Is PVP K-29/32 an Efficient Stabilizing Excipient in Amorphous Solid Dispersions Containing the Poorly Water-Soluble Drug—Bicalutamide?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ball Milling
2.2.2. Stability Studies of Solid Dispersions
2.2.3. Visual Inspection of the Sample
2.2.4. Powder X-Ray Diffraction
2.2.5. Dissolution Studies
2.2.6. Similarity Factor Calculation
3. Results and Discussion
3.1. Visual Inspection of the Samples
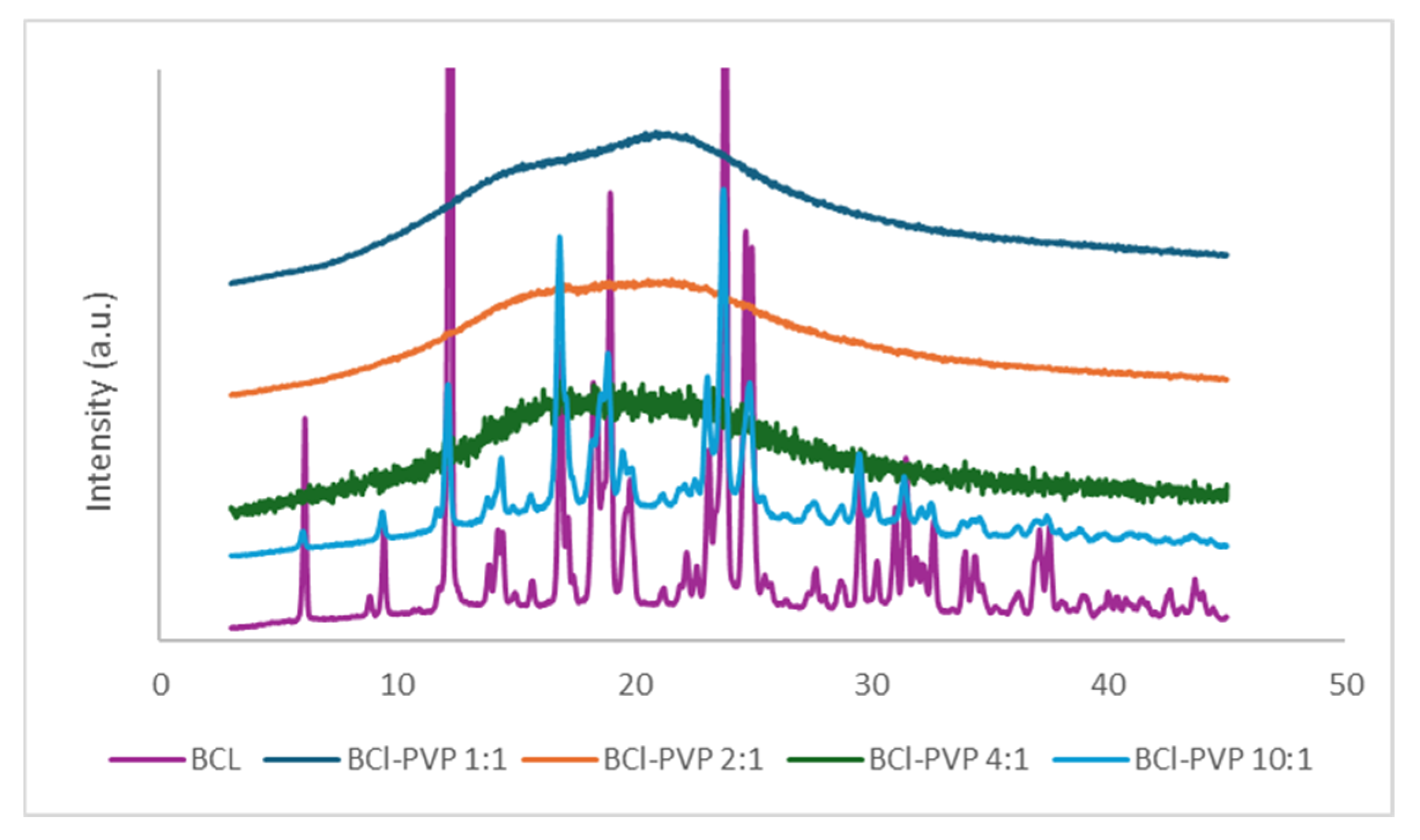
3.2. X-Ray Analysis
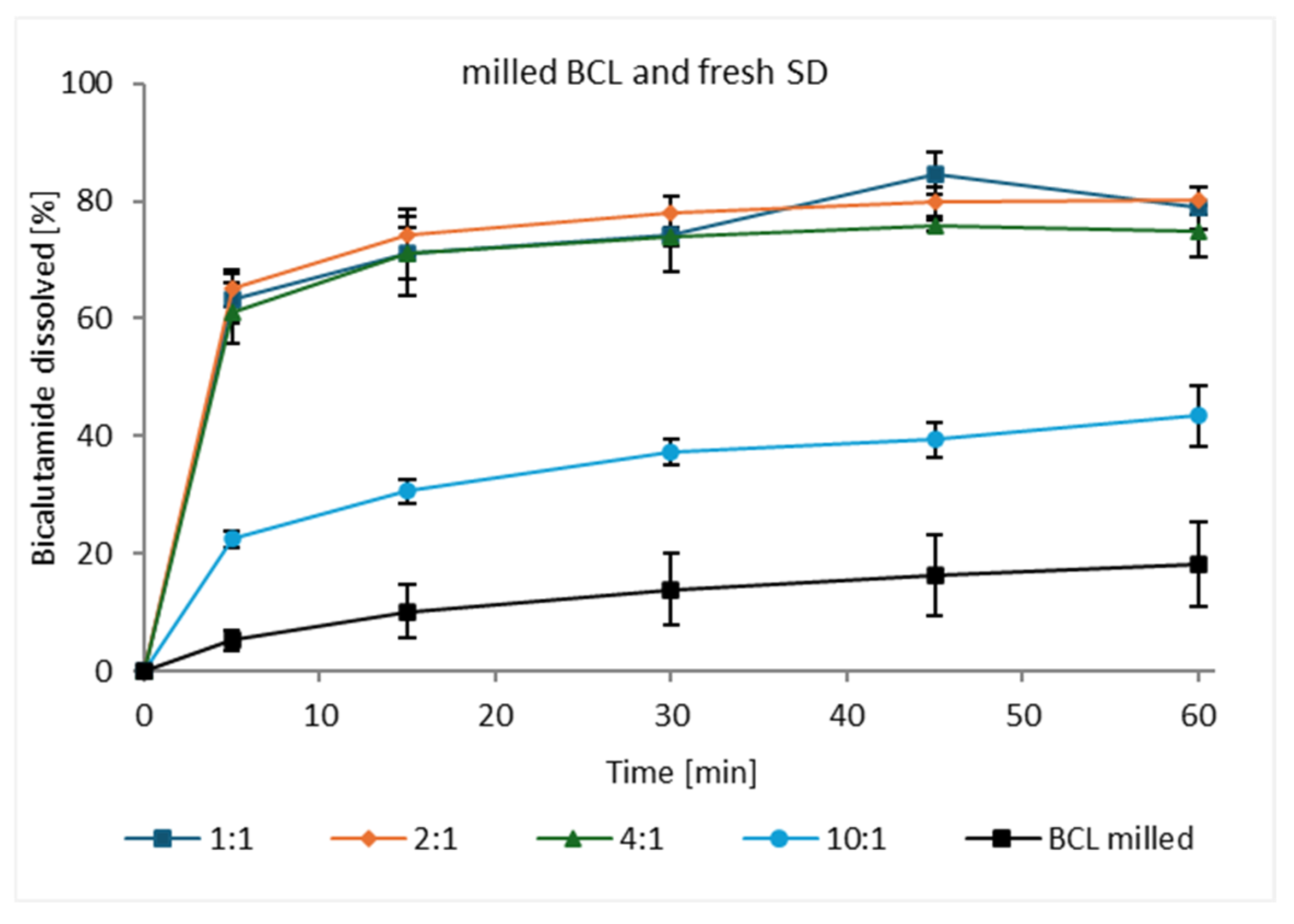
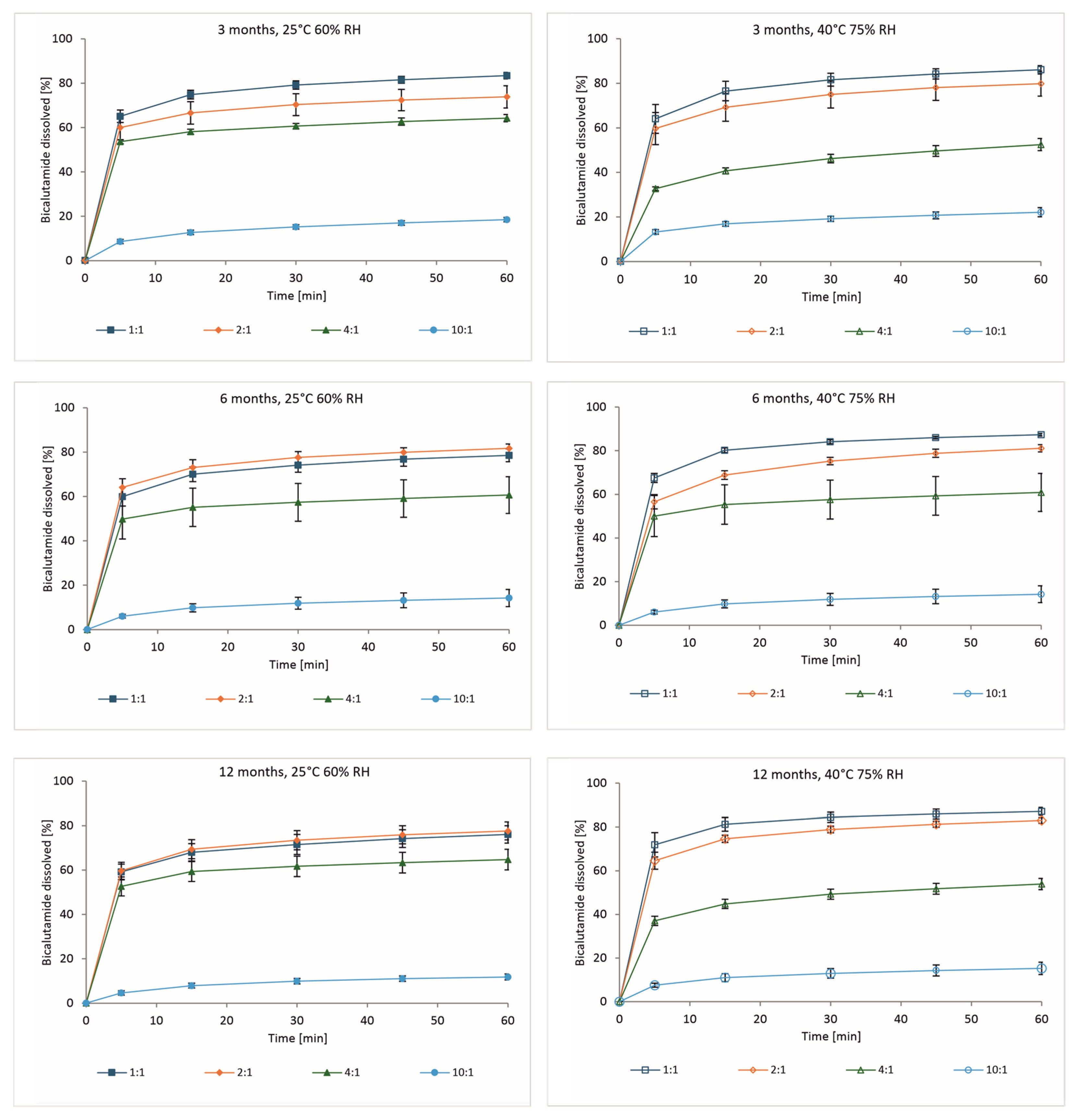
3.3. Dissolution of Bicalutamide from Solid Dispersions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, B.; Ryan, K.M.; Padrela, L. From batch to continuous—New opportunities for supercritical CO2 technology in pharmaceutical manufacturing. Eur. J. Pharm. Sci. 2019, 137, 104971. [Google Scholar] [CrossRef]
- Thiry, J.; Kok, M.G.; Collard, L.; Frère, A.; Krier, F.; Fillet, M.; Evrard, B. Bioavailability enhancement of itraconazole-based solid dispersions produced by hot melt extrusion in the framework of the Three Rs rule. Eur. J. Pharm. Sci. 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Patel, S.; Kou, X.; Hou, H.H.; Huang, Y.B.; Strong, J.C.; Zhang, G.G.Z.; Sun, C.C. Mechanical Properties and Tableting Behavior of Amorphous Solid Dispersions. J. Pharm. Sci. 2017, 106, 217–223. [Google Scholar] [CrossRef]
- Sruti, J.; Patra, C.N.; Swain, S.; Panigrahi, K.C.; Patro, A.P.; Beg, S.; Dinda, S.C.; Rao, M.E.B. Improvement in the dissolution rate and tableting properties of cefuroxime axetil by melt-granulated dispersion and surface adsorption. Acta Pharm. Sin. B 2013, 3, 113–122. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Rams-Baron, M.; Jachowicz, R.; Boldyreva, E.; Zhou, D.; Jamroz, W.; Paluch, M. Amorphous Drugs; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Autzen Virtanen, A.; Myślińska, M.; Healy, A.M.; Power, E.; Madi, A.; Sivén, M. The challenge of downstream processing of spray dried amorphous solid dispersions into minitablets designed for the paediatric population—A sustainable product development approach. Eur. J. Pharm. Sci. 2024, 196, 106752. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Blattner, S.M.; Hirsh, D.; Hoffmann, R.; Luebbert, C.; Schaefer, K. Development of Ternary Amorphous Solid Dispersions Manufactured by Hot-Melt Extrusion and Spray-Drying─Comparison of In Vitro and In Vivo Performance. Mol. Pharm. 2024, 21, 1309–1320. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Misztalewska-Turkowicz, I.; Wilczewska, A.Z.; Basa, A.; Winnicka, K. Solid Dispersions Obtained by Ball Milling as Delivery Platform of Etodolac, a Model Poorly Soluble Drug. Materials 2024, 17, 3923. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhu, W.; Zhang, X.; Xu, Y.; Di, L. Application of the combination of ball-milling and hot-melt extrusion in the development of an amorphous solid dispersion of a poorly water-soluble drug with high melting point. RSC Adv. 2019, 9, 22263–22273. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Pattnaik, S.; Swain, K.; De, P.K.; Saha, A.; Mazumdar, P.; Ghoshal, G. Physicochemical Characterization of Interaction of Ibuprofen by Solid-State Milling with Aluminum Hydroxide. Drug Dev. Ind. Pharm. 2008, 34, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef]
- Chutimaworapan, S.; Ritthidej, G.C.; Yonemochi, E.; Oguchi, T.; Yamamoto, K. Effect of Water-Soluble Carriers on Dissolution Characteristics of Nifedipine Solid Dispersions. Drug Dev. Ind. Pharm. 2000, 26, 1141–1150. [Google Scholar] [CrossRef]
- Betageri, G. Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int. J. Pharm. 1995, 126, 155–160. [Google Scholar] [CrossRef]
- Bley, H.; Fussnegger, B.; Bodmeier, R. Characterization and stability of solid dispersions based on PEG/polymer blends. Int. J. Pharm. 2010, 390, 165–173. [Google Scholar] [CrossRef]
- Ghanavati, R.; Taheri, A.; Homayouni, A. Anomalous dissolution behavior of celecoxib in PVP/Isomalt solid dispersions prepared using spray drier. Mater. Sci. Eng. C 2017, 72, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Zhang, J.; Lv, Y.; Liu, X.; Lv, G.; Ma, X. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef]
- Luebbert, C.; Stoyanov, E.; Sadowski, G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. X 2021, 3, 100070. [Google Scholar] [CrossRef]
- Doreth, M.; Löbmann, K.; Priemel, P.; Grohganz, H.; Taylor, R.; Holm, R.; de Diego, H.L.; Rades, T. Influence of PVP molecular weight on the microwave assisted in situ amorphization of indomethacin. Eur. J. Pharm. Biopharm. 2018, 122, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Alqurshi, A.; Chan, K.L.A.; Royall, P.G. In-situ freeze-drying—Forming amorphous solids directly within capsules: An investigation of dissolution enhancement for a poorly soluble drug. Sci. Rep. 2017, 7, 2910. [Google Scholar] [CrossRef]
- Li, Y.; Rantanen, J.; Yang, M.; Bohr, A. Molecular structure and impact of amorphization strategies on intrinsic dissolution of spray dried indomethacin. Eur. J. Pharm. Sci. 2019, 129, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of a nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: Physicochemical investigations. Colloids Surf. Physicochem. Eng. Asp. 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Le, Y.; Ji, H.; Chen, J.-F.; Shen, Z.; Yun, J.; Pu, M. Nanosized bicalutamide and its molecular structure in solvents. Int. J. Pharm. 2009, 370, 175–180. [Google Scholar] [CrossRef]
- Masiello, D.; Cheng, S.; Bubley, G.J.; Lu, M.L.; Balk, S.P. Bicalutamide Functions as an Androgen Receptor Antagonist by Assembly of a Transcriptionally Inactive Receptor. J. Biol. Chem. 2002, 277, 26321–26326. [Google Scholar] [CrossRef]
- Vega, D.R.; Polla, G.; Martinez, A.; Mendioroz, E.; Reinoso, M. Conformational polymorphism in bicalutamide. Int. J. Pharm. 2007, 328, 112–118. [Google Scholar] [CrossRef]
- Ren, F.; Jing, Q.; Tang, Y.; Shen, Y.; Chen, J.; Gao, F.; Cui, J. Characteristics of Bicalutamide Solid Dispersions and Improvement of the Dissolution. Drug Dev. Ind. Pharm. 2006, 32, 967–972. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhao, L.-J.; Wang, Y.-T. Synthesis and clinical application of small-molecule drugs approved to treat prostatic cancer. Eur. J. Med. Chem. 2023, 262, 115925. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Kurek, M.; Syrek, K.; Chmiel, K.; Paluch, M.; Jachowicz, R. Planetary ball milling and supercritical fluid technology as a way to enhance dissolution of bicalutamide. Int. J. Pharm. 2017, 533, 470–479. [Google Scholar] [CrossRef]
- Cockshott, I.D. Bicalutamide: Clinical Pharmacokinetics and Metabolism. Clin. Pharmacokinet. 2004, 43, 855–878. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, Committee for Medicinal Product for Human Use (CHMP), Guideline on the Investigation of Bioequivalence. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 19 May 2025).
- Hu, X.-R.; Gu, J.-M. N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-(4-fluorophenylsulfonyl)-2-hydroxy-2-methylpropionamide. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, o3897–o3898. [Google Scholar] [CrossRef]
- Gana, I.; Céolin, R.; Rietveld, I.B. Bicalutamide polymorphs I and II: A monotropic phase relationship under ordinary conditions turning enantiotropic at high pressure. J. Therm. Anal. Calorim. 2013, 112, 223–228. [Google Scholar] [CrossRef]
- Dhaked, D.K.; Jain, V.; Kasetti, Y.; Bharatam, P.V. Conformational polymorphism in bicalutamide: A quantum chemical study. Struct. Chem. 2012, 23, 1857–1866. [Google Scholar] [CrossRef]
- Yoshioka, M.; Hancock, B.C.; Zografi, G. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipitates. J. Pharm. Sci. 1995, 84, 983–986. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Liu, H.; Edgar, K.J.; Taylor, L.S. Understanding Polymer Properties Important for Crystal Growth Inhibition—Impact of Chemically Diverse Polymers on Solution Crystal Growth of Ritonavir. Cryst. Growth Des. 2012, 12, 3133–3143. [Google Scholar] [CrossRef]
- Patel, C.; Patel, Z.; Dani, H.; Zaveri, M. ALU-ALU Packaging Vs ALU-PVC Blister Packaging: Waste to Value—A Comparative Analysis. Int. J. Pharm. Sci. 2025, 3, 2810–2818. [Google Scholar] [CrossRef]
- Lamberti, M.; Escher, F. Aluminium Foil as a Food Packaging Material in Comparison with Other Materials. Food Rev. Int. 2007, 23, 407–433. [Google Scholar] [CrossRef]
- Yaren Çapkın, İ.; Gökelma, M. A review on characterization and recyclability of pharmaceutical blisters. Clean. Waste Syst. 2023, 4, 100082. [Google Scholar] [CrossRef]
- Fang, X.; Hu, Y.; Yang, G.; Shi, W.; Lu, S.; Cao, Y. Improving physicochemical properties and pharmacological activities of ternary co-amorphous systems. Eur. J. Pharm. Biopharm. 2022, 181, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; AbuDiak, O.A.; Jones, D.S. Physicochemical Characterization of Hot Melt Extruded Bicalutamide–Polyvinylpyrrolidone Solid Dispersions. J. Pharm. Sci. 2010, 99, 1322–1335. [Google Scholar] [CrossRef]
- Theil, F.; Milsmann, J.; Kyeremateng, S.O.; Anantharaman, S.; Rosenberg, J.; Van Lishaut, H. Extraordinary Long-Term-Stability in Kinetically Stabilized Amorphous Solid Dispersions of Fenofibrate. Mol. Pharm. 2017, 14, 4636–4647. [Google Scholar] [CrossRef]
- Lehmkemper, K.; Kyeremateng, S.O.; Bartels, M.; Degenhardt, M.; Sadowski, G. Physical stability of API/polymer-blend amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2018, 124, 147–157. [Google Scholar] [CrossRef]
- Antosik-Rogóż, A.; Szafraniec-Szczęsny, J.; Knapik-Kowalczuk, J.; Kurek, M.; Gawlak, K.; Paluch, M.; Jachowicz, R. How Does Long-Term Storage Influence the Physical Stability and Dissolution of Bicalutamide from Solid Dispersions and Minitablets? Processes 2022, 10, 1002. [Google Scholar] [CrossRef]

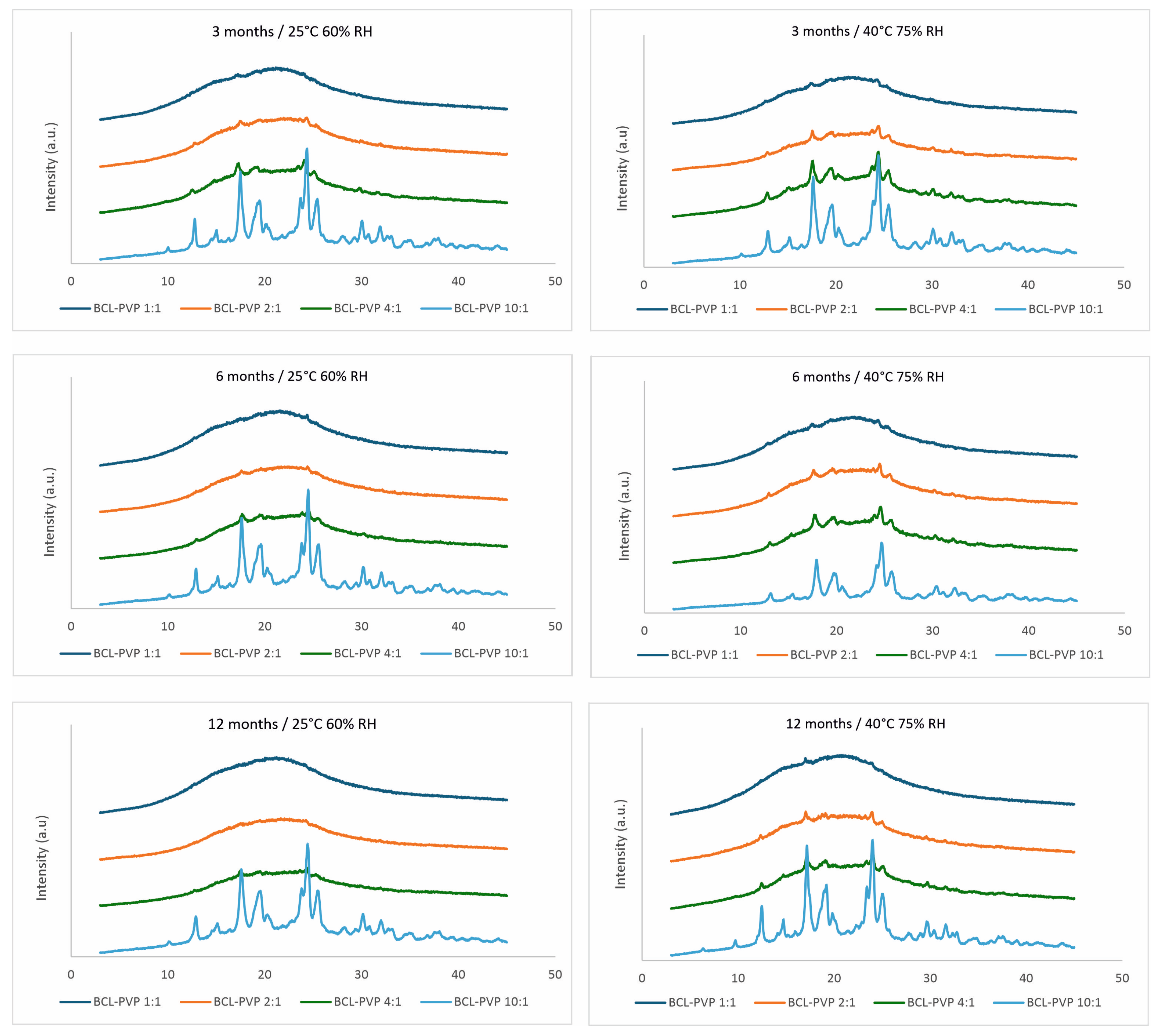

| Time (Months) | f2 Values | |||||||
| 25 °C/60% RH | 40 °C/75% RH | |||||||
| 1:1 | 2:1 | 4:1 | 10:1 | 1:1 | 2:1 | 4:1 | 10:1 | |
| 3 | 70.5 | 57.9 | 46.5 | 34.3 | 63.9 | 71.0 | 28.2 | 38.8 |
| 6 | 69.8 | 92.5 | 41.1 | 30.9 | 56.5 | 65.9 | 41.3 | 30.9 |
| 12 | 62.5 | 67.6 | 47.6 | 29.2 | 53.7 | 86.7 | 30.9 | 32.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antosik-Rogóż, A.; Szafraniec-Szczęsny, J.; Gawlak, K.; Mendyk, A. Is PVP K-29/32 an Efficient Stabilizing Excipient in Amorphous Solid Dispersions Containing the Poorly Water-Soluble Drug—Bicalutamide? Processes 2025, 13, 2714. https://doi.org/10.3390/pr13092714
Antosik-Rogóż A, Szafraniec-Szczęsny J, Gawlak K, Mendyk A. Is PVP K-29/32 an Efficient Stabilizing Excipient in Amorphous Solid Dispersions Containing the Poorly Water-Soluble Drug—Bicalutamide? Processes. 2025; 13(9):2714. https://doi.org/10.3390/pr13092714
Chicago/Turabian StyleAntosik-Rogóż, Agata, Joanna Szafraniec-Szczęsny, Karolina Gawlak, and Aleksander Mendyk. 2025. "Is PVP K-29/32 an Efficient Stabilizing Excipient in Amorphous Solid Dispersions Containing the Poorly Water-Soluble Drug—Bicalutamide?" Processes 13, no. 9: 2714. https://doi.org/10.3390/pr13092714
APA StyleAntosik-Rogóż, A., Szafraniec-Szczęsny, J., Gawlak, K., & Mendyk, A. (2025). Is PVP K-29/32 an Efficient Stabilizing Excipient in Amorphous Solid Dispersions Containing the Poorly Water-Soluble Drug—Bicalutamide? Processes, 13(9), 2714. https://doi.org/10.3390/pr13092714








