Pyrolytic Valorization of Polygonum multiflorum Residues: Kinetic, Thermodynamic, and Product Distribution Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physicochemical Analyses
2.3. TG-DTG Experiment
2.4. Pyrolysis Parameters and Performance Evaluation
2.5. Kinetic Analysis
2.5.1. Model-Free Methods
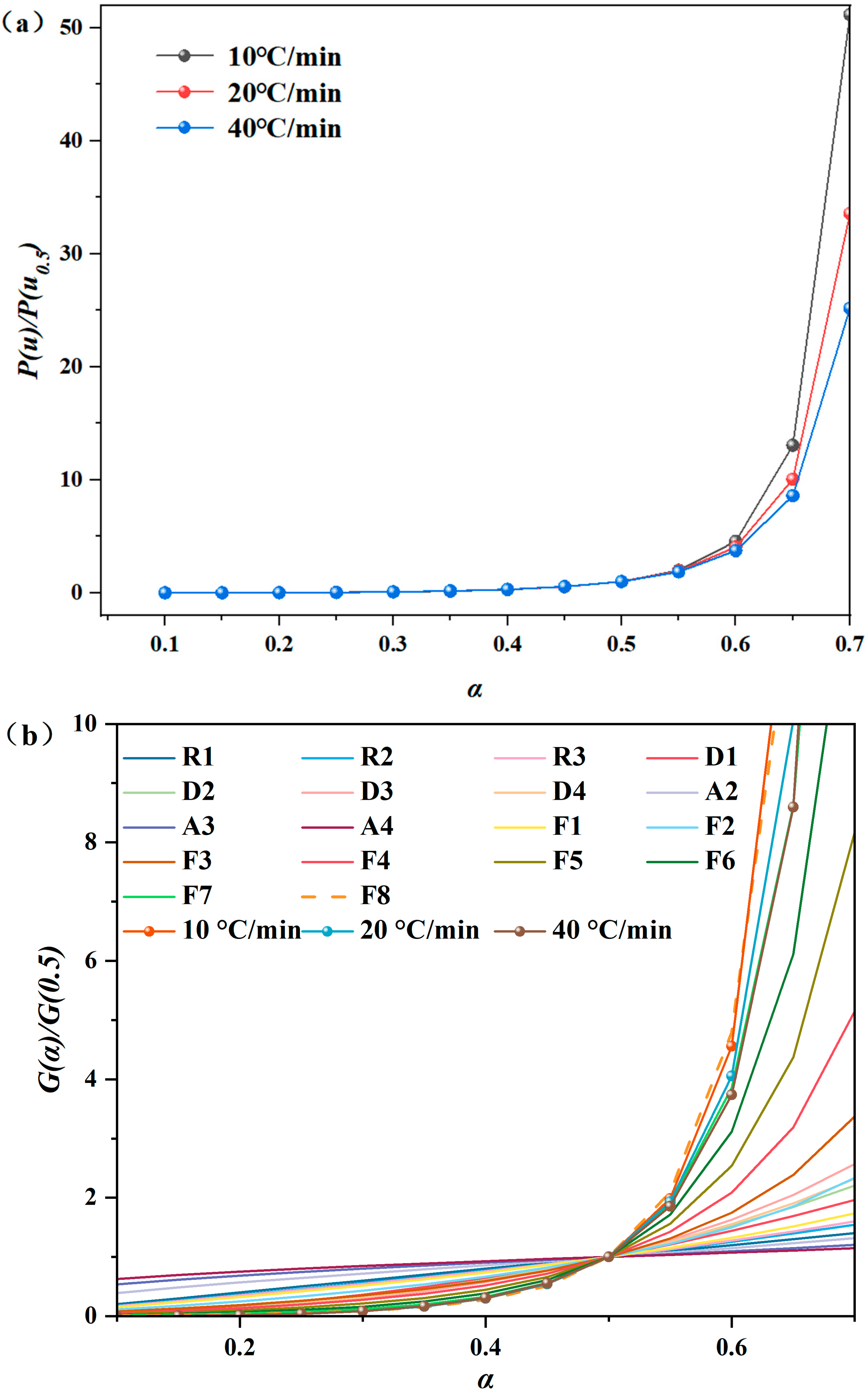
2.5.2. Master-Plots Method
2.6. Thermodynamic Parameter Estimation
2.7. TG-FTIR-GC/MS Experiment
2.8. Py-GC/MS Experiment
3. Results and Discussion
3.1. Physicochemical Properties and Pyrolysis Suitability of PM Residues
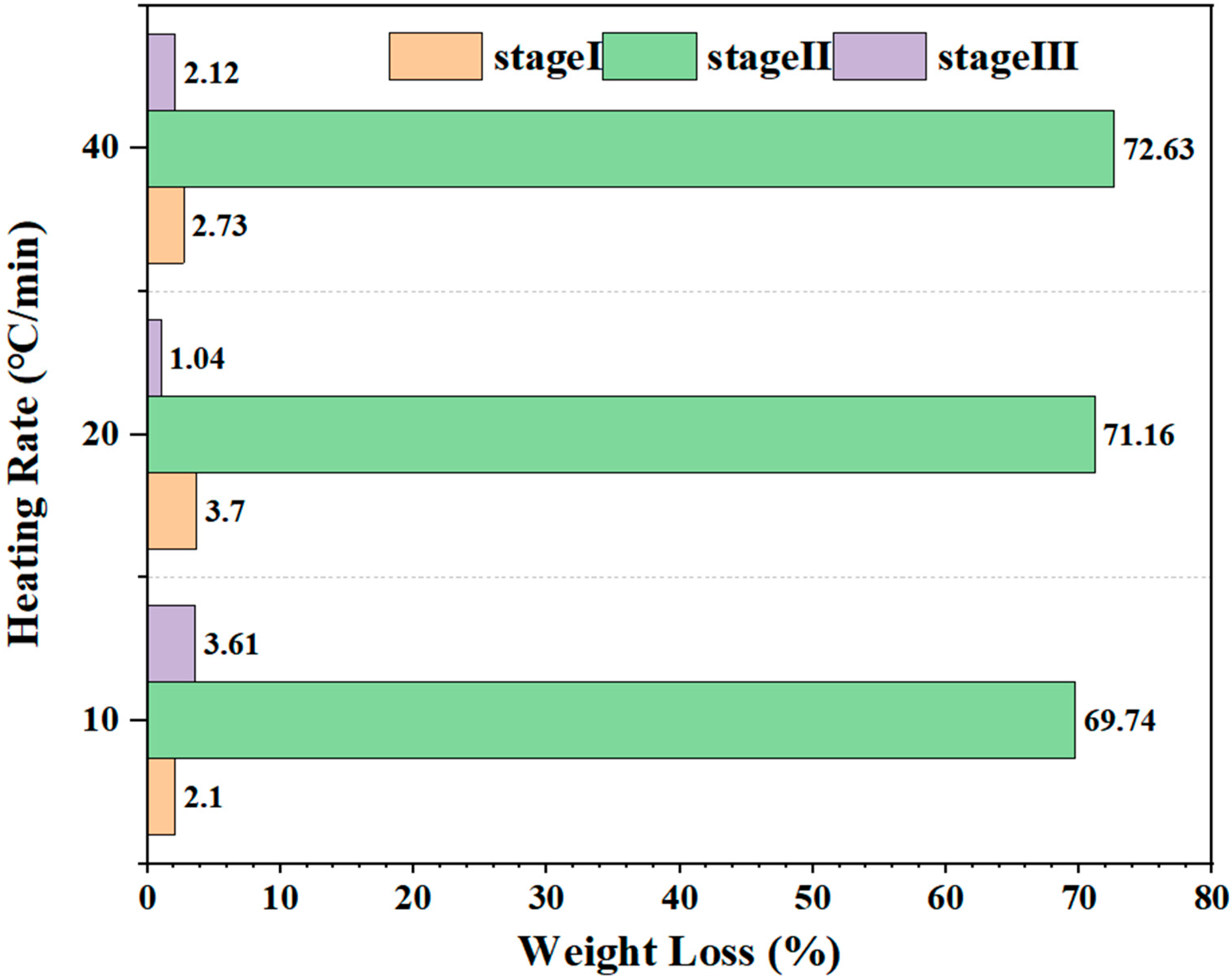
3.2. Thermal Decomposition Regime with Stage/Heating Rate-Specific Effects
3.3. Key Pyrolysis Performance Indicators
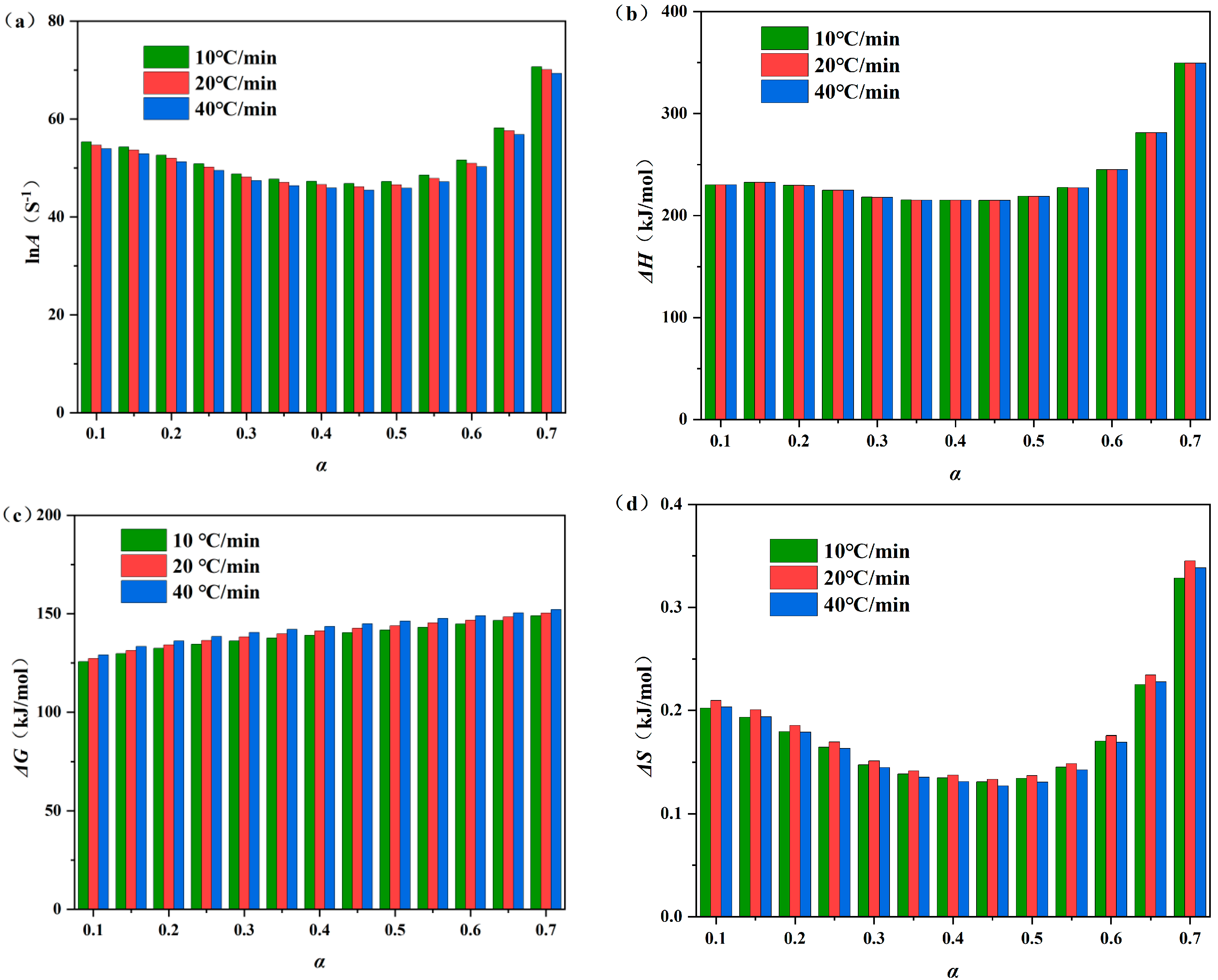
3.4. Kinetic Analysis: Apparent Activation Energy Trends
3.5. Thermal Decomposition Mechanisms
3.6. Thermodynamic Parameter Evolution
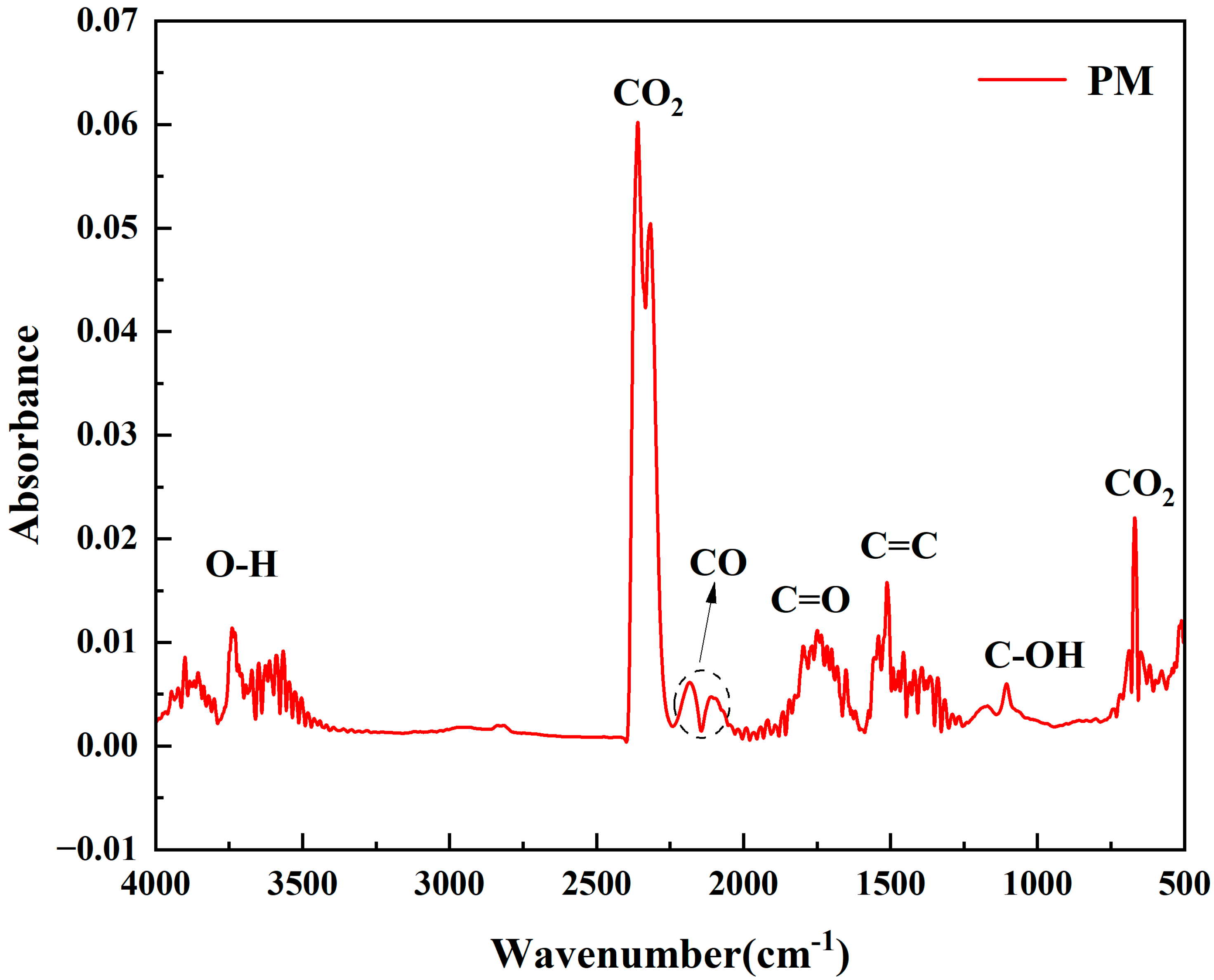
3.7. Real-Time Volatile Evolution During Slow Pyrolysis
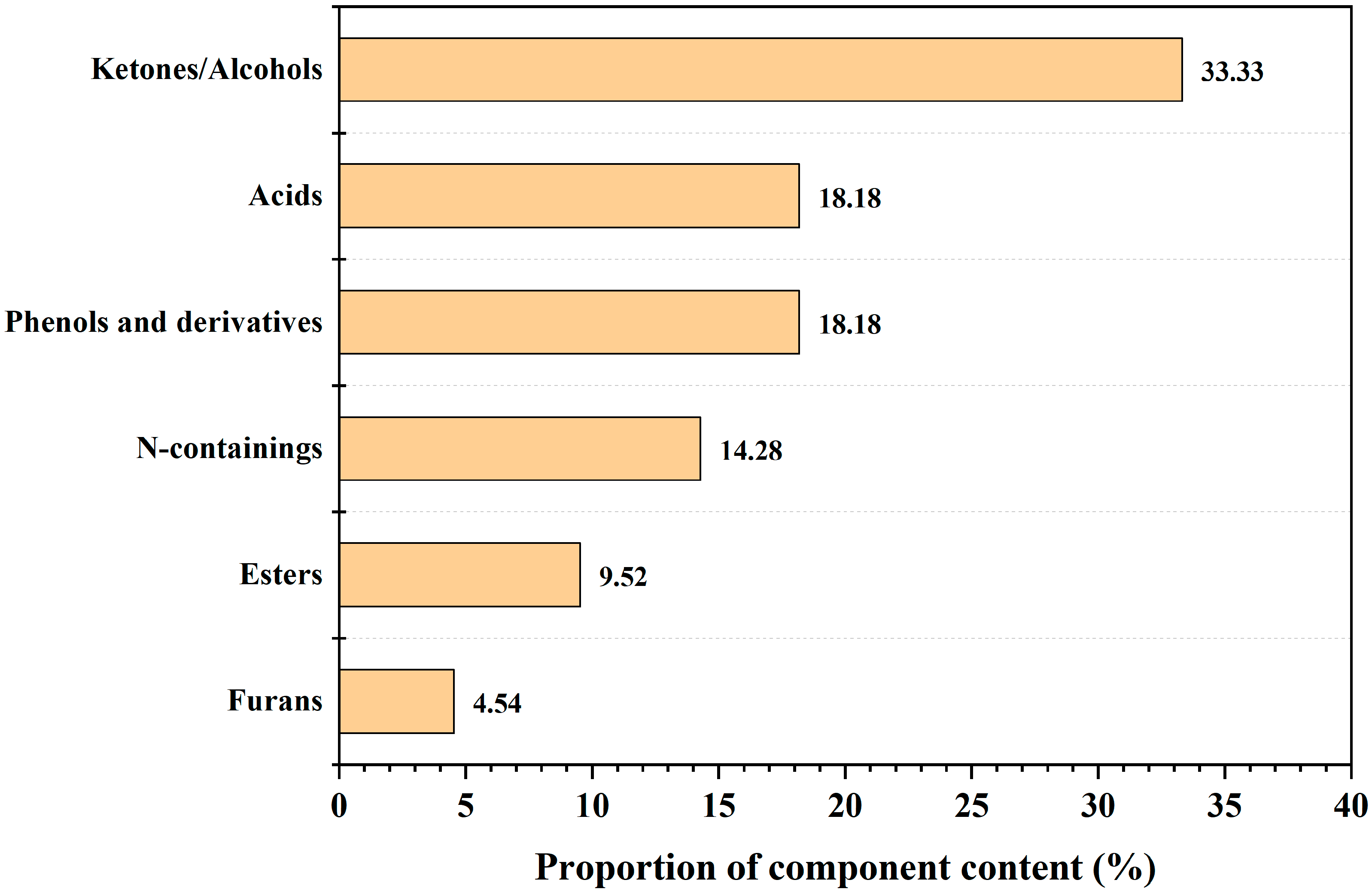
3.8. Fast-Pyrolysis Product Distribution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Mensah, P.; Yankson, E. Biomass energy as a catalyst for achieving global sustainability goals: Technological advancements and policy implications. Acad. Green Energy 2025, 2. [Google Scholar] [CrossRef]
- Konyannik, B.Y.; Lavie, J.D. Valorization techniques for biomass waste in energy Generation: A systematic review. Bioresour. Technol. 2025, 435, 132973. [Google Scholar] [CrossRef]
- Liuyang, Z.; Jishuo, L.; Kaili, X. Analysis of the pyrolysis and combustion behavior and product release characteristics of Chinese medicine residue under a nitrogen/oxygen atmosphere. Biomass Bioenergy 2025, 197, 107824. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Lee, T.; Cha, H.; Chen, W.-H.; Tsang, Y.F.; Kwon, E.E. Production of combustible gas via incorporating CO2 to pyrolysis of medicinal herbal waste. Ind. Crops Prod. 2024, 219, 119110. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Li, L.; Chen, X.; Lin, Z.; Yang, C.; Evrendilek, F.; Li, W.; Huang, W.; He, Y.; et al. Optimizing pyrolysis of herbal tea and Salvia miltiorrhiza residues for sustainable energy and product recovery. Chem. Eng. J. 2025, 513, 162694. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Huang, T.-Y.; Chung, Y.-Y.; Lin, W.-C.; Lin, H.-Y.; Chiu, H.-C.; Lee, S.-Y. Exosomes derived from Polygonum multiflorum-treated human dental pulp stem cells (hDPSCs): New approach in regenerative medicine. J. Drug Deliv. Sci. Technol. 2024, 99, 105941. [Google Scholar] [CrossRef]
- Gong, L.; Shen, X.; Huang, N.; Wu, K.; Li, R.; Liu, Y.; Zhang, H.; Chen, S.; Sun, R. Research progress on hepatotoxicity mechanism of polygonum multiflorum and its main components. Toxicon 2024, 248, 108040. [Google Scholar] [CrossRef]
- Liu, D.; Ye, D.; Li, T.; Zheng, Z.; Zhang, X. Practicability of using Polygonum japonicum as a P accumulator for P phytoextraction from soil amended with swine manure. Appl. Soil Ecol. 2017, 112, 11–17. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Wang, Y.; Hao, K. Microwave torrefaction of biomass waste: Fuel property evaluation and life cycle impact. Next Energy 2025, 9, 100380. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.; Arudra, P.; Al-Khattaf, S.S. Catalytic cracking of 1-butene to propylene using modified H-ZSM-5 catalyst: A comparative study of surface modification and core-shell synthesis. Appl. Catal. A Gen. 2017, 533, 109–120. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Ramachandran, S.; Subbiah, S. Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis. Bioresour. Technol. 2017, 233, 413–422. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Castro, J.D.S.; Camelo, E.R.; Sousa, H.O.; Oliveira, P.S.; Rodrigues, W.S.; Zhou, S.; Virgens, C.F. Impact of chemical treatment on the energy potential of Pachira aquatica A. fruit peel during pyrolysis. J. Anal. Appl. Pyrolysis 2025, 192, 107325. [Google Scholar] [CrossRef]
- Starink, M. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, P.; Fu, K.; Guo, W.; Li, H.; Hao, X.; Wang, J.; Yang, B.; Zhang, B.; Xie, T.; et al. Effect of reservoir moisture on underground in-situ pyrolysis of tar-rich coal: Experimental investigation and ReaxFF MD simulation. J. Anal. Appl. Pyrolysis 2025, 192, 107285. [Google Scholar] [CrossRef]
- Wu, F.; Huang, S.; Jiang, Q.; Jiang, G. Effects of pressure and heating rate on coal pyrolysis: A study in simulated underground coal gasification. J. Anal. Appl. Pyrolysis 2023, 175, 106179. [Google Scholar] [CrossRef]
- Rony, A.H.; Kong, L.; Lu, W.; Dejam, M.; Adidharma, H.; Gasem, K.A.M.; Zheng, Y.; Norton, U.; Fan, M. Kinetics, thermodynamics, and physical characterization of corn stover (Zea mays) for solar biomass pyrolysis potential analysis. Bioresour. Technol. 2019, 284, 466–473. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Tang, S.; Zhang, T. The nitrogen transformation behavior based on the pyrolysis products of wheat straw. Chin. J. Chem. Eng. 2024, 71, 58–65. [Google Scholar] [CrossRef]
- Li, S.; Hu, E.; Xu, G.; Liu, Z.; Zeng, Y.; Yu, J.; Zheng, G.; Pan, D.; Li, M.; Ma, Y. Rapid infrared co-pyrolysis performance of corn stover and polyurethane foam waste for upgrading oil yield and quality. Energy 2025, 333, 137258. [Google Scholar] [CrossRef]
- Bisen, D.; Lanjewar, R.; Alawa, B.; Chouhan, A.P.S.; Chakma, S. Kinetic analysis and fuel characterization with hydrocarbon distribution in pyro-oil produced from co-pyrolysis of rice husk and low-density polyethylene. J. Energy Inst. 2025, 121, 102175. [Google Scholar] [CrossRef]
- Hakeem, I.G.; Rathnayake, N.; Surapaneni, A.; Shah, K. Effects of mild acid pre-treatment on the co-pyrolysis behaviour of biosolids and wheat straw. Process Saf. Environ. Prot. 2024, 185, 375–391. [Google Scholar] [CrossRef]
- Gu, X.; Ma, X.; Li, L.; Liu, C.; Cheng, K.; Li, Z. Pyrolysis of poplar wood sawdust by TG-FTIR and Py–GC/MS. J. Anal. Appl. Pyrolysis 2013, 102, 16–23. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Pyrolysis behaviors of wood and its constituent polymers at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 78, 328–336. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Jia, D.; Liang, J.; Liu, J.; Chen, D.; Evrendilek, F.; Wen, T.; Cao, H.; Zhong, S.; Yang, Z.; He, Y. Insights into pyrolysis of ginger via TG-FTIR and Py-GC/MS analyses: Thermochemical behaviors, kinetics, evolved gas, and products. J. Anal. Appl. Pyrolysis 2024, 179, 106442. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Wu, X.; Zhang, J.; Evrendilek, D.E.; Liu, J.; Liang, G.; Li, W. Temperature- and heating rate-dependent pyrolysis mechanisms and emissions of Chinese medicine residues and numerical reconstruction and optimization of their non-linear dynamics. Renew. Energy 2021, 164, 1408–1423. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Liu, H.; Zhao, M.; Soomro, A.F.; Memon, M.Z.; Dupont, V. Thermogravimetric and kinetic analysis to discern synergy during the co-pyrolysis of microalgae and swine manure digestate. Biotechnol. Biofuels 2019, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Boubacar Laougé, Z.; Merdun, H. Kinetic analysis of Pearl Millet (Penissetum glaucum (L.) R. Br.) under pyrolysis and combustion to investigate its bioenergy potential. Fuel 2020, 267, 117172. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Taqvi, S.T.H.; Elkamel, A.; Liu, C.-G.; Xu, J.; Rahimuddin, S.A.; Gull, M. Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour. Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef]
- Askari, F.; Abdulkhani, A.; Azadfallah, M.; Hamzeh, Y.; Ashori, A. Development and characterization of furfural-based bio-resins from lignocellulosic waste for eco-friendly wood resins. Int. J. Adhes. Adhes. 2025, 140, 104014. [Google Scholar] [CrossRef]
- Kumar Mishra, R. Pyrolysis of low-value waste switchgrass: Physicochemical characterization, kinetic investigation, and online characterization of hot pyrolysis vapours. Bioresour. Technol. 2022, 347, 126720. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.M.; Borges, L.E.P.; Fraga, M.A. Lactic acid production from aqueous-phase selective oxidation of hydroxyacetone. J. Mol. Catal. A Chem. 2015, 400, 64–70. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, J.-B.; Li, W.-F.; Qiu, C.-X.; Hu, G.; Wang, S.-T.; Song, Y.-F.; Gao, H.-Y.; Liu, Y.; Wang, Q.; et al. In vivo hepatotoxicity screening of different extracts, components, and constituents of Polygoni Multiflori Thunb. in zebrafish (Danio rerio) larvae. Biomed. Pharmacother. 2020, 131, 110524. [Google Scholar] [CrossRef]


| Symbol | Mechanism | f(a) | G(a) |
|---|---|---|---|
| Diffusion | |||
| D1 | One-dimension diffusion | 1/(2a) | a2 |
| D2 | Two-dimension diffusion | [−ln(1 − a)]−1 | (1 − a)ln(1 − a) + a |
| D3 | Three-dimension diffusion | [(3/2)(1 − a)2/3]/[1−(1 − a)1/3] | [1 − (1 − a)1/3]2 |
| D4 | Four-dimension diffusion | [(3/2)(1 − a)1/3]/[1 − (1 − a)1/3] | (1 − 2a/3)−(1 − a)2/3 |
| Geometrical contraction | |||
| R2 | Contracting cylinder | 2(1 − a)1/2 | 1−(1 − a)1/2 |
| R3 | Contracting sphere | 3(1 − a)1/3 | 1−(1 − a)1/3 |
| Reaction order | |||
| F1 | First-order reaction | 1 − a | −ln(1 − a) |
| F2 | Second-order reaction | (1 − a)2 | (1 − a)−1 − 1 |
| F3 | Third-order reaction | (1 − a)3 | [(1 − a)−2 − 1]/2 |
| Fn | nth-order reaction | (1 − a)n | [(1 − a)(1−n) − 1]/(n − 1) |
| Power law | |||
| P2 | One-power law | 2a1/2 | a1/2 |
| P3 | Two-power law | 3a1/3 | a1/3 |
| P4 | Three-power law | 4a1/4 | a1/4 |
| Nucleation | |||
| A1.5 | Avrami–Erofeev | 1.5(1 − a)[−ln(1 − a)]1/3 | [−ln(1 − a)]2/3 |
| A2 | Avrami–Erofeev | 2(1 − a)[−ln(1 − a)]1/2 | [−ln(1 − a)]1/2 |
| A3 | Avrami–Erofeev | 3(1 − a)[−ln(1 − a)]2/3 | [−ln(1 − a)]1/3 |
| An | Avrami–Erofeev | n(1 − a)[−ln(1 − a)](n−1)/n | [−ln(1 − a)]1/n |
| Sample | Proximate Analysis (wt%) | Ultimate Analysis (wt%) | HHV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | V | Ash | FC | C | H | N | O | S | (MJ/kg) | |
| PM | 4.10 | 69.83 | 5.26 | 20.81 | 43.10 | 6.07 | 0.91 | 44.63 | 0.03 | 16.69 |
| β (°C/min) | Ti (°C) | Tp (°C) | −Rp (%/min) | −Rm (%/min) | Re (%) | CPI (10−5·%3·min−2·°C−3) |
|---|---|---|---|---|---|---|
| 10 | 254.0 | 291.8 | 6.95 | 0.79 | 24.36 | 9.72 |
| 20 | 263.9 | 302.0 | 13.31 | 1.58 | 23.89 | 31.9 |
| 40 | 270.0 | 311.8 | 27.14 | 3.26 | 22.27 | 124 |
| FWO | KAS | Starink | ||||
|---|---|---|---|---|---|---|
| 0.10 | 234.38 | 0.999 | 237.87 | 0.999 | 238.03 | 0.999 |
| 0.15 | 236.99 | 0.997 | 240.36 | 0.997 | 240.52 | 0.997 |
| 0.20 | 234.07 | 0.998 | 237.12 | 0.998 | 237.29 | 0.998 |
| 0.25 | 229.41 | 0.999 | 232.09 | 0.999 | 232.27 | 0.999 |
| 0.30 | 222.60 | 0.999 | 224.81 | 0.999 | 225.01 | 0.999 |
| 0.35 | 219.93 | 0.999 | 221.92 | 0.999 | 222.12 | 0.999 |
| 0.40 | 219.84 | 0.999 | 221.74 | 0.999 | 221.95 | 0.999 |
| 0.45 | 219.70 | 0.999 | 221.51 | 0.999 | 221.72 | 0.999 |
| 0.50 | 223.67 | 0.999 | 225.60 | 0.999 | 225.81 | 0.999 |
| 0.55 | 232.29 | 0.999 | 234.57 | 0.999 | 234.77 | 0.999 |
| 0.60 | 249.89 | 0.999 | 252.97 | 0.999 | 253.17 | 0.999 |
| 0.65 | 286.16 | 0.997 | 290.98 | 0.997 | 291.15 | 0.997 |
| 0.70 | 354.72 | 0.994 | 362.91 | 0.994 | 363.03 | 0.994 |
| Average | 243.36 | — | 246.50 | — | 246.68 | — |
| α | Ea | A (s−1) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (kJ/mol) |
|---|---|---|---|---|---|
| 0.1 | 234.38 | 5.52 × 1023 | 230.04 | 127.28 | 0.20 |
| 0.15 | 236.99 | 2.04 × 1023 | 232.52 | 131.35 | 0.19 |
| 0.2 | 234.07 | 3.77 × 1022 | 229.51 | 134.18 | 0.17 |
| 0.25 | 229.41 | 6.38 × 1021 | 224.79 | 136.37 | 0.16 |
| 0.3 | 222.60 | 8.08 × 1020 | 217.92 | 138.22 | 0.14 |
| 0.35 | 219.93 | 2.78 × 1020 | 215.21 | 139.79 | 0.13 |
| 0.4 | 219.84 | 1.76 × 1020 | 215.08 | 141.21 | 0.13 |
| 0.45 | 219.70 | 1.12 × 1020 | 214.89 | 142.57 | 0.13 |
| 0.5 | 223.67 | 1.69 × 1020 | 218.82 | 143.89 | 0.13 |
| 0.55 | 232.29 | 6.37 × 1020 | 227.39 | 145.28 | 0.14 |
| 0.6 | 249.89 | 1.37 × 1022 | 244.94 | 146.74 | 0.16 |
| 0.65 | 286.16 | 1.04 × 1025 | 281.13 | 148.34 | 0.22 |
| 0.7 | 354.72 | 2.80 × 1030 | 349.60 | 150.35 | 0.32 |
| Wavenumber (cm−1) | Functional Group | Possible Compounds |
|---|---|---|
| 4000–3500 | O-H | Water, alcohols, and carboxylic acids |
| 2400–2240 | C=O | CO2 |
| 2240–2020 | C-O | CO |
| 1900–1650 | C=O | Aldehydes, ketones, acids |
| 1650–1250 | C=C, benzene skeleton | Aromatics |
| 1250–1000 | C-O,O-H | Ethers, alcohols |
| 750–500 | C=O | CO2 |
| No | RT | Area (%) | Substance | Formula | MW | Class |
|---|---|---|---|---|---|---|
| 1 | 2.71 | 52.4 | 3-methyl-Furan | C5H6O | 82 | Furan |
| 2 | 3.43 | 17.23 | Benzene | C6H6 | 78 | Benzene |
| 3 | 7.82 | 19.68 | Furfural | C5H4O2 | 96 | Aldehyde |
| 4 | 9.09 | 5.02 | 4-[[(4-methylphenyl)sulfonyl]oxy]-Cyclohexanone | C13H16O4S | 268 | Ketones |
| 5 | 9.71 | 1.32 | 2-(9,12-octadecadienyloxy)-, (Z,Z)-Ethanol | C20H38O2 | 310 | Alcohol |
| 6 | 22.92 | 0.81 | 3-ethyl-5-(2-ethylbutyl)-Octadecane | C26H54 | 366 | Hydrocarbons |
| 7 | 23.83 | 0.86 | Methyl glycocholate, 3TMS derivative | C36H69NO6Si3 | 695 | Ester |
| 8 | 24.1 | 0.86 | 3-Desoxo-3,16-dihydroxy-12-desoxyphorbol 3,13,16,20-tetraacetate | C28H38O10 | 534 | Ester |
| 9 | 24.12 | 0.85 | Withaferin A | C28H38O6 | 470 | Withaferin |
| 10 | 25.49 | 1.17 | Oleic acid, 3-(octadecyloxy)propyl ester | C39H76O3 | 592 | Ester |
| 11 | 26.88 | 0.49 | 3-acetoxy-7,8-Epoxylanostan-11-ol | C32H54O4 | 502 | Alcohol |
| No | RT | Area (%) | Substance | Formula | MW | Class |
|---|---|---|---|---|---|---|
| 1 | 1.49 | 5.54 | Carbamic acid, monoammonium salt | CH6N2O2 | 78 | Carbamates |
| 2 | 1.97 | 1.17 | Ethenyl ester | C4H6O2 | 86 | Ester |
| 3 | 2.02 | 2.22 | 3-Cyclopentene-1,2-diol, cis- | C5H8O2 | 100 | Alcohols |
| 4 | 2.26 | 9.22 | Acetic acid | C2H4O2 | 60 | Acids |
| 5 | 2.48 | 12.3 | 2-Propanone, 1-hydroxy- | C3H6O2 | 74 | Ketones |
| 6 | 2.65 | 2.31 | Furan, 2,5-dimethyl- | C6H8O | 96 | Furan |
| 7 | 3.28 | 1.29 | Hydrazinecarboxylic acid, phenylmethyl ester | C8H10N2O2 | 166 | Ester |
| 8 | 3.56 | 3.05 | 3-Amino-2-oxazolidinone | C3H6N2O2 | 102 | Ketones |
| 9 | 4.44 | 9.49 | 2-Furanmethanol | C5H6O2 | 98 | Alcohols |
| 10 | 5.61 | 1.69 | 2-Cyclopenten-1-one, 2-hydroxy- | C5H6O2 | 98 | Ketones |
| 11 | 5.97 | 3.58 | 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 110 | Aldehyde |
| 12 | 6.36 | 12.81 | Phenol | C6H6O | 94 | Phenol |
| 13 | 7.13 | 7.33 | 3-methyl-1,2-Cyclopentanedione | C6H8O2 | 112 | Ketones |
| 14 | 7.49 | 4.0 | o-Cresol | C7H8O | 108 | Phenol |
| 15 | 7.84 | 7.35 | p-Cresol | C7H8O | 108 | Phenol |
| 16 | 8.49 | 0.41 | 3-ethyl-2-hydroxy-2-Cyclopenten-1-one | C7H10O2 | 126 | Ketones |
| 17 | 9.66 | 1.93 | Dodecanoic acid, 3-hydroxy- | C12H24O3 | 216 | Acids |
| 18 | 9.88 | 0.94 | Catechol | C6H6O2 | 110 | Phenol |
| 19 | 18.8 | 6.41 | n-Hexadecanoic acid | C16H32O2 | 256 | Acids |
| 20 | 20.49 | 2.77 | Oleic acid | C18H34O2 | 282 | Acids |
| 21 | 24.78 | 4.20 | 9,10-Anthracenedione, 1,8-dihydroxy-3-methoxy-6-methyl- | C16H12O5 | 284 | Anthraquinone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Chen, Y.; Chen, X.; Jia, D.; Evrendilek, F.; Liu, J. Pyrolytic Valorization of Polygonum multiflorum Residues: Kinetic, Thermodynamic, and Product Distribution Analyses. Processes 2025, 13, 2701. https://doi.org/10.3390/pr13092701
Huang J, Chen Y, Chen X, Jia D, Evrendilek F, Liu J. Pyrolytic Valorization of Polygonum multiflorum Residues: Kinetic, Thermodynamic, and Product Distribution Analyses. Processes. 2025; 13(9):2701. https://doi.org/10.3390/pr13092701
Chicago/Turabian StyleHuang, Jiawei, Yan Chen, Xin Chen, Dajie Jia, Fatih Evrendilek, and Jingyong Liu. 2025. "Pyrolytic Valorization of Polygonum multiflorum Residues: Kinetic, Thermodynamic, and Product Distribution Analyses" Processes 13, no. 9: 2701. https://doi.org/10.3390/pr13092701
APA StyleHuang, J., Chen, Y., Chen, X., Jia, D., Evrendilek, F., & Liu, J. (2025). Pyrolytic Valorization of Polygonum multiflorum Residues: Kinetic, Thermodynamic, and Product Distribution Analyses. Processes, 13(9), 2701. https://doi.org/10.3390/pr13092701







