Macauba Kernel Oil: Refining, Transesterification, and Density/Viscosity of Blends B15 to B20 with Mineral Diesel
Abstract
1. Introduction
2. Materials and Methods
- MKOH = weight of potassium hydroxide (g),
- Moil = weight of macauba kernel oil (g),
- %KOH = 1.5% (wt.) based on the macauba kernel oil mass,
- IA = Acidity index of the macauba kernel oil mass (mg NaOH/g),
- KOH purity = purity of the KOH provided by the supplier (85%), and
- MMeOH = weight of methanol (g).
- X = 15% and 20%,
- DensityBX (T) = density of the blend composed by X biodiesel: (1 − X) mineral diesel (vol.:vol.) at the determined Temperature (g.cm−3),
- ViscosityBX (T) = viscosity of the blend composed by X biodiesel: (1 − X) mineral diesel (vol.:vol.) at the determined Temperature (if dynamic (mPa·s), if kinematic (mm2/s)).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duque, T.S.; Barroso, G.M.; Borges, C.E.; Mendes, D.S.; da Silva, R.S.; Evaristo, A.B.; dos Santos, J.B. Current and Future Development of Acrocomia Aculeata Focused on Biofuel Potential and Climate Change Challenges. Sci. Rep. 2025, 15, 8120. [Google Scholar] [CrossRef]
- Nunes, Â.A.; Buccini, D.F.; Jaques, J.A.S.; Portugal, L.C.; Guimarães, R.C.A.; Favaro, S.P.; Caldas, R.A.; Carvalho, C.M.E. Effect of Acrocomia Aculeata Kernel Oil on Adiposity in Type 2 Diabetic Rats. Plant Foods Hum. Nutr. 2018, 73, 61–67. [Google Scholar] [CrossRef]
- Falótico, T.; Valença, T.; Verderane, M.P.; Santana, B.C.; Sirianni, G. Mapping Nut-cracking in a New Population of Wild Capuchin Monkeys (Sapajus Libidinosus) at Ubajara National Park, Brazil. Am. J. Primatol. 2024, 86, e23595. [Google Scholar] [CrossRef]
- Pires, T.P.; dos Santos Souza, E.; Kuki, K.N.; Motoike, S.Y. Ecophysiological Traits of the Macaw Palm: A Contribution towards the Domestication of a Novel Oil Crop. Ind. Crops. Prod. 2013, 44, 200–210. [Google Scholar] [CrossRef]
- de Araújo, V.C.R.; Silva, G.A.; Ramos, R.S.; Júnior, P.A.S.; Pereira, R.R.; Motoike, S.Y.; Picanço, M.C. Distribution and Attack of Pineapple Mealybug to Macauba Palm Acrocomia Aculeata. Int. J. Trop. Insect. Sci. 2021, 41, 2765–2773. [Google Scholar] [CrossRef]
- da Silva César, A.; de Azedias Almeida, F.; de Souza, R.P.; Silva, G.C.; Atabani, A.E. The Prospects of Using Acrocomia Aculeata (Macaúba) a Non-Edible Biodiesel Feedstock in Brazil. Renew. Sustain. Energy Rev. 2015, 49, 1213–1220. [Google Scholar] [CrossRef]
- Correia, F.d.S.; da Silva, W.B.; de Almeida, F.J.S.; Bulhões, K.d.S.; Leme, S.A.d.F. Analysis of the Proximate Composition, Bioactive Markers and Antioxidant Activity Present in the Mesocarp of Acrocomia Aculeata Fruit Harvested in the State of Mato Grosso. Rev. Virtual De Química 2022, 14, 207–213. [Google Scholar] [CrossRef]
- Solidario de Souza Benatti, G.; Buainain, A.M.; Cavalcante Filho, P.G.; Vargas-Carpintero, R.; Asveld, L.; Osseweijer, P. Macaw Palm (Acrocomia spp.): An Opportunity for Including Smallholders in Brazil’s Biodiesel Production. Clean. Circ. Bioeconomy 2025, 10, 100134. [Google Scholar] [CrossRef]
- da Silva, J.Q.; Santos, D.Q.; Fabris, J.D.; Harter, L.V.L.; Chagas, S.P. Light Biodiesel from Macaúba and Palm Kernel: Properties of Their Blends with Fossil Kerosene in the Perspective of an Alternative Aviation Fuel. Renew Energy 2020, 151, 426–433. [Google Scholar] [CrossRef]
- Wang, Z.; Osseweijer, P.; Posada, J.A. Human Health Impacts of Aviation Biofuel Production: Exploring the Application of Different Life Cycle Impact Assessment (LCIA) Methods for Biofuel Supply Chains. Processes 2020, 8, 158. [Google Scholar] [CrossRef]
- del Río, J.C.; Evaristo, A.B.; Marques, G.; Martín-Ramos, P.; Martín-Gil, J.; Gutiérrez, A. Chemical Composition and Thermal Behavior of the Pulp and Kernel Oils from Macauba Palm (Acrocomia aculeata) Fruit. Ind. Crops. Prod. 2016, 84, 294–304. [Google Scholar] [CrossRef]
- da Cruz, A.V.C.; Alencar, N.L.; de Almeida, A.L.S.; Lopes, C.G.R. Aspectos Que Influenciam a Escolha de Locais de Coleta Por Extrativistas de Macaúba No Cerrado Brasileiro. Front. J. Soc. Technol. Environ. Sci. 2021, 10, 101–113. [Google Scholar] [CrossRef]
- Sorita, G.D.; Favaro, S.P.; de Sousa Rodrigues, D.; da Silva Junior, W.P.; de Oliveira Leal, W.G.; Ambrosi, A.; Di Luccio, M. Aqueous Enzymatic Extraction of Macauba (Acrocomia aculeata) Pulp Oil: A Green and Sustainable Approach for High-Quality Oil Production. Food Res. Int. 2024, 182, 114160. [Google Scholar] [CrossRef]
- Braga, E.; Damasceno, L.; Barros de Sousa Silva, C.; Silva, L.; Cavalcante, M.; Barreto, C.; Silva, S.; Murilo Tavares de Luna, F.; Bertini, L.; Nascimento, T.; et al. 1H NMR and UV-Vis as Analytical Techniques to Evaluate Biodiesel Conversion and Oxidative Stability. Fuels 2024, 5, 107–122. [Google Scholar] [CrossRef]
- de Mesquita Figueredo, I.; Tavares de Luna, F.M.; Loureiro Cavalcante, C.; de Sousa Rios, M.A. Babassu Biodiesel Doped with Antioxidants: Assessment of Thermo-Oxidative Stability by Borchardt and Daniels Method. J. Am. Oil Chem. Soc. 2020, 97, 1355–1363. [Google Scholar] [CrossRef]
- Silva, C.; Sousa, B.; Nunes, J.; Malveira, J.; Marques, R.; Damasceno, L.; Braga, E.; Lessa, T.; Bertini, L.; Maciel, M.; et al. Evaluation of Babassu Cake Generated in the Extraction of the Oil as Feedstock for Biofuel Production. Processes 2023, 11, 585. [Google Scholar] [CrossRef]
- Chew, S.-C.; Tan, C.-P.; Nyam, K.-L. Optimization of Neutralization Parameters in Chemical Refining of Kenaf Seed Oil by Response Surface Methodology. Ind. Crops Prod. 2017, 95, 742–750. [Google Scholar] [CrossRef]
- Wang, T.; Johnson, L.A. Refining High-free Fatty Acid Wheat Germ Oil. J. Am. Oil Chem. Soc. 2001, 78, 71–76. [Google Scholar] [CrossRef]
- Farhoosh, R.; Einafshar, S.; Sharayei, P. The Effect of Commercial Refining Steps on the Rancidity Measures of Soybean and Canola Oils. Food Chem. 2009, 115, 933–938. [Google Scholar] [CrossRef]
- Piloto-Rodríguez, R.; Díaz-Domínguez, Y. Production Process, Methods of Extraction, and Refining Technologies of Unconventional Seed Oils. In Multiple Biological Activities of Unconventional Seed Oils; Elsevier: Amsterdam, The Netherlands, 2022; pp. 413–430. [Google Scholar]
- Lopresto, C.G.; De Paola, M.G.; Calabrò, V. Importance of the Properties, Collection, and Storage of Waste Cooking Oils to Produce High-Quality Biodiesel—An Overview. Biomass Bioenergy 2024, 189, 107363. [Google Scholar] [CrossRef]
- Penha, F.M.; Rezzadori, K.; Proner, M.C.; Zin, G.; Fogaça, L.A.; Petrus, J.C.C.; de Oliveira, J.V.; Di Luccio, M. Evaluation of Permeation of Macauba Oil and N-Hexane Mixtures through Polymeric Commercial Membranes Subjected to Different Pre-Treatments. J. Food Eng. 2015, 155, 79–86. [Google Scholar] [CrossRef]
- Nunes, A.A.; Favaro, S.P.; Galvani, F.; Miranda, C.H.B. Good Practices of Harvest and Processing Provide High Quality Macauba Pulp Oil. Eur. J. Lipid Sci. Technol. 2015, 117, 2036–2043. [Google Scholar] [CrossRef]
- Selvaraj, R.; Praveenkumar, R.; Moorthy, I.G. A Comprehensive Review of Biodiesel Production Methods from Various Feedstocks. Biofuels 2019, 10, 325–333. [Google Scholar] [CrossRef]
- García Cabrera, O.; Magalhães Grimaldi, L.; Grimaldi, R.; Paula Badan Ribeiro, A. Macauba (Acrocomia aculeata): Biology, Oil Processing, and Technological Potential. In Oilseed Crops-Uses, Biology and Production; IntechOpen: London, UK, 2023; ISBN 978-1-80356-171-4. [Google Scholar]
- da Silva Sousa, P.; Neto, F.S.; de Sousa Junior, P.G.; de Mattos, M.C.; de Sousa Rios, M.A.; da Fonseca, A.M.; Lomonaco, D.; dos Santos, J.C.S. Sustainable Biofuel Production from Fish Processing Waste: Lipase-catalyzed Hydroesterification of Tilapia Residual Oil. Biofuels Bioprod. Biorefining 2025. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, P.K.; Chintala, V.; Khatri, N.; Patel, A. Environment-Friendly Biodiesel/Diesel Blends for Improving the Exhaust Emission and Engine Performance to Reduce the Pollutants Emitted from Transportation Fleets. Int. J. Environ. Res. Public Health 2020, 17, 3896. [Google Scholar] [CrossRef]
- McCormick, R.L.; Fioroni, G.M.; Naser, N.; Luecke, J. Properties That Potentially Limit High-Level Blends of Biomass-Based Diesel Fuel. Energy Fuels 2024, 38, 8829–8841. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the Density and Viscosity of Biodiesel and the Influence of Biodiesel Properties on a Diesel Engine Fuel Supply System. J. Mar. Eng. Technol. 2021, 20, 299–311. [Google Scholar] [CrossRef]
- Santos, A.L.R.; Marinho, E.S.; Rufino Bezerra Neto, J.; Sousa, B.A.; Figueredo, I.M.; Luna, F.M.T.; Cavalcante, C.L.; Nascimento, T.L.; Rios, M.A.S.; de Lima-Neto, P. Study of Molecular Arrangement and Density Estimation of Soybean Oil Biodiesel-Diesel Blends Employing Molecular Dynamic Simulation. Fuel 2024, 377, 132760. [Google Scholar] [CrossRef]
- Hernández, E.A.; Sánchez-Reyna, G.; Ancheyta, J. Evaluation of Mixing Rules to Predict Viscosity of Petrodiesel and Biodiesel Blends. Fuel 2021, 283, 118941. [Google Scholar] [CrossRef]
- Biodieselbr Mistura de 25% de Biodiesel Ao Diesel Não é Factível No Momento, Diz Anfavea|BiodieselBR.Com. Available online: https://www.biodieselbr.com/noticias/qualidade/motor/mistura-de-25-de-biodiesel-ao-diesel-nao-e-factivel-no-momento-diz-anfavea-011124 (accessed on 20 July 2025).
- Brazilian National Agency for Petroleum, Natural Gas and Biofuels. Resolution No. 920/2023. Available online: https://atosoficiais.com.br/anp/resolucao-n-920-2023-estabelece-a-especificacao-do-biodiesel-e-as-obrigacoes-quanto-ao-controle-da-qualidade-a-serem-atendidas-pelos-agentes-economicos-que-comercializem-o-produto-em-territorio-nacional?origin=instituicao (accessed on 25 June 2025).
- Brazilian National Agency for Petroleum, Natural Gas and Biofuels. Resolution No. 909/2022. Available online: https://atosoficiais.com.br/anp/resolucao-n-909-2022-estabelece-a-especificacao-de-oleo-diesel-bx-a-b30-em-carater-autorizativo-nos-termos-dos-incisos-i-ii-e-iii-do-art-1o-da-resolucao-cnpe-no-3-de-21-de-setembro-de-2015?origin=instituicao (accessed on 25 June 2025).
- Brazilian National Agency for Petroleum, Natural Gas and Biofuels. Resolution No. 968/2024. Available online: https://atosoficiais.com.br/anp/resolucao-n-968-2024-estabelece-as-especificacoes-dos-oleos-diesel-destinados-a-veiculos-ou-equipamentos-dotados-de-motores-do-ciclo-diesel-e-as-obrigacoes-quanto-ao-controle-da-qualidade-a-serem-atendidas-pelos-agentes-economicos-que-comercializam-o-produto-em-territorio-nacional (accessed on 25 June 2025).
- Cravotto, C.; Fabiano-Tixier, A.-S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods 2022, 11, 3412. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz Métodos Físico-Químicos Para Análise de Alimentos, 4th ed.; 2008. Available online: https://www.ial.sp.gov.br/ial/publicacoes/livros/metodos-fisico-quimicos-para-analise-de-alimentos (accessed on 4 August 2025).
- American Oil Chemists’ Society AOCS CD3-25-Saponification Value of Fats and Oils. Available online: https://library.aocs.org/Cd-3-25/ (accessed on 4 August 2025).
- Sousa, B.A. de Óleo da semente de macaúba (Acrocomia aculeata): Extração, Obtenção de Biodiesel e Caracterização da Conversão e Viscosidade Cinemática. Dissertação (Mestrado em Engenharia Mecânica); Universidade Federal do Ceará: Fortaleza, Brazil, 2023. [Google Scholar]
- ASTM D7042-21a Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). Available online: https://store.astm.org/d7042-21a.html (accessed on 4 August 2025).
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Com-Pounds; 1992; Volume 30, ISBN 0471634042. Available online: https://books.google.com/books/about/Spectrometric_Identification_of_Organic.html?hl=pt-PT&id=umMvAAAAMAAJ (accessed on 28 June 2025).
- Sonvanshi, V.; Gandhi, K.; Ramani, A.; Sharma, R.; Seth, R. ATR-FTIR Coupled with Chemometric Techniques to Detect Vanaspati Ghee (Hydrogenated Vegetable Oil) Adulteration in Milk Fat. Results Chem. 2024, 7, 101343. [Google Scholar] [CrossRef]
- Do Nascimento, T.A.; Lopes, T.I.B.; Nazario, C.E.D.; Oliveira, S.L.; Alcantara, G.B. Vegetable Oils: Are They True? A Point of View from ATR-FTIR, 1H NMR, and Regiospecific Analysis by 13C NMR. Food Res. Int. 2021, 144, 110362. [Google Scholar] [CrossRef]
- Popescu, R.; Costinel, D.; Dinca, O.R.; Marinescu, A.; Stefanescu, I.; Ionete, R.E. Discrimination of Vegetable Oils Using NMR Spectroscopy and Chemometrics. Food Control. 2015, 48, 84–90. [Google Scholar] [CrossRef]
- Krivdin, L.B. Recent Advances in 1D and 2D Liquid-Phase and Solid-State NMR Studies of Biofuel. Renew Energy 2025, 243, 122592. [Google Scholar] [CrossRef]
- Doudin, K.I. Quantitative and Qualitative Analysis of Biodiesel by NMR Spectroscopic Methods. Fuel 2021, 284, 119114. [Google Scholar] [CrossRef]
- Mandarino, J.M.G.; Roessing, A.C. Tecnologia Para Produção Do Óleo de Soja: Descrição Das Etapas, Equipamentos, Produtos e Subprodutos, 2nd ed.; Embrapa Soja: Londrina, Brazil, 2015. [Google Scholar]
- Reis, H.F.A.F.; de Lima, L.P.; Perez, R. Palma No Brasil Viabilidade Da Produção de Óleo Ou Biodiesel? Rev. De Política Agrícola 2017, 26, 20–30. [Google Scholar]
- Lieb, V.M.; Schex, R.; Esquivel, P.; Jiménez, V.M.; Schmarr, H.-G.; Carle, R.; Steingass, C.B. Fatty Acids and Triacylglycerols in the Mesocarp and Kernel Oils of Maturing Costa Rican Acrocomia Aculeata Fruits. NFS J. 2019, 14–15, 6–13. [Google Scholar] [CrossRef]
- Magalhães, K.T.; de Sousa Tavares, T.; Nunes, C.A. The Chemical, Thermal and Textural Characterization of Fractions from Macauba Kernel Oil. Food Res. Int. 2020, 130, 108925. [Google Scholar] [CrossRef]
- Evaristo, A.B.; Grossi, J.A.S.; Carneiro, A.d.C.O.; Pimentel, L.D.; Motoike, S.Y.; Kuki, K.N. Actual and Putative Potentials of Macauba Palm as Feedstock for Solid Biofuel Production from Residues. Biomass Bioenergy 2016, 85, 18–24. [Google Scholar] [CrossRef]
- Trentini, C.P.; Cuco, R.P.; Cardozo-Filho, L.; Silva, C. da Extraction of Macauba Kernel Oil Using Supercritical Carbon Dioxide and Compressed Propane. Can J. Chem. Eng. 2019, 97, 785–792. [Google Scholar] [CrossRef]
- Favaro, S.P.; Cardoso, A.N.; Schultz, E.L.; da Conceição, L.D.H.C.S.; de Leal, W.G.O.; Pighinelli, A.L.M.T.; da Silva, B.R.; da Cruz, R.G.S. Armazenamento e Processamento Da Macaúba: Contribuições Para Manutenção Da Qualidade e Aumento Do Rendimento de Óleo Da Polpa. Embrapa Agroenergia 2018, 38. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1099911/armazenamento-e-processamento-da-macauba-contribuicoes-para-manutencao-da-qualidade-e-aumento-do-rendimento-de-oleo-da-polpa (accessed on 28 June 2025).
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil From Spent Coffee Grounds for Biofuel Production. Waste Biomass Valorization 2019, 10, 253–264. [Google Scholar] [CrossRef]
- Ciconini, G.; Favaro, S.P.; Roscoe, R.; Miranda, C.H.B.; Tapeti, C.F.; Miyahira, M.A.M.; Bearari, L.; Galvani, F.; Borsato, A.V.; Colnago, L.A.; et al. Biometry and Oil Contents of Acrocomia Aculeata Fruits from the Cerrados and Pantanal Biomes in Mato Grosso Do Sul, Brazil. Ind. Crops Prod. 2013, 45, 208–214. [Google Scholar] [CrossRef]
- Prado, R.G.; Almeida, G.D.; de Oliveira, A.R.; de Souza, P.M.T.G.; Cardoso, C.C.; Constantino, V.R.-L.; Pinto, F.G.; Tronto, J.; Pasa, V.M.D. Ethanolysis and Methanolysis of Soybean and Macauba Oils Catalyzed by Mixed Oxide Ca–Al from Hydrocalumite for Biodiesel Production. Energy Fuels 2016, 30, 6662–6670. [Google Scholar] [CrossRef]
- Razaq, Z.; Tousif, M.I.; Noureen, S.; Hussain, S.U.; Saleem, M.; Mehmood Khan, F.; Shaukat, U.; Riaz, H.; Zengin, G.; Hashem, A.; et al. Utilization of Opium Poppy Seed Oil for Biodiesel Production: A Parametric Characterization and Statistical Optimization. Heliyon 2024, 10, e36851. [Google Scholar] [CrossRef]
- Ivanova, M.; Hanganu, A.; Dumitriu, R.; Tociu, M.; Ivanov, G.; Stavarache, C.; Popescu, L.; Ghendov-Mosanu, A.; Sturza, R.; Deleanu, C.; et al. Saponification Value of Fats and Oils as Determined from 1H-NMR Data: The Case of Dairy Fats. Foods 2022, 11, 1466. [Google Scholar] [CrossRef]
- Wen, C.; Shen, M.; Liu, G.; Liu, X.; Liang, L.; Li, Y.; Zhang, J.; Xu, X. Edible Vegetable Oils from Oil Crops: Preparation, Refining, Authenticity Identification and Application. Process Biochem. 2023, 124, 168–179. [Google Scholar] [CrossRef]
- Paula, R.S.F.; Figueredo, I.M.; Vieira, R.S.; Nascimento, T.L.; Cavalcante, C.L.; Machado, Y.L.; Rios, M.A.S. Castor–Babassu Biodiesel Blends: Estimating Kinetic Parameters by Differential Scanning Calorimetry Using the Borchardt and Daniels Method. SN Appl. Sci. 2019, 1, 884. [Google Scholar] [CrossRef]
- de Figueredo, I.M.; de Rios, M.A.S.; Cavalcante, C.L.; Luna, F.M.T. Effects of Amine and Phenolic Based Antioxidants on the Stability of Babassu Biodiesel Using Rancimat and Differential Scanning Calorimetry Techniques. Ind. Eng. Chem. Res. 2020, 59, 18–24. [Google Scholar] [CrossRef]
- Rangel, N.V.P.; da Silva, L.P.; Pinheiro, V.S.; Figueredo, I.M.; Campos, O.S.; Costa, S.N.; Luna, F.M.T.; Cavalcante, C.L., Jr.; Marinho, E.S.; de Lima-Neto, P.; et al. Effect of Additives on the Oxidative Stability and Corrosivity of Biodiesel Samples Derived from Babassu Oil and Residual Frying Oil: An Experimental and Theoretical Assessment. Fuel 2021, 289, 119939. [Google Scholar] [CrossRef]
- European Committee for Standardization EN 14103:2020-Fat and Oil Derivatives-Fatty Acid Methyl Esters (FAME)-Determination of Ester. Available online: https://standards.iteh.ai/catalog/standards/cen/2eb3696f-7f13-49f2-846e-0ebd8f4c42ea/en-14103-2020 (accessed on 4 August 2025).
- Navarro-Díaz, H.J.; Gonzalez, S.L.; Irigaray, B.; Vieitez, I.; Jachmanián, I.; Hense, H.; Oliveira, J.V. Macauba Oil as an Alternative Feedstock for Biodiesel: Characterization and Ester Conversion by the Supercritical Method. J. Supercrit. Fluids 2014, 93, 130–137. [Google Scholar] [CrossRef]
- Mekonnen, K.D.; Endris, Y.A.; Abdu, K.Y. Alternative Methods for Biodiesel Cetane Number Valuation: A Technical Note. ACS Omega 2024, 9, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A Comprehensive Review on Properties of Edible and Non-Edible Vegetable Oil-Based Biodiesel: Composition, Specifications and Prediction Models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Lanjekar, R.D.; Deshmukh, D. A Review of the Effect of the Composition of Biodiesel on NO x Emission, Oxidative Stability and Cold Flow Properties. Renew. Sustain. Energy Rev. 2016, 54, 1401–1411. [Google Scholar] [CrossRef]
- de Rodrigues, J.A.; de Cardoso, F.P.; Lachter, E.R.; Estevão, L.R.M.; Lima, E.; Nascimento, R.S.V. Correlating Chemical Structure and Physical Properties of Vegetable Oil Esters. J. Am. Oil Chem. Soc. 2006, 83, 353–357. [Google Scholar] [CrossRef]
- Fernández-Coppel, I.A.; Barbosa-Evaristo, A.; Corrêa-Guimarães, A.; Martín-Gil, J.; Navas-Gracia, L.M.; Martín-Ramos, P. Life Cycle Analysis of Macauba Palm Cultivation: A Promising Crop for Biofuel Production. Ind. Crops Prod. 2018, 125, 556–566. [Google Scholar] [CrossRef]
- Colombo, C.A.; Chorfi Berton, L.H.; Diaz, B.G.; Ferrari, R.A. Macauba: A Promising Tropical Palm for the Production of Vegetable Oil. OCL 2018, 25, D108. [Google Scholar] [CrossRef]
- ANP-Agência Nacional do Petróleo, Gás Natural e Biocombustíveis. Boletim Trimestral de Preço e Volumes de Combustíveis—ANP. Available online: https://www.gov.br/anp/pt-br/centrais-de-conteudo/publicacoes/boletins-anp/boletins/btpvc-1 (accessed on 4 August 2025).
- de Lopes, D.C.; Steidle Neto, A.J.; Mendes, A.A.; Pereira, D.T.V. Economic Feasibility of Biodiesel Production from Macauba in Brazil. Energy Econ. 2013, 40, 819–824. [Google Scholar] [CrossRef]
- Esteban, B.; Riba, J.-R.; Baquero, G.; Rius, A.; Puig, R. Temperature Dependence of Density and Viscosity of Vegetable Oils. Biomass Bioenergy 2012, 42, 164–171. [Google Scholar] [CrossRef]
- Corsino, V.; Ruiz-Díez, V.; Sánchez-Rojas, J.L. Smart Density and Viscosity Sensing Based on Edge Machine Learning and Piezoelectric MEMS for Edible Oil Monitoring. Sens. Actuators A Phys. 2025, 385, 116258. [Google Scholar] [CrossRef]
- Almeida, K.M.; de Medeiros, E.P.; Gomes, J.P.; de Sousa, E.P.; Santos, J.W. dos Caracterização Físico-Química de Misturas de Óleos Vegetais Para Fins Alimentares. Rev. Verde De Agroecol. E Desenvolv. Sustentável 2013, 8, 218–222. [Google Scholar]
- Brock, J.; Nogueira, M.R.; Zakrzevski, C.; de Corazza, F.C.; Corazza, M.L.; de Oliveira, J.V. Determinação Experimental Da Viscosidade e Condutividade Térmica de Óleos Vegetais. Ciência E Tecnol. De Aliment. 2008, 28, 564–570. [Google Scholar] [CrossRef]
- Miyasaki, F.V. Determinação Experimental Da Densidade e Viscosidade de Misturas BX a Partir de Biodiesel Produzido Do Óleo de Pinhão Manso. Graduação (Engenharia de Energia); Universidade Estadual Paulista: Rosana, Brazil, 2021. [Google Scholar]
- Suh, H.K.; Lee, C.S. A Review on Atomization and Exhaust Emissions of a Biodiesel-Fueled Compression Ignition Engine. Renew. Sustain. Energy Rev. 2016, 58, 1601–1620. [Google Scholar] [CrossRef]
- Mishra, S.; Bukkarapu, K.R.; Krishnasamy, A. A Composition Based Approach to Predict Density, Viscosity and Surface Tension of Biodiesel Fuels. Fuel 2021, 285, 119056. [Google Scholar] [CrossRef]
- Yuan, W.; Hansen, A.C.; Zhang, Q.; Tan, Z. Temperature-dependent Kinematic Viscosity of Selected Biodiesel Fuels and Blends with Diesel Fuel. J. Am. Oil Chem. Soc. 2005, 82, 195–199. [Google Scholar] [CrossRef]
- Zhang, P.; Su, X.; Chen, H.; Geng, L.; Zhao, X. Assessing Fuel Properties Effects of 2,5-Dimethylfuran on Microscopic and Macroscopic Characteristics of Oxygenated Fuel/Diesel Blends Spray. Sci. Rep. 2020, 10, 1427. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Oduwale, A.I.; Owootomo, Y.; Obisesan, O.R.; Elugoke, S.E.; Durodola, S.S.; Akintunde, S.B.; Oluwafemi, O.S. Biodiesel Potential of Used Vegetable Oils Transesterified with Biological Catalysts. Energy Rep. 2020, 6, 2861–2871. [Google Scholar] [CrossRef]
- Ocanha, V.F.; Ferreira Pinto, L.; Zanette, A.F. Determinação Experimental e Aplicação de Modelo Numérico Da Densidade e Viscosidade de Blendas Diesel, Biodiesel e Óleo Vegetal. Res. Soc. Dev. 2022, 11, e299111234405. [Google Scholar] [CrossRef]
- Battisti, G.; Júnior, E.S.; Pozzo, D.M.D.; Santos, R.F. Comparação Das Características Físico-Químicas Do Biodiesel de Citronela e Eucalipto Com o Biodiesel Da Soja. Acta Iguazu 2017, 173–180. Available online: https://e-revista.unioeste.br/index.php/actaiguazu/article/view/18492 (accessed on 29 June 2025).
- Schaffner, R.D.A.; Júnior, E.S.; Dal Pozzo, D.M.; Santos, R.F.; Neves, A.C. Obtenção e Caracterização de Biodiesel de Diferentes Óleos Vegetais. Rev. Bras. De Energ. Renov. 2019, 8, 623–628. [Google Scholar] [CrossRef]
- Farias, J.G. Análise de Desgaste de Um Pistão de Bomba de Injeção a Diesel Combinando Ensaio Experimental e Simulação Por Elementos Finitos. Master’s Thesis, Universidade Tecnológica Federal do Paraná, Curitiba, Brazil, 2016. [Google Scholar]
- Milano, J.; Shamsuddin, A.H.; Silitonga, A.S.; Sebayang, A.H.; Siregar, M.A.; Masjuki, H.H.; Pulungan, M.A.; Chia, S.R.; Zamri, M.F.M.A. Tribological Study on the Biodiesel Produced from Waste Cooking Oil, Waste Cooking Oil Blend with Calophyllum Inophyllum and Its Diesel Blends on Lubricant Oil. Energy Rep. 2022, 8, 1578–1590. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel Fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Feitosa, F.X.; Fernandes, F.A.N.; Santiago, R.S.; de Sant’Ana, H.B. Densities and Viscosities of Binary Mixtures of Babassu Biodiesel + Cotton Seed or Soybean Biodiesel at Different Temperatures. J. Chem. Eng. Data 2010, 55, 5305–5310. [Google Scholar] [CrossRef]








| Fuel | Density (g/cm3), 40 °C | Viscosity (cSt), 40 °C | Kinematic Viscosity Range of Blends (cSt), 40 °C |
|---|---|---|---|
| Mineral diesel | 0.8172 | 2.8821 | - |
| Sunflower oil biodiesel | 0.8672 | 4.0303 | 3.4322–3.7926 (2–75 vol.% biodiesel) 1 |
| Soybean oil biodiesel | 0.8677 | 3.9713 | 3.4315–3.7786 (2–75 vol.% biodiesel) 2 |
| Corn oil biodiesel | 0.8672 | 4.1769 | 3.4589–3.9091 (2–75 vol.% biodiesel) 3 |
| Canola oil biodiesel | 0.8658 | 4.3401 | 3.4368–4.0318 (2–75 vol.% biodiesel) 4 |
| Cottonseed oil biodiesel | 0.8668 | 4.0568 | 3.4384–3.8250 (2–75 vol.% biodiesel) 5 |
| Waste palm oil biodiesel | 0.8576 | 4.2802 | 3.4501–4.0717 (2–75 vol.% biodiesel) 6 |
| Kernel Ground (g) | Extracted Oil (mL) | Yield 1 |
|---|---|---|
| 70 | 37.9 | 54 |
| 105 | 69.0 | 66 |
| 70 | 48.4 | 69 |
| 35 | 15.7 | 45 |
| 35 | 15.5 | 44 |
| 58.64 | 22.8 | 39 |
| 145.38 | 76.7 | 53 |
| 61.62 | 24.6 | 40 |
| 64.62 | 24.1 | 37 |
| Parameter | Result |
|---|---|
| Acidity index (mg NaOH/g) | 7.49 |
| Saponification index (mg KOH/g) 2 | 565.44 |
| Density at 20 °C (kg/m3) | 919.9 |
| Kinematic viscosity at 40 °C (mm2/s) | 27.72 |
| Macauba Kernel Oil | Acidity Index (mg NaOH/g) |
|---|---|
| Before the refining process | 7.49 |
| After the degumming process | 2.83 |
| After the neutralization process | 0.13 |
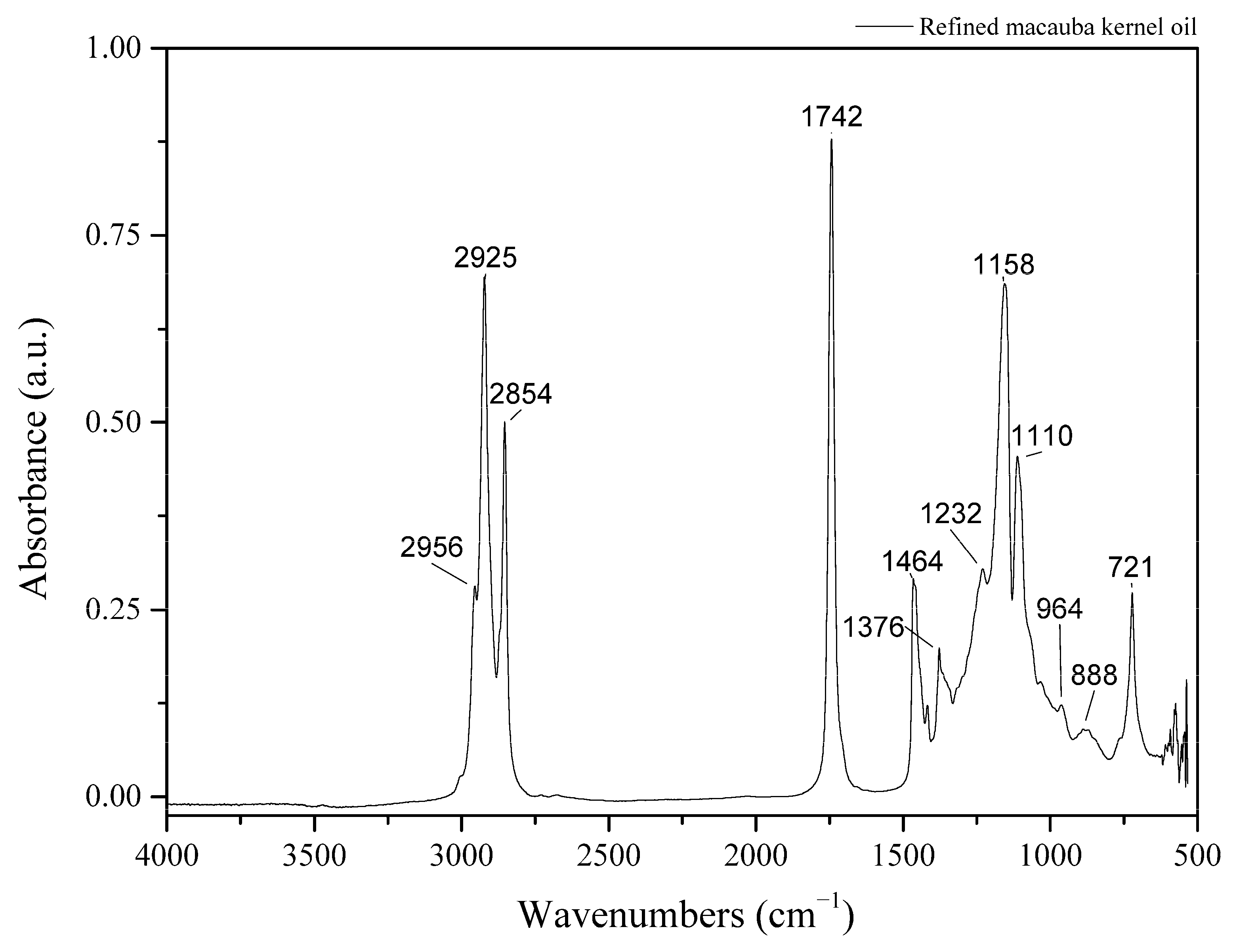
| Wavenumber (cm−1) | Assignments |
|---|---|
| 2956 | Asymmetrical stretching (νas CH2 and CH3) C-H stretching [11] |
| 2925 | Asymmetrical stretching (νas CH2) (C-H) cis bonds [11,41,42] |
| 2854 | Symmetrical stretching (νs CH2) [11,41,42] |
| 1742 | Ester carbonyl (-C-C=O-) group [11,41,42] |
| 1464 | In-plane bending or scissoring (δs CH2) [11,41,42] |
| 1376 | Symmetrical bending vibration δs CH3 [11,41,42] |
| 1232 | C–O stretching in O–C(=O)–CH2 of ester C–O stretching [11] |
| 1158 | Out-of-plane bending or wagging (ω CH2) or Out-of-plane bending or twisting (τ CH2) [11,41,42] |
| 1110 | ν C-O ester [11,41,42] |
| 964 | C–H bending of a trans double bond C–H bending [11] |
| 888 | =CH wagging vibration in the plan =CH wagging vibration [11] |
| 721 | In-plane bending or rocking (ρ CH2) [11,41,42] |
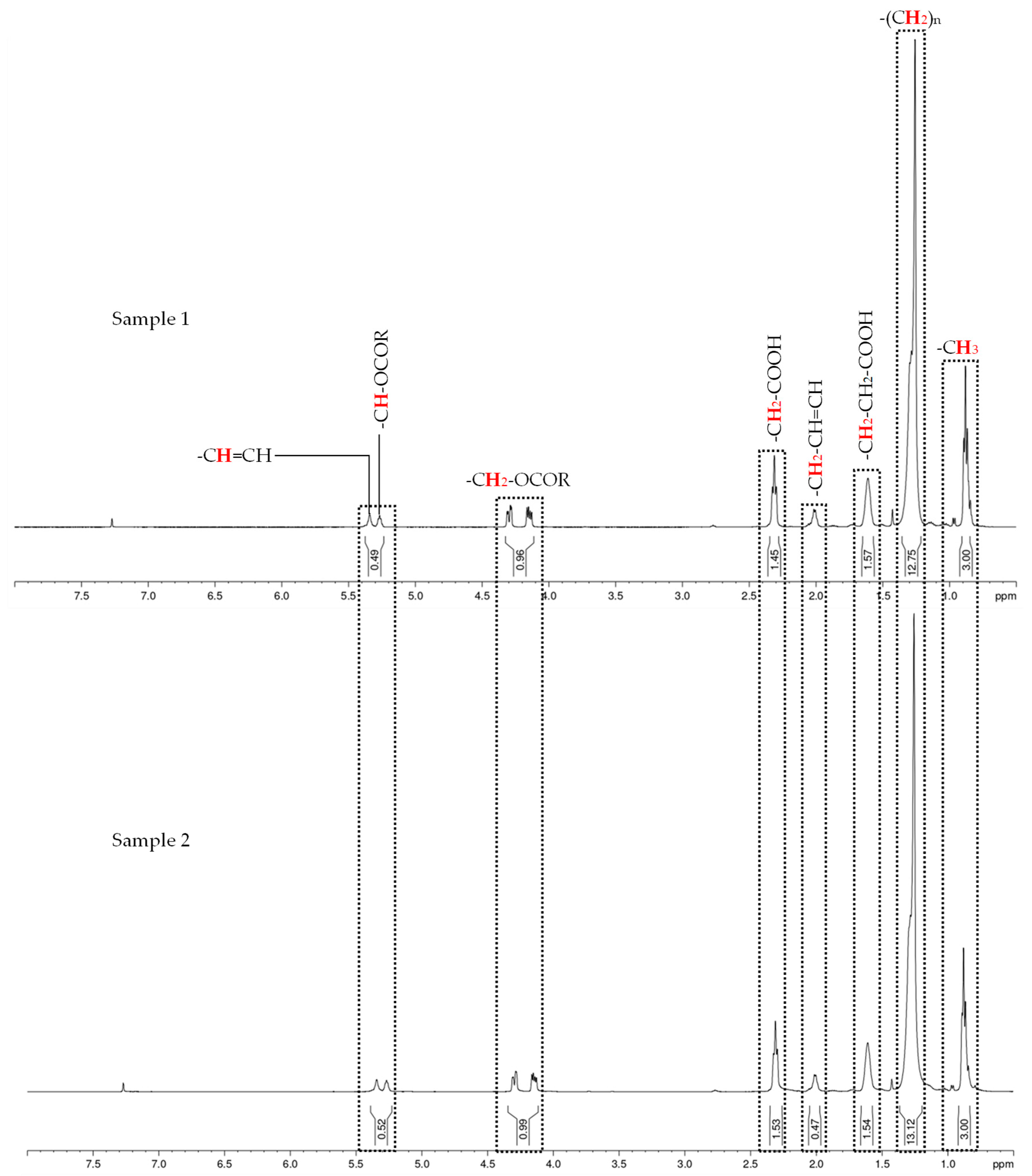
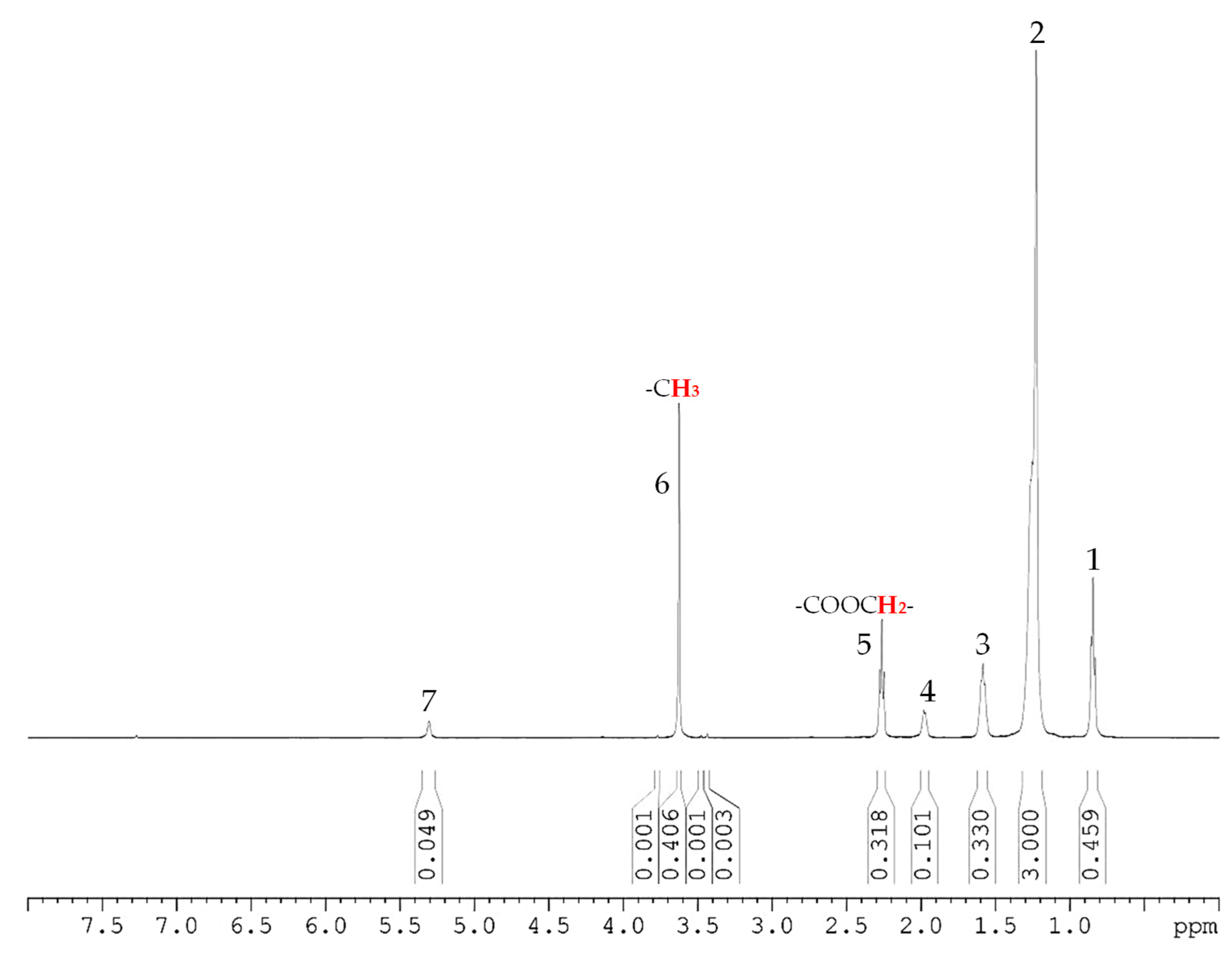
| Fatty Acid Methyl Esters | Content (%) | |
|---|---|---|
| Caprylic acid methyl ester | CH3(CH2)6COOCH3 | 5.57 |
| Capric acid methyl ester | CH3(CH2)8COOCH3 | 4.36 |
| Lauric acid methyl ester | CH3(CH2)10COOCH3 | 44.45 |
| Myristic acid methyl ester | CH3(CH2)12COOCH3 | 11.87 |
| Palmitic acid methyl ester | CH3(CH2)14COOCH3 | 8.32 |
| Stearic acid methyl ester | CH3(CH2)16COOCH3 | 2.88 |
| Oleic acid methyl ester | CH3(CH2)7CH=CH(CH2)7COOCH3 | 19.84 |
| Linoleic Acid Methyl Ester | CH3(CH2)3(CH2CH=CH)2(CH2)7COOCH3 | 2.71 |
| Oil Extraction Process | ||||
| Macauba kernel crushed (mass) | Oil extracted (volume) | Hexane (volume) a | ||
| 1142 g | 689 mL | 5519 mL | ||
| 1.5 ton * | 0.91 m3 * | 7.25 m3 * | ||
| Degumming Process | ||||
|---|---|---|---|---|
| Oil extracted (volume) | Water (volume) b | Oil degummed (volume) | ||
| 0.000689 m3 | 0.000345 m3 | 0.000416 m3 | ||
| 0.91 m3 * | 0.45 m3 * | 0.55 m3 * | ||
| Transesterification Process | ||||
| Oil degummed (volume) | MeOH (volume) | KOH (mass) | Water (volume) c | Biodiesel (volume) |
| 0.000416 m3 | 0.000203 m3 | 0.000013 kg | 0.000063 m3 | 0.000373 m3 |
| 0.55 m3 * | 0.27 m3 * | 0.02 kg * | 0.08 m3 * | 0.49 m3 * |
| Temperature (°C) | η (mPa·s) 1 | ν (mm2/s) 2 | ρ (g/cm3) |
|---|---|---|---|
| 20 | 59.81 | 65.02 | 0.9199 |
| 40 | 25.07 | 27.72 | 0.9041 |
| 100 | 5.31 | 6.16 | 0.8590 |
| Sample | Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | 60 °C | 80 °C | 90 °C | 100 °C | |
| ρ (g/cm3) | ρ (g/cm3) | ρ (g/cm3) | ρ (g/cm3) | ρ (g/cm3) | ρ (g/cm3) | ρ (g/cm3) | ρ (g/cm3) | |
| B16 | 0.8519 | 0.8444 | 0.8378 | 0.8303 | 0.8240 | 0.8104 | 0.8038 | 0.7966 |
| B17 | 0.8521 | 0.8446 | 0.8380 | 0.8305 | 0.8242 | 0.8106 | 0.8040 | 0.7967 |
| B18 | 0.8524 | 0.8448 | 0.8382 | 0.8307 | 0.8244 | 0.8108 | 0.8041 | 0.7969 |
| B19 | 0.8526 | 0.8450 | 0.8384 | 0.8309 | 0.8246 | 0.8110 | 0.8043 | 0.7971 |
| Sample | Result | Temperature | |||||||
| 20 °C | 30 °C | 40 °C | 50 °C | ||||||
| η c (mPa·s) | ν d (mm2/s) | η (mPa·s) | ν (mm2/s) | η (mPa·s) | ν (mm2/s) | η (mPa·s) | ν (mm2/s) | ||
| Biodiesel | Average | 3.7588 | 4.3161 | 2.9701 | 3.4405 | 2.4014 | 2.8065 | 1.9890 | 2.3533 |
| SD * | 0.2479 | 0.2770 | 0.1858 | 0.2093 | 0.1638 | 0.1858 | 0.1336 | 0.1366 | |
| Diesel | Average | 3.5605 | 4.1938 | 2.7874 | 3.3119 | 2.2514 | 2.6982 | 1.8668 | 2.2689 |
| SD * | 0.0006 | 0.0062 | 0.0020 | 0.0005 | 0.0117 | 0.0127 | 0.0008 | 0.0222 | |
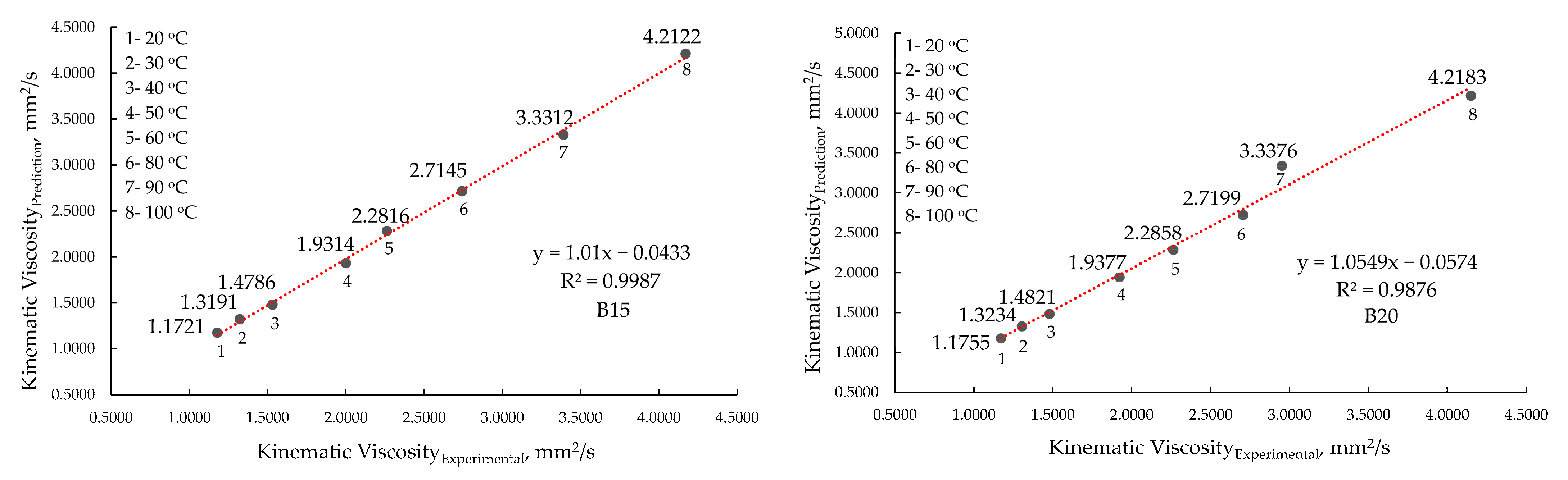
| B15 a | Average | 3.5504 | 4.1702 | 2.8678 | 3.3908 | 2.2999 | 2.7431 | 1.8805 | 2.2634 |
| SD * | 0.0024 | 0.0029 | 0.0017 | 0.0021 | 0.0191 | 0.0215 | 0.0005 | 0.0006 | |
| B20 b | Average | 3.5370 | 4.1488 | 2.7772 | 2.9519 | 2.2692 | 2.7058 | 1.8842 | 2.2655 |
| SD * | 0.0020 | 0.0023 | 0.0011 | 0.5763 | 0.0234 | 0.0262 | 0.0004 | 0.0005 | |
| Sample | Result | Temperature | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 60 °C | 80 °C | 90 °C | 100 °C | ||||||
| η (mPa·s) | ν (mm2/s) | η (mPa·s) | ν (mm2/s) | η (mPa·s) | ν (mm2/s) | η (mPa·s) | ν (mm2/s) | ||
| Biodiesel | Average | 1.7165 | 2.0389 | 1.2757 | 1.5376 | 1.1380 | 1.3913 | 0.9971 | 1.2310 |
| SD * | 0.0351 | 0.0455 | 0.0291 | 0.0503 | 0.0156 | 0.0186 | 0.0052 | 0.0062 | |
| Diesel | Average | 1.5693 | 1.9124 | 1.1856 | 1.4682 | 1.0465 | 1.3064 | 0.9224 | 1.1617 |
| SD * | 0.0001 | 0.0002 | 0.0225 | 0.0259 | 0.0002 | 0.0002 | 0.0002 | 0.0003 | |
| B15 | Average | 1.6525 | 2.0009 | 1.2182 | 1.5313 | 1.0643 | 1.3235 | 0.9398 | 1.1789 |
| SD * | 0.0009 | 0.0011 | 0.0001 | 0.0520 | 0.0001 | 0.0001 | 0.0003 | 0.0004 | |
| B20 | Average | 1.5857 | 1.9235 | 1.2011 | 1.4807 | 1.0520 | 1.3061 | 0.9359 | 1.1724 |
| SD * | 0.0002 | 0.0001 | 0.0003 | 0.0004 | 0.0035 | 0.0002 | 0.0116 | 0.0141 | |
| Sample | Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | 60 °C | 80 °C | 90 °C | 100 °C | |
| η (mPa·s) | η (mPa·s) | η (mPa·s) | η (mPa·s) | η (mPa·s) | η (mPa·s) | η (mPa·s) | η (mPa·s) | |
| B16 | 3.5922 | 2.8167 | 2.2754 | 1.8863 | 1.5929 | 1.2000 | 1.0612 | 0.9344 |
| B17 | 3.5942 | 2.8185 | 2.2769 | 1.8876 | 1.5943 | 1.2009 | 1.0621 | 0.9351 |
| B18 | 3.5962 | 2.8203 | 2.2784 | 1.8888 | 1.5958 | 1.2018 | 1.0630 | 0.9359 |
| B19 | 3.5981 | 2.8221 | 2.2799 | 1.8900 | 1.5973 | 1.2027 | 1.0639 | 0.9366 |
| Sample | Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | 60 °C | 80 °C | 90 °C | 100 °C | |
| ν (mm2/s) | ν (mm2/s) | ν (mm2/s) | ν (mm2/s) | ν (mm2/s) | ν (mm2/s) | ν (mm2/s) | ν (mm2/s) | |
| B16 | 4.2134 | 3.3325 | 2.7155 | 2.2824 | 1.9326 | 1.4793 | 1.3200 | 1.1728 |
| B17 | 4.2146 | 3.3338 | 2.7166 | 2.2833 | 1.9339 | 1.4800 | 1.3208 | 1.1735 |
| B18 | 4.2158 | 3.3350 | 2.7177 | 2.2841 | 1.9352 | 1.4807 | 1.3217 | 1.1742 |
| B19 | 4.2170 | 3.3363 | 2.7188 | 2.2850 | 1.9364 | 1.4814 | 1.3225 | 1.1748 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, B.; Figueredo, I.; Brito, D.; Dorneles, M.; Sousa, E.; Nascimento, T.; Cunha, F.A.; Luna, F.M.T.; Cavalcante, C.L., Jr.; Rios, M. Macauba Kernel Oil: Refining, Transesterification, and Density/Viscosity of Blends B15 to B20 with Mineral Diesel. Processes 2025, 13, 2637. https://doi.org/10.3390/pr13082637
Sousa B, Figueredo I, Brito D, Dorneles M, Sousa E, Nascimento T, Cunha FA, Luna FMT, Cavalcante CL Jr., Rios M. Macauba Kernel Oil: Refining, Transesterification, and Density/Viscosity of Blends B15 to B20 with Mineral Diesel. Processes. 2025; 13(8):2637. https://doi.org/10.3390/pr13082637
Chicago/Turabian StyleSousa, Bruna, Igor Figueredo, Débora Brito, Mauricio Dorneles, Eva Sousa, Tassio Nascimento, Francisco Assis Cunha, Francisco Murilo T. Luna, Célio L. Cavalcante, Jr., and Maria Rios. 2025. "Macauba Kernel Oil: Refining, Transesterification, and Density/Viscosity of Blends B15 to B20 with Mineral Diesel" Processes 13, no. 8: 2637. https://doi.org/10.3390/pr13082637
APA StyleSousa, B., Figueredo, I., Brito, D., Dorneles, M., Sousa, E., Nascimento, T., Cunha, F. A., Luna, F. M. T., Cavalcante, C. L., Jr., & Rios, M. (2025). Macauba Kernel Oil: Refining, Transesterification, and Density/Viscosity of Blends B15 to B20 with Mineral Diesel. Processes, 13(8), 2637. https://doi.org/10.3390/pr13082637








