1. Introduction
Waterflooding has been a widely used practice in the oil industry for secondary hydrocarbon recovery since the early 20th century [
1]. Before field implementation, compatibility tests are often conducted to prevent formation damage caused by the hydration and swelling of clay layers. Low-salinity water (LSW) injection emerged as a common practice for oil recovery in reservoirs as early as the 1930s, when formation compatibility tests with fresh water and low-salinity water became a standard requirement. Since 1955, studies have examined the impact of fresh water and LSW on clay-containing core samples, focusing on oil recovery and residual oil saturation [
1,
2,
3].
Bernard [
2] conducted experiments to evaluate the effects of fresh water and low-salinity water on clayey core samples. He observed that fresh water increased oil recovery during core flooding in samples with clay content, but the pressure drops also increased as oil recovery progressed. They attributed this phenomenon to clay swelling and the generation of fine particles from clay hydration, which enhanced oil recovery. In petroleum reservoirs, three common clay types (montmorillonite, illite, and kaolinite) are frequently encountered, along with other forms such as attapulgite, sericite, chlorite, and anauxite [
1,
2]. In the late 20th century, the influence of carbonates, such as natural soda with calcium, magnesium, or sulfate, was identified in reservoir formations [
3]. Interestingly, the improvement in oil recovery was attributed not to the presence of specific ions but to the removal of oil films from sand grains [
3]. These findings have shaped the understanding of how water chemistry and clay interactions influence oil recovery, laying the groundwork for modern practices in low-salinity waterflooding and enhanced oil recovery (EOR).
Several works have been extended on freshwater in the last two decades, and low saline water is generally referred to as low-salinity water (LSW). Modification of injected water ions or chemical composition to improve the recovery performance, which is attributed to different proposed mechanisms of interaction of these ions with the fluid and reservoir, leads to different names like smart water injection and water-engineered ions. LSW injection has been experimentally demonstrated to be successful for the recovery of oil in different reservoirs (sandstone and carbonate) [
4,
5]. Therefore, LSW injection has been combined with different recovery methodologies to enhance recovery performance or control the shortcomings of the system, such as fine migration. Typical combinations of LSW with other recovery methods include CO
2, surfactant, alkali, polymer, and nanoparticles (NPs).
Chaturvedi et al. [
6] discussed the impact of water salinity on the absorption of CO
2 in the solution and the interfacial tension (IFT) of the CO
2 solution in the reservoir. The pressure and temperature are inversely related to the solubility of CO
2 in the system. In summary, LSW increases the potential of CO
2 in oil recovery through increased absorption, reduced IFT, and increased CO
2 loading [
6]. Ion exchange and surface charge interactions between reservoir rocks and fluids during water-alternating gas (WAG) CO
2–LSW injection play a crucial role in enhancing oil recovery. Numerical simulation is used to evaluate the dissolution of the rock mineralogy, formation, and injected brine composition during CO
2–LSW injection [
7,
8,
9]. Naderi and Simjoo [
7] coupled fluid flow and a geochemical model of the ion-exchange mechanism through compositional numerical simulation to explain the wettability alteration by Na
+ and Ca
2+ ions and dissolution of the rock mineral (calcite).
IFT is reported to vary inversely with the salinity of the solution until optimum salinity is achieved, as noted in several studies. It has been observed in different experiments that LSW improves oil recovery through ion exchange and wettability alteration. However, the IFT increases, which is disadvantageous for oil recovery [
10,
11]. This trend has been explained using the drift-diffusion theory by Farhadi et al. [
12]. Rostami et al. [
13] described that the movement of polar oil molecules toward the oil–water interface is driven by electrostatic attraction (drift) while being counteracted by a concentration gradient (diffusion). By applying this model to dynamic IFT data, they calculated the IFT reduction rate and the time required for the establishment of the electric double layer (EDL). Similar findings were reported by Sarma et al. [
14], further validating the influence of salinity on IFT behavior and its implications for oil recovery efficiency. Surfactants play a crucial role in enhancing the efficiency of LSW by addressing key limitations such as a high IFT, poor oil mobilization, and suboptimal wettability conditions. When combined with LSW, surfactants exhibit enhanced performance due to the synergistic effects of ion exchange, reduced surfactant adsorption, and improved surface charge interactions [
15,
16]. Therefore, surfactant combinations reduce the IFT of different salinities to the ultra-low value, as demonstrated by Roldán-Carrillo et al. [
11]. Souayeh et al. [
10] demonstrated that surfactant improves the compatibility of LSW and the temperature limit of the surfactant in the reservoir system. The wettability of oil-aged calcite is improved through the displacement of the carboxylic group by polyethoxylated nonionic molecules [
10,
11].
The impact of divalent ions (Ca
2+ and Mg
2+) is very significant compared to the salinity of the solution [
11]. Sarma et al. [
14] highlighted that Ca
2+ and Mg
2+ play a crucial role in modifying surface charges at the rock–fluid interface, directly influencing the wettability alteration in LSW applications. The active surfaces in the interface are reduced by the low concentration of cations, which increases the IFT [
11]. Wettability alteration toward a more water-wet state has been observed with the injection of a tenfold dilution of engineered water (EW) containing varying compositions of Ca
2+, Mg
2+, and an ionic benzene sulfonic acid surfactant [
4,
17]. Olayiwola and Dejam [
18] observed that alternating injections of LSW and surfactant shifted the wettability of the reservoir toward a water-wet state. Alkaline surfactant flooding with LSW creates a soap emulsion through the reaction of the alkali with the acidic component of the crude oil.
Despite the efficient recovery of oil during the injection of LSW, controlling the front mobility during flooding, fluctuating flow, flow direction, viscous fingering, unsteady flow, film formation, and trapped oil are still a problem [
19,
20,
21]. Therefore, further study is extended to the application of LSW with polymer or alkaline surfactant polymer (ASP) flooding. Polymer injection enhances oil recovery by increasing the viscosity, which improves the sweep efficiency, but it is sensitive to salinity and temperature [
19]. Abdullahi et al. [
19] attributed the performance of the LSW with polymer flooding to the uncoiling and stretching of partially hydrolyzed polyacrylamide (HPAM) molecules, which increases the viscosity and viscoelasticity of the fluid. Adsorption of polymer on the rock surface is minimized by LSW. Sharma et al. [
21] explained that the displacement mechanisms and pore-scale phenomena during flooding with LSW and the slightly miscible chemicals are still unclear. Therefore, micro- and macro-scale experiments are used to demonstrate the displacement mechanism and oil recovery. Injection of polymer after LSW to control the mobility results in a slight increase in the recovery. However, the alkali–polymer injection after injecting polymer further increases the recovery by reacting with the acid component of crude oil.
Application of LSW is not limited to the aforementioned chemical methods. It is further extended to different NPs. Unlike other chemicals, NPs behave differently in their physical and chemical properties. LSW and alkaline flooding are very efficient for oil recovery, but fine migration develops from the high pH, which leads to formation damage [
22]. Therefore, further study is required for the optimization of salinity and pH for effective flooding. Assef et al. [
22] proposed NP treatment of colloidal particles to alter the zeta potential (ZP) and increase particle retention during alkaline flooding with LSW. The interaction between LSW and silica NPs creates an amorphous silicate structure and a crystal structure, which creates disjoining pressure in the reservoir. Wettability alteration of the rock is attributed to the amorphous structure of the silica NPs in brine solution, which serves as a bridge between the oil droplet and rock surface [
23]. The adsorption of NPs onto the quartz surface is reduced in LSW/deionized water (DIW) compared to synthetic seawater (SSW), which contains higher ionic components [
24]. The ionic component increases the electrostatic repulsion between the NPs and SSW [
17,
24]. Fakir et al. [
25] evaluated the effectiveness of the LSW–surfactant–NPs combination for enhanced oil recovery (EOR) in tight carbonate reservoirs.
The LSW application for oil recovery has been combined with different chemical recovery methods. Therefore, several mechanisms have been ascribed to the effectiveness and efficiency of LSW to synthesize other chemicals. Most of these methods are an effort to reduce the capillary number, which is directly related to residual oil saturation. The fines produced during LSW injection can be explained using the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory due to van der Waals interactions and electrostatic repulsion, while some approaches use the swelling and hydration of clay during flooding [
22]. The electric double layer (EDL) of ions, surface charge, surface ion exchange, and carbonate dissolution in the reservoir system are used to explain the fluid interactions, which lower the IFT and improve the wettability with the rock surface. Disjoining pressure, in addition to the aforementioned mechanisms, is responsible for wettability alterations during the injection of NPs. This work examined both the physical and chemical techniques that describe the fluid–fluid and fluid–solid interactions in the reservoir during the injection of LSW. In summary, a detailed explanation and analysis of the different mechanisms of injection of LSW with other chemical methods are provided in this work for future study and industrial application.
This work is organized in the order of LSW with CO2 injection, LSW with surfactant/alkali, LSW with alkali, surfactant, and polymer (ASP), and LSW with NPs/surfactant.
2. Low-Salinity Water (LSW) and CO2 Mechanisms
Climate change, driven in part by increasing petroleum industry activities, has become a major environmental concern. As a result, CO
2 injection into reservoirs for enhanced oil recovery (EOR) or carbon sequestration has gained significant attention in the oil industry. CO
2 is widely used to improve the mobility ratio by reducing oil viscosity, making it an effective agent for EOR [
26]. At the minimum miscibility pressure (MMP) and specific temperature, CO
2 becomes completely miscible with oil, a condition typically determined through slim-tube experiments [
27]. The miscibility of CO
2 in a reservoir is driven by two primary mechanisms: gas vaporization and condensation. However, during immiscible injections, oil recovery is primarily achieved through oil swelling and the dissolution of CO
2 in the oil.
The integration of low-salinity water (LSW) with CO
2 water-alternating gas (WAG) injection has been extensively studied to further enhance oil recovery. Dang et al. [
28] implemented a model coupled with multiphase flow equations to examine the advantages of LSW–CO
2 WAG. Their model demonstrated the geochemical reactions occurring in the system and highlighted the synergy between the processes. Awolayo et al. [
8] conducted a comprehensive study on fluid–rock interactions during low-salinity brine–CO
2 flooding in carbonate reservoirs. Their work highlighted the importance of geochemical reactions and wettability alteration in optimizing EOR processes, providing valuable insights into the synergy between low-salinity water and CO
2 injection. Similarly, Al-Saedi et al. [
29] conducted core flooding experiments and spontaneous imbibition tests using various brine compositions and cores. Their study measured key parameters such as minimum miscibility pressure, pH changes, CO
2 solubility in brine, and contact angles, providing insights into the mechanisms driving EOR. Al-Abri et al. [
26] further explored the effectiveness of three different brines in enhancing oil recovery using Berea sandstone cores. Their core flooding experiments simulated CO
2–LSWAG as a tertiary EOR approach, emphasizing the importance of brine composition in optimizing recovery.
Experimental studies have also focused on wettability alteration, a critical mechanism in EOR. Teklu et al. [
30] conducted carbonate and sandstone core flooding experiments, demonstrating through contact angle measurements that the proposed process achieves significant wettability alteration. Naderi and Simjoo [
7] used modeling to study the mechanics of wettability alteration due to ion exchange and mineral dissolution. They discussed the data required for compiling and implementing such models, including rock composition, oil composition, and reservoir fluid properties. Moradpour et al. [
31] expanded on this by conducting experimental studies on EOR mechanisms in carbonates. They identified wettability alteration as the primary mechanism, enhanced by increased CO
2 solubility in low-salinity water. Their results emphasized the need to optimize brine salinity for effective EOR and explored the use of different gas types, such as N
2.
The mechanics of EOR by CO
2–LSWAG have been further elucidated by Gandomkar et al. [
32], who discussed various mechanisms, including interfacial tension (IFT) reduction, oil swelling, CO
2 solubility, and wettability alteration. Their results showed that including CO
2 as a phase in the system decreases the IFT between all phases, while CO
2 dissolution increases acidity, promoting calcite dissolution. Al-Bayati et al. [
33] investigated the impact of core heterogeneity on the miscible CO
2 WAG process. By vertically and horizontally stacking core samples with differing permeabilities, they found that CO
2 WAG was more successful in secondary mode than in tertiary mode, regardless of the core configuration. Oil recovery increased when the permeabilities of the core layers were closer in value.
Optimization of CO
2 WAG injection parameters has also been a focus of research. Van and Chon [
34] conducted core flood experiments to determine the optimal salinity and slug size for CO
2 WAG injections in Berea sandstone cores. They first determined the minimum miscibility pressure to ensure experiments were conducted under miscible conditions. AlQuraishi et al. [
35] compared the effectiveness of LSW and high-salinity water (HSW) in Berea and Bentheimer sandstone cores. Their results showed that LSW was more effective in Berea cores with higher kaolinite contents, while HSW performed better in Bentheimer cores with less clay. Al-Shalabi et al. [
36] used simulations to predict the effects of combining LSW and CO
2 injections, testing various injection modes such as continuous gas injection and simultaneous water-alternating gas (SWAG). Their results highlighted the advantages of using LSW and CO
2 together, with SWAG outperforming other modes as a tertiary recovery method.
Ramanathan et al. [
37] tested the success of CO
2–LSWAG using grey Berea sandstone cores, comparing three brines: formation brine, seawater, and low-salinity water. Contact angle measurements were used to quantify wettability before and after the injections. Their results showed that aging cores for longer periods led to higher recovery, with the lowest contact angles observed for low-salinity brines. Finally, Bastos et al. [
38] discussed the mechanics of oil recovery due to CO
2 injection under non-miscible conditions, emphasizing the benefits of combining CO
2 with LSW. Their experimental procedure, carried out through reservoir simulations, concluded that oil recovery improves even below the minimum miscibility pressure (MMP) when CO
2 is not miscible. They also noted that mineral reactions were more pronounced for CO
2–LSWAG than for CO
2 flooding, although their model neglected mineral dissolution.
2.1. Solubility of CO2 in Brine and LSW
WAG is the best method for LSW and CO
2 injection due to the difference in oil, water, and CO
2 densities, which can result in the fingering effect in a reservoir. Recently, several experiments and numerical studies have been performed to demonstrate the economic potential and recovery mechanism of LSW with CO
2 water alternating gas (WAG) [
39]. It has been reported in several studies that the solubility of CO
2 in brine decreases with increasing salinity, which makes LSW a suitable candidate for WAG [
40]. The main mechanism of trapping CO
2 bubbles encapsulated in water layers is referred to as physio-sorption, which enhances the reaction of water with CO
2 to produce carbonic acid (H
2CO
3), as shown in [
6].
Chaturvedi et al. [
6] concluded, based on their experimental results, that the solubility of CO
2 reduces in water with an increasing concentration of salt and decreasing pressure. The solubility of CO
2 is reduced by salting-out of the high salinity [
6,
26]. CO
2 solubility reduces the IFT of CO
2–water at lower pressure [
6]. Al-Abri et al. [
26] explained that the displacement front of the water during LSW–CO
2 WAG is stabilized by the mobility ratio. The effect of different ions on solubility is demonstrated using the pressure decay (
Figure 1). The reduction in solubility of CO
2 in aqueous solutions is attributed to the increasing number of free ions in the solution, which reduces the number of interactions with CO
2 molecules [
26]. In addition, the hydration process of the ions is impacted by the ionic radius, which is reduced as the ionic radius increases.
Mg
2+ ions are smaller than both Na
+ and K
+ ions, yet the pressure decay indicates that the solubility of MgCl
2 is lower than that of Na
+ but higher than that of K
+ due to the salting–out phenomenon. However,
Table 1 shows that the solubility of CO
2 increases with an increase in the ionic radius of the cations in the order K
+ > Mg
2+ > Na
+.
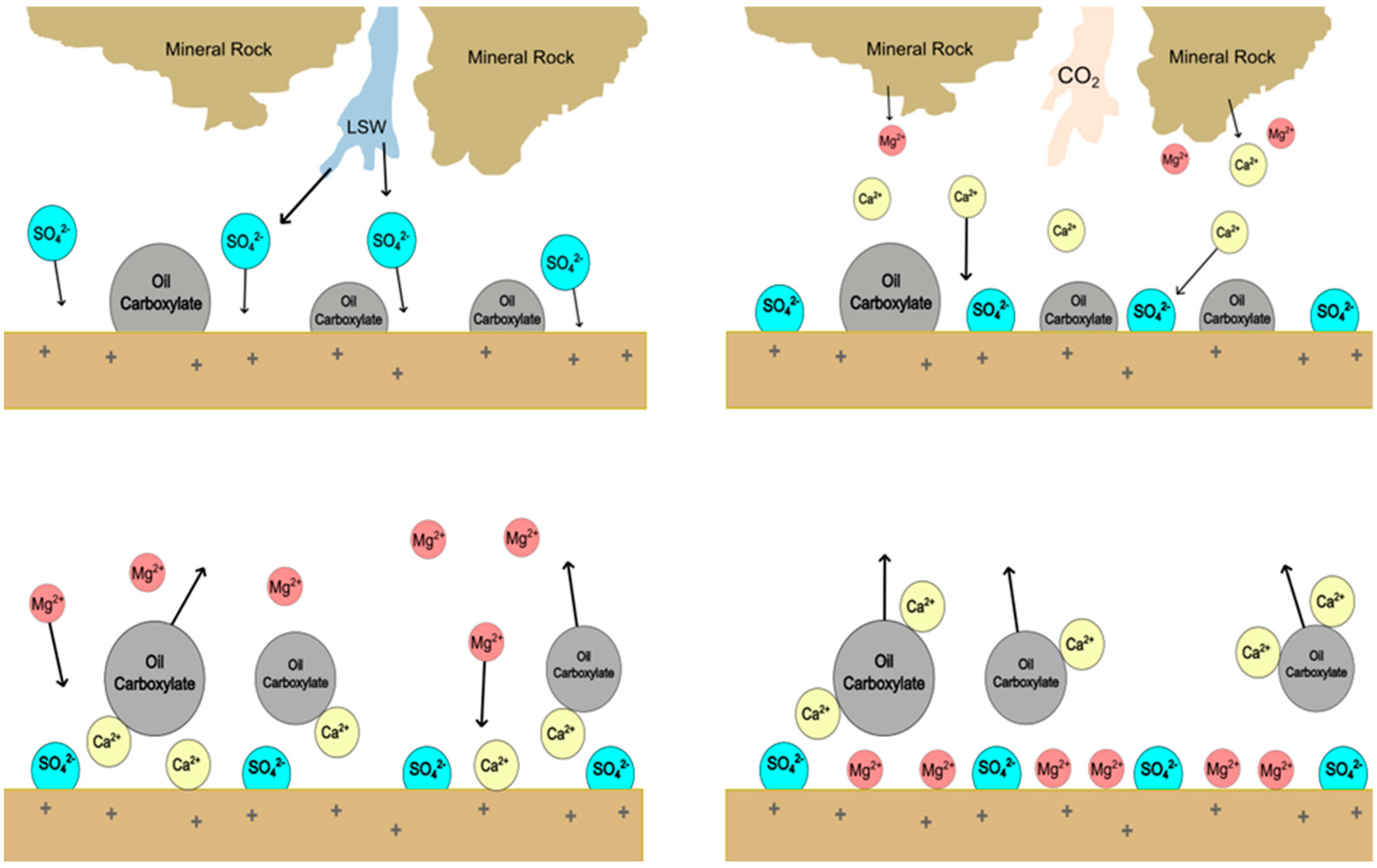
2.2. CO2–LSW’s Impact on Wettability
The wettability of rock is impacted by several factors, which have been highlighted by different studies, such as temperature, brine composition, rock mineralogy, and crude oil content. Al-Abri et al. [
26] described that wettability toward a water-wet state is increased more by LSW (NaCl)–CO
2 than by KCl and MgCl
2. Multicomponent ion exchange (MIE) of cations with the clay surface is assumed to be responsible for this wettability behavior. The MIE, which is considered to be one of the mechanisms contributing to wettability alteration during LSW, is conceptualized in
Figure 2.
CO
32− has great potential to react with Ca
2+ and Mg
2+ ions to form carbonate salt. The proton H
+ has a strong affinity to be adsorbed onto a negatively charged surface as follows [
41]:
The dissolution of CO
2 in water forms carbonic acid, which reduces the pH of the solution and enhances the MIE by modifying the clay surface with H
+, leading to an increased attraction of Mg
2+, K
+, and Na
+. Replacement of the divalent ions (Ca
2+ and Mg
2+) on the clay surface by monovalent ions (Na
+) to alter the wettability toward a water-wet state is responsible for the higher oil recovery in experiments with LSW–CO
2 injections [
27,
28]. H
+ enhances the dissolution of calcite and dolomite during the injection of LSW–CO
2, but the reaction rate is lower in dolomite than in calcite [
7,
28]. The dissolution of calcite by HCO
3− increases the amount of Ca
2+ in the injected brine. Therefore, Ca
2+ is attracted to the clay surface as Na
+, which stabilizes the clay, is removed [
28].
2.3. CO2–LSW’s Impact on the IFT
Injection of CO
2 reduces the IFT of oil–brine solutions, which is similar in LSW–CO
2 injection [
26,
40,
42]. Carbonic acid, formed by the dissolution of CO
2, disrupts the stability of the interphase and the hydrogen bond between the negatively charged, polar component of the crude oil [
26]. More ions are available at the interphase to interact with the polar component of the oil and brine. Olayiwola and Dejam [
43] explained that the double-layer region of a fluid surface is made of free charges that balance opposite charges and potential drop (net orientation of the dipoles). The surface charges in the double layer are increased by the carbonic acid formed during the dissolution of CO
2 in brine. Therefore, the reduction in the IFT is attributed to the increasing surface charge by HCO
3−, calcite, and dolomite dissolution.
Figure 3 illustrates the effect of salinity and CO
2 on oil-brine IFT.
In summary, LSW–CO
2 injection reduces the IFT and viscosity and alters the wettability toward a water-wet state, but the predominant mechanism responsible for oil recovery is wettability alteration. The monovalent LSW with CO
2 has approximately the same IFT as divalent LSW–CO
2, but the wettability shifts more toward a water-wet state in monovalent than in divalent LSW. However, CO
2 injection also extracts light hydrocarbon components from crude oil, thereby altering the physical properties and flow behavior of the residual oil [
44,
45]. CO
2 extraction leads to an increase in the viscosity of the residual oil, and the extraction capacity improves with increasing pressure but diminishes as temperature rises [
46,
47].
3. Low-Salinity Water (LSW) and Surfactant, Alkali, and Polymer Mechanisms
The effectiveness of LSW can be significantly enhanced by integrating chemical enhanced oil recovery (CEOR) techniques such as surfactants, alkalis, and polymers. These chemical additives modify interfacial properties, improve sweep efficiency, and stabilize emulsions, ultimately leading to higher oil recovery. Recent studies have demonstrated that the synergistic effects of LSW with surfactants and polymers contribute to wettability alteration, a reduced IFT, and improved mobility control, making it a promising approach for optimizing oil displacement under various reservoir conditions [
48,
49]. Salinity optimization is important for the effectiveness of surfactant injection in reservoirs.
Recent studies have demonstrated the significant benefits of combining LSW with surfactant-enhanced methods to improve oil recovery in carbonate reservoirs. In particular, the effectiveness of low-salinity surfactant (LSS) flooding has been compared to conventional LSW to assess its impact on IFT reduction, wettability alteration, and oil displacement efficiency. Belhaj et al. [
50] investigated surfactant-enhanced smart waterflooding using an anionic alkyl polyglucoside carboxylate in 1% diluted seawater. The surfactant significantly reduced interfacial tension, improving oil mobilization compared to low-salinity water alone. Contact angle measurements indicated a shift from oil-wet to intermediate-wet conditions, further improving oil displacement. Core flooding tests demonstrated that adding 0.2 wt.% surfactant to low-salinity water increased oil recovery by 9.11%. They further applied smart waterflooding with the same anionic surfactant, which shifted rock wettability toward a more water-wet state, enhancing oil displacement. These findings emphasize that the synergy between surfactants and low-salinity water enhances the overall efficiency of oil recovery by optimizing wettability and reducing capillary forces. The incremental recovery observed underscores the need for further research into surfactant selection and concentration optimization to maximize recovery efficiency while minimizing operational costs.
The retention of surfactants in porous media is a critical factor affecting the economics and efficiency of the hybrid LSW process. Surfactant retention varies widely depending on several factors, including surfactant structure, mineralogy, salinity, pH, microemulsion viscosity, crude oil properties, co-solvent presence, and mobility control [
51]. Surfactant retention occurs through several key mechanisms, including precipitation in the presence of divalent ions, diffusion into dead-end pores, adsorption onto rock surfaces, and partitioning into the oil phase. These processes alter the composition of the injected slug, ultimately reducing displacement efficiency [
52]. The retention values for both surfactant–polymer (SP) and alkali–surfactant–polymer (ASP) floods typically range from 0.01 to 0.37 mg/g of rock [
51]. The types of surfactant retention during LSW can be categorized based on the surfactant’s interaction with the reservoir rock and fluids. Adsorption is a primary mechanism of retention, where surfactants adhere to the rock surface. This process is influenced by factors such as the surfactant’s chemical structure, rock mineralogy, and brine composition. For instance, in low-salinity environments, surfactant adsorption can be reduced, which is advantageous for the EOR process. Additionally, the presence of divalent ions in the brine can affect surfactant adsorption, with higher Ca
2+/Na
+ ratios leading to increased surfactant adsorption. The reduced surfactant retention in low-salinity environments contributes to improved surfactant solubility and effectiveness in reducing the IFT between oil and water phases [
53]. In order to achieve effective IFT reduction at minimal cost, it is common practice to use a surfactant concentration ranging from 0.1 to 0.5 wt.% in the injected brine for both laboratory experiments and field applications [
54]. It was reported in published studies by Pourafshary and Moradpour [
55] and Kerunwa [
56] that surfactant retention in an LSW environment was considerably lower—up to ten times less—than in conventional surfactant flooding, primarily due to the reduced cation concentration. This finding underscores the economic benefit of injecting surfactants at lower ionic strengths, as it minimizes retention losses and allows for a more cost-efficient surfactant application.
Alkali is used as a co-surfactant in the alkali–surfactant (AS) process [
57]. AS is more effective in high-acid-number crude oil recovery due to the formation of surfactant by the reaction of alkali with certain acidic components of the crude oil, and it reduces surfactant adsorption onto the rock surface. Alkali reduced the IFT of crude oil (total acid number (TAN) 2.683 mg KOH/g) by 50% during an LSW–alkali flooding experiment, while surfactant reduced the IFT to one third of the initial value [
58]. The main mechanisms involved during the two processes (LSW–alkali and LSW–surfactant) are IFT reduction and wettability alteration. Alkali is very stable, irrespective of the salinity of formation water, compared to surfactant, but its performance depends on the reaction with the carboxylic acids (COOH-) of crude oil [
57,
59]. The chemical reaction of alkali with organic acid (XA) of the crude oil can be expressed as follows:
Therefore, the effectiveness of alkali to reduce the IFT and improve the recovery of oil depends on the interaction of the TAN present in crude oil. The addition of alkali to surfactant at critical micelle concentration (CMC) does not significantly reduce the IFT, but it slightly increases at a higher concentration of alkali [
57]. Esfandiarian et al. [
57] explained that the minimum IFT is achieved when the ratio of ionized acid to un-ionized acid is equal, while the reversal phenomenon is attributed to the salting-out effect, which reduces the concentration of ionized acid and the formation of micelles. Surfactant modifies the surface from hydrophilic to hydrophobic through the interaction of its CH2 groups, the electric double layer (EDL), and the dipole–dipole interaction [
43]. Increasing the concentration of alkali with surfactant increases the number of cations and OH- ions present at the interface or the EDL. Lin et al. [
60] attributed the diffusion-controlled adsorption and re-equilibration relaxations at the interface to the strong cohesive force between hydrocarbon and polar groups, which are increased by the introduction of alkali. Alkali reacts with the carboxylic acid of crude oil to improve the hydrophobic interaction with crude oil, thereby reducing the IFT. However, increasing the alkali concentration beyond the equilibrium state reduces the hydrophobic interaction and increases the IFT [
43]. The positively charged surfactant head groups react with the excess OH- ions of the alkali through electrostatic adsorption, which reduces the amount of surfactant available at the surface. However, a negatively charged surface increases the amount of negative ions at the surface, which might result in weak dipole–dipole interaction [
43,
61]. Esfandiarian et al. [
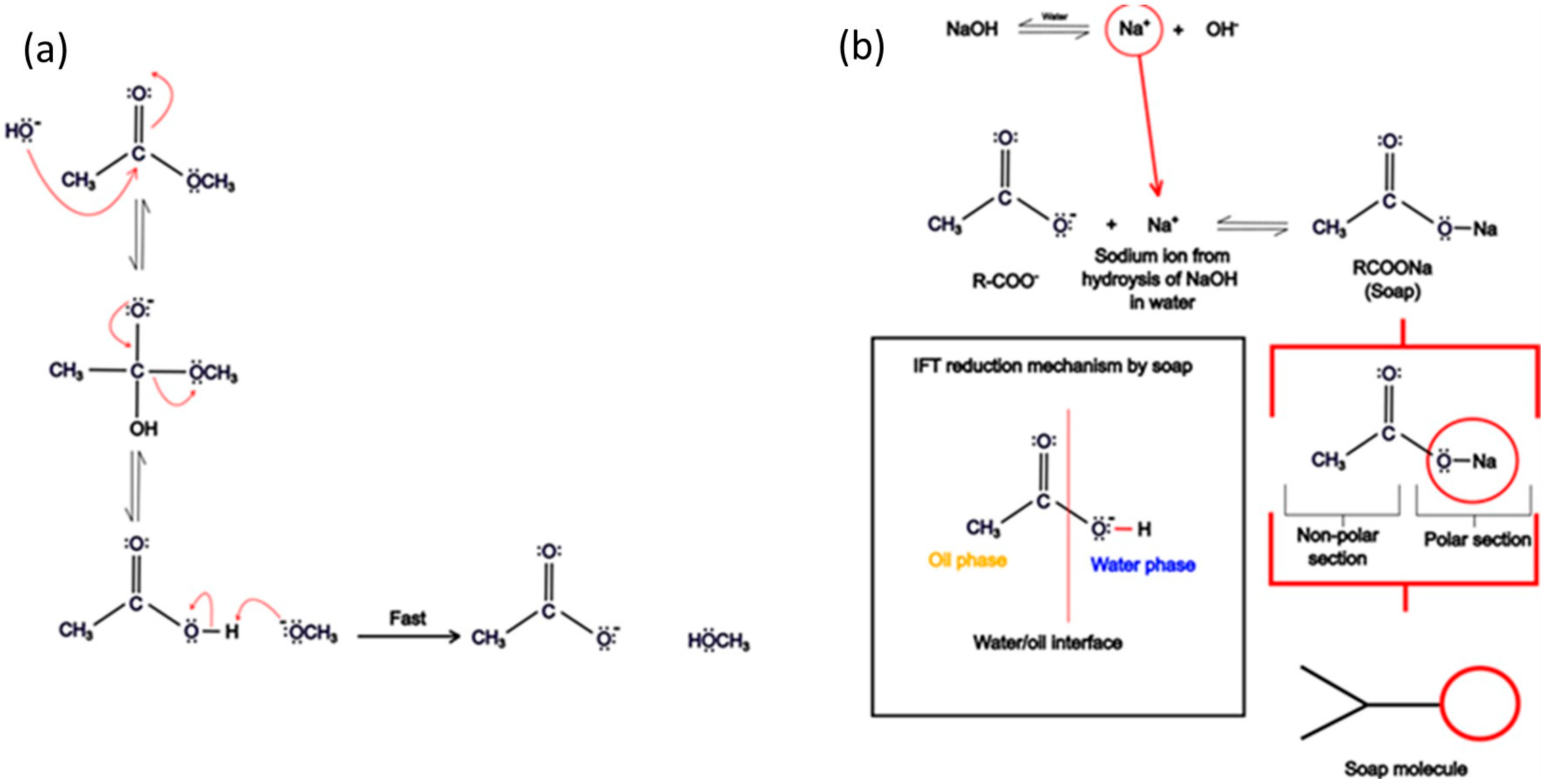
57] explained that hydrolysis of ester in the crude oil by alkali, which produces alcohol and carboxylic acid, can be observed using FTIR, as shown in
Figure 4.
This reaction leads to the three-step formation of acyl-oxygen cleavage, which reacts with Na
+ ion to form soap, as shown in
Figure 5.
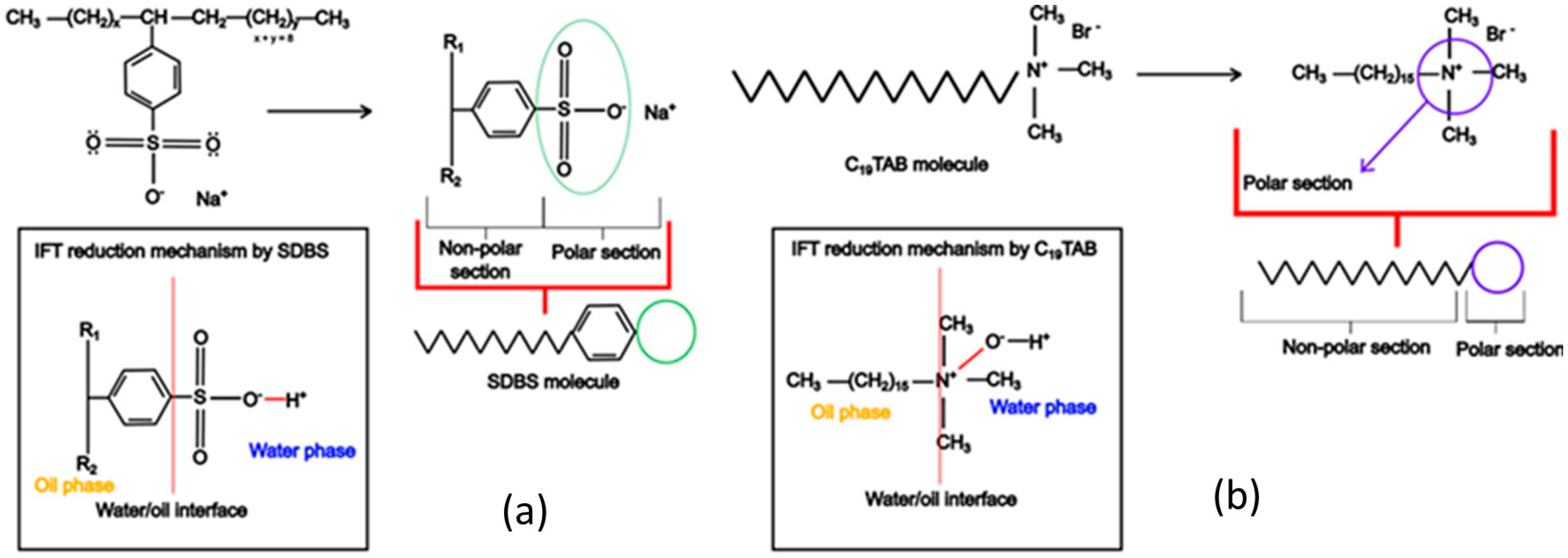
In addition to the hydrolysis of esters observed in the alkali solution, the SDBS solution increases the hydrolysis of esters. Thus, the accumulation of SDBS and soap molecules at the interface increases the amount of positive and negative ions at the EDL to decrease the IFT, as shown in
Figure 6. Meanwhile, CTAB hydrolyzes in water to release Br- ions from the cationic surfactant, along with OH- ions, which accumulate at the interface (
Figure 6). In summary, a high concentration of alkali results in excess OH- ions at the interface, which react with the available cationic surfactant, while the force of repulsion dominates the reaction with anionic surfactant.
Injection of polymer into a reservoir modifies the mobility ratio by increasing the viscosity of the injected fluids. However, polymers are unstable at high temperatures and in high-salinity flooding, which results in polymer breakdown. LSW synchronizes effectively with polymers to modify the wettability, improve oil recovery, and reduce shear sensitivity, thereby improving polymer viscoelasticity, but an optimum amount of polymer is required for effective application [
20,
62].
Abdullahi et al. [
19] conducted an experiment to demonstrate the effectiveness of LSW-augmented polymer injections in a sand-pack-with-clay system. The carboxylate and amide functional groups of hydrolyzed polyacrylamides (HPAM) polymer identified by the Fourier transform infrared (FTIR) instrument are available for ion–dipole interaction with freshwater, seawater, and brine ions, especially Ca
2+ and Mg
2+ [
19,
62]. However, these interactions weaken the N-H and C=O bonds of HPAM and break the molecules to reduce viscosity. The hydrolysis of HPAM in deionized water (DIW) produces a carboxylic group, which reacts with the cations to result in screening of polymer charges and increases the viscosity by shrinking and coiling the expanded HPAM chains [
19,
62].
In contrast to hydrolysis of HPAM in DIW, the HPAM chain is constricted by the cations of seawater or brine, especially divalent ions, to reduce the viscosity [
19]. Hence, HPAM viscosity continues to decline until the critical shear rate is reached, when the HPAM ions stop interacting with the ions of the brine and seawater. The influence of salinity on the viscoelastic properties of the polymer is important for enhanced oil recovery. Abdullahi et al. [
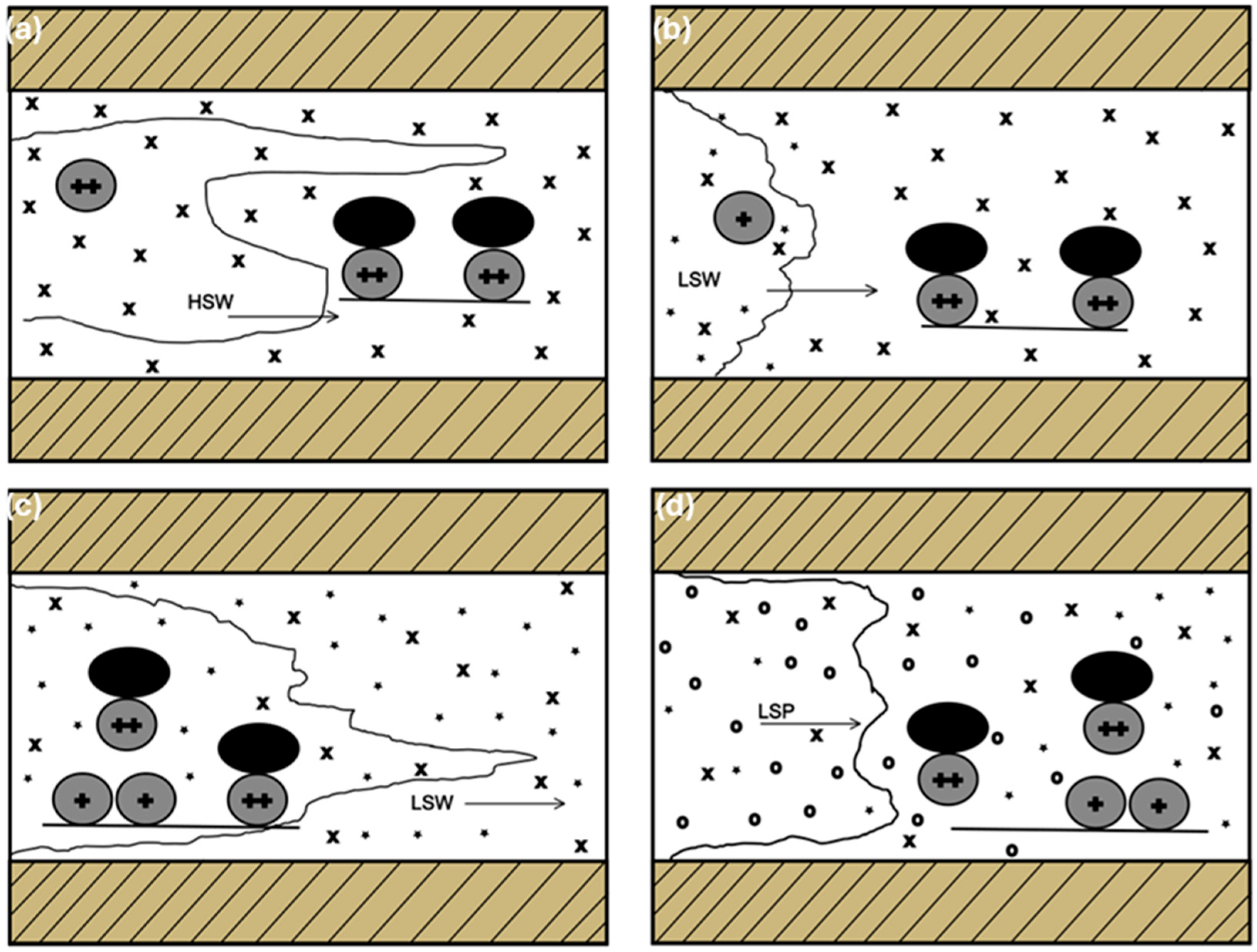
19] explained that the gel strength of the polymer is increased by the strong ionic components of the brine, which causes isolation and clogging in the pore system.
Figure 7 illustrates the flow pattern of a polymer injection or LSW and brine (high salinity) in a porous system.
Saeed and Jadhawar [
62] explained that LSW injection with polymer reduces the extent of polymer degradation and adsorption onto the rock surface. Adsorption onto the clay surface is increased by the presence of multivalent cations in the brine. Carbonate rocks have a positive surface that attracts the carboxylic group of the HPAM, thereby influencing the interaction between LSW and carbonate, while sandstone with a high clay content screens the negative charge with the cations attached to the surface. Saeed and Jadhawar [
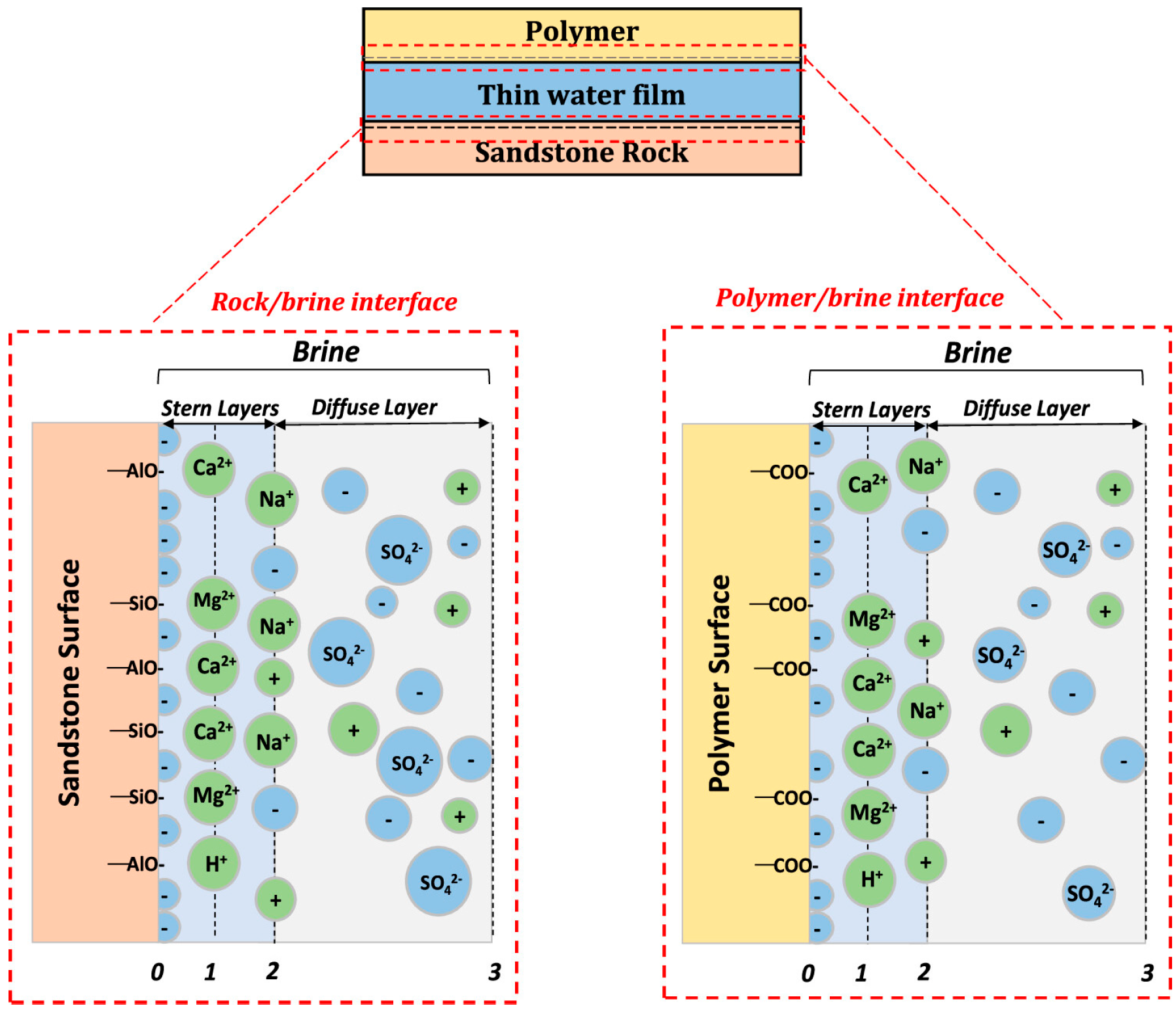
62] utilized surface complexation modeling to describe the mechanism of adsorption involved between the ions in the fluid and in the solid surface using the EDL interface with the solid–fluid interface (triple interface), as shown in
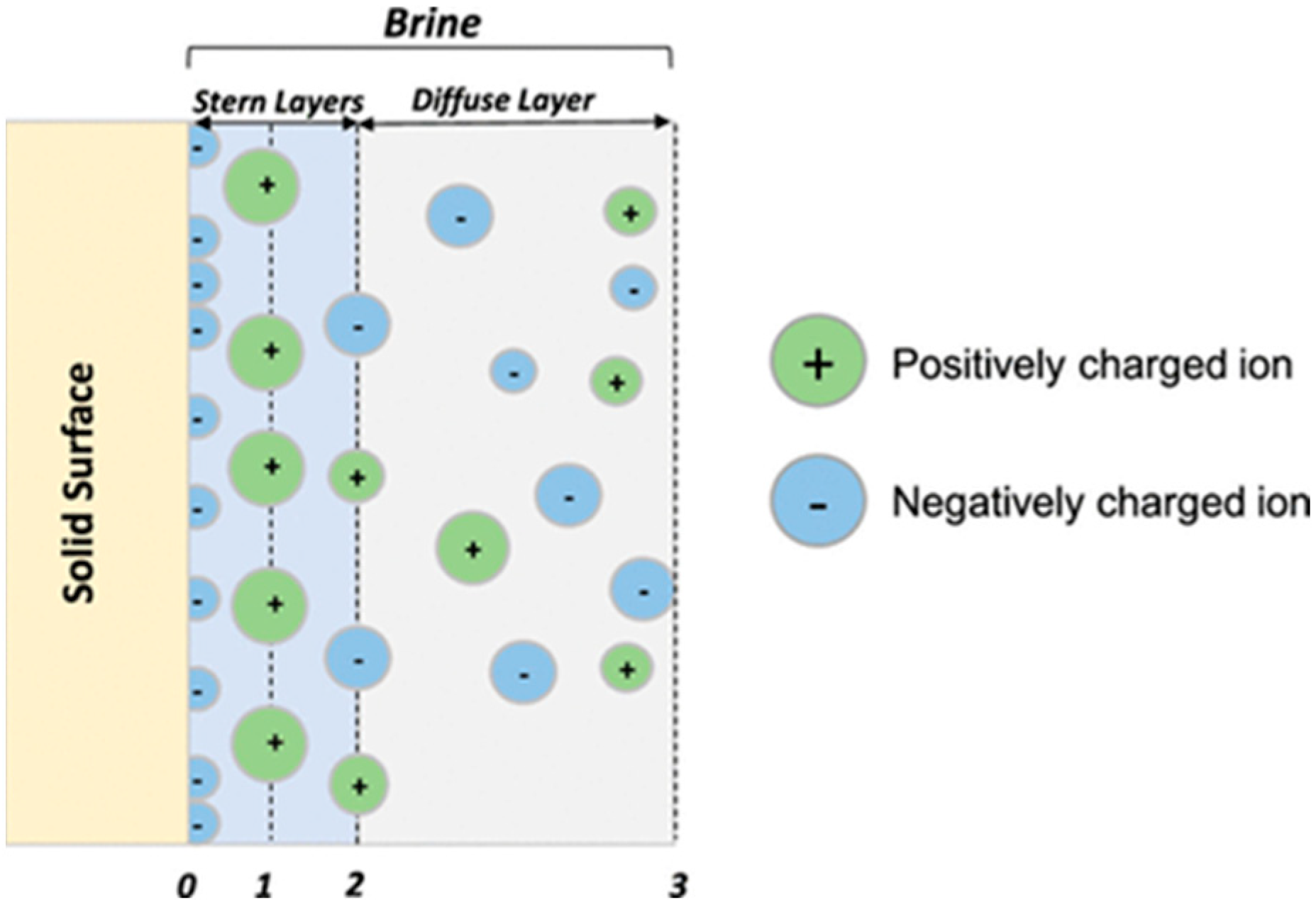
Figure 8. Thus, the triple-layer interface includes the electrical interfacial layer, which is made up of the Stern layer (inner and outer Helmholtz) and the diffuse layer, as well as the solid layer interface.
The carboxyl group produced during the hydrolysis of the polymer determines the amount of adsorption surface ions, as proposed by the triple-interface. Some reactions of the polymer surface with brine are as follows [
62]:
The experimental results obtained by Saeed and Jadhawar [
62] show that the measured zeta potential shifts toward a negative value and the viscosity increases as the polymer concentration increases, which is caused by the repulsion between the polymer chains. Increasing the polymer concentration increases the amount of carboxyl group surface available for adsorption in the triple interface. Therefore, more active negative ions are available to enhance the zeta potential toward a more negative value, as shown in
Figure 9.
4. LSW with NPs and Other Enhancers
Injection of NPs into reservoirs has become widespread in the petroleum industry in the last two decades due to their special physical and chemical properties. Their large surface area to volume ratio property is responsible for the wettability alteration of the rock, viscosity modification of oil and water, and interfacial tension [
63,
64]. It is generally observed during different studies that fine particles are produced when LSW is injected into a clayey–sandy reservoir, which reduces the permeability and causes formation damage [
18,
65]. Olayiwola and Dejam [
23] explained that the injection of NPs into a reservoir for EOR is not as effective as other chemical methods. However, the performance of oil recovery is improved with the combination of LSW and surfactant. Therefore, NPs are dispersed in different aqueous solutions of LSW or surfactant to form a stable colloidal solution that prevents clogging. In addition, the properties of NPs are enhanced to improve oil recovery. The Derjaguin–Landau–Verwey–Overbeek (DLVO) and non-DLVO theories are responsible for the stability of NPs [
23,
43]. High-salinity water reduces the surface charges of NPs (zeta potential), causing the clogging of NPs, while LSW increases the surface charges to increase the zeta potential. In addition to the surface charge of the surfactant, stearic force (non-DLVO) and hydration force also adsorb onto the particle surface of NPs. High-salinity water with a variety of charges and ions screens the particles and causes coagulation of NPs. Therefore, LSW increases the surface potential through proton exchange [
23,
66]. One of the key characteristics of nano-emulsions is their stability. Unstable nano-emulsions exhibit stronger attractive forces between their components, which makes them prone to flocculation and coagulation. To ensure stability, the repulsive forces must outweigh the attractive forces. This can be achieved either by enhancing steric repulsion, where surfactant molecules adsorb onto the particle surfaces, preventing them from coming into close contact, or by electrostatic stabilization, where the distribution of charged species influences particle interactions [
25]. Yousefvand and Jafari [
66] attributed the stability of NPs in deionized water to the formation of hydroxyl group (OH-) on the silanol group and in anionic hydrolyzed polyacrylamide (HPAM) to the stronger repulsive force between the polymer and particles by hydrogen bonding. The lower zeta potential of silica NPs in deionized water not only implies the stability of NPs but also indicates the charges on the NPs classified as anionic NPs [
23,
43,
66]. Fakir et al. [
25] reported that silica NPs with cationic surfactant in LSW form a stable nano-emulsion that has the potential to be used for oil recovery from tight heterogeneous reservoirs. The oil–brine interfacial tension (IFT) initially decreases by 90% upon adding surfactant to the low-salinity water (LSW) and then further decreases by an additional 90% when nanoparticles (NPs) are incorporated into the LSW–surfactant mixture. It has been observed that the NPs become unstable and precipitate with increasing concentrations of salinity.
With the effective application of NPs in the reservoirs, it is expected that oil recovery will increase with increasing concentration. However, optimal recovery is obtained at the optimum concentration of NPs, which requires proper studies for different fields [
18,
66,
67]. Therefore, this work elaborates on the mechanisms involved during the injection of LSW with NPs and other chemical enhancers or stabilizers for EOR.
The application of NPs has been attributed to its ability to alter the wettability, modify the viscosity, and reduce the IFT. Some experimental results have shown that NPs do not have any influence on the recovery. Therefore, an in-depth study to increase the intrinsic understanding of the mechanism involved in the interaction between NPs, brine, and rock has been conducted [
23]. Olayiwola and Dejam [
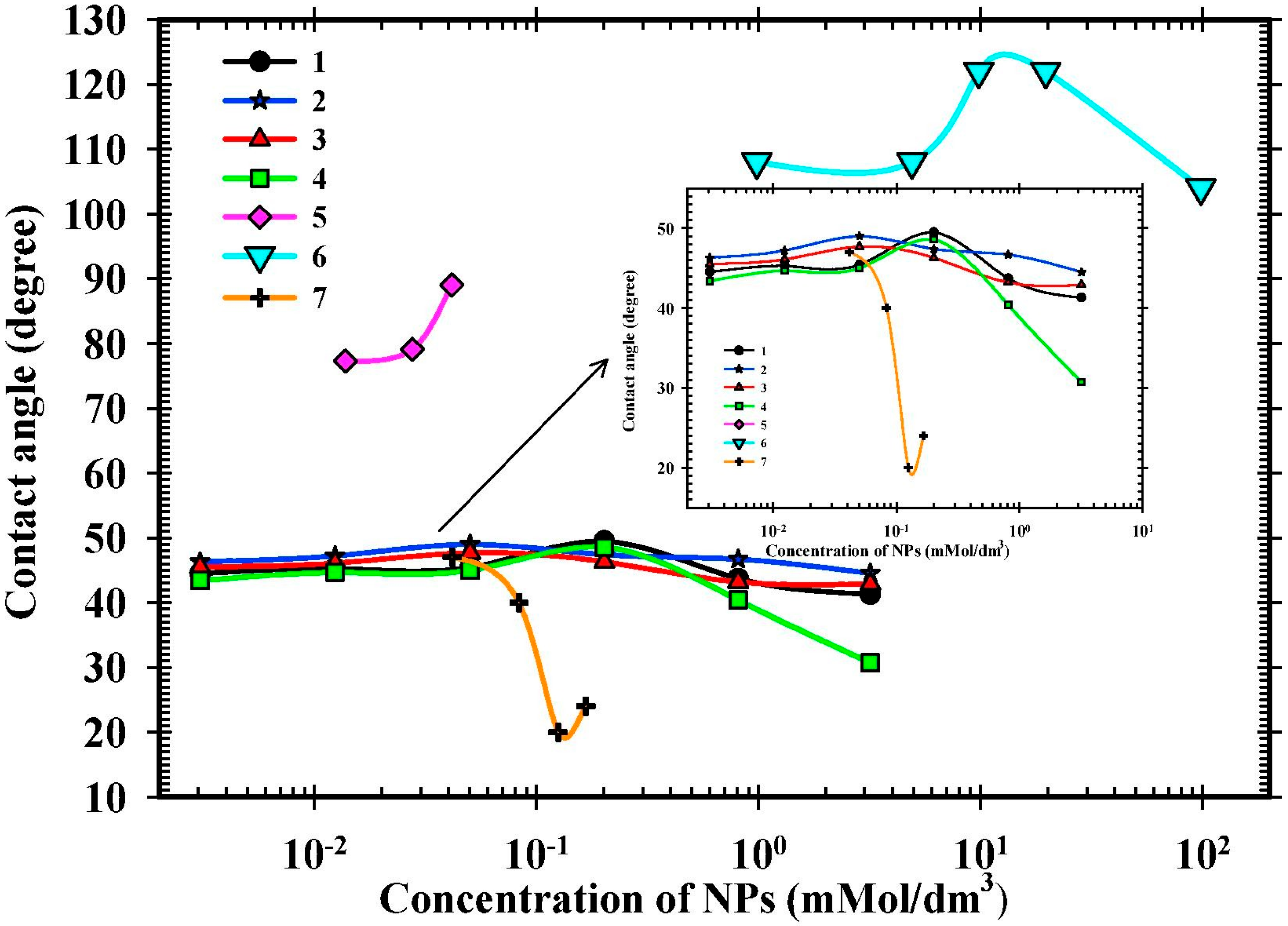
23] reported that the contact angle varies in different patterns with increasing concentrations of NPs, as shown in
Figure 10, which can be directly attributed to the reduction of recovery at high concentrations of NPs.
Vafaei et al. [
70] observed a similar trend in the aqueous solution of NPs and attributed it to the polarization of the thioglycolic acidic functional group of the NPs. The capillary properties may be affected by particle–fluid and electrostatic interaction, which increases the surface area as the distance between the NPs decreases. Lim et al. [
71] showed that NPs create two contact lines (outer and inner) in LSW when observed under a microscope, as shown in
Figure 11. The wedge region in the contact angle is strongly affected by NPs in the aqueous phase due to the structural effect. The structural disjoining is increased with increasing NP volume fractions. The experimental investigation by Fakir et al. [
25] concluded that silica NPs significantly reduce the oil–brine IFT by around 90%, confirming it to be suitable for EOR application.
Olayiwola and Dejam [
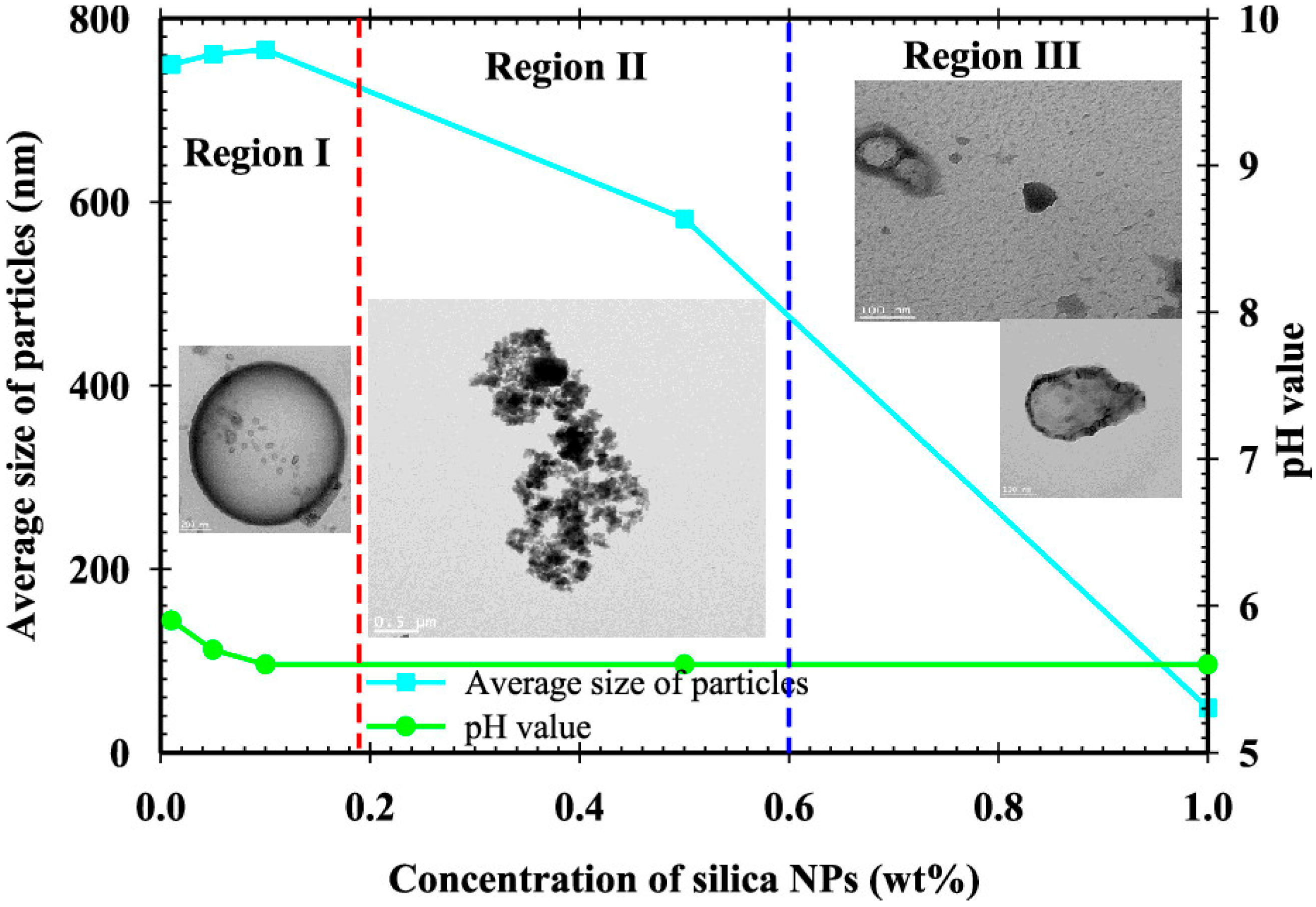
61] classified the forms of silica NPs in saline/aqueous solutions into three regions, as shown in
Figure 12. The amorphous structure in
Figure 13b,c is attributed to the structural form of silica NPs in LSW, as shown in
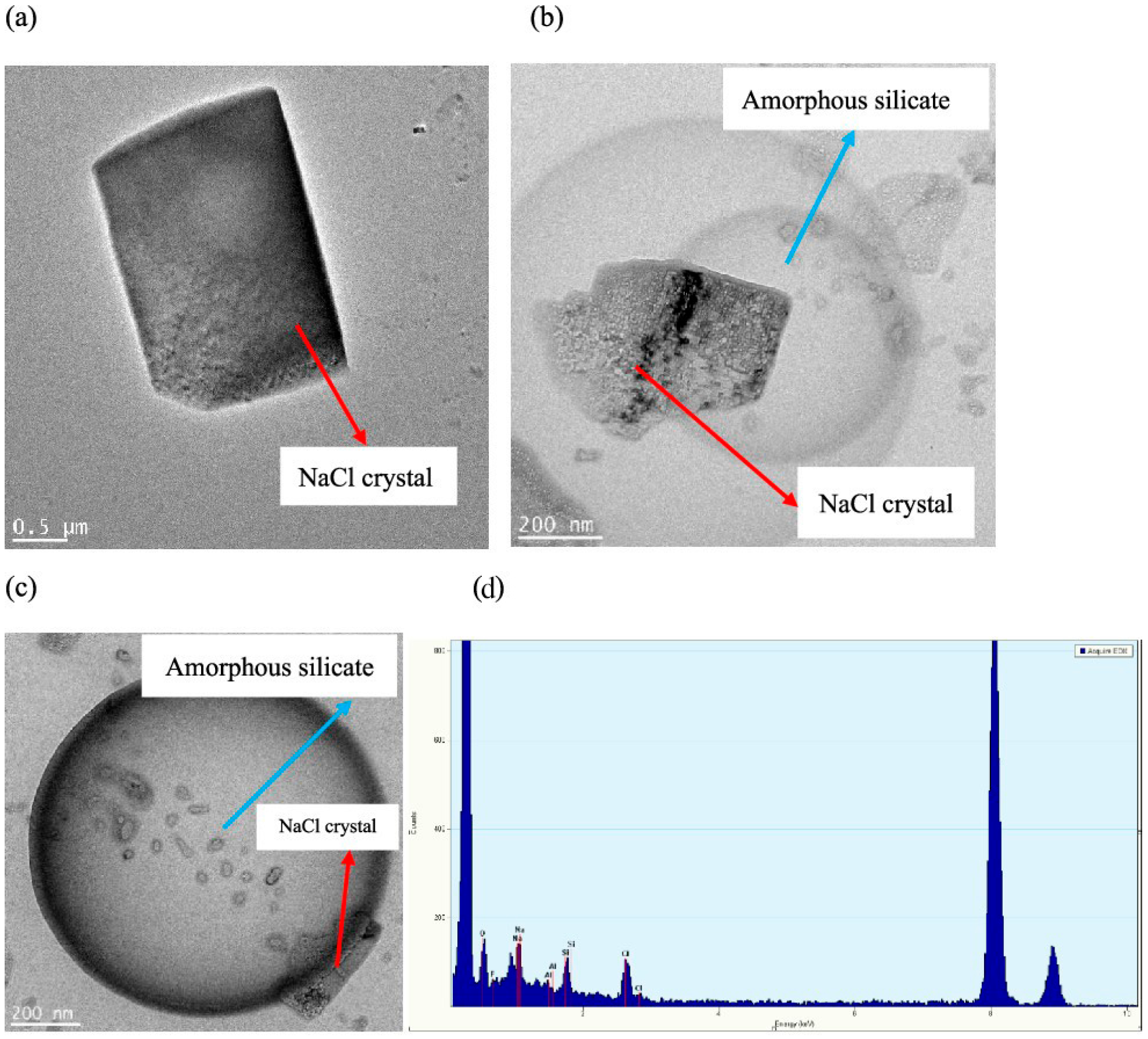
Figure 13. NPs are polyfilaments with functional hairs on the surface, which react with the hydrogen or hydroxyl ions of the electrolytes in the solution. The interaction of saline ions with NPs creates some complex structures, such as silicate in silica and aluminate in alumina NPs, which dissolve in water to form the amorphous structure. Therefore, the outer and inner contact lines, which modify the wettability, can be attributed to the amorphous structure of NPs in LSW.
However, under high-salinity conditions and at high concentrations of NPs, a crystal form of NPs (
Figure 14) has been reported in several studies [
61,
72,
73]. It is important to highlight these properties of NPs due to their influence on the wettability alteration of rock and viscosity modification. Experimental studies have shown that brine viscosity increases with increasing concentrations of NPs, which is attributed to the formation of complex functional groups of NPs, as shown in
Figure 11 and
Figure 13b. Increasing the viscosity of the brine increases the oil recovery [
74].
Olayiwola and Dejam [
23] demonstrated the influence of an aqueous solution (LSW) and surfactant injection on the recovery of oil during NP injection. The alternating injection of NPs with surfactant and LSW directly altered the wettability toward water-wet and neutral-wet states. In addition to wettability alteration, the IFT also plays a crucial role in the recovery of oil.
Figure 15 is a schematic of the mechanism involved during the process. Amorphous NPs are deposited on the water of the porous system, which peels the oil films coating the wall. Crystal is deposited alongside the amorphous NPs, which block the pore system. LSW and surfactant increase the dissolution of the crystal structure to create more amorphous NPs, which are transported to prevent formation damage [
27].
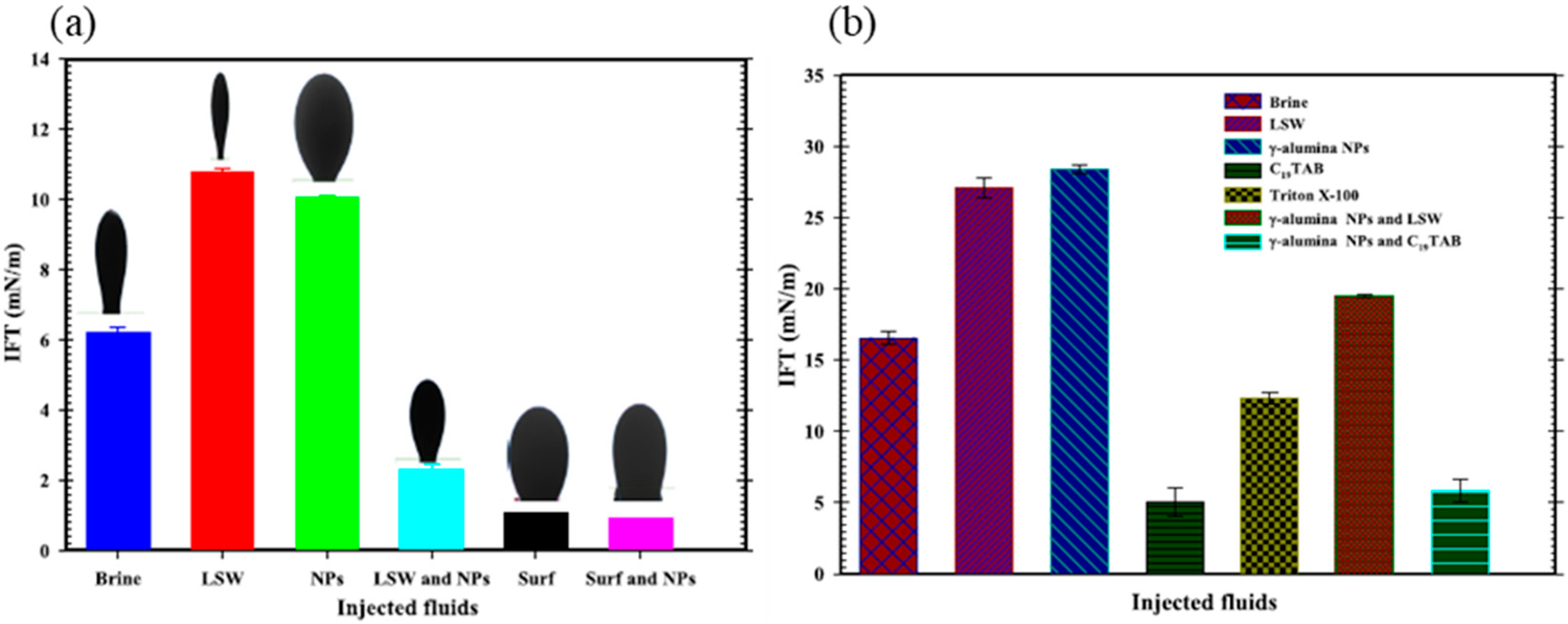
Moreover, NPs greatly reduce the IFT of LSW with oil but have little impact on surfactant, as shown in
Figure 16. Olayiwola and Dejam [
23] attributed the interaction of NPs with brine to the dipole–dipole interaction of the ions and the structural effects of the particles, which creates a discontinuous structure in the boundary to form a wedge. Olayiwola and Dejam (2020) explained that the cohesive force, in addition to the EDL and dipole–dipole interaction, is responsible for the lower IFT of the mixture, which increases oil recovery [
43]. Dordzie and Dejam [
63] explained that the synergy between alternating LSW with alumina NPs can increase oil recovery from a coreflooding experiment by reducing the wettability toward neutral, but it is ineffective when alternating with CTAB, as the wettability is increased toward an oil-wet state and a higher IFT. The increase in recovery is attributed to the positive capillary pressure caused by wettability alteration of the carbonate reservoir, which helps to detach oil droplets from the calcite surface, and to the higher brine viscosity due to viscosity modification by alumina NPs [
63]. The hetero-component (asphaltene) of crude oil interacts with NPs to reduce oil viscosity.
5. Summary
This article provided an in-depth analysis of LSW injection and its integration with various EOR techniques, emphasizing its effectiveness in improving oil displacement and recovery efficiency. The discussion covered the fundamental mechanisms of LSW, including ion exchange, wettability alteration, and IFT reduction, which contribute to increased oil mobilization.
One of the key insights discussed was the synergy between LSW and carbon dioxide injection. The combination of LSW with CO2 enhances oil recovery by promoting wettability alteration and reducing the IFT, while also supporting carbon sequestration efforts. The role of multicomponent ion exchange and carbonate dissolution was highlighted, demonstrating how CO2–LSW injection leads to improved oil displacement efficiency.
The article also explored the combination of LSW with chemical EOR methods, such as surfactants, alkalis, and polymers. The integration of surfactants with LSW was shown to significantly lower the IFT, facilitating oil mobilization. ASP flooding was discussed as a means to further improve oil recovery by reducing surfactant adsorption and promoting in situ soap formation. Polymer augmentation was noted for its ability to enhance the sweep efficiency of LSW by increasing fluid viscosity and modifying flow behavior in reservoirs.
Additionally, the role of nanoparticles in LSW-based EOR strategies was examined. Nanoparticles improve oil recovery by altering wettability, modifying viscosity, and reducing the IFT. The structural effects of NPs in brine, their interaction with rock surfaces, and their ability to stabilize emulsions were discussed as key factors influencing their efficiency. The article also emphasized the importance of optimizing the nanoparticle concentration to maximize recovery while minimizing formation damage.
Overall, this article presented a comprehensive review of LSW injection in combination with CO2, surfactants, polymers, alkalis, and nanoparticles. The discussion highlighted the various mechanisms driving oil recovery improvements, the challenges associated with each method, and the potential for future research and field applications. By integrating LSW with advanced EOR techniques, the oil industry can enhance recovery efficiency while addressing environmental concerns and operational limitations.