. var. franchetii based on quantitative analysis of Q-marker combined with chemometrics and machine learning algorithms. J. Chem. 2021, 2021, 8502929. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Yang, S.; Tao, A.; Li, J. Advancements in the extraction, characterization and function activities of polysaccharides from Physalis L.: A review. Int. J. Biol. Macromol. 2025, 303, 140685. [Google Scholar] [CrossRef]
- Luque-García, J.L.; Luque de Castro, M.D. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- González-Silva, N.; Nolasco-González, Y.; Aguilar-Hernández, G.; Sáyago-Ayerdi, S.G.; Villagrán, Z.; Acosta, J.L.; Montalvo-González, E.; Anaya-Esparza, L.M. Ultrasound-assisted extraction of phenolic compounds from Psidium cattleianum Leaves: Optimization using the response surface methodology. Molecules 2022, 27, 3557. [Google Scholar] [CrossRef]
- Keskin Çavdar, H.; Avşar, S. Ultrasonic extraction of Inula viscosa: Enhancing antioxidant bioactivity and its application in sunflower oil as an antioxidant. Ultrason. Sonochem. 2024, 109, 106992. [Google Scholar] [CrossRef]
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Ruiz, K.; Hamieh, T.; Chemat, F. Extraction of green absolute from thyme using ultrasound and sunflower oil. Resour.-Effic. Technol. 2017, 3, 12–21. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Mazova, N.; Popova, V.; Stoyanova, A. Phytochemical composition and biological activity of Physalis spp.: A mini-review. Food Sci. Appl. Biotechnol. 2020, 3, 56–70. [Google Scholar] [CrossRef]
- Yang, M.; Wang, S.; Zhou, R.; Zhao, Y.; He, Y.; Zheng, Y.; Gong, H.; Wang, W. Optimization and component identification of ultrasound-assisted extraction of functional compounds from waste blackberry (Rubus fruticosus Pollich) seeds. J. Sci. Food Agric. 2024, 104, 9169–9179. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Zhang, R.; Wang, X.; Yu, H.; Shi, X.; Yi, J.; Li, J.; Qi, Q.; Dong, R.; Li, Q. Ultrasound-assisted extraction of polyphenols from Phyllanthi Fructus: Comprehensive insights from extraction optimization and antioxidant activity. Ultrason. Sonochem. 2024, 111, 107083. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Feng, M.; Tiliwa, E.S.; Yan, W.; Wei, B.; Zhou, C.; Ma, H.; Wang, B.; Chang, L. Multi-frequency power ultrasound green extraction of polyphenols from Pingyin rose: Optimization using the response surface methodology and exploration of the underlying mechanism. LWT-Food Sci. Technol. 2022, 156, 113037. [Google Scholar] [CrossRef]
- Huo, H.; Bao, H.; Yin, H. Optimization of bioactive polyphenols recovery from Flammulina velutipes stem waste using nonionic surfactant-integrated ultrasound-assisted extraction. Ultrason. Sonochem. 2025, 119, 107408. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Haque, S.K.M. Application of combined Box–Behnken design with response surface methodology and desirability function in optimizing pectin extraction from fruit peels. J. Sci. Food Agric. 2024, 104, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Xie, C.; Ma, Y.; Miao, Y.; Chen, X.; Gong, H.; Wang, J. Optimization and component identification of ultrasound-assisted extraction of polyphenols from coriander (Coriandrum sativum L.) and evaluation of polyphenol content changes and antioxidant activity during storage. Separations 2025, 12, 217. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Chernonosov, A.A.; Kuznetsov, A.A.; Petrova, N.V.; Krivenko, D.A.; Chernysheva, O.A.; Wang, W.; Erst, A.S. Identification of flavonoids in the leaves of Eranthis longistipitata (Ranunculaceae) by liquid chromatography with high-resolution mass spectrometry (LC-HRMS). Plants 2021, 10, 2146. [Google Scholar] [CrossRef] [PubMed]
- Barnaba, C.; Larcher, R.; Nardin, T.; Dellacassa, E.; Nicolini, G. Glycosylated simple phenolic profiling of food tannins using high resolution mass spectrometry (Q-Orbitrap). Food Chem. 2018, 267, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Alonso-Carrillo, N.; Aguilar-Santamaría, M.d.l.Á.; Vernon-Carter, E.J.; Jiménez-Alvarado, R.; Cruz-Sosa, F.; Román-Guerrero, A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crops Prod. 2017, 103, 213–221. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Chu, J.; Ming, Y.; Cui, Q.; Zheng, N.; Yang, S.; Li, W.; Gao, H.; Zhang, R.; Cheng, X. Efficient extraction and antioxidant activity of polyphenols from Antrodia cinnamomea. BMC Biotechnol. 2022, 22, 9. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, B.; Stephen Chen, Z.; Wang, L.; Tang, W.; Huang, C. Recent technologies for the extraction and separation of polyphenols in different plants: A Review. J. Renew. Mater. 2022, 10, 1471–1490. [Google Scholar] [CrossRef]
- Gavrila, A.I.; Damian, E.J.; Rosca, A.; Calinescu, I.; Hodosan, C.; Popa, I. Optimization of microwave-assisted extraction of polyphenols from Crataegus monogyna L. Antioxidants . Antioxidants 2025, 14, 357. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Lu, X.-X.; Yang, X.-T.; Jiang, L.-J.; Cui, Q. An efficient approach for the extraction of polyphenols from pomegranate peel using the green solvent and profiling by UPLC-Q-TOF-MS/MS analysis. Microchem. J. 2024, 205, 111421. [Google Scholar] [CrossRef]
- Slimani, C.; Fadil, M.; Rais, C.; El-hanafi, L.; Benjelloun, M.; Shin, H.-M.; Giesy, J.P.; Lazraq, A.; Abulmeaty, M.M.A.; Almutairi, K.M.; et al. Extraction of natural antioxidants from Moroccan saffron (Crocus sativus L.) using ultrasound-assisted extraction: An optimization approach with box-behnken design. Ultrason. Sonochem. 2025, 120, 107448. [Google Scholar] [CrossRef] [PubMed]
- Medina-Plaza, C.; Beaver, J.W.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Impact of temperature, ethanol and cell wall material composition on cell wall-anthocyanin interactions. Molecules 2019, 24, 3350. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Bao, H.; Li, J.; Li, H. Optimization and antioxidant evaluation of clean and efficient recovery of polyphenols from Phellodendron amurense waste leaves via ultrasound-assisted extraction. Ultrason. Sonochem. 2025, 120, 107512. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Y.; Wang, S.; Wang, W.; Yu, N.; Gong, H.; Ni, Z. Optimization of functional compounds extraction from Ginkgo biloba seeds using response surface methodology. Qual. Assur. Saf. Crop. 2022, 14, 102–112. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, S.; Liu, M.; Tian, S. Simultaneous process optimization of ultrasound-assisted extraction of polyphenols and ellagic acid from pomegranate (Punica granatum L.) flowers and its biological activities. Ultrason. Sonochem. 2021, 80, 105833. [Google Scholar] [CrossRef]
- Boateng, I.D.; Kumar, R.; Daubert, C.R.; Flint-Garcia, S.; Mustapha, A.; Kuehnel, L.; Agliata, J.; Li, Q.; Wan, C.; Somavat, P. Sonoprocessing improves phenolics profile, antioxidant capacity, structure, and product qualities of purple corn pericarp extract. Ultrason. Sonochem. 2023, 95, 106418. [Google Scholar] [CrossRef]
- Liu, C.; Qiao, L.; Gao, Q.; Zhang, F.; Zhang, X.; Lei, J.; Ren, M.; Xiao, S.; Kuang, J.; Deng, S.; et al. Total biflavonoids extraction from Selaginella chaetoloma utilizing ultrasound-assisted deep eutectic solvent: Optimization of conditions, extraction mechanism, and biological activity in vitro. Ultrason. Sonochem. 2023, 98, 106491. [Google Scholar] [CrossRef]
- Su, J.; Li, P.; Tang, X.; Chen, Z.; Peng, Q.; Li, H.; Zhang, Q.; Lu, S.; Wen, F.; Qin, H.; et al. Ultrasonic extraction of polyphenols from roots of Ilex asprella: Structural characterization, antioxidant, antimicrobial, anti-inflammatory, and anti-hyperlipidemic activities. Ultrason. Sonochem. 2025, 120, 107462. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Mazova, N.; Ivanova, T.; Petkova, N.; Stoyanova, M.; Stoyanova, A.; Ercisli, S.; Assouguem, A.; Kara, M.; Alshawwa, S.Z.; et al. Phytonutrient composition of two phenotypes of Physalis alkekengi L. fruit. Horticulturae 2022, 8, 373. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhang, J.; Song, X.; Zhang, Z.; Li, J.; Li, S. Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J. Ethnopharmacol. 2018, 211, 197–206. [Google Scholar] [CrossRef]
- Chen, L.-X.; Xia, G.-Y.; Liu, Q.-Y.; Xie, Y.-Y.; Qiu, F. Chemical constituents from the calyces of Physalis alkekengi var. franchetii. Biochem. Syst. Ecol. 2014, 54, 31–35. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wu, D.; Tang, L.; Cao, X.; Xin, Y. In vitro effects on intestinal bacterium of physalins from Physalis alkekengi var. Francheti. Fitoterapia 2012, 83, 1460–1465. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; Gong, Q.; Liu, X.; Deng, F. Metabolic profiling of different parts of Physalis alkekengi L. var. franchetii (Mast.) Makino based on UPLC-Q-Orbitrap-HRMS coupled with bioactivity assays. J. Pharmaceut. Biomed. 2024, 249, 116388. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, B.; Wang, B.; Zhang, H. In vivo comprehensive metabolite profiling of esculetin and esculin derived from chicory in hyperuricemia rats using ultra-high-performance liquid chromatography coupled with quadrupole-orbitrap high-resolution mass spectrometry. J. Sep. Sci. 2023, 47, e2300664. [Google Scholar] [CrossRef]
- Buszewski, B.; Walczak, J.; Žuvela, P.; Liu, J.J. Non-target analysis of phospholipid and sphingolipid species in egg yolk using liquid chromatography/triple quadrupole tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Li, X.; Liang, J.; Qin, S.; Li, J.; Zhang, A.; Xu, L.; Tang, D.; Li, F. Characterization of volatile flavour compounds and characteristic flavour precursors in poultry eggs based on multi-omics and machine learning. Food Chem. 2025, 489, 144840. [Google Scholar] [CrossRef] [PubMed]
- Adesegun, S.A.; Alabi, S.O.; Olabanji, P.T.; Coker, H.A.B. Evaluation of antioxidant potential of Melanthera scandens. J. Acupunct. Meridian 2010, 3, 267–271. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Li, C.; Wang, E.; Elshikh, M.S.; Alwahibi, M.S.; Wang, W.; Wu, G.; Shen, Y.; Abbasi, A.M.; Shan, S. Extraction and purification of total flavonoids from Gnaphalium affine D. Don and their evaluation for free radicals’ scavenging and oxidative damage inhabitation potential in mice liver. Arab. J. Chem. 2021, 14, 103006. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Wu, X.; Wang, Z.; Liang, B.; Gao, Y.; Dai, Y.; Wu, Q. Preparation of Physalis alkekengi L. calyx total flavonoids-chitosan composite film and its effect on preservation of chilled beef. Int. J. Biol. Macromol. 2024, 283, 137768. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Rosado-Souza, L.; Sampathkumar, A.; Fernie, A.R. The relationship between cell wall and postharvest physiological deterioration of fresh produce. Plant Physiol. Bioch. 2024, 210, 108568. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Tech. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Mouhoubi, K.; Boulekbache-Makhlouf, L.; Madani, K.; Palatzidi, A.; Perez-Jimenez, J.; Mateos-Aparicio, I.; Garcia-Alonso, A. Phenolic compounds and antioxidant activity are differentially affected by drying processes in celery, coriander and parsley leaves. Int. J. Food Sci. Tech. 2022, 57, 3467–3476. [Google Scholar] [CrossRef]
- Joseph Bassey, E.; Cheng, J.-H.; Sun, D.-W. Comparative elucidation of bioactive and antioxidant properties of red dragon fruit peel as affected by electromagnetic and conventional drying approaches. Food Chem. 2024, 439, 138118. [Google Scholar] [CrossRef]




| Independent Variable | Coded Symbol | Level | ||
|---|---|---|---|---|
| 1 | 0 | −1 | ||
| Ethanol concentration (%) | X1 | 60 | 50 | 40 |
| Liquid-to-material ratio (mL/g) | X2 | 50 | 40 | 30 |
| Extraction time (min) | X3 | 40 | 30 | 20 |
| Extraction temperature (°C) | X4 | 70 | 60 | 50 |
| Time (min) | B: Acetonitrile | A: 0.1% HCOOH Aqueous Solution |
|---|---|---|
| 0–2 | 5 | 95 |
| 2–6 | 30 | 70 |
| 6–7 | 30 | 70 |
| 7–12 | 78 | 22 |
| 12–14 | 78 | 22 |
| 14–17 | 95 | 5 |
| 17–20 | 95 | 5 |
| 20–21 | 5 | 95 |
| Run | X1 (%) | X2 (mL/g) | X3 (Min) | X4 (°C) | Actual Value (mg GAE/g) | Predicted Value (mg GAE/g) |
|---|---|---|---|---|---|---|
| 1 | 40 | 40 | 30 | 70 | 10.32 | 10.27 |
| 2 | 60 | 40 | 30 | 50 | 9.63 | 9.65 |
| 3 | 50 | 30 | 30 | 70 | 10.42 | 10.42 |
| 4 | 40 | 50 | 30 | 60 | 10.18 | 10.13 |
| 5 | 50 | 50 | 30 | 70 | 10.36 | 10.37 |
| 6 | 50 | 50 | 20 | 60 | 9.91 | 9.91 |
| 7 | 50 | 40 | 40 | 70 | 9.95 | 9.95 |
| 8 | 50 | 30 | 40 | 60 | 9.79 | 9.75 |
| 9 | 50 | 30 | 30 | 50 | 9.92 | 9.96 |
| 10 | 50 | 50 | 30 | 50 | 10.03 | 10.09 |
| 11 | 40 | 40 | 30 | 50 | 9.82 | 9.74 |
| 12 | 50 | 40 | 20 | 50 | 9.71 | 9.69 |
| 13 | 60 | 40 | 20 | 60 | 9.48 | 9.44 |
| 14 | 50 | 40 | 30 | 60 | 10.69 | 10.65 |
| 15 | 50 | 50 | 40 | 60 | 9.98 | 9.97 |
| 16 | 50 | 40 | 30 | 60 | 10.61 | 10.65 |
| 17 | 60 | 50 | 30 | 60 | 9.72 | 9.71 |
| 18 | 40 | 40 | 40 | 60 | 9.47 | 9.57 |
| 19 | 50 | 40 | 30 | 60 | 10.68 | 10.65 |
| 20 | 50 | 40 | 30 | 60 | 10.63 | 10.65 |
| 21 | 60 | 30 | 30 | 60 | 9.81 | 9.84 |
| 22 | 60 | 40 | 40 | 60 | 9.52 | 9.49 |
| 23 | 40 | 40 | 20 | 60 | 9.77 | 9.86 |
| 24 | 50 | 40 | 30 | 60 | 10.65 | 10.65 |
| 25 | 50 | 30 | 20 | 60 | 10.07 | 10.04 |
| 26 | 50 | 40 | 20 | 70 | 10.22 | 10.22 |
| 27 | 60 | 40 | 30 | 70 | 9.82 | 9.86 |
| 28 | 40 | 30 | 30 | 60 | 9.92 | 9.92 |
| 29 | 50 | 40 | 40 | 50 | 9.75 | 9.73 |
| Source | Sum of Squares | DF | Mean-Squared Value | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 3.92 | 14 | 0.2800 | 83.12 | <0.0001 | ** |
| X1 | 0.1875 | 1 | 0.1875 | 55.67 | <0.0001 | ** |
| X2 | 0.0052 | 1 | 0.0052 | 1.55 | 0.2341 | |
| X3 | 0.0408 | 1 | 0.0408 | 12.12 | 0.0037 | * |
| X4 | 0.4144 | 1 | 0.4144 | 123.04 | <0.0001 | ** |
| X1X2 | 0.0306 | 1 | 0.0306 | 9.09 | 0.0093 | * |
| X1X3 | 0.0289 | 1 | 0.0289 | 8.58 | 0.0110 | * |
| X1X4 | 0.0240 | 1 | 0.0240 | 7.13 | 0.0183 | * |
| X2X3 | 0.0306 | 1 | 0.0306 | 9.09 | 0.0093 | * |
| X2X4 | 0.0072 | 1 | 0.0072 | 2.15 | 0.1651 | |
| X3X4 | 0.0240 | 1 | 0.0240 | 7.13 | 0.0183 | * |
| X12 | 1.191 | 1 | 1.191 | 566.25 | <0.0001 | ** |
| X22 | 0.2888 | 1 | 0.2888 | 85.74 | <0.0001 | ** |
| X32 | 1.77 | 1 | 1.77 | 525.25 | <0.0001 | ** |
| X42 | 0.3461 | 1 | 0.3461 | 102.76 | <0.0001 | ** |
| Residual | 0.0472 | 14 | 0.0034 | |||
| Lack of fit | 0.0427 | 10 | 0.0043 | 3.81 | 0.1046 | Not significant |
| Pure error | 0.0045 | 4 | 0.0011 | |||
| Cor total | 3.97 | 28 | ||||
| R2 | 0.9881 | |||||
| Radj2 | 0.9762 | |||||
| CV (%) | 0.5787 |
| NO. | Name | Classification | Ionization Mode | RT (min) | Formula | Predicted | Measured | Delta Mass (ppm) | MS/MS (m/z) | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| A: Flavonoids | ||||||||||
| 1 | Apigenin a [34] | Apigenin derivatives | [M−H]− | 9.854 | C15H10O5 | 270.05282 | 270.05283 | 0.04 | 151.00290, 117.03364 | 82.36 |
| 2 | Cosmosiin a [35] | [M−H]− | 7.291 | C21H20O10 | 432.10565 | 432.10577 | 0.27 | 269.04559, 268.03775 | 75.89 | |
| 3 | Isovitexin b | [M+H]+ | 6.599 | C21H20O10 | 432.10565 | 432.10533 | −0.72 | 415.10175, 397.09218, 313.07013, 283.05969 | 81.53 | |
| 4 | Hydroxy- genkwanin b | Baicalein derivatives | [M+H]+ | 9.948 | C16H12O6 | 300.06339 | 300.06269 | −2.31 | 287.05069, 286.04666, 153.01767 | 78.96 |
| 5 | Homoplantaginin b | [M+H]+ | 7.33 | C22H22O11 | 462.11621 | 462.11547 | −1.61 | 301.07007, 286.04657, 161.56418 | 75.46 | |
| 6 | Cynaroside a [2] | Luteolin derivatives | [M−H]− | 7.316 | C21H20O11 | 448.10056 | 448.10062 | 0.14 | 285.04050, 284.03275, 151.00319 | 81.36 |
| 7 | Luteolin-4′-O-glucoside a [2] | [M−H]− | 7.523 | C21H20O11 | 448.10056 | 448.10078 | 0.5 | 369.05130, 285.04065, 135.04442 | 82.58 | |
| 8 | Pectolinarigenin b | [M−H]− | 11.586 | C17H14O6 | 314.07904 | 314.07899 | −0.15 | 299.05167, 298.04837, 283.02490 | 79.50 | |
| 9 | Vicenin II b | [M-H]− | 5.763 | C27H30O15 | 594.15847 | 594.15896 | 0.83 | 575.14117, 503.12006, 473.10941, 353.06699 | 82.36 | |
| 10 | Luteolin a [35] | [M−H]− | 6.842 | C15H10O6 | 286.04774 | 286.04776 | 0.06 | 151.00304, 133.02873, 107.01301 | 78.68 | |
| 11 | Vicenin III b | [M−H]− | 9.328 | C26H28O14 | 564.14791 | 564.14847 | 1.01 | 519.15125, 443.13525, 401.08781 | 78.33 | |
| 12 | 2″-O-β-L-Galactopyranosylorientin b | [M+H]+ | 5.524 | C27H30O16 | 610.15338 | 610.15294 | −0.73 | 593.23419, 449.10721, 287.05453 | 73.24 | |
| 13 | Luteolin 7-rutinoside b | [M−H]− | 6.59 | C27H30O15 | 594.15847 | 594.15917 | 1.17 | 430.09146, 285.04056, 254.03275 | 71.51 | |
| 14 | Diosmetin b | Diosmetin derivatives | [M−H]− | 9.995 | C16H12O6 | 300.06339 | 300.06325 | −0.46 | 284.03268, 190.90388, 151.0110 | 76.14 |
| 15 | Diosmetin-7-O-β-D-glucopyranoside b | [M−H]− | 7.38 | C22H22O11 | 462.11621 | 462.1164 | 0.4 | 446.08563, 299.05612, 284.03293, 283.02478 | 72.31 | |
| 16 | 5,7,3′-Trihydroxy-6,4′,5′-trimethoxyflavone b | Others | [M+H]+ | 11.432 | C18H16O8 | 360.08452 | 360.08394 | −1.6 | 346.06757, 345.05988, 301.07010, 153.05444, 151.03883 | 70.26 |
| 17 | Sinensetin b | [M+H]+ | 11.735 | C20H20O7 | 372.1209 | 372.12031 | −1.59 | 358.10406, 343.08066, 312.09845 | 71.49 | |
| 18 | Eupafolin b | [M+H]+ | 10.653 | C16H12O7 | 316.0583 | 316.05779 | −1.63 | 302.04141, 299.05426, 271.05963, 151.03883 | 73.29 | |
| 19 | Nobiletin a [2] | [M+H]+ | 11.209 | C21H22O8 | 402.13147 | 402.13074 | −1.8 | 388.11447, 373.09100, 343.22592 | 84.56 | |
| B: Flavonols | ||||||||||
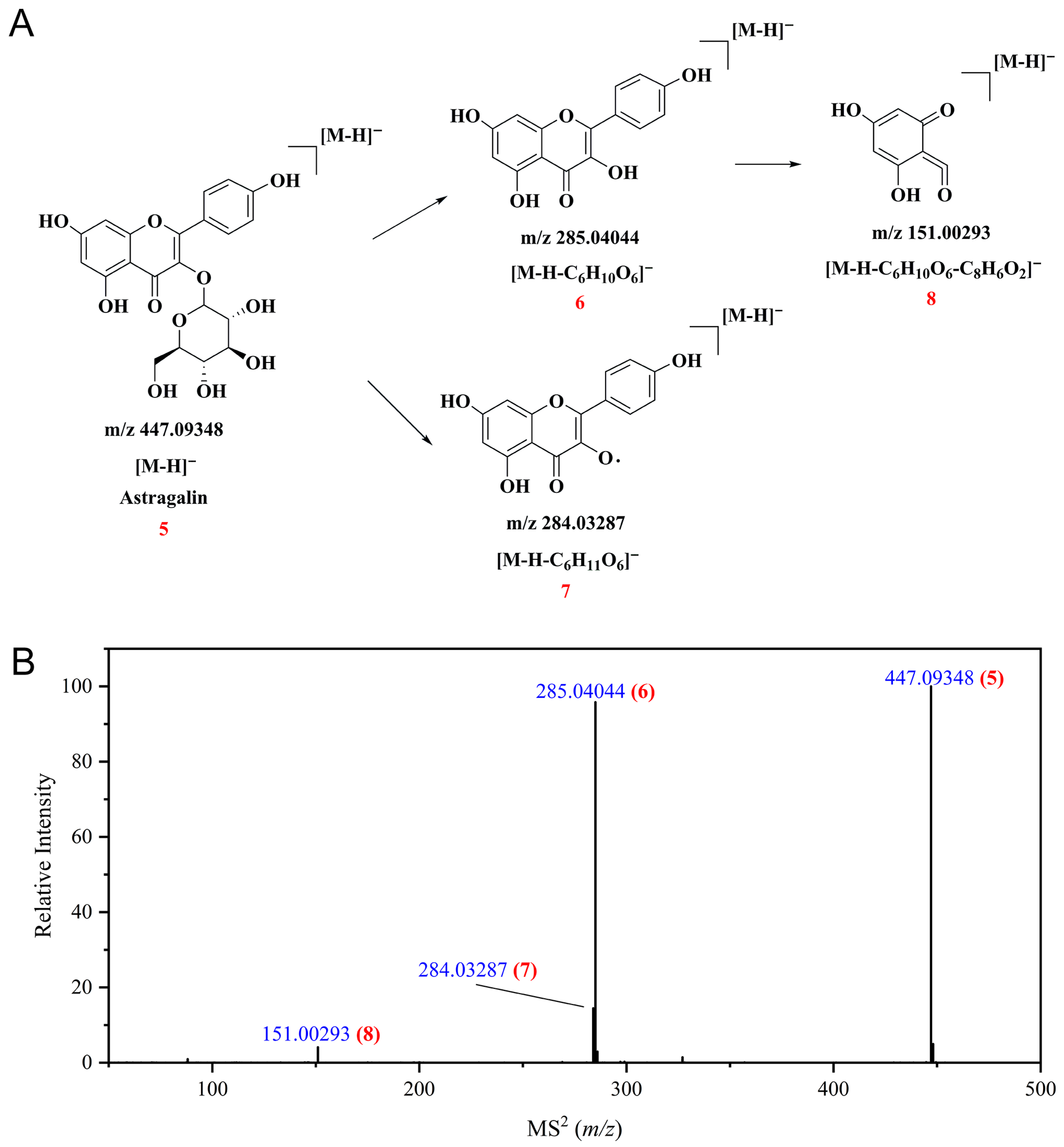
| 20 | Astragalin a [2] | Quercetin derivatives | [M−H]− | 6.82 | C21H20O11 | 448.10056 | 448.1008 | 0.53 | 429.08334, 285.04044, 284.03287, 151.00293 | 72.89 |
| 21 | Isoquercitrin a [2] | [M+H]+ | 6.738 | C21H20O12 | 464.09548 | 464.09478 | −1.51 | 315.04922, 303.04922, 145.04932 | 81.14 | |
| 22 | Hyperoside a [9] | [M−H]− | 6.766 | C21H20O12 | 464.09548 | 464.09569 | 0.46 | 301.03510, 300.02753, 151.00294 | 86.25 | |
| 23 | Quercetin a [34] | [M+H]+ | 6.736 | C15H10O7 | 302.04265 | 302.04201 | −2.12 | 285.03864, 219.06436, 153.01799 | 94.37 | |
| 24 | Tiliroside b | [M+H]+ | 8.558 | C30H26O13 | 594.13734 | 594.13666 | −1.15 | 577.20520, 416.14600, 291.08575, 287.05450, 147.04391 | 71.67 | |
| 25 | Rutin a [35] | [M−H]− | 6.549 | C27H30O16 | 610.15338 | 610.1535 | 0.19 | 301.03528, 300.02737, 151.00290 | 96.12 | |
| 26 | Quercetin-3-O-β-D-glucose-7-O-β-D-gentiobioside b | [M−H]− | 4.799 | C33H40O22 | 788.20112 | 788.20234 | 1.55 | 625.14160, 463.08853, 301.03540 | 74.24 | |
| 27 | Kaempferol-7-O-β-D-glucopyranoside b | Kaempferol derivatives | [M+H]+ | 6.749 | C21H20O11 | 448.10056 | 448.09991 | −1.45 | 287.05447, 270.05148, 153.01784 | 84.64 |
| 28 | Kaempferol a [46] | [M−H]− | 9.069 | C15H10O6 | 286.04774 | 286.04785 | 0.4 | 267.02969, 151.00298 | 86.11 | |
| 29 | Afzelin b | [M+H]+ | 7.648 | C21H20O10 | 432.10565 | 432.10512 | −1.21 | 287.05450, 271.05960, 151.11168 | 76.98 | |
| 30 | Leucoside b | [M−H]− | 6.621 | C26H28O15 | 580.14282 | 580.14308 | 0.46 | 543.17456, 447.09277, 285.04077, 284.03250, 151.00284 | 75.69 | |
| 31 | Kaempferol-3-gentiobioside b | [M−H]− | 5.582 | C27H30O16 | 610.15338 | 610.15411 | 1.18 | 447.09357, 446.08575, 563.27118, 285.04059 | 72.78 | |
| 32 | Nicotiflorin b | [M+H]+ | 6.857 | C27H30O15 | 594.15847 | 594.15781 | −1.11 | 449.10699, 287.05435,147.06490 | 74.56 | |
| 33 | Isorhamnetin b | Others | [M−H]− | 10.717 | C16H12O7 | 316.0583 | 316.0583 | 0 | 300.02762, 287.05621, 165.01871 | 82.03 |
| 34 | Fisetin b | [M+H]+ | 6.75 | C15H10O6 | 286.04774 | 286.04717 | −1.97 | 270.05154, 255.10130, 121.06470 | 99.32 | |
| 35 | Morin b | [M+H]+ | 6.496 | C15H10O7 | 302.04265 | 302.04193 | −2.39 | 285.12283, 257.04382, 153.01796 | 97.32 | |
| C: Phenolic acids | ||||||||||
| 36 | Vanillic acid a [2] | Hydroxybenzoic acids | [M−H]− | 5.853 | C8H8O4 | 168.04226 | 168.04157 | −4.13 | 152.01082, 139.03932, 124.01575 | 86.33 |
| 37 | Salicylic acid b | [M+H]+ | 5.061 | C7H6O3 | 138.03169 | 138.03161 | −0.61 | 121.02849, 111.04427, 93.03389 | 81.21 | |
| 38 | Protocatechuic acid b | [M−H]− | 3.948 | C7H6O4 | 154.02661 | 154.02587 | −4.79 | 109.02859, 91.01786 | 75.65 | |
| 39 | Methyl gallate b | [M−H]− | 6.654 | C8H8O5 | 184.03717 | 184.03664 | −2.92 | 168.00578, 165.01874, 149.54288, 124.01579 | 73.25 | |
| 40 | 4-Amino-3-hydroxybenzoic acid b | [M+H]+ | 1.143 | C7H7NO3 | 153.04259 | 153.04252 | −0.45 | 136.03918, 109.05241, 92.04987 | 97.17 | |
| 41 | 4-Methoxysalicylic acid b | [M−H]− | 3.07 | C8H8O4 | 168.04226 | 168.0417 | −3.33 | 149.02376, 123.04437, 122.02872 | 70.61 | |
| 42 | p-Coumaric acid b | [M+H]+ | 5.681 | C9H8O3 | 164.04734 | 164.04726 | −0.52 | 147.04385, 137.05957, 135.04395 | 78.93 | |
| 43 | Propylparaben b | [M−H]− | 7.728 | C10H12O3 | 180.07864 | 180.07806 | −3.22 | 153.09145, 151.07579, 135.08072, 120.05722 | 83.21 | |
| 44 | Syringic acid a [2] | [M−H]− | 5.926 | C9H10O5 | 198.05282 | 198.05242 | −2.03 | 182.02158, 166.99803, 153.05508, 138.03154, 123.00797 | 72.87 | |
| 45 | (2E)-3-(4-Hydroxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]acrylamide a [46] | Hydroxycinnamic acids | [M+H]+ | 8.256 | C17H17NO3 | 283.12084 | 283.12038 | −1.65 | 266.17459, 147.04390, 121.06489 | 97.10 |
| 46 | Cryptochlorogenic acid a [2] | [M−H]− | 5.338 | C16H18O9 | 354.09508 | 354.09521 | 0.35 | 317.62381, 191.05566, 179.03450, 173.04504 | 77.58 | |
| 47 | Chlorogenic acid a [47] | [M−H]− | 5.463 | C16H18O9 | 354.09508 | 354.09512 | 0.12 | 336.67032, 191.05548, 179.03432, 173.04485 | 77.76 | |
| 48 | Ferulic acid a [48] | [M−H]− | 7.103 | C10H10O4 | 194.05791 | 194.05735 | −2.9 | 178.02657, 149.06009, 134.03654 | 85.06 | |
| 49 | Methyl 4-hydroxy-3-methoxycinnamate b | [M+H]+ | 7.719 | C11H12O4 | 208.07356 | 208.07332 | −1.13 | 191.07005, 181.08571, 177.05444, 145.05959 | 75.33 | |
| 50 | Sinapic acid b | [M+H]+ | 5.243 | C11H12O5 | 224.06847 | 224.06827 | −0.93 | 207.06480, 175.03867, 147.04376, 119.04914 | 90.36 | |
| 51 | 1-Caffeoylquinic acid b | [M-H]− | 4.511 | C16H18O9 | 354.09508 | 354.09519 | 0.3 | 191.05565, 179.03445, 135.04442 | 78.87 | |
| 52 | 2-Hydroxycinnamic acid b | [M−H]− | 7.012 | C9H8O3 | 164.04734 | 164.04673 | −3.73 | 135.00790, 119.04932, 118.72409 | 72.15 | |
| 53 | Caffeic acid a [35] | [M+H]+ | 5.777 | C9H8O4 | 180.04226 | 180.04212 | −0.78 | 163.03876, 145.02826, 135.04396 | 80.77 | |
| 54 | Rosmarinic acid b | [M+H]+ | 7.54 | C18H16O8 | 360.08452 | 360.08395 | −1.57 | 251.06993, 181.04936, 163.03879, 145.02829 | 81.75 | |
| D: Dihydroflavonoids | ||||||||||
| 55 | Naringenin b | Dihydroflavonols | [M−H]− | 9.888 | C15H12O5 | 272.06847 | 272.06827 | −0.75 | 253.05063, 151.00368, 117.03459 | 78.96 |
| 56 | Didymin b | [M+H]+ | 5.21 | C28H34O14 | 594.19486 | 594.19428 | −0.96 | 449.10712, 287.05444, 153.01824 | 73.09 | |
| E: Isoflavones | ||||||||||
| 57 | Iridin b | Isoflavones | [M−H]− | 9.012 | C24H26O13 | 522.13734 | 522.13759 | 0.47 | 359.07727, 358.06967, 343.04602, 344.05377, 329.23343 | 70.98 |
| F: Other Polyphenolics | ||||||||||
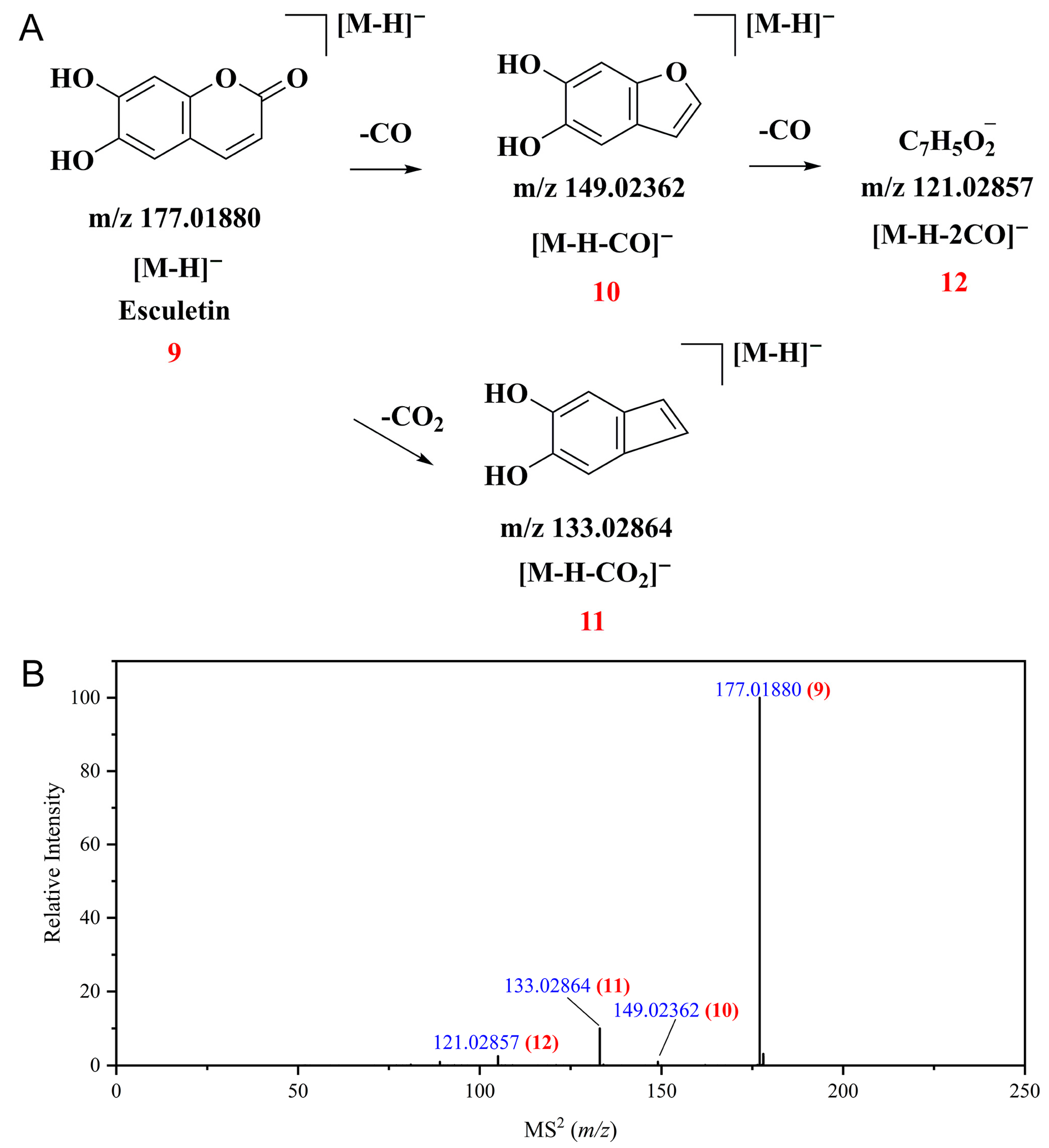
| 58 | Esculetin a [49] | Coumarin derivatives | [M−H]− | 5.779 | C9H6O4 | 178.02661 | 178.02596 | −3.36 | 158.90924, 133.02864, 105.03358, 89.03854 | 85.72 |
| 59 | 5,7-Dihydroxy-4-methylcoumarin b | [M+H]+ | 7.04 | C10H8O4 | 192.04226 | 192.04204 | −1.14 | 175.03882, 161.05952, 151.03882, 123.04411, 95.04951 | 76.21 | |
| 60 | Esculin b | [M−H]− | 4.875 | C15H16O9 | 340.07943 | 340.07931 | −0.36 | 177.01865, 176.01111, 133.02878 | 99.17 | |
| 61 | Salidroside b | Phenylethanoid glycosides | [M−H]− | 4.724 | C14H20O7 | 300.1209 | 300.12083 | −0.24 | 179.05545, 176.35088, 161.04488, 119.03410, 89.02330 | 84.61 |
| 62 | Forsythoside E b | [M−H]− | 4.524 | C20H30O12 | 462.17373 | 462.17388 | 0.32 | 317.12265, 309.11725, 293.13855, 179.07007, 147.06500, 129.05455 | 74.27 | |
| 63 | Aurantioobtusin β-D-glucoside b | Others | [M+H]+ | 9.071 | C23H24O12 | 492.12678 | 492.12632 | −0.94 | 475.17490, 331.08060, 316.05716, 299.05426 | 80.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Wang, Z.; Xu, X.; He, Y.; Gong, H.; Chen, X.; Wang, J. Optimized Extraction and Component Identification of Physalis alkekengi L. Calyx Polyphenols and Antioxidant Dynamics During Thermal Processing. Processes 2025, 13, 2793. https://doi.org/10.3390/pr13092793
Yuan H, Wang Z, Xu X, He Y, Gong H, Chen X, Wang J. Optimized Extraction and Component Identification of Physalis alkekengi L. Calyx Polyphenols and Antioxidant Dynamics During Thermal Processing. Processes. 2025; 13(9):2793. https://doi.org/10.3390/pr13092793
Chicago/Turabian StyleYuan, Heng, Ziyi Wang, Xingyu Xu, Yu He, Hao Gong, Xuehong Chen, and Jun Wang. 2025. "Optimized Extraction and Component Identification of Physalis alkekengi L. Calyx Polyphenols and Antioxidant Dynamics During Thermal Processing" Processes 13, no. 9: 2793. https://doi.org/10.3390/pr13092793
APA StyleYuan, H., Wang, Z., Xu, X., He, Y., Gong, H., Chen, X., & Wang, J. (2025). Optimized Extraction and Component Identification of Physalis alkekengi L. Calyx Polyphenols and Antioxidant Dynamics During Thermal Processing. Processes, 13(9), 2793. https://doi.org/10.3390/pr13092793






