The Hydrochar Pre-Coupled Butyrate-Degrading Microbiome Assists the Bioenergy Production from Brewing Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrates
2.2. Preparation of HTC
2.3. Batch Experiment Design
2.4. Determination of Key Physicochemical Properties
2.5. Microbial Community Analysis
2.6. Quantitative PCR Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Effects of Different Enhancement Methods on Biogas and Methane Production
3.2. Changes in Organic Matter in MFLW During AD
3.3. Microbial Diversity and Quantitative Analysis
3.3.1. Microbial Diversity Indices and PCoA Analysis
3.3.2. Microbial Community Composition of Bacteria and Archaea
3.3.3. Absolute Quantification of Bacteria and Archaea
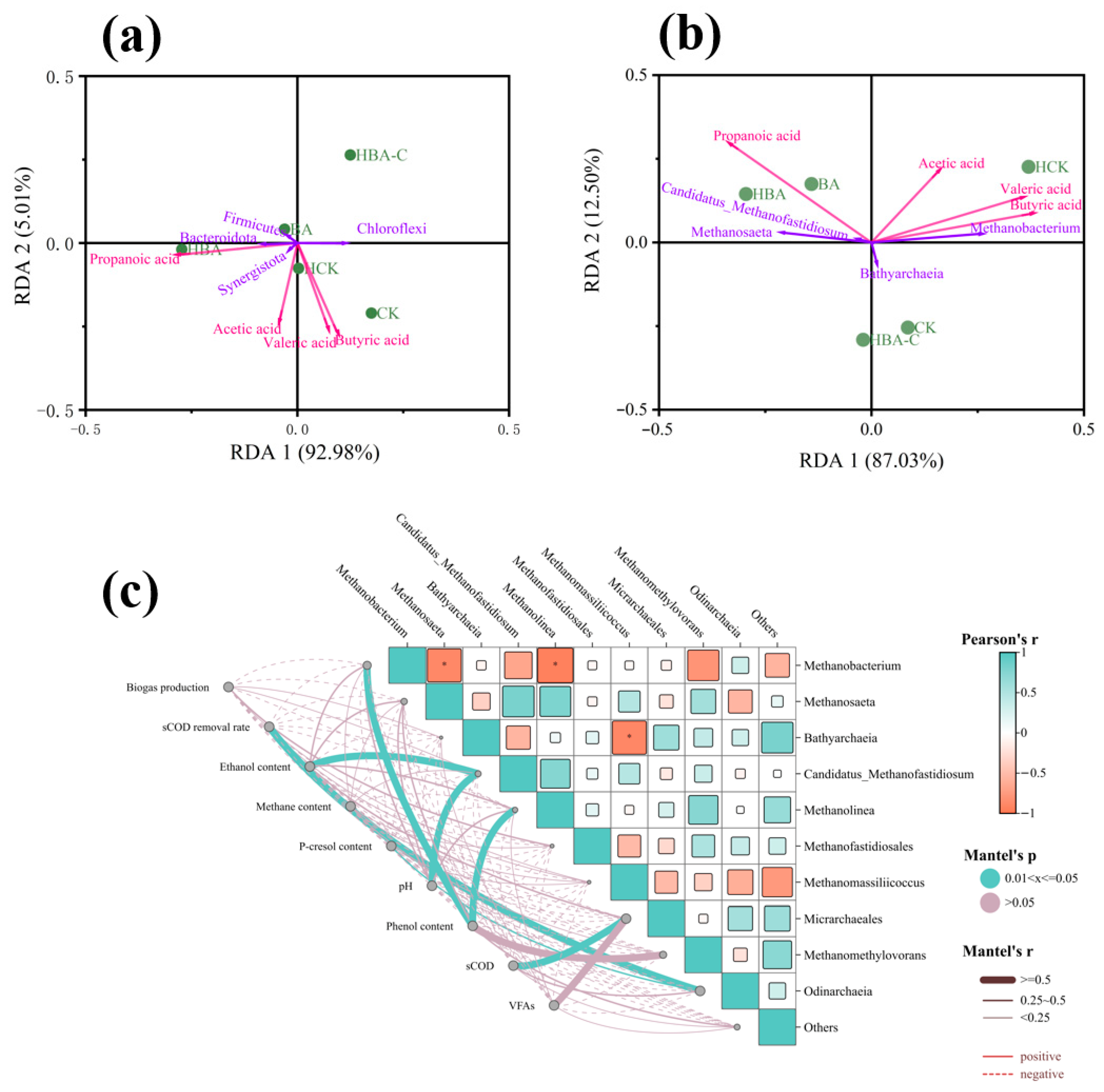
3.3.4. Analysis of Microbial and Environmental Factor Correlations
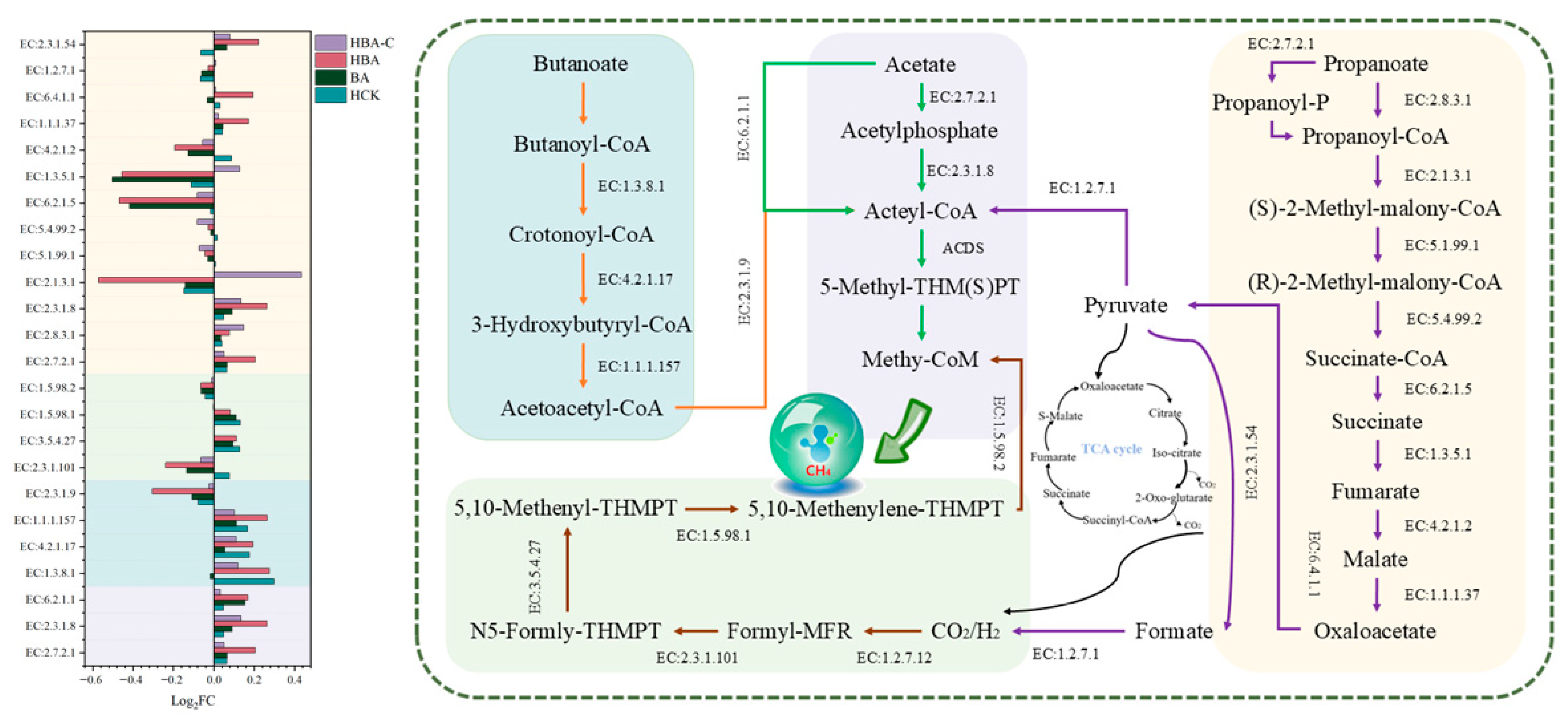
3.3.5. Acid Metabolic Pathways and Relative Abundance of Key Enzymes
3.4. Practical Implications and Economic Considerations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minnalkodi Senguttuvan, K.R.; Sellappa, K.; Kuppusamy, S. Performance Evaluation of the Electro-Fenton Process for Distillery Wastewater Treatment. Sustainability 2024, 16, 6512. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Ye, M.; Zha, X.; He, R. Effects of Fe-modified digestate hydrochar at different hydrothermal temperatures on anaerobic digestion of swine manure. Bioresour. Technol. 2024, 395, 130393. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, X.; Song, Y.; Lv, X.; Sun, Y. Effects of different concentrations of butyrate on microbial community construction and metabolic pathways in anaerobic digestion. Bioresour. Technol. 2023, 377, 128845. [Google Scholar] [CrossRef]
- Bueno, B.E.; Soares, L.A.; Quispe-Arpasi, D.; Sakamoto, I.K.; Zhang, Y.; Varesche, M.B.; Ribeiro, R.; Tommaso, G. Anaerobic digestion of aqueous phase from hydrothermal liquefaction of Spirulina using biostimulated sludge. Bioresour. Technol. 2020, 312, 123552. [Google Scholar] [CrossRef]
- Fan, Q.; Fan, X.; Fu, P.; Li, Y.; Zhao, Y.; Hua, D. Anaerobic digestion of wood vinegar wastewater using domesticated sludge: Focusing on the relationship between organic degradation and microbial communities (archaea, bacteria, and fungi). Bioresour. Technol. 2022, 347, 126384. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite triggering enhanced direct interspecies electron transfer: A scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef]
- Shi, Z.; Campanaro, S.; Usman, M.; Treu, L.; Basile, A.; Angelidaki, I.; Zhang, S.; Luo, G. Genome-centric metatranscriptomics analysis reveals the role of hydrochar in anaerobic digestion of waste activated sludge. Environ. Sci. Technol. 2021, 55, 8351–8361. [Google Scholar] [CrossRef]
- Bu, J.; Wei, H.; Wang, Y.; Cheng, J.; Zhu, M. Biochar boosts dark fermentative H2 production from sugarcane bagasse by selective enrichment/colonization of functional bacteria and enhancing extracellular electron transfer. Water Res. 2021, 202, 117440. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Usman, M.; He, J.; Chen, H.; Zhang, S.; Luo, G. Combined microbial transcript and metabolic analysis reveals the different roles of hydrochar and biochar in promoting anaerobic digestion of waste activated sludge. Water Res. 2021, 205, 117679. [Google Scholar] [CrossRef]
- Ren, S.; Usman, M.; Tsang, D.C.W.; O-Thong, S.; Angelidaki, I.; Zhu, X.; Zhang, S.; Luo, G. Hydrochar-facilitated anaerobic digestion: Evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environ. Sci. Technol. 2020, 54, 5755–5766. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- He, Y.; Wang, S.; Shen, C.; Wang, Z.; Liu, Y.; Meng, X.; Li, X.; Zhao, X.; Chen, J.; Xu, J. Biochar accelerates methane production efficiency from Baijiu wastewater: Some viewpoints considering direct interspecies electron transfer. Chem. Eng. J. 2024, 497, 154527. [Google Scholar] [CrossRef]
- Cui, Y.; Mao, F.; Zhang, J.; He, Y.; Tong, Y.; Peng, Y. Biochar enhanced high-solid mesophilic anaerobic digestion of food waste: Cell viability and methanogenic pathways. Chemosphere 2021, 272, 129863. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Shen, C.; Wang, S.; Liu, Y.; Chen, S.; Li, X.; Zhao, X.; Chen, J.; Shi, J. Enhancing anaerobic digestion efficiency using biochars: Mechanisms and material perspectives. Renew. Energy 2026, 256, 124147. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Luo, G. The Role of Hydrochar in Promoting Methane Production from Anaerobic Digestion with Different Inocula. Fermentation 2023, 9, 433. [Google Scholar] [CrossRef]
- Wang, R.; Peng, P.; Song, G.; Zhao, Z.; Yin, Q. Effect of corn stover hydrochar on anaerobic digestion performance of its associated wastewater. Environ. Pollut. 2022, 315, 120430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Z.; Liu, K.; Si, B.; Yang, G.; Tian, C.; Zhang, Y. Multi-cycle anaerobic digestion of hydrothermal liquefaction aqueous phase: Role of carbon and iron based conductive materials in inhibitory compounds degradation, microbial structure shaping, and interspecies electron transfer regulation. Chem. Eng. J. 2023, 454, 140019. [Google Scholar] [CrossRef]
- Usman, M.; Shi, Z.; Cai, Y.; Zhang, S.; Luo, G. Microbial insights towards understanding the role of hydrochar in enhancing phenol degradation in anaerobic digestion. Environ. Pollut. 2023, 330, 121779. [Google Scholar] [CrossRef]
- Rea, V.G.; Bueno, B.E.; Cerqueda-García, D.; Sierra, J.D.M.; Spanjers, H.; van Lier, J.B. Degradation of p-cresol, resorcinol, and phenol in anaerobic membrane bioreactors under saline conditions. Chem. Eng. J. 2022, 430, 132672. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Q.; Luo, X.; Yan, S.; Sun, Q.; Zheng, Y.; Zhen, G. In-depth exploration of microbial electrolysis cell coupled with anaerobic digestion (MEC-AD) for methanogenesis in treating protein wastewater at high organic loading rates. Energy Convers. Manag. 2025, 323, 119152. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, H.; Yao, Y.; Jing, C.; Liu, R.; Niu, Q.; Lu, H. Overcoming methanogenesis barrier to acid inhibition and enhancing PAHs removal by granular biochar during anaerobic digestion. Chem. Eng. J. 2023, 477, 147229. [Google Scholar] [CrossRef]
- Zhou, G.; Huang, X.; Zhang, S.; Xiang, Z.; Wei, J.; Ma, S.; Teng, X.; Zheng, Z. Volatile fatty acids (VFAs) production from sludge and chicken manure anaerobic co-fermentation: Effects of mixing ratio and microbial mechanisms. J. Environ. Chem. Eng. 2024, 12, 114014. [Google Scholar] [CrossRef]
- Yan, S.; Wang, M.; Zhang, S.; Tong, Z.; Li, S.; Yong, X.; Zhang, X.; Zhou, J. Fe-doped hydrochar facilitating simultaneous methane production and pharmaceutical and personal care products (PPCPs) degradation in co-anaerobic digestion of municipal sludge and food waste. Chem. Eng. J. 2023, 474, 146001. [Google Scholar] [CrossRef]
- You, J.; Farghali, M.; Osman, A.I.; Yoshida, G.; Ihara, I. Mechanisms of biochar-mediated reduction of antibiotic-resistant bacteria and biogas production enhancement in anaerobic digesters. Biochem. Eng. J. 2024, 211, 109465. [Google Scholar] [CrossRef]
- Usman, M.; Shi, Z.; Ji, M.; Ren, S.; Luo, G.; Zhang, S. Microbial insights towards understanding the role of hydrochar in alleviating ammonia inhibition during anaerobic digestion. Chem. Eng. J. 2021, 419, 129541. [Google Scholar] [CrossRef]
- Guo, M.; Wei, S.; Guo, M.; Li, M.; Qi, X.; Wang, Y.; Jia, X. Potential mechanisms of propionate degradation and methanogenesis in anaerobic digestion coupled with microbial electrolysis cell system: Importance of biocathode. Bioresour. Technol. 2024, 400, 130695. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Luo, C.; Shao, L.; He, P. Biochar alleviates combined stress of ammonium and acids by firstly enriching Methanosaeta and then Methanosarcina. Water Res. 2016, 90, 34–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, N.; Guo, B.; Mohammed, A.; Zhang, L.; Liu, Y. Conductive biofilms in up-flow anaerobic sludge blanket enhanced biomethane recovery from municipal sewage under ambient temperatures. Bioresour. Technol. 2022, 361, 127658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhang, Y.; Zou, H.; Zheng, Y.; Guo, R.; Fu, S. Understanding the mechanisms behind enhanced anaerobic digestion of corn straw by humic acids. Bioresour. Technol. 2022, 359, 127454. [Google Scholar] [CrossRef]
- Wang, F.; He, Z.; Tang, C.; Zhou, A.; Liu, W.; Ren, Y.; Li, Z.; Wang, A. Aluminum chloride enhances the production of short-chain fatty acids from waste activated sludge: Insights to performance, mechanism, and implications. J. Water Process. Eng. 2024, 57, 104668. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Liu, K.; Li, Q.; Li, W.; Fan, J.; Wang, S.; Shi, F.; Zuo, X.; Li, P. Relationship between the effects of heat pre-treatment on anaerobic performance of pig manure and the microbial variation within reactors. Chem. Eng. J. 2023, 461, 141991. [Google Scholar] [CrossRef]
- Muñoz-Páez, K.M.; Ramos-Arechiga, K.Y.; Buitrón, G. Ex-situ biogas enrichment by hydrogenotrophic methanogens at low H2/CO2 ratios: Effect of empty bed residence time. Fuel 2025, 381, 133330. [Google Scholar] [CrossRef]
- Franchi, O.; Cabrol, L.; Chamy, R.; Rosenkranz, F. Correlations between microbial population dynamics, bamA gene abundance and performance of anaerobic sequencing batch reactor (ASBR) treating increasing concentrations of phenol. J. Biotechnol. 2020, 310, 40–48. [Google Scholar] [CrossRef]
- Guo, Y.; Askari, N.; Smets, I.; Appels, L. A review on co-metabolic degradation of organic micropollutants during anaerobic digestion: Linkages between functional groups and digestion stages. Water Res. 2024, 256, 121598. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Chen, Z.; Gong, H.; Guo, X.; Yu, H.; Chen, L. Enhancement of anaerobic digestion by adding elemental sulfur. Bioresour. Technol. 2025, 416, 131820. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Green, H.; Tao, W. Reversibility of propionic acid inhibition to anaerobic digestion: Inhibition kinetics and microbial mechanism. Chemosphere 2020, 255, 126840. [Google Scholar] [CrossRef] [PubMed]



| Groups | Hydrochar Addition (g) | Inoculum (mL) | Substrate (mL) | Hydrochar Load (g) | sCOD (mg/L) | System Solid Load (g TS/L) |
|---|---|---|---|---|---|---|
| CK | 0 | 150 (R1) | 150 | 0 | 13,345.86 ± 433.07 | 6.81 |
| HCK | 3 | 150 (R1) | 150 | 3 | 13,904.72 ± 506.90 | 6.81 |
| BA | 3 | 150 (R2) | 150 | 3 | 13,403.27 ± 482.87 | 6.81 |
| HBA | 3 | 150 (R2) | 150 | 3 | 13,495.40 ± 432.04 | 6.81 |
| HBA-C | 0 | 150 (R3) | 150 | 3 | 13,348.88 ± 408.27 | 6.81 |
| Y0 | Rm | λ | R2 | Measured Final Methane Yield | |
|---|---|---|---|---|---|
| mL | mL day−1 | day | mL | ||
| CK | 896.89 ± 3.56 | 127.12 ± 7.51 | 0.33 ± 0.08 | 0.99 | 944.55 ± 20.58 |
| HCK | 911.99 ± 1.87 | 145.90 ± 6.20 | 0.17 ± 0.05 | 0.97 | 970.45 ± 3.40 |
| BA | 955.25 ± 2.08 | 197.46 ± 5.57 | 0.06 ± 0.02 | 0.99 | 1030.01 ± 27.53 |
| HBA | 938.38 ± 1.76 | 214.56 ± 2.65 | 0.22 ± 0.04 | 0.99 | 1017.10 ± 8.94 |
| HBA-C | 975.83 ± 1.50 | 213.21 ± 1.79 | 0 | 0.95 | 1090.15 ± 22.49 |
| Chao1 (Bacteria/ Archaea) | Shannon | Simpson | Coverage | |
|---|---|---|---|---|
| CK | 4905.81/398.673 | 8.57553/3.13641 | 0.986846/0.723664 | 0.97745/0.998971 |
| HCK | 3818.7/280.26 | 8.01142/2.32419 | 0.98176/0.55253 | 0.981531/0.999233 |
| BA | 4689.37/422.383 | 8.55799/2.98412 | 0.984866/0.725788 | 0.979359/0.999018 |
| HBA | 4151.39/349.939 | 8.16553/3.08905 | 0.98055/0.766452 | 0.981043/0.999131 |
| HBA-C | 5353.57/442.022 | 8.76358/3.36802 | 0.988092/0.763719 | 0.974541/0.998592 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Z.; Wang, X.; Shen, C.; He, Y.; Li, S.; Chen, J.; Wang, S.; Zhuang, W.; Meng, X.; et al. The Hydrochar Pre-Coupled Butyrate-Degrading Microbiome Assists the Bioenergy Production from Brewing Wastewater. Processes 2025, 13, 2634. https://doi.org/10.3390/pr13082634
Li X, Wang Z, Wang X, Shen C, He Y, Li S, Chen J, Wang S, Zhuang W, Meng X, et al. The Hydrochar Pre-Coupled Butyrate-Degrading Microbiome Assists the Bioenergy Production from Brewing Wastewater. Processes. 2025; 13(8):2634. https://doi.org/10.3390/pr13082634
Chicago/Turabian StyleLi, Xiaoyong, Zhi Wang, Xi Wang, Caihong Shen, Yun He, Shiru Li, Jinmeng Chen, Shilei Wang, Wei Zhuang, Xingyao Meng, and et al. 2025. "The Hydrochar Pre-Coupled Butyrate-Degrading Microbiome Assists the Bioenergy Production from Brewing Wastewater" Processes 13, no. 8: 2634. https://doi.org/10.3390/pr13082634
APA StyleLi, X., Wang, Z., Wang, X., Shen, C., He, Y., Li, S., Chen, J., Wang, S., Zhuang, W., Meng, X., Cai, Y., Xu, J., & Ying, H. (2025). The Hydrochar Pre-Coupled Butyrate-Degrading Microbiome Assists the Bioenergy Production from Brewing Wastewater. Processes, 13(8), 2634. https://doi.org/10.3390/pr13082634








