Metal Phosphomolybdate-Catalyzed Condensation of Furfural with Glycerol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Phosphomolybdate Salts
2.3. Catalytic Runs
- A0 = GC peak initial area of furfural (time = t0);
- Ai = GC peak instantaneous area of furfural (time = t);
- ∑Ap = total sum of GC peak corrected areas of the products.
2.4. Characterization of Metal Phosphomolybdate Salt Catalysts
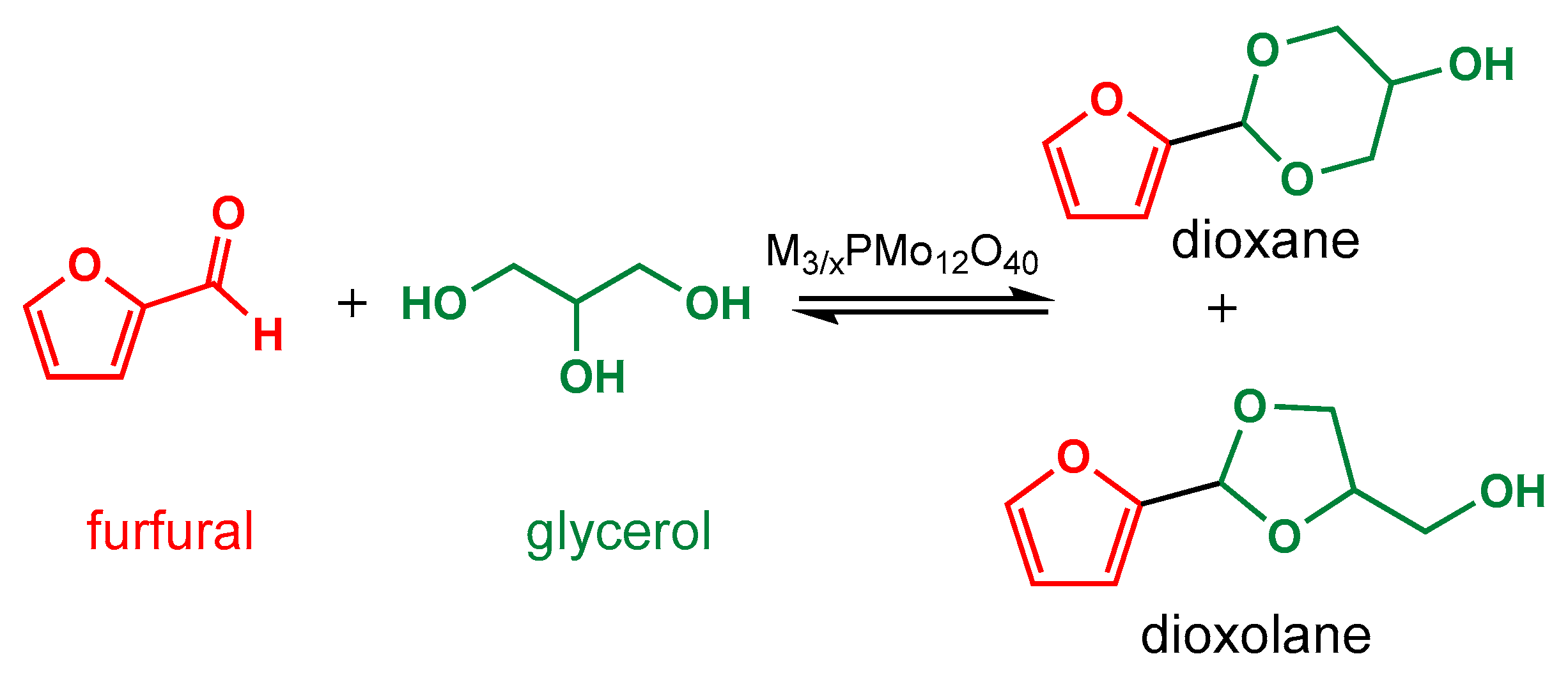
2.5. Identification of Main Reaction Products
3. Results and Discussion
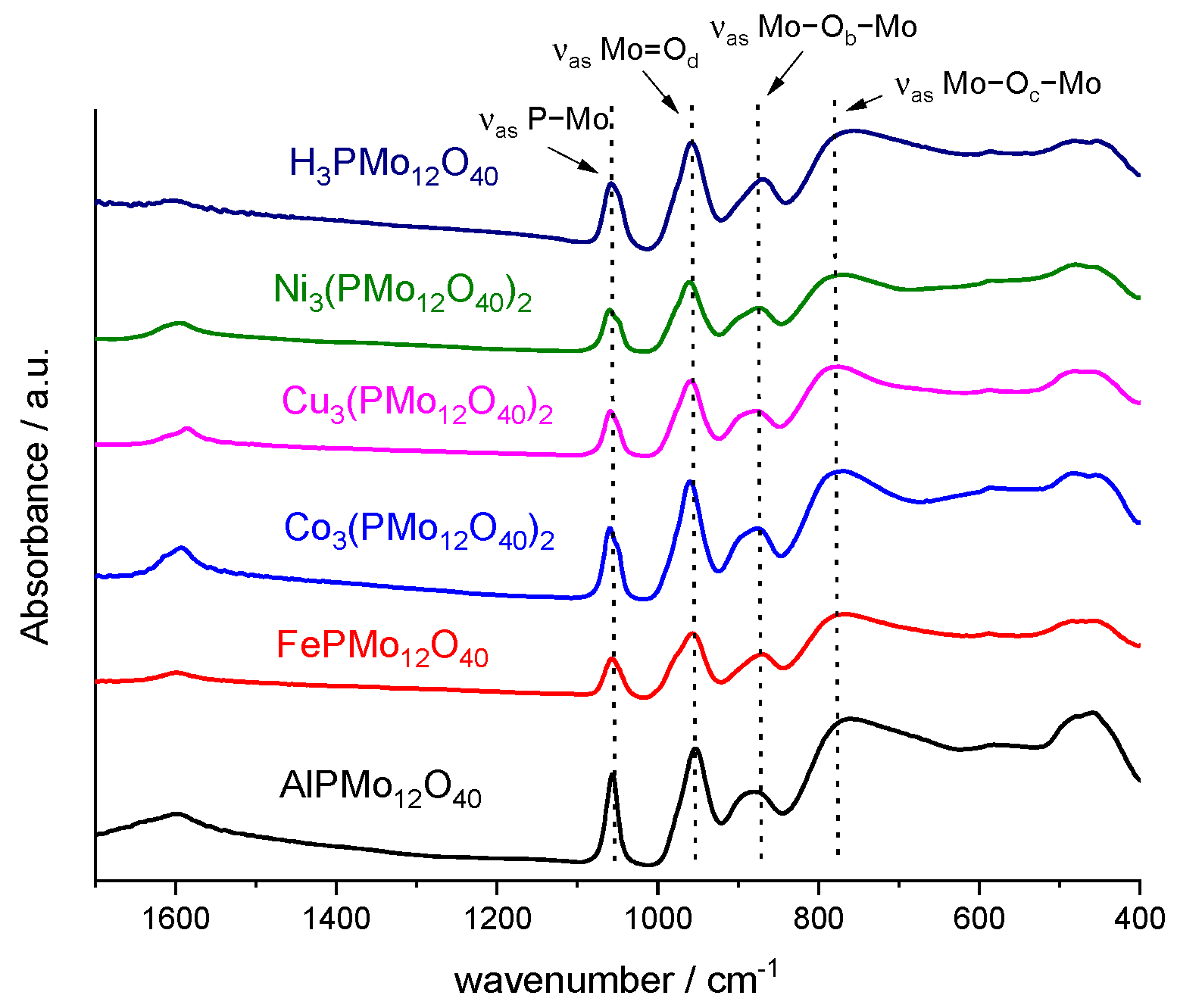
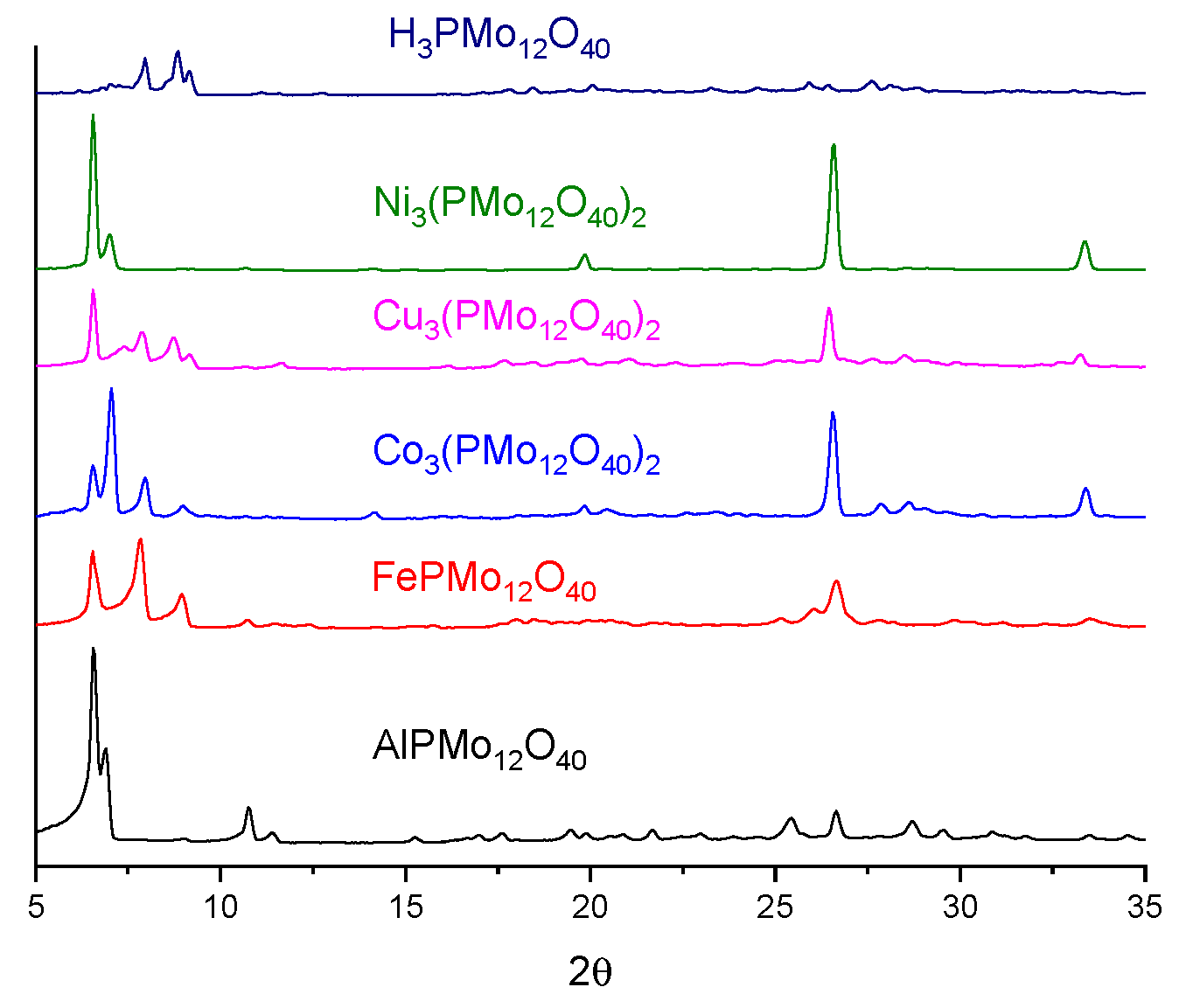
3.1. Characterization of Catalysts
3.2. Catalyst Tests
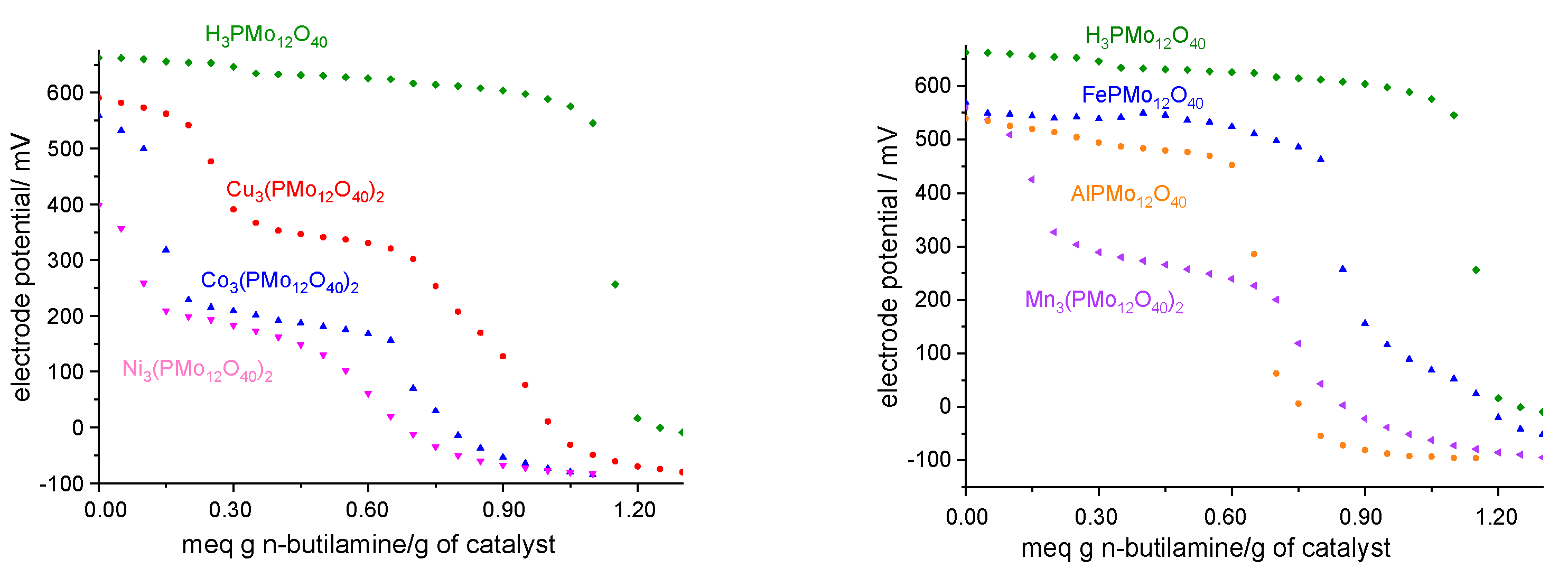
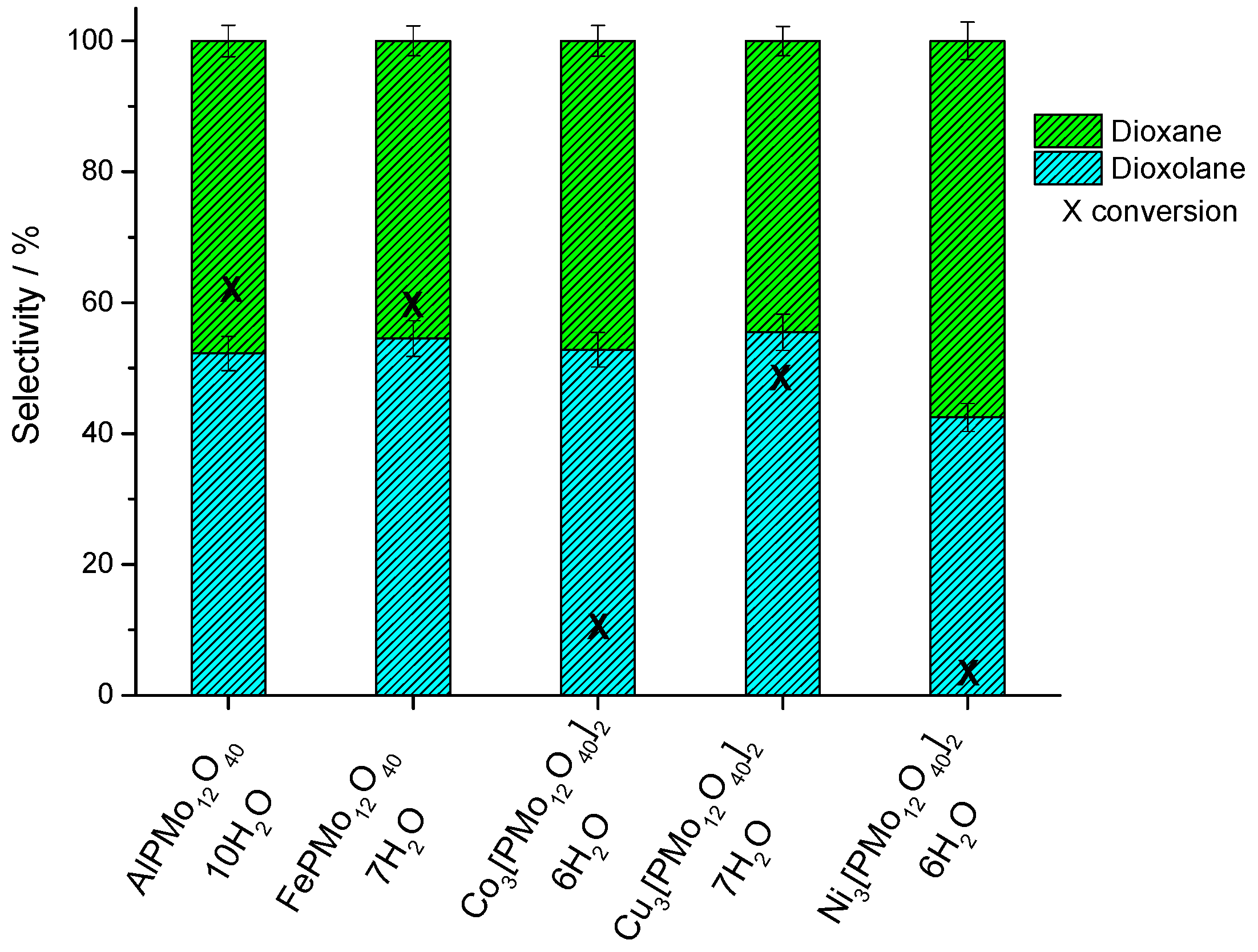
3.2.1. Effect of Catalyst Nature
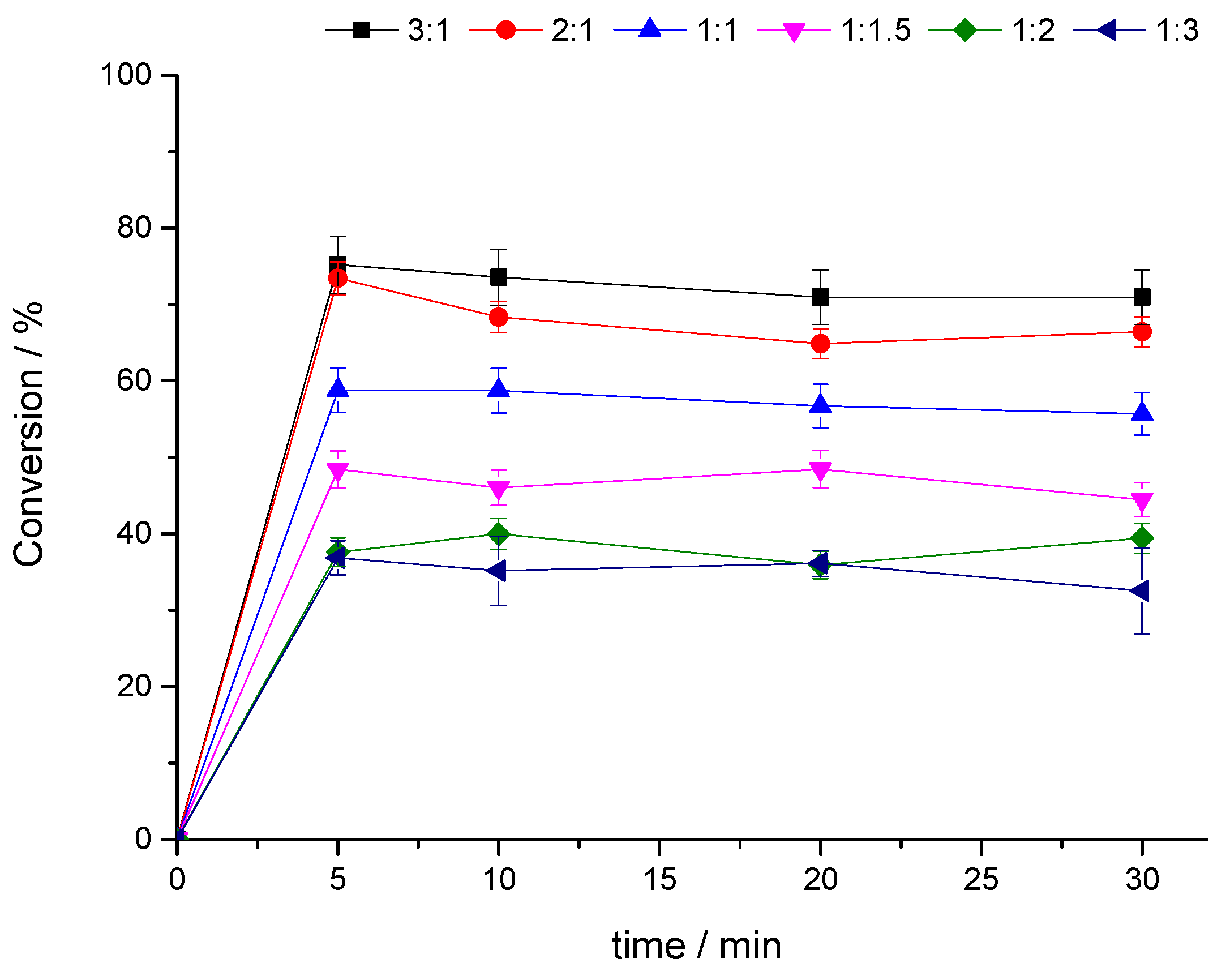
3.2.2. Study of the Influence of the Furfural: Glycerol Molar Ratio on Aluminum Phosphomolybdate-Catalyzed Condensation Reactions
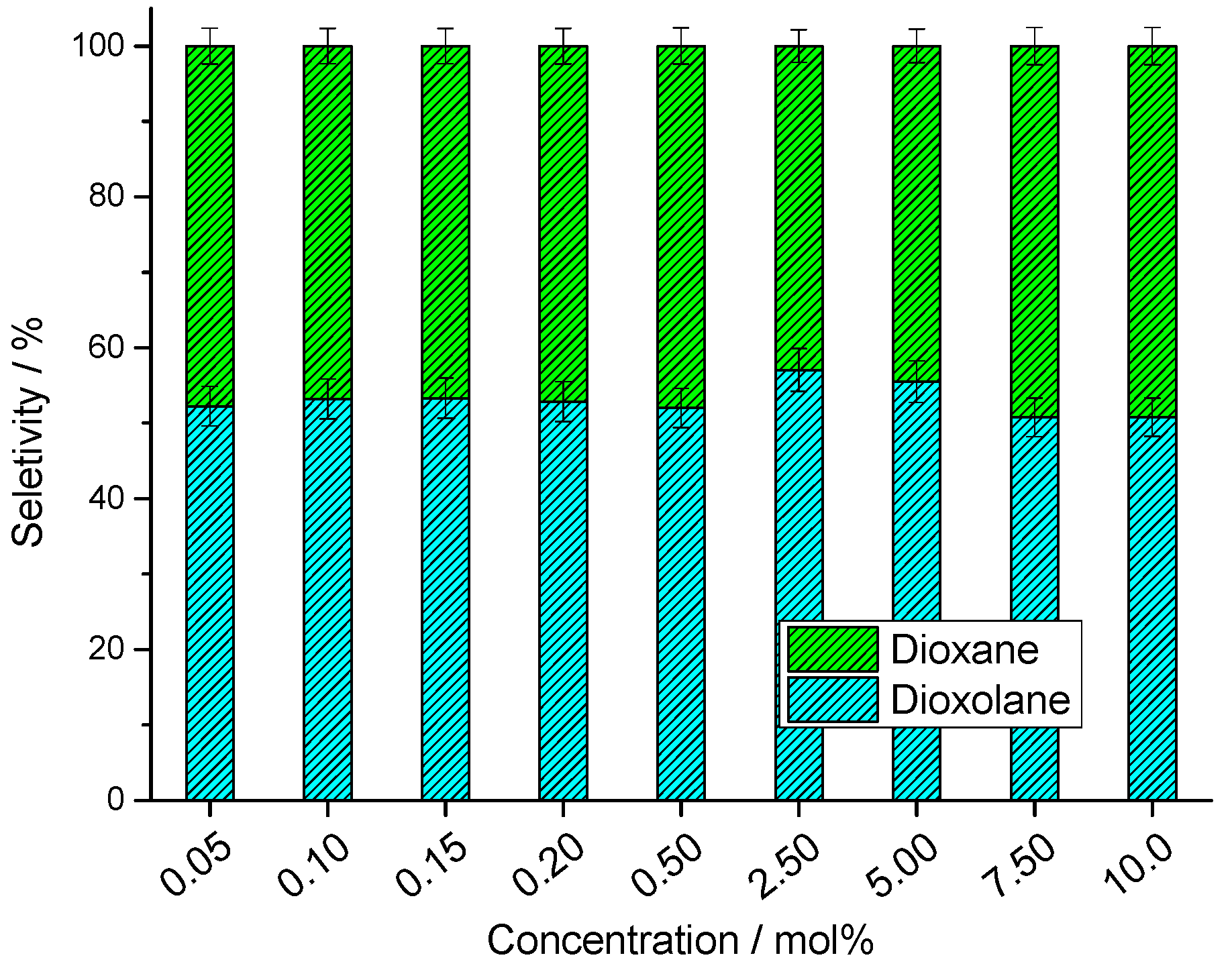
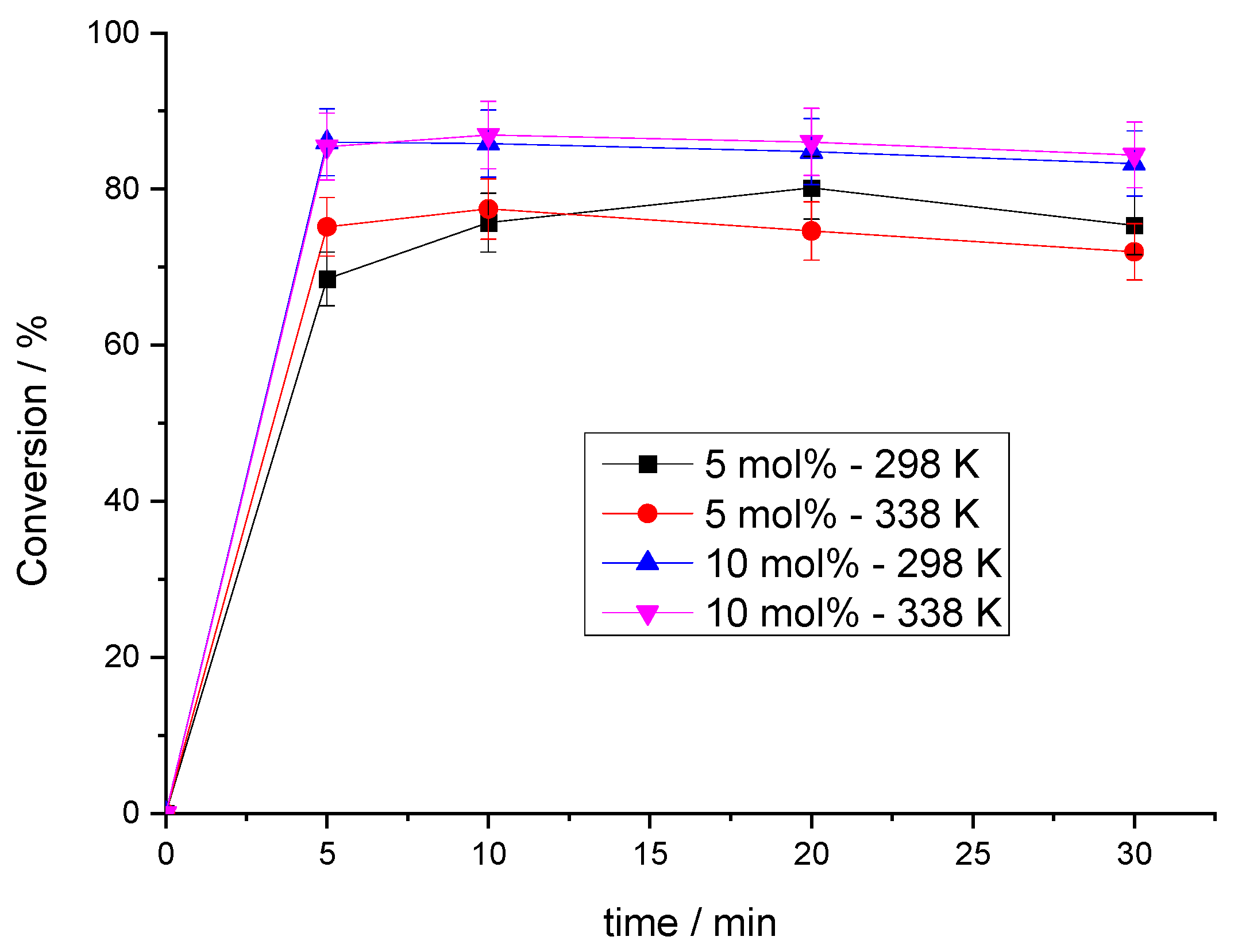
3.2.3. Influence of Catalyst Loading
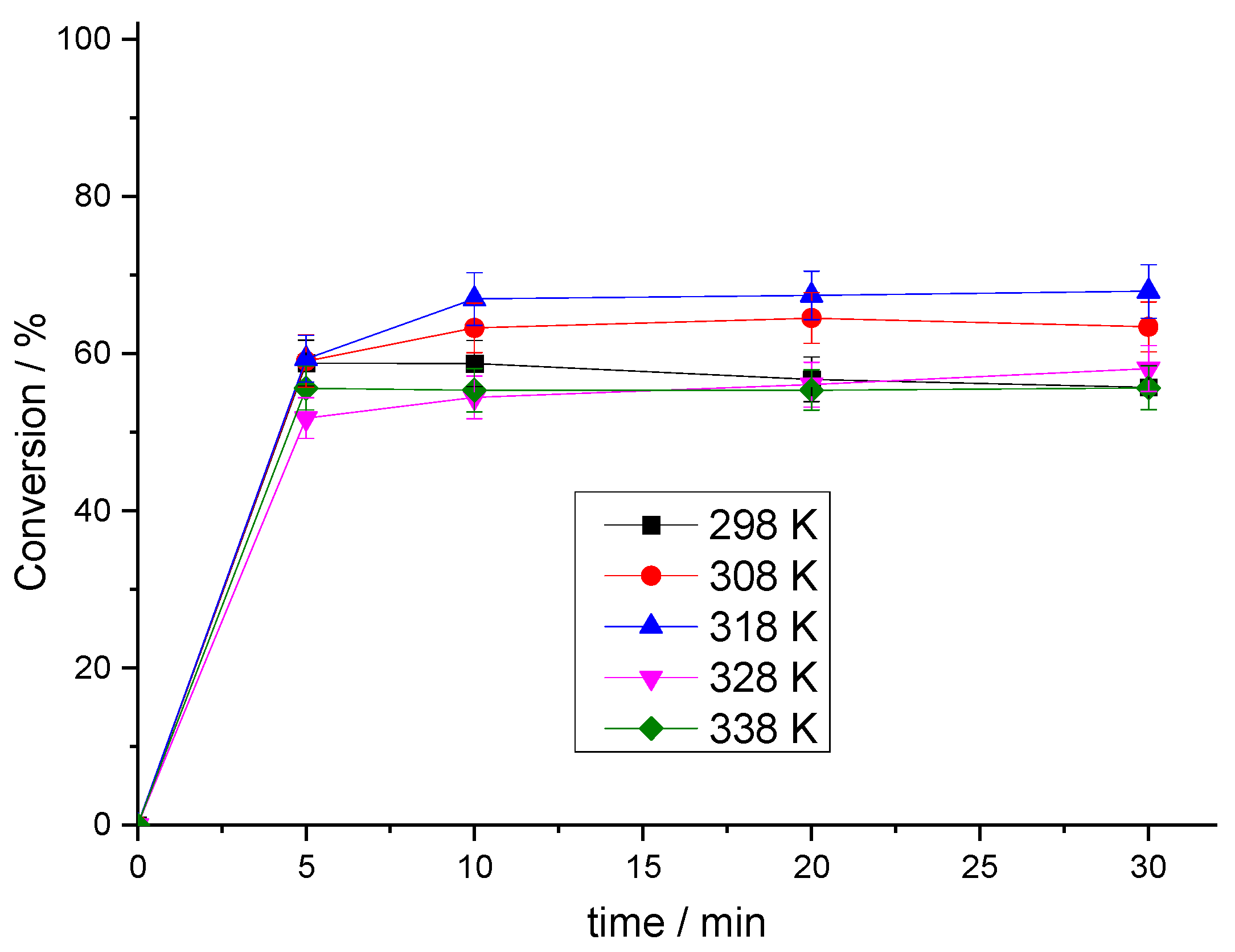
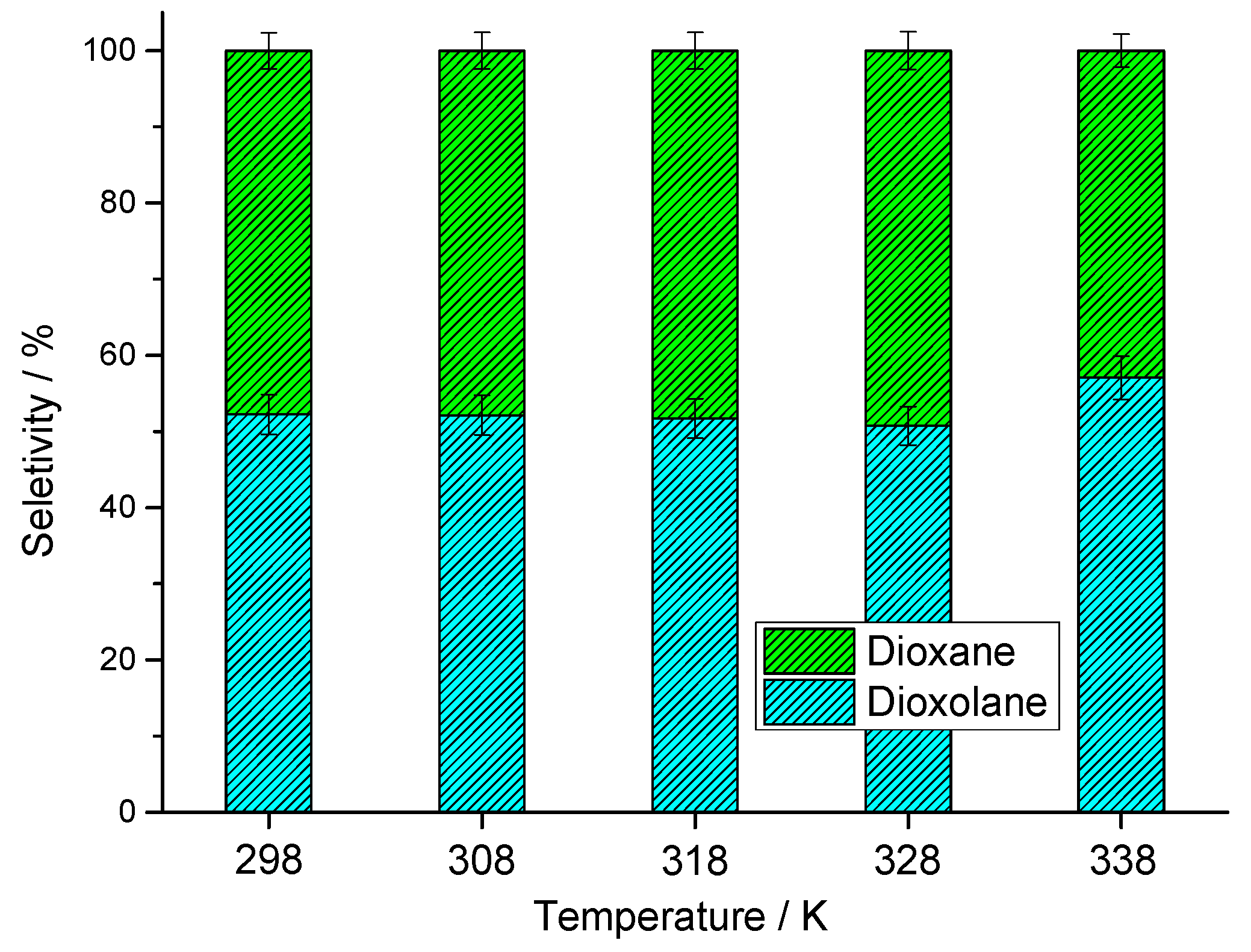
3.2.4. Effect of Reaction Temperature
3.2.5. Reusability of the Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FT-IR | Fourier transform infrared spectroscopy |
| GC-MS | The gas chromatography–mass spectrometry |
| GC-FID | The gas chromatography–flame ionization detector |
| HPAs | Heteropolyacids |
| MS-QP | Mass spectrometry–quadrupole analyzer |
| XRD | X-ray diffraction |
References
- Bozel, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- de Carvalho, E.G.; Rodrigues, F.D.A.; Monteiro, R.S.; Ribas, R.M.; da Silva, M.J. Experimental design and economic analysis of 5-hydroxymethylfurfural synthesis from fructose in acetone-water system using niobium phosphate as catalyst. Biomass Convers. Biorefin. 2018, 8, 635–646. [Google Scholar] [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. A review on cellulose and its utilization from agro-industrial waste. Drug Invent. Today 2018, 10, 89–94. [Google Scholar]
- Ye, L.; Han, Y.; Wang, X.; Lu, X.; Qi, X.; Yu, H. Recent progress in furfural production from hemicellulose and its derivatives: Conversion mechanism, catalytic system, solvent selection. Mol. Catal. 2021, 515, 111899. [Google Scholar] [CrossRef]
- Pardo Cuervo, O.H.; Romanelli, G.P.; Cubillos, J.A.; Rojas, H.A.; Martínez, J.J. Selective Catalytic Dehydration of Xylose to Furfural and Fructose and Glucose to 5-Hydroximethylfurfural (HMF) Using Preyssler Heteropolyacid. ChemistrySelect 2020, 5, 4186–4193. [Google Scholar] [CrossRef]
- Azlan, N.S.M.; Yap, C.L.; Gan, S.; Rahman, M.B.A. Recent advances in the conversion of lignocellulosic biomass and its degraded products to levulinic acid: A synergy of Brønsted-Lowry acid and Lewis acid. Ind. Crops Prod. 2022, 181, 114778. [Google Scholar] [CrossRef]
- Cousin, E.; Namhaed, K.; Péres, Y.; Cognet, P.; Delmas, M.; Hermansyah, H.; Aroua, M.K. Towards efficient and greener processes for furfural production from biomass: A review of the recent trends. Sci. Total Environ. 2022, 847, 157599. [Google Scholar] [CrossRef]
- Brown, D.E. Lignocellulose hydrolysis. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1983, 300, 305–322. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value-Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; No. DOE/GO-102004-1992; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2004. [Google Scholar]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.; Zhou, Q.; Zhang, L. Furfural production from biomass residues: Current technologies, challenges, and future prospects. Biomass Bioenergy 2022, 161, 106458. [Google Scholar] [CrossRef]
- Rubio-Caballero, J.M.; Saravanamurugan, S.; Maireles-Torres, P.; Riisager, A. Acetalization of furfural with zeolites under benign reaction conditions. Catal. Today 2014, 234, 233–236. [Google Scholar] [CrossRef]
- da Silva, M.J.; Ribeiro, C.A.J.; Rodrigues, A.A.; Silva, T.A. Fuel Bioadditives Synthesis from Furfural Glycerol Condensation over Vanadium-Substituted Cesium Phosphomolybdate Salts. Catal. Lett. 2024, 154, 3251–3263. [Google Scholar] [CrossRef]
- Bohmer, N.; Roussiere, T.; Kuba, M.; Schunk, S.A. Valorisation of glycerol as renewable feedstock: Comparison of the exploration of chemical transformation methods aided by high throughput experimentation. Comb. Chem. High Throughput Screen. 2012, 15, 123–135. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Kirchhecker, S.; Dell’Acqua, A.; Angenvoort, A.; Spannenberg, A.; Ito, K.; Tin, S.; Taden, A.; de Vries, J.G. HMF–glycerol acetals as additives for the debonding of polyurethane adhesives. Green Chem. 2021, 23, 957–965. [Google Scholar] [CrossRef]
- Castellanos-Blanco, N.; Taborda, G.; Cobo, M. An Efficient Acetalization Method for Biomass-Derived Furfural with Ethanol Using γ-Al2O3-Supported Catalysts. ChemistrySelect 2020, 5, 3458–3470. [Google Scholar] [CrossRef]
- Dodson, J.R.; Avellar, T.; Athayde, J.; Mota, C.J. Glycerol acetals with antioxidant properties. Pure Appl. Chem. 2024, 86, 905–911. [Google Scholar] [CrossRef]
- He, J.; Qiang, Q.; Bai, L.; Su, W.; Yu, H.; Liu, S.; Li, C. Acetalization strategy in biomass valorization: A review. Ind. Chem. Mater. 2024, 2, 30–56. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gosai, K.A.; Bhatt, A.S.; Kumaresan, S.; Lee, S.M.; Bajaj, H.C. Clay catalyzed rapid valorization of glycerol towards cyclic acetals and ketals. RSC Adv. 2015, 5, 83985–83996. [Google Scholar] [CrossRef]
- Guerrero-Ruíz, F.; Yara-Varon, E.; González, M.D.; Torres, M.; Salagre, P.; Canela-Garayoa, R.; Cesteros, Y. Use of biobased crude glycerol, obtained biocatalytically, to obtain biofuel additives by catalytic acetalization of furfural using SAPO catalysts. Fuel 2022, 319, 123803. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sawamura, N.; Iwamoto, M. Highly effective acetalization of aldehydes and ketones with methanol on siliceous mesoporous material. Tetrahedron Lett. 1998, 39, 9457–9460. [Google Scholar] [CrossRef]
- Wegenhart, B.L.; Abu-Omar, M.M. A Solvent-free method for making dioxolane and dioxane from the biorenewables glycerol and furfural catalyzed by oxorhenium (V) oxazoline. Inorg. Chem. 2010, 49, 4741–4743. [Google Scholar] [CrossRef]
- Zaher, S.; Christ, L.; Abd El Rahim, M.; Kanj, A.; Karamé, I. Green acetalization of glycerol and carbonyl catalyzed by FeCl3·6H2O. Mol. Catal. 2017, 438, 204–213. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Ramalingam, R.J.; Al-Lohedan, H.A.; Khoerunnisa, F.; Ling, T.C.; Ng, E.P. Selective synthesis of dioxolane biofuel additive via acetalization of glycerol and furfural enhanced by MCM-41-alanine bifunctional catalyst. Fuel 2021, 288, 119573. [Google Scholar] [CrossRef]
- Oger, N.; Lin, Y.F.; Le Grognec, E.; Rataboul, F.; Felpin, F.X. Graphene-promoted acetalisation of glycerol under acid-free conditions. Green Chem. 2016, 18, 1531–1537. [Google Scholar] [CrossRef]
- da Silva, M.J.; Ribeiro, C.J.A.; de Araújo, E.N.; Torteloti, I.M. Acetalization of alkyl alcohols with benzaldehyde over cesium phosphomolybdovanadate salts. Processes 2023, 11, 2220. [Google Scholar] [CrossRef]
- Patel, A.; Pithadia, D. Low temperature synthesis of bio-fuel additives via valorisation of glycerol with benzaldehyde as well as furfural over a novel sustainable catalyst, 12-tungstosilicic acid anchored to ordered cubic nano-porous MCM-48. Appl. Catal. A Gen. 2020, 602, 117729. [Google Scholar] [CrossRef]
- da Silva, M.J.; Teixeira, M.G. Assessment on the double role of the transition metal salts on the acetalization of furfural: Lewis and Brønsted acid catalysts. Mol. Catal. 2018, 461, 40–47. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts supported on mesostructured silica. Appl. Catal. A Gen. 2018, 549, 207–215. [Google Scholar] [CrossRef]
- da Silva, M.J.; Lopes, N.P.G.; Bruziquesi, C.G.O. Furfural acetalization over Keggin heteropolyacid salts at room temperature: Effect of cesium doping. React. Kinet Mech. Catal. 2021, 133, 913–931. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Heteropoly acids and related compounds as catalysts for fine chemical synthesis. Catal. Rev. 1995, 37, 311–352. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M.; Okuhara, T. Catalytic Chemistry of Heteropoly Compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Omwoma, S.; Gore, C.T.; Ji, Y.; Hu, C.; Song, Y.-F. Environmentally benign polyoxometalate materials. Coord. Chem. Rev. 2015, 286, 17–29. [Google Scholar] [CrossRef]
- Timofeeva, M.N. Acid catalysis by heteropoly acids. Appl. Catal. A 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; da Silva, M.J.; Ferreira, S.O.; da Silva, R.C.; Silva, T.A.; de Araújo, E.N.D. Assessment of the metal exchanged phosphomolybdic acid salt-catalyzed nerol oxidation reactions with hydrogen peroxide. Mol. Catal. 2023, 545, 113221. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Shang, J.; Ren, Y.; Yue, B.; He, H. H3PMo12O40 Immobilized on amine functionalized SBA-15 as a catalyst for aldose epimerization. Materials 2020, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.J.; Liberto, N.A. Soluble and solid supported Keggin heteropolyacids as catalysts in reactions for biodiesel production; challenges and recent advances. Curr. Org. Chem. 2016, 20, 1263–1283. [Google Scholar] [CrossRef]
- Balaga, V.; Pedada, J.; Friedrich, H.B.; Singh, S. Tunning surface composition of cesium exchanged phosphomolybdic acid catalyst of C-H activation bonds of toluene to benzaldehyde at room temperature. J. Mol. Catal. A 2016, 425, 116–123. [Google Scholar] [CrossRef]
- Mouheb, L.; Dermeche, L.; Essayem, N.; Rabia, C. Keggin-type mixed polyoxomolybdates catalyzed cyclohexanone oxidation by hydrogen peroxide: In situ IR pyridine adsorption. Catal. Lett. 2020, 150, 3327–3334. [Google Scholar] [CrossRef]
- da Silva, M.J.; Andrade Ribeiro, C.J. Cs4PMo11VO40-catalyzed glycerol ketalization to produce solketal: An efficient bioadditives synthesis method. Processes 2024, 12, 854. [Google Scholar] [CrossRef]



| Catalyst | pH |
|---|---|
| AlPMo12O40 10 H2O | −3.0 |
| FePMo12O40 7 H2O | −4.2 |
| Co3(PMo12O40)2 6 H2O | −2.9 |
| Cu3(PMo12O40)2 7 H2O | −2.8 |
| Ni3(PMo12O40)2 6 H2O | −0.6 |
| Acetonitrile | 8.4 |
| Run | Conversion/% | Recovery Rate/% |
|---|---|---|
| 1 | 90 | 90 |
| 2 | 87 | 88 |
| 3 | 88 | 88 |
| 4 | 87 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.J.d.; Andrade, P.H.d.S.; Miranda, L.D. Metal Phosphomolybdate-Catalyzed Condensation of Furfural with Glycerol. Processes 2025, 13, 2665. https://doi.org/10.3390/pr13082665
Silva MJd, Andrade PHdS, Miranda LD. Metal Phosphomolybdate-Catalyzed Condensation of Furfural with Glycerol. Processes. 2025; 13(8):2665. https://doi.org/10.3390/pr13082665
Chicago/Turabian StyleSilva, Márcio José da, Pedro Henrique da Silva Andrade, and Luiza Diogo Miranda. 2025. "Metal Phosphomolybdate-Catalyzed Condensation of Furfural with Glycerol" Processes 13, no. 8: 2665. https://doi.org/10.3390/pr13082665
APA StyleSilva, M. J. d., Andrade, P. H. d. S., & Miranda, L. D. (2025). Metal Phosphomolybdate-Catalyzed Condensation of Furfural with Glycerol. Processes, 13(8), 2665. https://doi.org/10.3390/pr13082665








