Optimization of Ultrasound-Assisted Extraction of Polyphenols from Rowan (Sorbus aucuparia L.): A Response Surface Methodology Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. UAE
2.3. Determination of Bioactive Compounds by HPLC
2.4. Box–Behnken Experimental Design and Statistical Analyses
3. Results
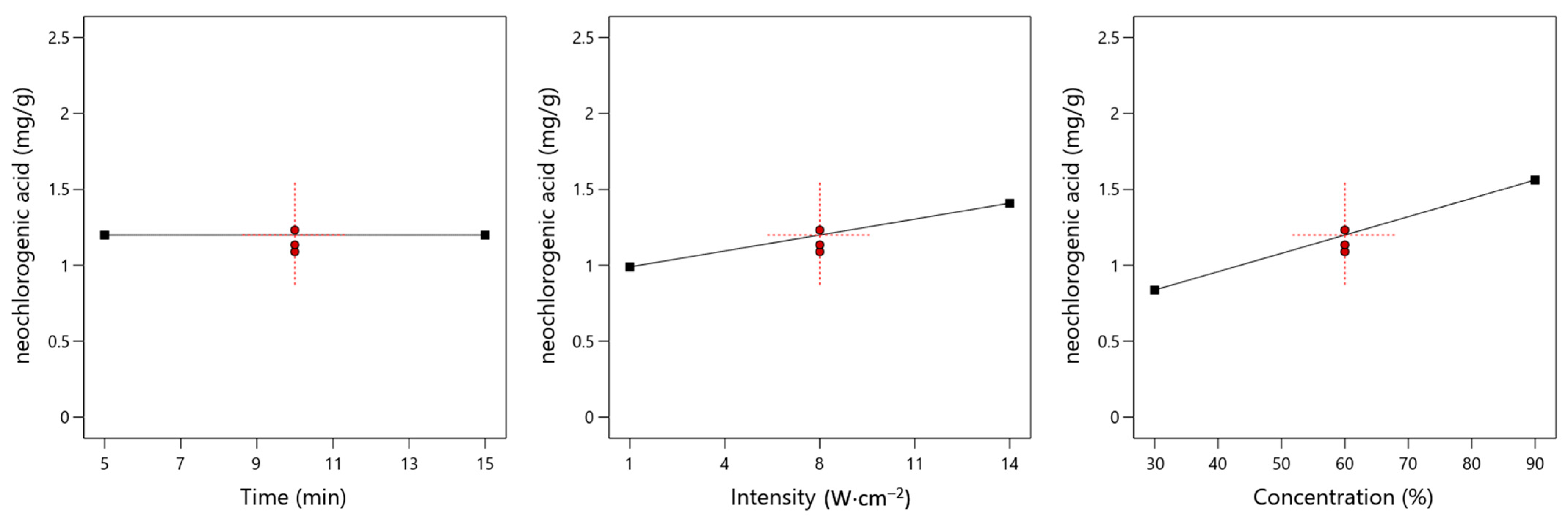
3.1. Neochlorogenic Acid
3.2. Chlorogenic Acid
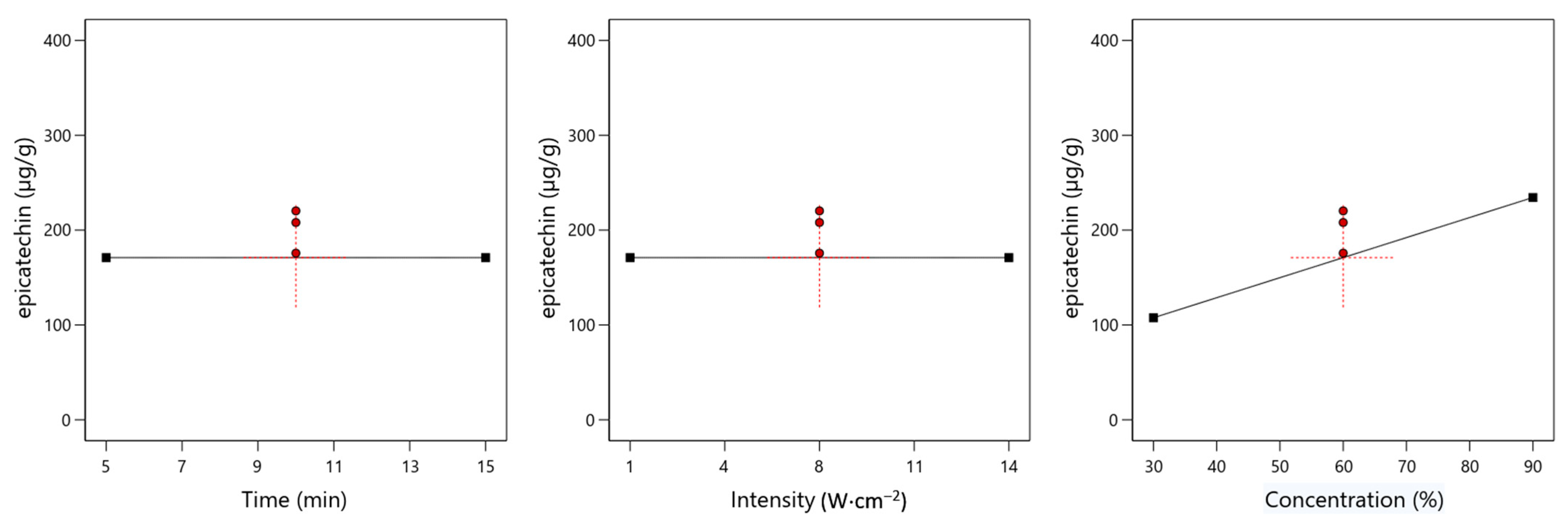
3.3. Epicatechin
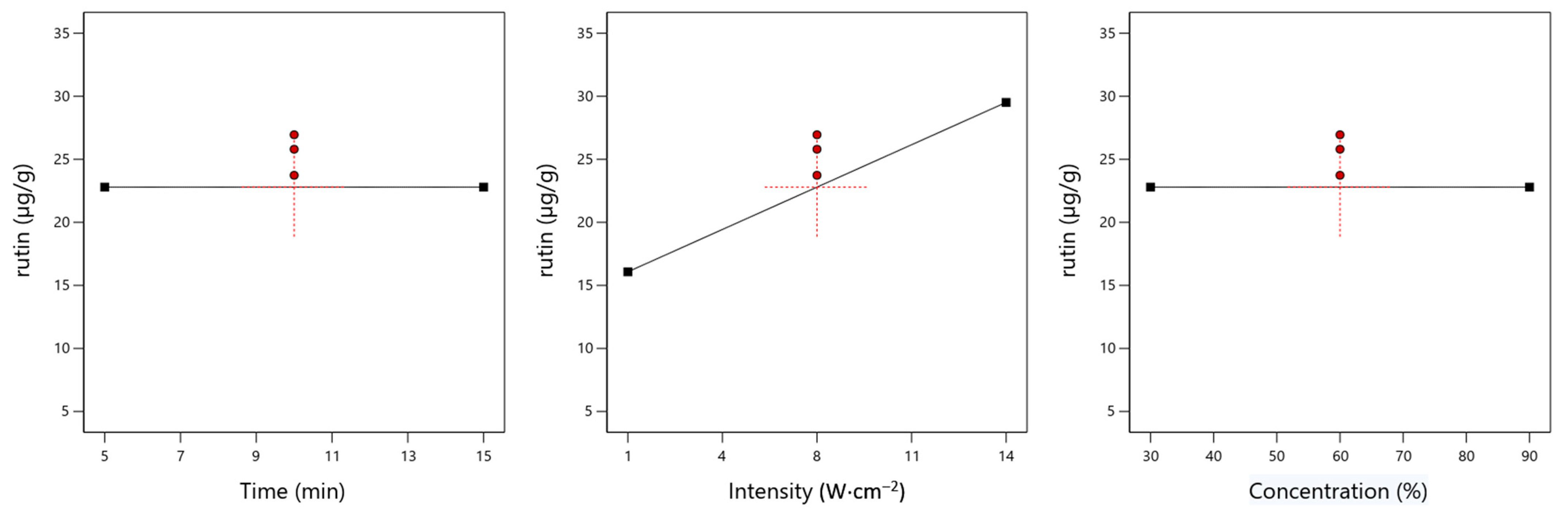
3.4. Rutin
3.5. Optimization of Process Conditions and Model Validation
4. Discussion of Results
4.1. Effect of Time on the Yield of Specific Chemical Compounds
4.2. Effect of Ultrasound Intensity on the Yield of Specific Chemical Compounds
4.3. Effect of Ethanol Concentration on the Yield of Specific Chemical Compounds
4.3.1. Solubility and Polarity
4.3.2. The Effect of Ethanol Concentration on the Presence of Individual Polyphenolic Compounds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bugała, W. Drzewa i Krzewy Iglaste, 1st ed.; Rolnicze i Leśne: Warszawa, Poland, 2004. [Google Scholar]
- Olczyk, M.; Geszprych, A. Rośliny jadalne i lecznicze z rodzaju Sorbus L. Postępy Fitoterapii 2017, 18, 278–285. [Google Scholar] [CrossRef]
- Rushforth, K. Trees: Of Britain & Europe; HarperCollins: London, UK, 1999. [Google Scholar]
- Arvinte, O.M.; Senila, L.; Becze, A.; Amariei, S. Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties. Plants 2023, 12, 3225. [Google Scholar] [CrossRef]
- Aladedunye, F.; Matthäus, B. Phenolic extracts from Sorbus aucuparia (L.) and Malus baccata (L.) berries: Antioxidant activity and performance in rapeseed oil during frying and storage. Food Chem. 2014, 159, 273–281. [Google Scholar] [CrossRef]
- Bobinaite, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Rimantas Venskutonis, P. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of the extracts isolated from the pomace of rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 136, 109310. [Google Scholar] [CrossRef]
- Bujor, A.; Miron, A.; Luca, S.V.; Skalicka-Wozniak, K.; Silion, M.; Ancuceanu, R.; Dinu, M.; Girard, C.; Demougeot, C.; Totoson, P. Metabolite profiling, arginase inhibition and vasorelaxant activity of Cornus mas, Sorbus aucuparia and Viburnum opulus fruit extracts. Food Chem. Toxicol. 2019, 133, 110764. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Mellenthin, A. Identification and quantitation of flavonols in rowanberry (Sorbus aucuparia L.) juice. Eur. Food Res. Technol. 2001, 213, 12–17. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry Phenolics: Compositional Analysis and Bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Krska, B.; Kiprovski, B.; Veberic, R. Bioactive Components and Antioxidant Capacity of Fruits from Nine Sorbus Genotypes. J. Food Sci. 2017, 82, 647–658. [Google Scholar] [CrossRef]
- Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars. Molecules 2018, 23, 2593. [Google Scholar] [CrossRef]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel insight into biological activity and phytochemical composition of Sorbus aucuparia L. fruits: Fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef]
- Cristea, E.; Ghendov-Mosanu, A.; Patras, A.; Socaciu, C.; Pintea, A.; Tudor, C.; Sturza, R. The Influence of Temperature, Storage Conditions, pH, and Ionic Strength on the Antioxidant Activity and Color Parameters of Rowan Berry Extracts. Molecules 2021, 26, 3786. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Gilbert-López, B.; Mendiola, J.A.; Quirantes-Piné, R.; Segura-Carretero, A.; Ibánez, E. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 2016, 37, 1938–1946. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Lai, J.; Qu, L.; Li, X.; Yang, Y.; Shen, L. A green and recyclable ternary deep eutectic solvent for extracting flavonol glycoside from Ginkgo leaves: Mechanism insights based on molecular level. J. Mol. Liq. 2024, 406, 125053. [Google Scholar] [CrossRef]
- Lai, J.; Zhou, P.; Li, X.; Lu, Y.; Wang, Y.; Yuan, H.; Yang, Y. Ultrasound-assisted deep eutectic solvent extraction of flavonol glycosides from Ginkgo biloba: Optimization of efficiency and mechanism. Ultrason. Sonochem. 2025, 114, 107254. [Google Scholar] [CrossRef]
- Meng, Y.; Zou, W.; Guo, T.; Zhang, L.; Zhang, P.; Shu, P. Optimization of ultrasound-assisted deep eutectic solvent extraction, characterization, and bioactivities of flavonoids from Cercis glabra leaves. Ultrason. Sonochem. 2025, 120, 107434. [Google Scholar] [CrossRef]
- Wu, J.; Xie, P.; Hao, W.; Lu, D.; Qi, Y.; Mi, Y. Ionic liquids as electrolytes in aluminum electrolysis. Front. Chem. 2022, 10, 1014893. [Google Scholar] [CrossRef]
- Ferreira, B.L.; Junior, T.K.; Block, J.M.; Granato, D.; Nunes, I.L. Innovative approach for obtaining phenolic compounds from guava (Psidium guajava L.) coproduct using ionic liquid ultrasound-assisted extraction (IL-UAE). Biocatal. Agric. Biotechnol. 2021, 38, 102196. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Han, Y.; Wang, X.; Sheng, Z. Optimization of process conditions for ionic liquid-based ultrasound- enzyme-assisted extraction of resveratrol from Polygonum Cuspidatum. Ultrason. Sonochem. 2024, 108, 106973. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castello, E.; Rodriguez-Lopez, A.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Kobus, Z.; Buczaj, A.; Pecyna, A.; Kapica, J.; Findura, P.; Kocira, S. Application of Response Surface Method in Pulsed Ultrasound-Assisted Extraction of Complex Plant Materials—A Case Study on Cannabis sativa L. Appl. Sci. 2023, 13, 760. [Google Scholar] [CrossRef]
- Plawgo, M.; Kocira, S.; Bohata, A. Multi-Objective Optimization of the Green Extraction Conditions of Bio-Active Compounds from a Levisticum officinale WDJ Koch: Pareto Optimality and Compromise Solutions for Process Management. Agric. Eng. 2024, 28, 137–165. [Google Scholar] [CrossRef]
- Krzywicka, M.; Kobus, Z. Effect of the Shape of Ultrasonic Vessels on the Chemical Properties of Extracts from the Fruit of Sorbus aucuparia. Appl. Sci. 2023, 13, 7805. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M. Energy Aspects of Flavonoid Extraction from Rowanberry Fruits Using Pulsed Ultrasound-Assisted Extraction. Energies 2023, 16, 4966. [Google Scholar] [CrossRef]
- Orsavová, J.; Juríková, T.; Bednaříková, R.; Mlček, J. Total Phenolic and Total Flavonoid Content, Individual Phenolic Compounds and Antioxidant Activity in Sweet Rowanberry Cultivars. Antioxidants 2023, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Gaivelyte, K.; Jakstas, V.; Razukas, A.; Janulis, V. Variation in the contents of neochlorogenic acid, chlorogenic acid and three quercetin glycosides in leaves and fruits of Rowan (Sorbus) species and varieties from collections in Lithuania. Nat. Prod. Commun. 2013, 8, 1105–1110. [Google Scholar] [CrossRef]
- Šavikin, K.P.; Zdunić, G.M.; Krstić-Milošević, D.B.; Šircelj, H.J.; Stešević, D.D.; Pljevljakušić, D.S. Sorbus aucuparia and Sorbus aria as a Source of Antioxidant Phenolics, Tocopherols, and Pigments. Chem. Biodivers. 2017, 14, e1700329. [Google Scholar] [CrossRef]
- Mrkonjic, Z.; Nadjpal, J.; Beara, I.; Aleksic-Sabo, V.; Cetojevic-Simin, D.; Mimica-Dukic, N.; Lesjak, M. Phenolic profiling and bioactivities of fresh fruits and jam of Sorbus species. J. Serbian Chem. Soc. 2017, 82, 651–664. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S. Development and validation of ultrasound assisted extraction (UAE) and HPLC-DAD method for determination of polyphenols in dry beans (Phaseolus vulgaris). J. Food Compos. Anal. 2020, 85, 103334. [Google Scholar] [CrossRef]
- Repajić, M.; Zorić, M.; Magnabosca, I.; Pedisić, S.; Dragović-Uzelac, V.; Elez Garofulić, I. Bioactive Power of Black Chokeberry Pomace as Affected by Advanced Extraction Techniques and Cryogrinding. Molecules 2025, 30, 3383. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Sun, J.; Qiu, S.; Chu, B.; Fang, R.; Zheng, F. Degradation kinetics and isomerization of 5-O-caffeoylquinic acid under ultrasound: Influence of epigallocatechin gallate and vitamin C. Food Chem. X 2021, 12, 100147. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, J.; Xu, D.; Wang, S.; Yuan, Y.; Cao, Y. Investigation of (+)-catechin stability under ultrasonic treatment and its degradation kinetic modeling. J. Food Process Eng. 2018, 41, e12904. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of high-intensity ultrasound process parameters on the phenolic compounds recovery from araticum peel. Ultrason. Sonochem. 2019, 50, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Baite, T.N.; Mandal, B.; Purkait, M.K. Ultrasound assisted extraction of gallic acid from Ficus auriculata leaves using green solvent. Food Bioprod. Process. 2021, 128, 1–11. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Chen, J.C.; Liu, D.H.; Ye, X.Q. Simultaneous extraction of phenolic compounds of citrus peel extracts: Effect of ultrasound. Ultrason. Sonochem. 2009, 16, 57–62. [Google Scholar] [CrossRef]
- Isaikina, N.V.; Kalinkina, G.I.; Razina, T.G.; Zueva, E.P.; Rybalkina, O.Y.; Ulirich, A.V.; Fedorova, E.P.; Shilova, A.B. Sorbus aucuparia L. Fruit Is a Source of the Drug for Increasing the Efficiency of Tumor Chemotherapy. Russ. J. Bioorganic Chem. 2018, 44, 899–905. [Google Scholar] [CrossRef]
- Hu, W.; Guo, T.; Jiang, W.J.; Dong, G.L.; Chen, D.W.; Yang, S.L.; Li, H.R. Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cynaroside extracted from flower buds of Lonicera japonica. Chin. J. Nat. Med. 2015, 13, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Ahmmed, M.K.; Rashidinejad, A. Exploring efficient extraction methods: Bioactive compounds and antioxidant properties from New Zealand damson plums. Food Biosci. 2023, 55, 103057. [Google Scholar] [CrossRef]
- Koch, W.; Kukuła-Koch, W.; Czop, M.; Helon, P.; Gumbarewicz, E. The Role of Extracting Solvents in the Recovery of Polyphenols from Green Tea and Its Antiradical Activity Supported by Principal Component Analysis. Molecules 2020, 25, 2173. [Google Scholar] [CrossRef] [PubMed]
| Compounds | RT (min) | R2 | Calibration Range (µg) | LOD (µg) | LOQ (µg) |
|---|---|---|---|---|---|
| Gallic acid | 1.859 | 0.99986 | 0.078–0.156 | 0.0021 | 0.0065 |
| Neochlorogenic acid | 2.592 | 0.99912 | 0.1–0.2 | 0.0026 | 0.0078 |
| Chlorogenic acid | 3.927 | 0.99985 | 0.026–0.052 | 1.4 × 10−5 | 4.3 × 10−5 |
| Vanillic acid | 5.455 | 0.99990 | 0.0834–0.1668 | 0.0033 | 0.0101 |
| Epicatechin | 6.167 | 0.99987 | 0.0592–0.1184 | 0.0004 | 0.0012 |
| Trans-ferulic acid | 9.650 | 0.99987 | 0.0854–0.1708 | 0.00241 | 0.00731 |
| Rutin | 9.858 | 0.99987 | 0.076–0.152 | 0.00294 | 0.00891 |
| Quercetin | 17.153 | 0.99986 | 0.0858–0.1716 | 0.0007 | 0.0021 |
| Cinnamic acid | 17.652 | 0.99987 | 0.092–0.184 | 0.0018 | 0.0053 |
| Run | X1 Time [min.] | X2 Ultrasound Intensity [W/cm2] | X3 Concentration [%] |
|---|---|---|---|
| 1. | 5 | 14.00 | 60 |
| 2. | 5 | 7.65 | 30 |
| 3. | 5 | 1.30 | 90 |
| 4. | 5 | 14.00 | 60 |
| 5. | 10 | 7.65 | 30 |
| 6. | 10 | 7.65 | 90 |
| 7. | 10 | 14.00 | 60 |
| 8. | 10 | 7.65 | 60 |
| 9. | 10 | 14.00 | 60 |
| 10. | 10 | 1.30 | 30 |
| 11. | 10 | 7.65 | 90 |
| 12. | 15 | 7.65 | 60 |
| 13. | 15 | 7.65 | 30 |
| 14. | 15 | 1.30 | 90 |
| 15. | 15 | 1.30 | 60 |
| (a) | ||||||||
| Run | Factor 1 Time [min] | Factor 2 Ultrasound Intensity [W/cm2] | Factor 3 Concentration [%] | Response 1 Gallic Acid [µg/g] | Response 2 Neochlorogenic Acid [µg/g] | Response 3 Chlorogenic Acid [µg/g] | Response 4 Vanillic Acid [µg/g] | Response 5 Epicatechin [µg/g] |
| 1 | 5 | 14.00 | 60 | 8.91 ± 0.17 | 1219.25 ± 42.3 | 1982.45 ± 24.4 | 6.42 ± 0.05 | 197.70 ± 37.5 |
| 2 | 10 | 7.65 | 60 | 3.81 ± 0.05 | 1134.39 ± 6.86 | 2007.83 ± 165 | 5.87 ± 0.02 | 175.70 ± 33.9 |
| 3 | 10 | 1.30 | 90 | 0.00 | 1464.93 ± 2.8 | 2099.98 ± 17.2 | 14.46 ± 0.13 | 221.55 ± 0.69 |
| 4 | 10 | 14.00 | 90 | 0.00 | 2025.33 ± 3.27 | 2957.83 ± 15.6 | 18.45 ± 0.2 | 330.51 ± 13.7 |
| 5 | 10 | 7.65 | 60 | 4.45 ± 0.2 | 1089.29 ± 4.7 | 1965.58 ± 17.6 | 5.48 ± 0.08 | 220.32 ± 12.5 |
| 6 | 15 | 7.65 | 90 | 0.00 | 2365.44 ± 2.72 | 3409.68 ± 10.1 | 19.84 ± 1.4 | 349.94 ± 13.4 |
| 7 | 10 | 14.00 | 30 | 19.15 ± 1.3 | 998.05 ± 21.45 | 0.00 | 0.00 | 42.23 ± 2.87 |
| 8 | 10 | 7.65 | 60 | 2.90 ± 0.32 | 1231.70 ± 6.45 | 1920.29 ± 22.4 | 5.82 ± 0.09 | 208.02 ± 1.87 |
| 9 | 15 | 14.00 | 60 | 9.27 ± 0.45 | 1271.07 ± 4.45 | 0.00 | 6.42 ± 0.34 | 138.68 ± 1.21 |
| 10 | 10 | 1.30 | 30 | 6.78 ± 0.23 | 445.12 ± 8.32 | 0.00 | 0.00 | 55.50 ± 0.46 |
| 11 | 5 | 7.65 | 30 | 15.74 ± 2.67 | 2165.95 ± 34.7 | 3113.36 ± 28.9 | 19.31 ± 2.32 | 327.41 ± 21.9 |
| 12 | 5 | 7.65 | 90 | 0.00 | 650.35 ± 9.76 | 0.00 | 0.00 | 71.65 ± 2.6 |
| 13 | 15 | 7.65 | 30 | 15.77 ± 3.24 | 0.00 | 0.00 | 0.00 | 41.24 ± 1.78 |
| 14 | 5 | 1.30 | 60 | 0.00 | 708.28 ± 9.48 | 1344.29 ± 15.6 | 0.00 | 130.32 ± 3.32 |
| 15 | 15 | 1.30 | 60 | 1.43 ± 0.21 | 1218.10 ± 12.34 | 0.00 | 5.65 ± 0.34 | 54.23 ± 0.98 |
| (b) | ||||||||
| Run | Factor 1 Time [min] | Factor 2 Ultrasound Intensity [W/cm2] | Factor 3 Concentration [%] | Response 6 Trans-Ferulic Acid [µg/g] | Response 7 Rutin [µg/g] | Response 8 Quercetin [µg/g] | Response 9 Cinnamic Acid [µg/g] | |
| 1 | 5 | 14.00 | 60 | 7.46 ± 0.1 | 29.94 ± 0.57 | 0.00 | 22.18 ± 2.4 | |
| 2 | 10 | 7.65 | 60 | 6.38 ± 0.12 | 23.73 ± 0.69 | 15.89 ± 4.7 | 25.42 ± 2.6 | |
| 3 | 10 | 1.30 | 90 | 5.78 ± 0.5 | 18.31 ± 0.54 | 0.00 | 0.00 | |
| 4 | 10 | 14.00 | 90 | 7.16 ± 0.6 | 25.81 ± 0.5 | 11.82 ± 0.16 | 22.87 ± 1.3 | |
| 5 | 10 | 7.65 | 60 | 6.45 ± 0.16 | 25.80 ± 0.21 | 21.53 ± 2.2 | 25.67 ± 1.8 | |
| 6 | 15 | 7.65 | 90 | 5.41 ± 0.32 | 27.83 ± 0.46 | 5.51 ± 0.9 | 8.51 ± 1.22 | |
| 7 | 10 | 14.00 | 30 | 0.00 | 34.25 ± 0.37 | 21.01 ± 1.34 | 0.00 | |
| 8 | 10 | 7.65 | 60 | 6.66 ± 0.46 | 26.95 ± 0.67 | 15.83 ± 2.89 | 45.61 ± 3.88 | |
| 9 | 15 | 14.00 | 60 | 6.73 ± 0.52 | 26.22 ± 0.54 | 52.05 ± 4.78 | 44.39 ± 4.12 | |
| 10 | 10 | 1.30 | 30 | 0.00 | 6.58 ± 0.1 | 0.00 | 0.00 | |
| 11 | 5 | 7.65 | 30 | 5.46 ± 0.67 | 20.82 ± 0.32 | 7.88 ± 0.36 | 7.45 ± 0.4 | |
| 12 | 5 | 7.65 | 90 | 0.00 | 9.09 ± 0.12 | 0.00 | 0.00 | |
| 13 | 15 | 7.65 | 30 | 0.00 | 29.01 ± 0.48 | 13.55 ± 1.45 | 0.00 | |
| 14 | 5 | 1.30 | 60 | 4.78 ± 0.38 | 15.98 ± 0.68 | 0.00 | 0.00 | |
| 15 | 15 | 1.30 | 60 | 5.74 ± 0.51 | 21.59 ± 32 | 11.47 ± 1.38 | 21.67 ± 1.89 | |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 5.17 | 3 | 1.72 | 38.33 | <0.0001 | significant |
| X2 | 0.3517 | 1 | 0.3517 | 7.83 | 0.0173 | |
| X3 | 1.05 | 1 | 1.05 | 23.35 | 0.0005 | |
| X1X3 | 3.77 | 1 | 3.77 | 83.81 | <0.0001 | |
| Residual | 0.4942 | 11 | 0.0449 | |||
| Lack of Fit | 0.4836 | 9 | 0.0537 | 10.14 | 0.0929 | not significant |
| Pure Error | 0.0106 | 2 | 0.0053 | |||
| Cor Total | 5.66 | 14 | ||||
| R2 = 0.9127; adj. R2 = 0.8889; CV = 17.68; Adeq Precision = 24.3453; Predicted R2 = 0.8027 | ||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 14.22 | 2 | 7.11 | 9.77 | 0.0030 | significant |
| X3 | 3.58 | 1 | 3.58 | 4.93 | 0.0465 | |
| X1X3 | 10.64 | 1 | 10.64 | 14.62 | 0.0024 | |
| Residual | 8.73 | 12 | 0.7275 | |||
| Lack of Fit | 8.73 | 10 | 0.8726 | 455.30 | 0.0022 | significant |
| Pure Error | 0.0038 | 2 | 0.0019 | |||
| Cor Total | 22.95 | 14 | ||||
| R2 = 0.6196; adj. R2 = 0.5562; CV = 61.51; Adeq Precision = 12.0595; Predicted R2 = 0.3821 | ||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 111,800 | 2 | 55,911.29 | 13.37 | 0.0009 | significant |
| X3 | 32,166.34 | 1 | 32,166.34 | 7.69 | 0.0168 | |
| X1X3 | 79,656.25 | 1 | 79,656.25 | 19.05 | 0.0009 | |
| Residual | 50,166.67 | 12 | 4180.56 | |||
| Lack of Fit | 49,104.17 | 10 | 4910.42 | 9.24 | 0.1015 | not significant |
| Pure Error | 1062.49 | 2 | 531.25 | |||
| Cor Total | 162,000 | 14 | ||||
| R2 = 0.6903; adj. R2 = 0.6387; CV = 37.81; Adeq Precision = 14.1465; Predicted R2 = 0.4417 | ||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 361.21 | 1 | 361.21 | 10.31 | 0.0068 | significant |
| X2 | 361.21 | 1 | 361.21 | 10.31 | 0.0068 | |
| Residual | 455.53 | 13 | 35.04 | |||
| Lack of Fit | 450.22 | 11 | 40.93 | 15.41 | 0.0625 | not significant |
| Pure Error | 5.31 | 2 | 2.66 | |||
| Cor Total | 816.75 | 14 | ||||
| R2 = 0.4423; adj. R2 = 0.3994; CV = 25.97; Adeq Precision = 6.2174; Predicted R2 = 0.2789 | ||||||
| Optimized Condition | R2 | Adj. R2 | Pred. R2 | Adj. R2 − Pred. R2 | CV | Adeq. Precision | Comments |
|---|---|---|---|---|---|---|---|
| Neochlorogenic acid | 0.913 | 0.889 | 0.803 | 0.086 | 17.68 | 24.35 | Correct model |
| Chlorogenic acid | 0.620 | 0.556 | 0.382 | 0.174 | 61.51 | 12.06 | No match |
| Epicatechin | 0.690 | 0.639 | 0.442 | 0.197 | 37.81 | 14.14 | High CV Low R2 |
| Rutin | 0.442 | 0.3992 | 0.279 | 0.121 | 25.97 | 6.22 | High CV Low R2 |
| Optimized Condition | Extraction Variables | Response | Yield of Extraction | ||||
|---|---|---|---|---|---|---|---|
| Time [min.] | Ultrasound Intensity [W/cm2] | Concentration [%] | Predicted | Experimental | Predictive | ||
| Neochlorogenic acid | 5.0 | 14.0 | 30.0 | Neochlorogenic acid | 2.01 mg/g | 1.75 mg/g | 87.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobus, Z.; Krzywicka, M.; Lakatošová, J.; Ivanišová, E. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Rowan (Sorbus aucuparia L.): A Response Surface Methodology Approach. Processes 2025, 13, 2778. https://doi.org/10.3390/pr13092778
Kobus Z, Krzywicka M, Lakatošová J, Ivanišová E. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Rowan (Sorbus aucuparia L.): A Response Surface Methodology Approach. Processes. 2025; 13(9):2778. https://doi.org/10.3390/pr13092778
Chicago/Turabian StyleKobus, Zbigniew, Monika Krzywicka, Jana Lakatošová, and Eva Ivanišová. 2025. "Optimization of Ultrasound-Assisted Extraction of Polyphenols from Rowan (Sorbus aucuparia L.): A Response Surface Methodology Approach" Processes 13, no. 9: 2778. https://doi.org/10.3390/pr13092778
APA StyleKobus, Z., Krzywicka, M., Lakatošová, J., & Ivanišová, E. (2025). Optimization of Ultrasound-Assisted Extraction of Polyphenols from Rowan (Sorbus aucuparia L.): A Response Surface Methodology Approach. Processes, 13(9), 2778. https://doi.org/10.3390/pr13092778









