1. Introduction
Currently, access to metals is fundamental for humanity’s technological development [
1]. In this way, the hydrometallurgical process has shown to be a viable alternative for metal recovery [
2]. This process has been studied to extract copper from chalcopyrite (CuFeS
2); nevertheless, the refractory and passive characteristics of the mineral are a challenging task for the scientific community [
3]. Alternative processes have been proposed to minimize these difficulties with interesting results, such as using pyrite as a catalyst and reductor agent [
4]; however, pyrite must be pretreated with silver, which causes an increase in the operating process cost. Furthermore, to achieve high copper extraction, oxidizing agents are required [
5]. The role of this agent is to avoid passivation caused by Fe
3+, O
2, H
2O
2 and others such as
,
, O
3 and
[
6,
7].
Additionally, most of the alternatives operate in an acidic medium, mainly sulfuric and hydrochloric acid, where the best results are obtained with hydrochloric acid, leading to a problematic corrosive medium. A less harmful alternative is employing ionic liquids, since refractory metal oxides can be leached with less corrosion, facilitating the extraction with solvents [
8]. In this sense, organic solvents (methanol, ethanol, acetic acid, acetone, and ethylene glycol) are promising leaching systems in acidic mediums, achieving around 90% extraction of copper in five h [
9]. These processes have lacked development beyond a pilot scale because they have not shown solid economic viability. On the other hand, the gases generated during chalcopyrite leaching have not been analyzed by qualitative and quantitative methods, regardless of the possibility of hydrogen formation with positive implications in economic profitability [
10,
11].
Czech and Troczynski [
12] demonstrated hydrogen generation by aluminum leaching, achieving around 1200 cm
3 in 60 min using the water-soluble inorganic salts KCl and NaCl [
12]. The critical issue limiting the efficiency of hydrogen generation is the formation of a dense oxide film, limiting the access to the aluminum surface; also, the aluminum dissolution represents an increase in the cost of the process [
13]. Furthermore, there are other options for hydrogen generation that do not depend on hydrocarbon, like the photo-split of water [
14]. This process includes a photocatalyst, water, and solar radiation as an energy source since this process is thermodynamically non-spontaneous [
15]. In the literature, values around 3.6 eV for the formation of H
2 using a photocatalyst of SiC have been reported [
16]. Similarly, in the leaching process, there is a catalyst in an aqueous medium, with the concentrate acting as the catalyst, and the oxidizing agent as the energy source.
Wu and Lee [
17] investigated the formation of hydrogen with Cu deposited in TiO
2 as a photocatalyst, finding that the oxidation of metallic copper occurs in the reaction with considerable hydrogen generation. The works of photocatalysts and chalcopyrite leaching have the presence of copper in common. During the formation of hydrogen by water splitting assisted with a photocatalyst, the Cu performed as a reducing agent. Xu and Sun [
11] studied the hydrogen production with CuO deposited on TiO
2, finding that leaching of the copper in the catalyst occurs, diminishing the amount of hydrogen generation. This Cu leaching represents a problem since the catalyst cannot be used for extended periods, thereby losing economic viability. This phenomenon in the leaching of chalcopyrite is adequate since the dissolution of copper is desired to be recovered later and simultaneously be capable of generating hydrogen. The process is improved by using an oxidizing agent like hydrogen peroxide.
Likewise, adding methanol favors chalcopyrite dissolution and the leaching of chalcopyrite. Here, we investigate the leaching of chalcopyrite concentrate with methanol and sulfuric acid using hydrogen peroxide as an oxidizing agent. Simultaneously, the hydrogen contained in the gas phase was quantified. The project deals with two goals: extraction of copper and hydrogen generation.
2. Materials and Methods
The chalcopyrite concentrates employed in this study were provided by COZAMIN from the CAPSTONE COPPER unit mine localized in Zacatecas, México, with average size particle of 200 µm. The metallic content in percentage is Fe 23%, Cu 21%, Pb 0.001%, Ag 243 ppm, and Au 11.3 g/ton; the phases present in raw chalcopyrite concentrate were determined by analysis of X-ray diffraction (XRD). The leaching experiments were carried out in a 1 L hermetic reactor with a gas sampler and magnetic stirring. All chemicals were reagent grade (J. T. Baker), and deionized water was used to form the solutions.
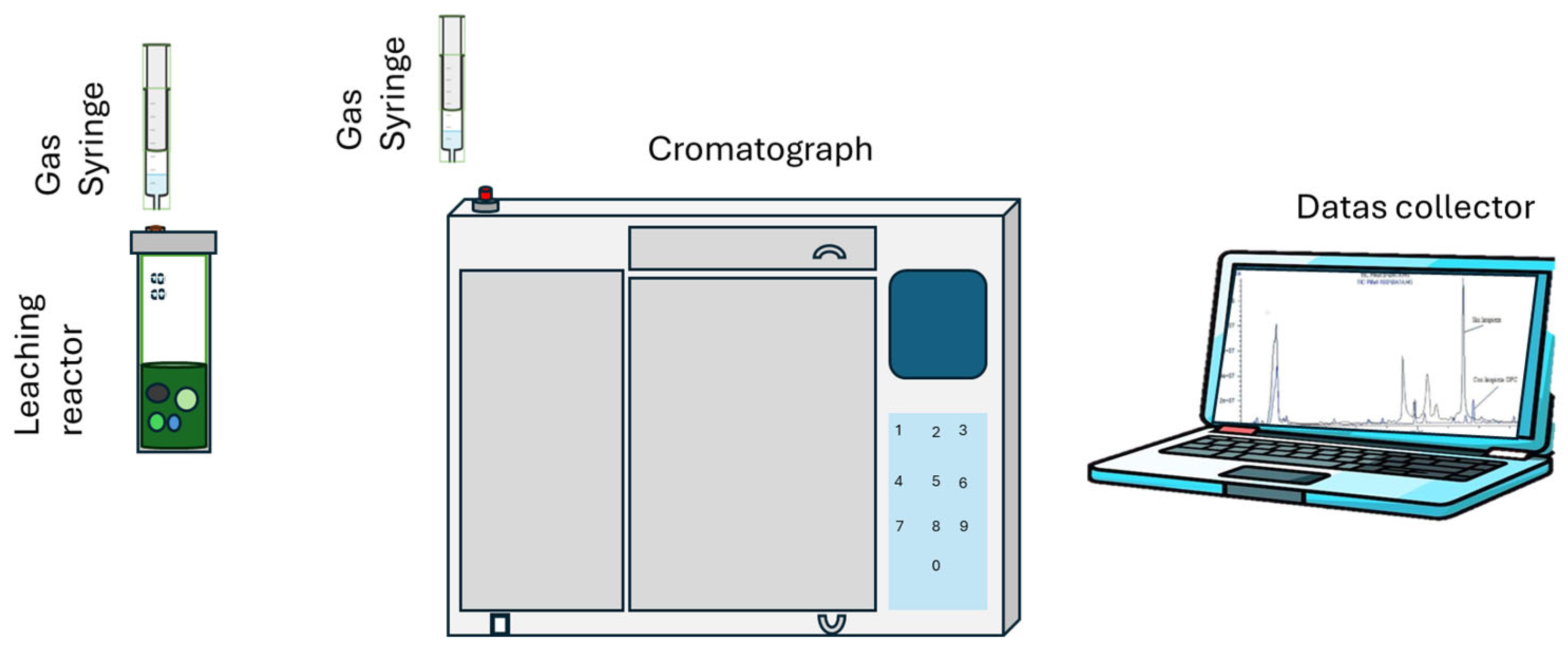
Figure 1 illustrates the experimental procedure of the leaching and quantification of gas by chromatography. Leaching tests were performed using sulfuric acid/methanol solutions and added hydrogen peroxide (30%
v/v) as the oxidizing agent. The leaching base solution was prepared with 140 mL of a 2 M sulfuric acid solution, 60 mL of methanol, and the corresponding amount of H
2O
2 to achieve the desired concentration. The final volume solution was 260 mL by adding deionized water at desired temperatures.
First, the required amount of concentrate was added to the leaching mixture. Aliquots of the leaching liquor were taken at pre-set times to determine the amount of dissolved iron and copper; the quantity of iron and copper was determined by atomic absorption spectrophotometer brand BUNSEN. The raw concentrate and residue were analyzed by X-ray diffraction (Bruker, D8 Advance, Austria Vienna) to set the initial phase in raw concentrate and formed phases, in a range of 10° to 70° in the 2θ scale degree, with a step of 0.02°, equipped with Cu Kα radiation (λ = 0.154 nm). The gas phase was analyzed in an Agilent 7890A gas chromatograph with two capillary columns Agilent CP 7430. These columns were connected in parallel to a thermal conductivity detector (TCD) and to a flame ionization detector (FID). Samples of 1 mL were extracted manually with the gas syringe and injected into the gas chromatograph. Nitrogen was used as a carrier, and the following temperature sequence was used: (a) at 50 °C for 6.5 min, (b) using a 15 °C/min ramp until reaching 250 °C, and (c) at 250 °C for 6 min. The Cu (I) ion determinations were carried out in a Varian Cary5000 spectrophotometer. Diluted samples 1:100 of the pregnant solution were analyzed in a quartz cell in the 200 to 800 nm range.
3. Results
3.1. Oxidizing Agent Effect
One of the most relevant variables in leaching is whether an oxidizing agent is incorporated, because they are usually expensive; therefore, the amount of this reagent can define the economic viability of the process. In this sense,
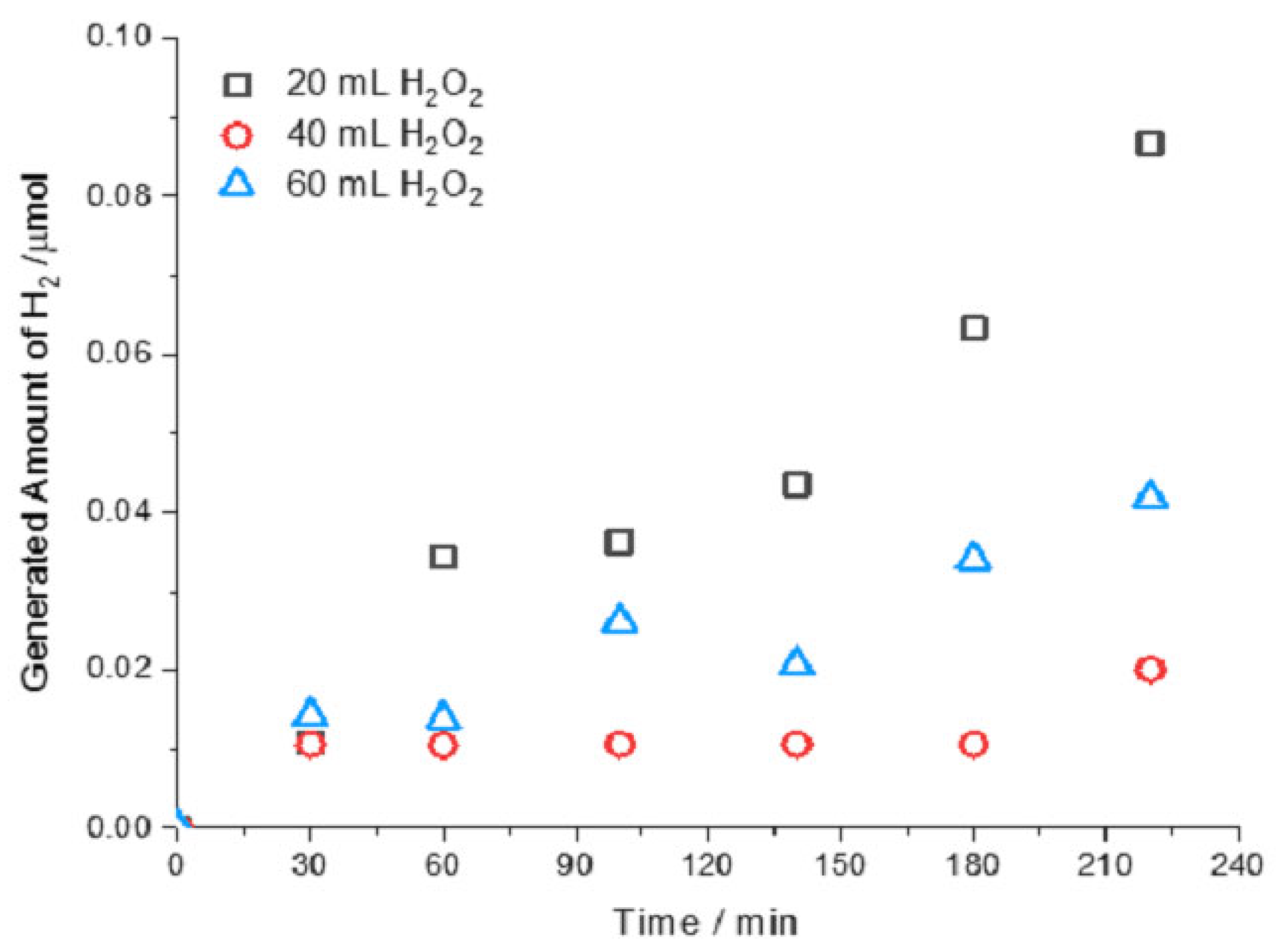
Figure 2 shows the amount of hydrogen generated versus time in the leaching of 10 g of chalcopyrite concentrate, with 140 mL of 1 M solution of H
2SO
4, and 60 mL of methanol at room temperature with different amounts of H
2O
2.
The results show hydrogen production through chalcopyrite leaching, supporting the generation of molecular hydrogen in chalcopyrite concentrate leaching. An increased hydrogen generation with time was observed; meanwhile, the largest hydrogen production (around 0.086 µmol) was achieved with the lowest quantity of hydrogen peroxide (20 mL). Increasing this H2O2 quantity, hydrogen production dropped. This behavior could be due to the dismutation properties of hydrogen peroxide, where hydroxyl radicals are generated and they increase with hydrogen peroxide, reacting with cuprous ions in a similar way to the Fenton reaction, which lead to quick oxidation of the cuprous ions formed in chalcopyrite leaching, limiting the hydrogen formation.
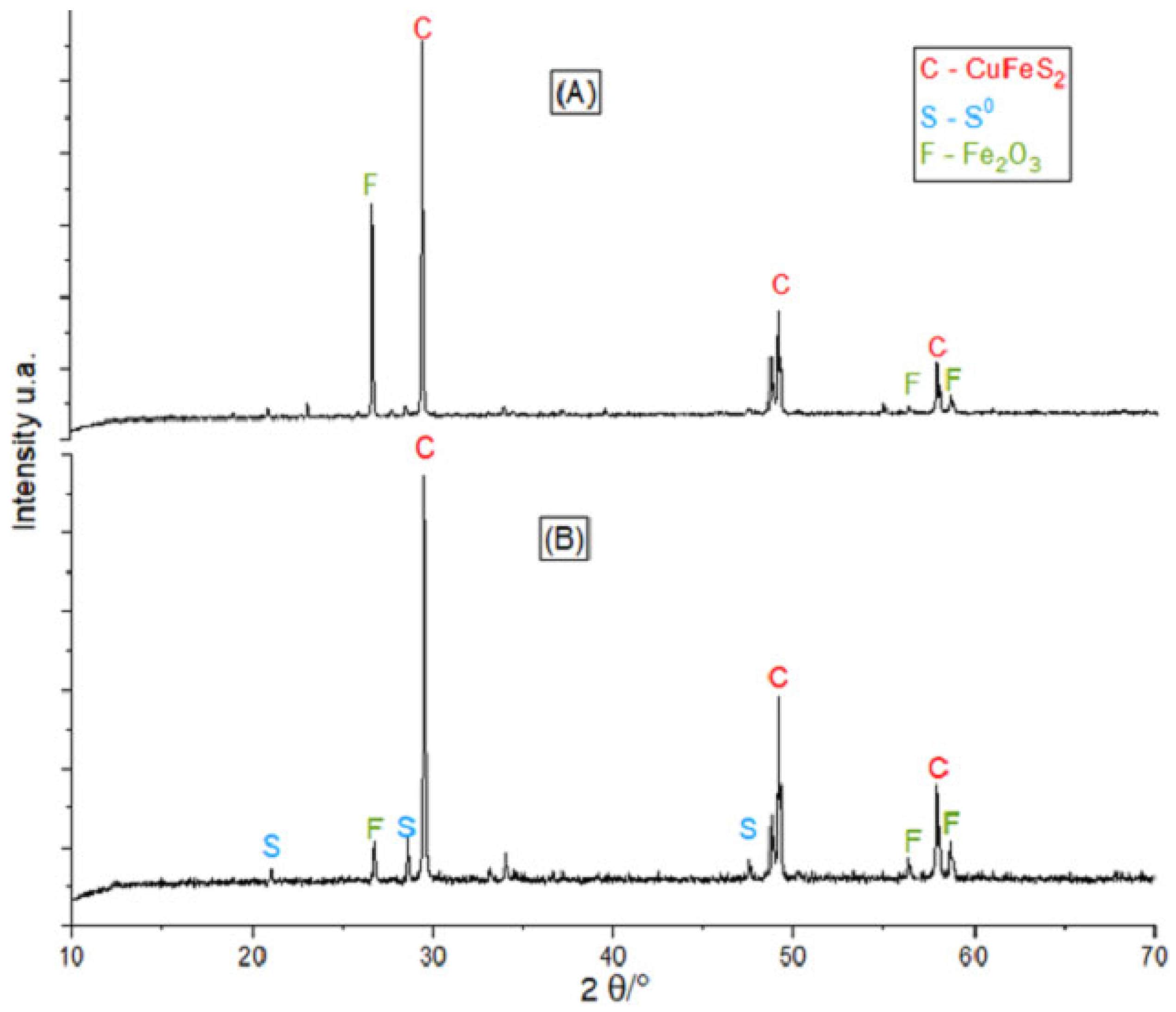
Analysis of the X-ray diffractograms (
Figure 3) PDF 00-037-0471, of raw chalcopyrite concentrate (
Figure 3A) showed CuFeS
2 and Fe
2O
3 as the main phases, but sulfur was not detected. For the solid leaching residues (
Figure 3B), the corresponding peaks of sulfur, Fe
2O
3, and no reacted chalcopyrite were detected, but in
Figure 3A they are not present, since they are products of the leaching.
Hence, with the species detected by XRD, the analysis of gas chromatography only found molecular hydrogen, and in atomic absorption spectrophotometry of the liquids, an overall reaction is proposed in Equation (1). The reaction establishes that copper in the chalcopyrite is like a cuprous ion [
18] oxidized to a cupric ion and iron in ferric oxide, thereby providing selective leaching.
Thermodynamic Analysis
Gibbs free energy is a thermodynamic variable and determines whether a reaction is spontaneous under constant pressure and temperature conditions. One way to calculate Gibbs free energy is using the difference between the Gibbs free energy of formation for the products and reactants, as in Equation (2). Then, the Gibbs free energy was calculated considering the formation of the Gibbs energy (
Table 1) of the species involved in Equation (1); if the result is negative, the reaction is spontaneous.
Substituting the Gibbs free energy values into Equation (2):
The calculations yield a negative value, meaning the reaction will occur in experimental conditions.
The amount of dissolved copper can affect hydrogen formation in chalcopyrite leaching. In this sense, Bashiri et al. [
14] found that the generation of hydrogen by the photocatalysis of water splitting with a TiO
2 containing copper catalysts improved the coexistence of Cu
+ and Cu
2+ ions due to the oxidation of the cuprous ions to cupric ions, as demonstrated in Equation (3). Similarly, it is proposed that the on-chalcopyrite dissolution of the copper first dissolved in cuprous form after the oxidation of copper (I) to copper (II) occurs by the reduction in the hydrogen ion to molecular hydrogen, as shown in Equation (3). This behavior suggests that the overall reaction performs in several steps.
On the other hand, the cuprous ion also reacts with oxygen peroxide, producing hydroxyl radical and hydroxide ion (Equation (4)) [
19]. In this reaction, the oxygen peroxide competes with hydrogen ions, limiting the generation of molecular hydrogen, but the chalcopyrite dissociation increases.
Then, the leached chalcopyrite concentrate amount can be related to hydrogen production.
Figure 4 shows the percentage of copper extracted versus time of 10 g of chalcopyrite concentrate with 140 mL of 1M H
2SO
4 solution, 60 mL of methanol, and different amounts of H
2O
2 (20, 40, and 60 mL). An ascending behavior in the extraction of copper for all tests is noticeable, obtaining a higher extraction of around 65% with 60 mL of hydrogen peroxide, continuing with 40 mL of H
2O
2 with 61% extraction, and reaching 55% extraction with 20 mL of H
2O
2. Proportional behavior between copper extraction and the amount of hydrogen peroxide is appreciated. With 20 mL of hydrogen peroxide, the highest hydrogen formation and the lowest copper extraction are achieved, employing a different H
2O
2 quantity probably favors Equation (4).
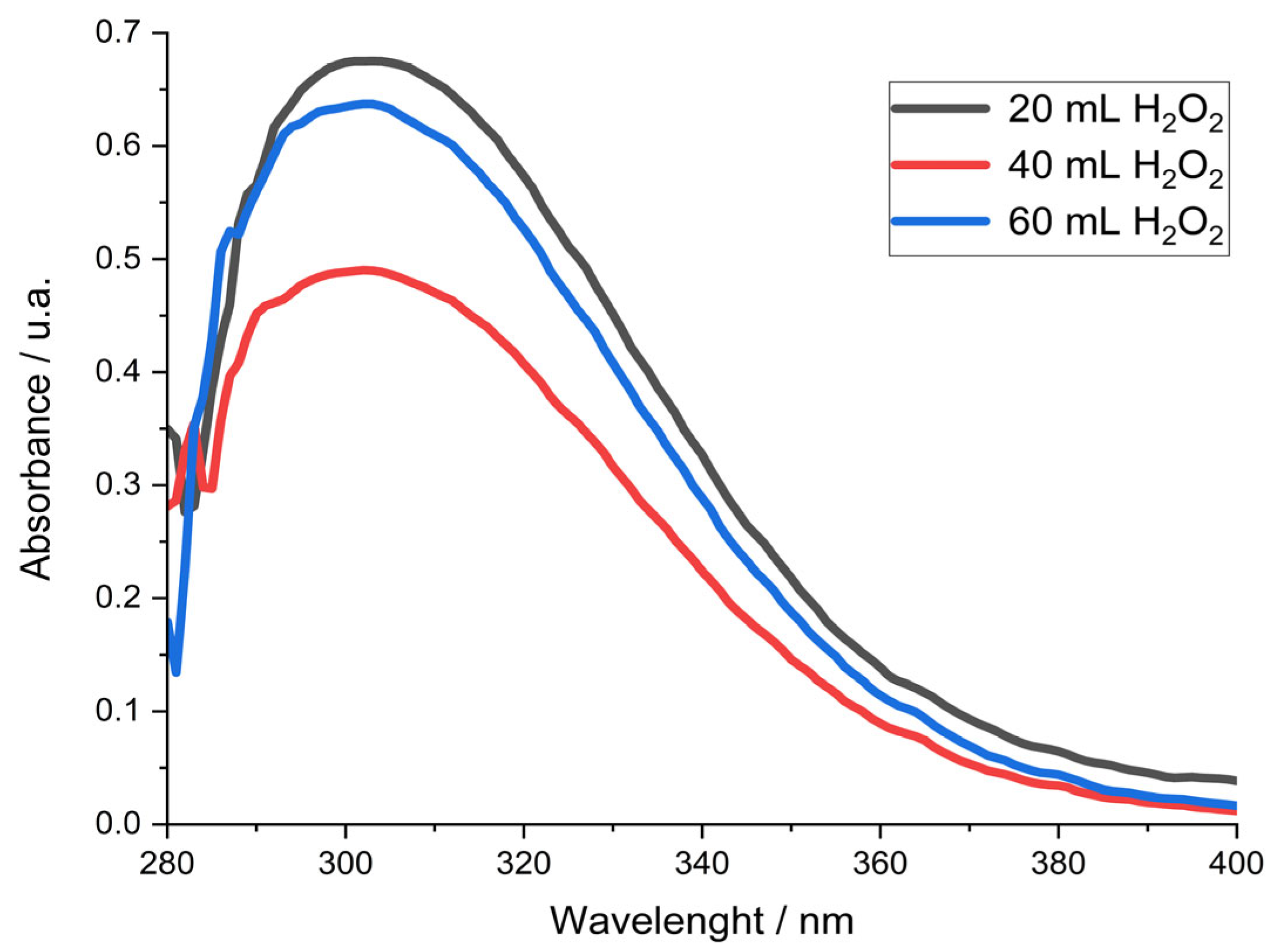
On other hand, Solis-Marcial and Lapidus (2014) [
20] used the UV-Vis spectrophotometry technique to detect that at a wavelength of 300 nm, a characteristic peak of the Cu (I) ion, is presented. Where the height is directly associated with the concentration of Cu (I). For the determination of Cu (I) ion, experiments were performed using different amounts of H
2O
2, samples were taken and analyzed with a UV-Vis spectrophotometer to determine where the maximum value of absorbance is located. The spectra of each sample are reported in
Figure 5, observing a peak of around 300 nm, which is characteristic of the Cu (I) ion, this corroborates that Cu (I) is present in this system.
The quantity of Cu (I) ion is quantified using the Equation (5), obtained in the curve of Solís-Marcial and Lapidus (2014) [
20], the results are shown in
Table 2.
3.2. Effect of the Chalcopyrite Concentrate Amount
Another variable that can affect hydrogen generation is the amount of chalcopyrite concentrate.
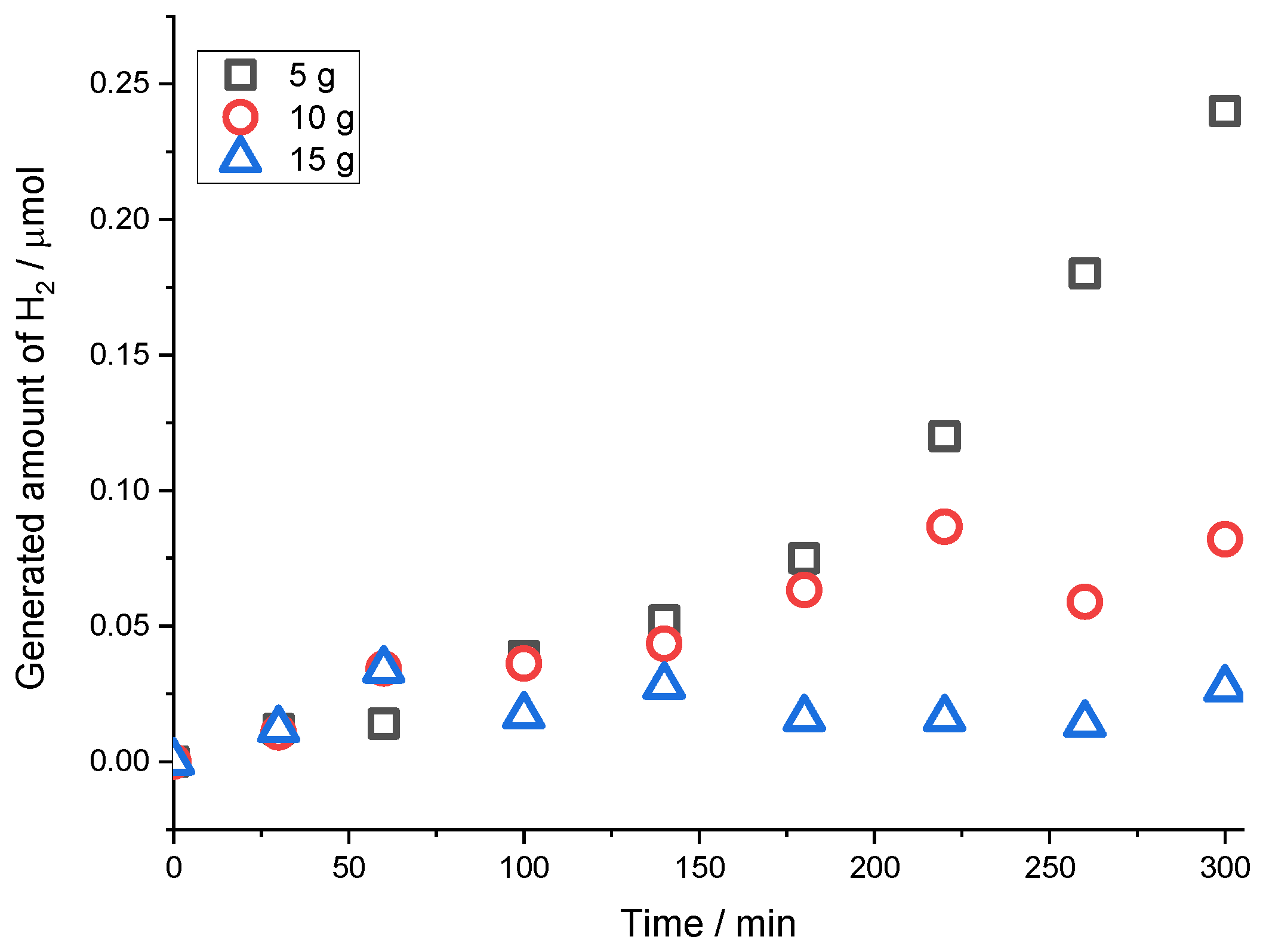
Figure 6 shows the generated H
2 versus time on the leaching of 5 g, 10 g, and 15 g of chalcopyrite concentrate in a medium of 140 mL of 1 M of a H
2SO
4 solution, 60 mL of methanol, and 20 mL of H
2O
2. Hydrogen production increases with the reduction in chalcopyrite concentrate. This behavior may be due to the fact that, the amounts of leaching reagents such as H
2O
2 and sulfuric acid are insufficient to dissolve a larger amount of chalcopyrite concentrate [
21,
22,
23].
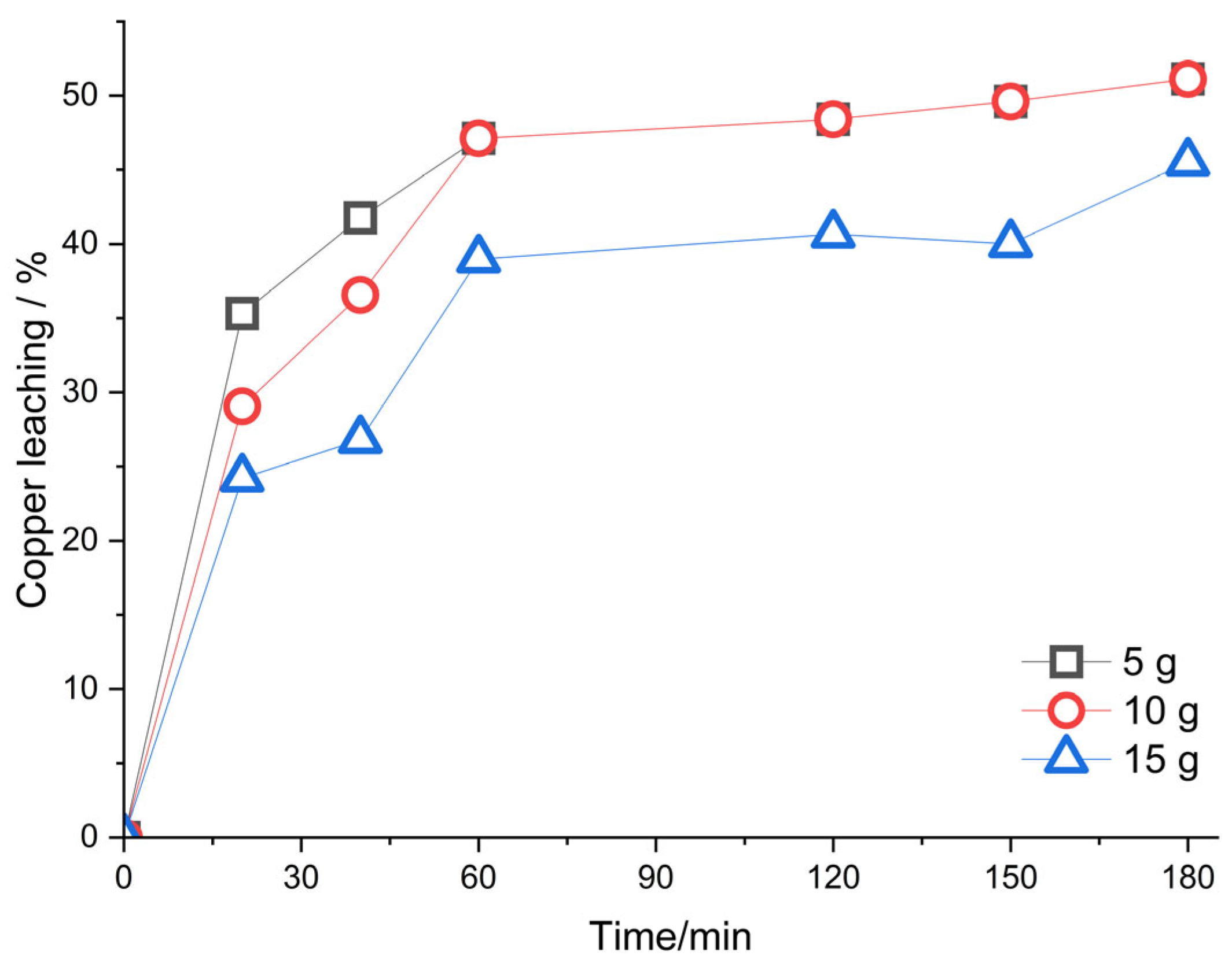
Figure 7 shows the percentage of copper extraction under the same conditions as the previous experiment. The copper extraction percentages are 51, 50, and 45% for 5, 10, and 15 g of chalcopyrite, respectively. With 5 and 10 g of concentrate, the leaching behavior is similar. Whereas with 5 g of concentrate, the collection of copper is slightly higher for shorter times. For 15 g of concentrate, the copper extraction was the lowest, caused by an insufficient amount of reagents. With 5 g of concentrate, the highest hydrogen generation and copper extraction is seen. Allegedly, with these conditions, Equation (4) is fulfilled. Conversely, with different amounts of hydrogen peroxide, the relationship does not exist, and the reaction proceeds in several steps.
3.3. Effect of H2SO4 Concentration
The study also investigated the influence of different initial sulfuric acid concentrations.
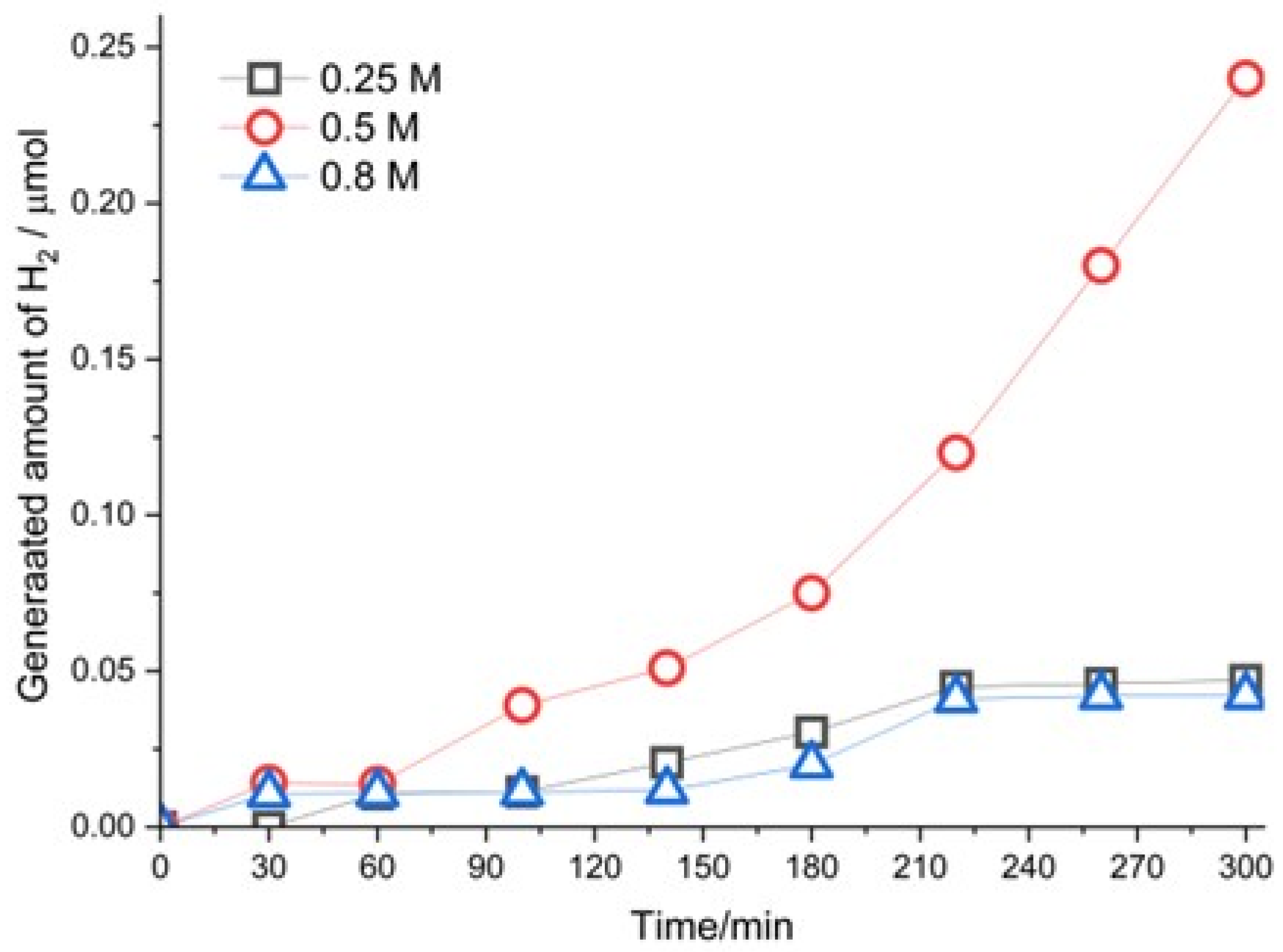
Figure 8 shows the amount of hydrogen generated over time by leaching 5 g of chalcopyrite concentrate in 60 mL of methanol, 20 mL of H
2O
2, and 140 mL of H
2SO
4 solution with different concentrations. All the profiles show an ascending hydrogen production over time; the H
2SO
4 concentration of 0.5 M produced the highest amount of hydrogen, 0.24 µmoles in 300 min. The behavior of hydrogen production with the other two acid concentrations is very similar, achieving 0.05 µmoles of hydrogen. There is an optimal acid concentration for hydrogen generation. It was previously postulated that hydrogen generation is dependent on the stability of the cuprous ion. In this regard, Navon et al. (1997) [
24] determined that cuprous ion stabilization depends on pH, since to low [H
+] does not achieve the minimum amount of ligand to complex Cu (I), and when the [H
+] increases, Cu (I) oxidates to Cu (II), thereby decreasing hydrogen generation. Due to this reason, an optimal production rate of hydrogen is observed in the concentration range.
The amount of copper extracted was monitored as well.
Figure 9 shows the percentage of copper extraction vs. time of 5 g of chalcopyrite concentrate, 20 mL of H
2O
2, 60 mL of methanol, and 140 mL of H
2SO
4 solutions with different concentrations (0.25 M, 0.5 M, and 0.8 M). The extraction percentage increases throughout the experiment and is directly proportional to the acid concentration. The highest copper extraction was 90% when adding an acid concentration of 0.8 M. Around 50% copper extraction was reported with concentrations of 0.25 and 0.5 M for the acid solution.
4. Discussion
Because it is more environmentally friendly, leaching has been suggested as an alternative for obtaining metals; a factor that has not been studied much in this context is the gases generated. During the leaching of chalcopyrite concentrate with sulfuric acid, hydrogen peroxide, and methanol, molecular hydrogen is one of the gases produced. The solid residue contained iron oxide, elemental sulfur, and chalcopyrite, while ionic copper was present in the solution. Given the reagents and products detected in the experiments, the global leaching reaction is proposed (Equation (1)). With a ΔG of −119.66 kJ/mol, the thermodynamic analysis of this equation indicates that the reaction is spontaneous. The hydrogen generation depends on the initial amount of hydrogen peroxide, where the highest hydrogen generation is achieved with the lowest amount of hydrogen peroxide (20 mL), followed by 60 mL, and then 40 mL. There is no straightforward behavior between hydrogen generation and the initial amount of hydrogen peroxide during chalcopyrite leaching, possibly due to the nature of the steps involved. In the first step of the chalcopyrite dissolution, the cuprous ion is released from the chalcopyrite, reacting with the hydrogen ions, and reducing them to molecular hydrogen. In this sense, the hydrogen peroxide reacts directly with the cuprous ion forming hydroxyl radicals with high oxidizing power that favors the dissolution of chalcopyrite. The above would explain why hydrogen generation is limited with a higher amount of hydrogen peroxide, and the dissolution of chalcopyrite is favored. Hydrogen generation by water splitting by photocatalysis using methanol as a sacrificial agent is similar to the leaching system proposed in this work, where the chalcopyrite concentrate has the role of the photocatalyst. One of the disadvantages of photocatalysis is that the catalyst is leached, losing its catalytic activity and decreasing its economic viability. The more a mineral dissolves through leaching, the more economically viable the process becomes.
During the experiments for chalcopyrite concentrate, the highest hydrogen generation was achieved with the lowest amount of concentrate (5 g). Allegedly, with the higher amount of concentrate diminishing the copper extraction percent, the amount of solid is higher than solvents, resulting in insufficient amounts of necessary reagents in the solution [
19], hindering leaching. Regarding the experiments conducted for the initial concentration of sulfuric acid (0.25 M, 0.5 M, and 0.8 M), an optimal hydrogen generation was produced with 0.5 M. Nonetheless, for the extraction of copper from chalcopyrite concentrate with a greater sulfuric acid concentration, more copper was extracted. This phenomenon could be because the dissociation of hydrogen peroxide is favored with a higher concentration of hydrogen ions, thereby causing a greater amount of hydroxyl radicals and accelerating the oxidation of the cuprous ion.
5. Conclusions
The gases generated in the leaching process of chalcopyrite concentrate have lacked chemical characterization and quantification; these gases can be harmful or beneficial. In this work, it was found that only one is formed, hydrogen, a value-added product that provides greater profitability to the leaching of chalcopyrite concentrate since two products, such as copper and hydrogen, can be obtained. The study of the economic viability and scaling of the process is part of a subsequent work.
Based on the reagents and products generated, the leaching reaction was proposed. The thermodynamic analysis agrees with a spontaneous chemical reaction (ΔG = −119.66 kJ/mol). From this perspective, the leaching process offers two benefits, industrial and environmental, given that in addition to copper extraction, it generates molecular hydrogen, one of the cleanest fuels. The highest amount of hydrogen produced was 0.24 μmol in 300 min with the initial conditions of 0.5 M of H2SO4 solution, 5 g of chalcopyrite concentrate, 20 mL of H2O2, and 60 mL of methanol. In turn, the leaching reaction was dependent on the initial concentrations of the reagents.
The leaching process involves several steps to produce cuprous ions, which then act as the reducing agent to generate molecular hydrogen from hydrogen ions. In this sense, during the leaching, two competitive reactions for the cuprous ion exist: with the hydrogen ion generating molecular hydrogen, and when it reacts with hydrogen peroxide to form hydroxyl radicals and favors the chalcopyrite dissolution.
Author Contributions
Conceptualization, O.J.S.M. and B.S.R.; methodology, A.T.L. and J.R.R.C.; software, J.P.R.-L. and J.A.H.M.; validation, J.P.R.-L., A.T.L. and R.Z.G.; formal analysis, J.P.R.-L. and B.S.R.; investigation, O.J.S.M. and J.A.H.M.; resources, O.J.S.M. and A.T.L.; data curation, J.P.R.-L. and B.S.R.; writing—original draft preparation, O.J.S.M.; writing—review and editing, J.P.R.-L.; visualization, J.A.H.M.; supervision, R.Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Research Program and the Project Management System of the National Polytechnic Institute for the financial support received.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, L.; Liu, H.; Wang, Z.; Lu, Q.; Zhao, Y.; Zhou, F.; Zhang, Y.; Lu, W.; Yang, B.; Zhu, Q.; et al. Advances in electrolytic copper foils: Fabrication, microstructure, and mechanical properties. Rare Met. 2025, 44, 757–792. [Google Scholar] [CrossRef]
- Long, H.; Tan, X.; Ni, S.; Ma, A.; Li, S.; Zhu, D. Ammoniacal leaching behavior and regularity of zinc ash. Int. J. Chem. Eng. 2023, 21, 895–906. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Chi, X.; Liu, H.; Xia, J.; Chen, H.; Yu, X.; Weng, W.; Zhong, S. Breaking the Fe3O4-wrapped copper microstructure to enhance copper-slag separation. Int. J. Min. Met. Mat. 2024, 31, 2312–2325. [Google Scholar] [CrossRef]
- Solis-Marcial, O.J.; Nájera-Bastida, A.; Bañuelos, J.E.; Valdés-Martínez, O.U.; Luevano, L.A.; Serrano-Rosales, B. Chalcopyrite Leaching Kinetics in the Presence of Methanol. Int. J. React. Eng. 2019, 17, 20190081. [Google Scholar] [CrossRef]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate-chloride and sulfate nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar] [CrossRef]
- McCluskey, A.; Lawrance, G.A.; Leitch, S.K.; Owen, M.P.; Hamilton, I.C. Ionic liquids and metal ions: From green chemistry to ore refining. In Ionic Liquids: Industrial Application to Green Chemistry; Rogers, R.D., Seddon, K.R., Eds.; American Chemical Society: Washington, DC, USA, 2002; pp. 199–212. [Google Scholar]
- Solís-Marcial, O.J.; Lapidus, G.T. Improvement of chalcopyrite dissolution in acid media using polar organic solvents. Hydrometallurgy 2013, 131–132, 120–126. [Google Scholar] [CrossRef]
- Kale, P.; Gangal, A.C.; Edla, R.; Sharma, P. Investigation of hydrogen storage behavior of silicon nanoparticle. Int. J. Hydrogen Energy 2012, 37, 3741–3747. [Google Scholar] [CrossRef]
- Xu, S.; Sun, D.D. Significant improvement of photocatalytic hydrogen generation rate over TiO2 with deposited CuO. Int. J. Hydrogen Energy 2009, 34, 6096–6104. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen massive corrosion of deformed aluminum in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Ho, C.Y.; Huang, C.H. Enhancement of hydrogen generation using waste aluminum cans hydrolysis in low alkaline de-ionized water. Int. J. Hydrogen Energy 2016, 41, 3741–3747. [Google Scholar] [CrossRef]
- Bashiri, R.; Mohamed, N.M.; Kait, C.F.; Sufian, S. Hydrogen production from water photospliting using Cu/TiO2 nanoparticles: Effect of hydrolysis rate and reaction medium. Int. J. Hydrogen Energy 2015, 40, 6021–6037. [Google Scholar] [CrossRef]
- Han, Z.; Eisenberg, R. Fuel from Water: The Photochemical Generation of Hydrogen from Water. Acc. Chem. Res. 2014, 47, 2537–2544. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Yang, C.; Rao, W. Hydrogen generation due to water splitting on Si-terminated 4H-Sic (0001) surfaces. Surf. Sci. 2018, 668, 68–72. [Google Scholar] [CrossRef]
- Wu, N.L.; Lee, M.S. Enhanced TiO2 photocatalysis by Cu in hydrogen production from aqueous methanol solution. Int. J. Hydrogen Energy 2004, 29, 1601–1605. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid. Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- Gunther, M.R.; Hanna, P.M.; Mason, R.P.; Cohen, M.S. Hydoxyl Radical Formation from Cuprous Ion and Hydrogen Peroxide: Spin-Trapping Study. Arch. Biochem. Biophys. 1995, 316, 515–522. [Google Scholar] [CrossRef]
- Solís-Marcial, O.J.; Lapidus, G.T. Chalcopyrite leaching in alcoholic acid media. Hydrometallurgy 2014, 147–148, 54–58. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Zhu, L.; Liu, B.; Zhou, K.; Xu, J.; Guo, C. Eco-friendly oxidation leaching from chalcopyrite powder and kinetics assisted by sodium chloride in organic acid media. Adv. Powder Technol. 2022, 33, 103547. [Google Scholar] [CrossRef]
- Tian, J.; Wu, D.; Li, S.; Ma, W.; Wang, R. Effect of process variables on leaching behavior and kinetics of silver element from waste photovoltaic modules. Sep. Purif. Technol. 2024, 335, 126062. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, G.; Yang, H.; Chen, G.; Qiu, X. Agglomeration-aggregation and leaching properties of mechanically active chalcopyrite. Trans. Nonferrous Met. Soc. China 2021, 31, 1465–1474. [Google Scholar] [CrossRef]
- Navon, N.; Masarwa, A.; Cohen, H.; Meyerstein, D. pH dependence of stability constants of copper (I) complexes with fumaric and maleic acids in aqueous solutions. Inorganica Chim. Acta 1997, 261, 29–35. [Google Scholar] [CrossRef]
Figure 1.
Scheme of the experimental procedure to analyze the gases.
Figure 1.
Scheme of the experimental procedure to analyze the gases.
Figure 2.
Hydrogen concentration (µmol) versus time for a solution of 1 M H2SO4, with 60 mL of methanol, 10 g of chalcopyrite, with a variation of H2O2.
Figure 2.
Hydrogen concentration (µmol) versus time for a solution of 1 M H2SO4, with 60 mL of methanol, 10 g of chalcopyrite, with a variation of H2O2.
Figure 3.
Diffractogram of (A) raw concentrate without leaching, (B) leaching residues with 10 g of chalcopyrite concentrate, 20 mL H2O2, 60 mL of methanol, 140 mL of 1M H2SO4.
Figure 3.
Diffractogram of (A) raw concentrate without leaching, (B) leaching residues with 10 g of chalcopyrite concentrate, 20 mL H2O2, 60 mL of methanol, 140 mL of 1M H2SO4.
Figure 4.
Percentage of copper extracted versus time for a solution of 1 M H2SO4 with 60 mL of methanol, 10 g of chalcopyrite concentrate, with a variation of H2O2.
Figure 4.
Percentage of copper extracted versus time for a solution of 1 M H2SO4 with 60 mL of methanol, 10 g of chalcopyrite concentrate, with a variation of H2O2.
Figure 5.
UV-Vis spectra of the leach solution at different amount of H2O2 of the pregnant solution with dissolution 1:100 of the leaching using 160 mL of 1 M H2SO4 with 60 mL of methanol, 10 g of chalcopyrite concentrate, with a variation of H2O2.
Figure 5.
UV-Vis spectra of the leach solution at different amount of H2O2 of the pregnant solution with dissolution 1:100 of the leaching using 160 mL of 1 M H2SO4 with 60 mL of methanol, 10 g of chalcopyrite concentrate, with a variation of H2O2.
Figure 6.
Hydrogen amount (µmol) versus time for a solution of 1 M of H2SO4 with 60 mL of methanol, with 20 mL of H2O2, with different amounts of chalcopyrite concentrate.
Figure 6.
Hydrogen amount (µmol) versus time for a solution of 1 M of H2SO4 with 60 mL of methanol, with 20 mL of H2O2, with different amounts of chalcopyrite concentrate.
Figure 7.
Percentage of extracted copper vs time, with 140 mL H2SO4 1M, 60 mL methanol, 20 mL H2O2 and amount different of chalcopyrite concentrate.
Figure 7.
Percentage of extracted copper vs time, with 140 mL H2SO4 1M, 60 mL methanol, 20 mL H2O2 and amount different of chalcopyrite concentrate.
Figure 8.
Amount of H2 produced vs time of 5 g of chalcopyrite concentrate with 20mL H2O2, 60 mL methanol, and 140 mL of H2SO4 at different concentrations.
Figure 8.
Amount of H2 produced vs time of 5 g of chalcopyrite concentrate with 20mL H2O2, 60 mL methanol, and 140 mL of H2SO4 at different concentrations.
Figure 9.
Copper extraction percentage vs time of 5 g of chalcopyrite concentrate, 20 mL H2O2, 60 mL methanol, and 140 mL of H2SO4 at different concentrations.
Figure 9.
Copper extraction percentage vs time of 5 g of chalcopyrite concentrate, 20 mL H2O2, 60 mL methanol, and 140 mL of H2SO4 at different concentrations.
Table 1.
Gibbs free energy of formation.
Table 1.
Gibbs free energy of formation.
| Substance | ΔG°f/kJ mol−1 |
|---|
| H+ | 0.00 |
| H2 | 0.00 |
| H2O2 | −120.42 |
| Cu2+ | 65.52 |
| CuFeS2 | −179.20 |
| H2O | −273.14 |
| Fe2O3 | −742.68 |
| S0 | 0.00 |
Table 2.
Concentration of Cu (I) with the amount of H2O2.
Table 2.
Concentration of Cu (I) with the amount of H2O2.
| H2O2 (mL) | Absorbance | [Cu(I)] (ppm) |
|---|
| 20 | 0.68 | 8256 |
| 40 | 0.48 | 5828 |
| 60 | 0.62 | 7528 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).