New Evidence of the Effect of Post-Combustion During Roasting/Reduction of Nickel-Bearing Lateritic Ores
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical-Physical Characteristics of the Lateritic Ore
2.2. Characteristics of the Experimental Facility
2.3. Analytical Techniques
2.4. Methodology for Determining Kinetic Behavior
- (a)
- Verification of compliance and stability with the furnace operating conditions.
- (b)
- Preparation of 9 samplers, previously coded with the number of each even-numbered furnace chamber, containing 200 mL of ammonia-carbonate liquor with an NH3 concentration of 80–85 g/L.
- (c)
- Determination of the mass of each sampler with the 200 mL of previously added liquor.
- (d)
- Addition of an argon flow to each sampler to prevent the reoxidation process of the previously reduced samples taken from the even-numbered hearths.
- (e)
- Collection of reduced ore samples from the even-numbered furnace hearts, from H-0 to H-16, with a 10 min interval between each sampler.
- (f)
- Determination of the mass of reduced ore using the weight difference method.
- (g)
- Leaching of the reduced mineral taken from each furnace hearth for 2 h with the ammonia-carbonate liquor with an NH3 concentration of 80–85 g/L (liquid/solid ratio of 10:1) in stirred-tank reactors.
- (h)
- Filtering and vacuum-washing the suspensions (formulated in g) with 200 mL of ammonia-carbonate solutions at (7 and 3%) and distilled water, respectively.
- (i)
- Drying the filtered solid in the oven at 150 °C for 2 h.
- (j)
- Cooling the dry solid to room temperature and grinding it until everything had a particle size of less than 0.150 mm.
- (k)
- Homogenization of the ground solid and characterize the elements of interest in a certified analytical laboratory.
- (l)
- Plotting the amount of residual Ni (%) of each of the leached solid samples as a function of time (min).
2.5. Methodology to Identify the Mechanism That Describes the Roasting/Reduction Process
3. Results and Discussions
3.1. Chemical-Physical Characteristics of the Reduced Mineral
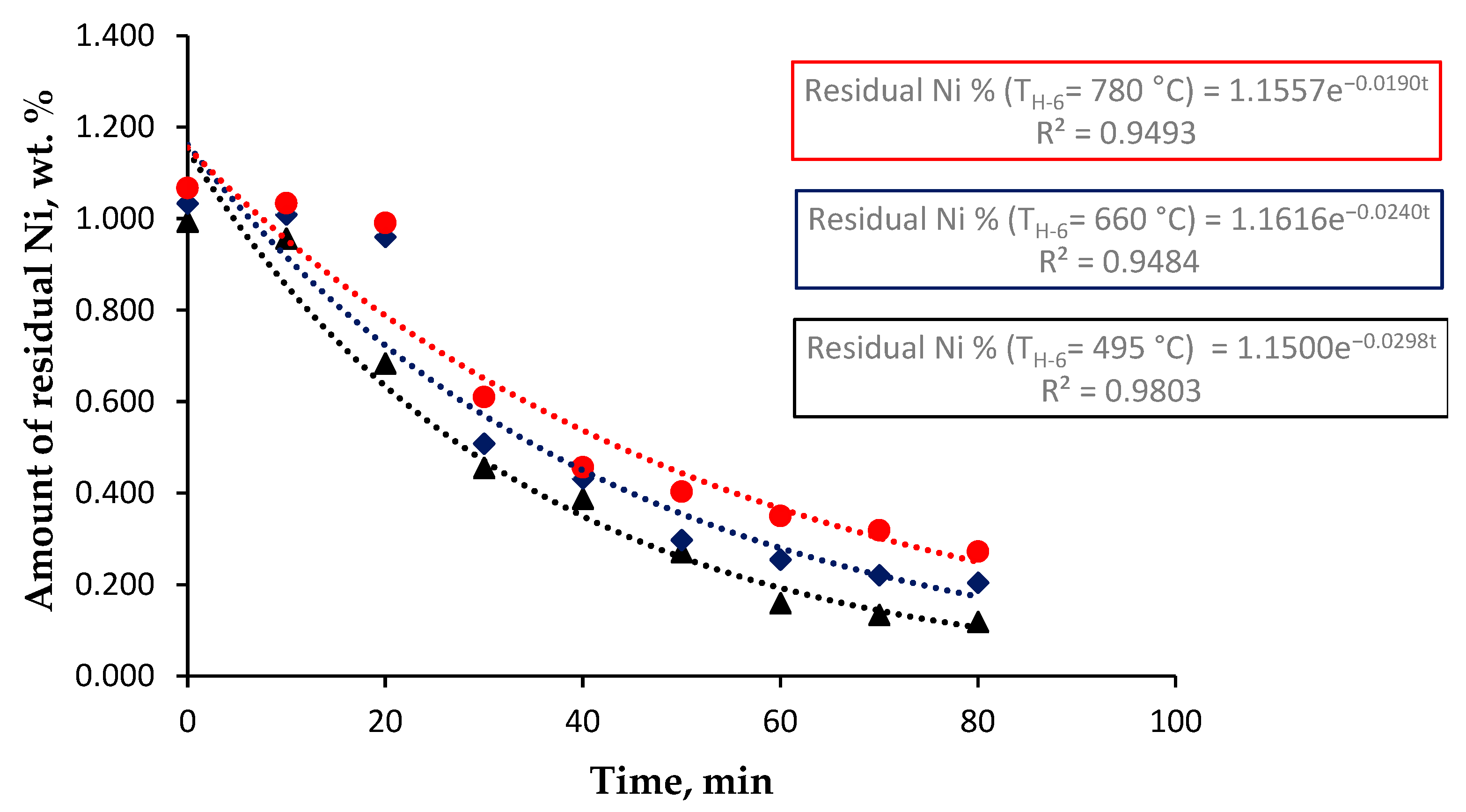
3.2. Effect of Post-Combustion Air on the Reaction Rate Constant of the Roasting/Reduction Process
3.3. Effect of Post-Combustion Air on the Granulometric Behavior of the Reduced Ore
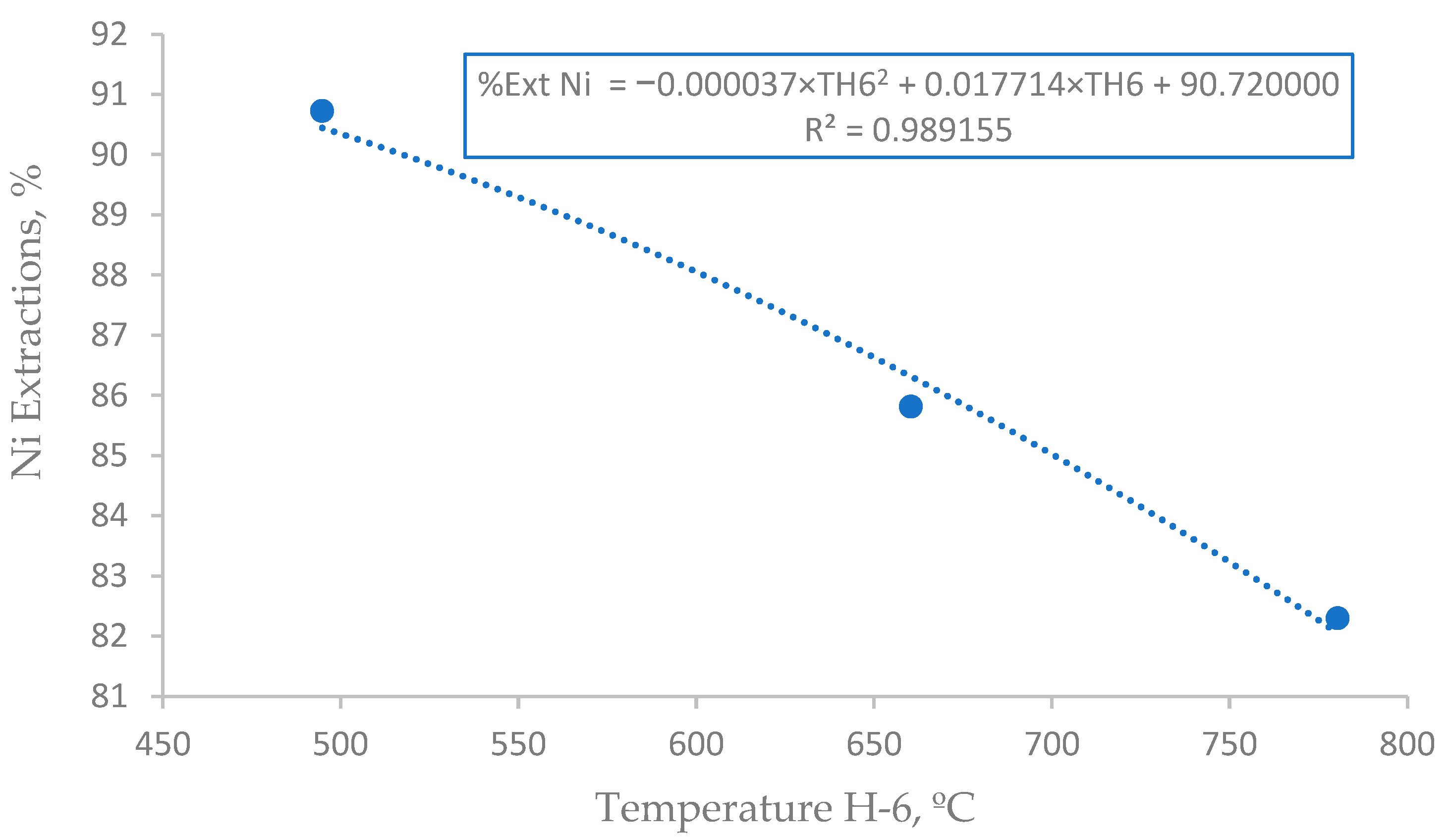
3.4. Effect of Post-Combustion Air on Nickel Extraction
4. Conclusions
- ▪
- A decrease in the reaction rate of the roasting/reduction process (k decreases 7.0938% for every 57 °C of temperature increase in H-6).
- ▪
- A considerable increase in the average particle size, making the diffusive processes controlling the process difficult.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Time, min | 1 | 2 | 3 | Average |
|---|---|---|---|---|
| 0 | 0.980 | 1.010 | 0.990 | 0.993 |
| 10 | 0.950 | 0.980 | 0.940 | 0.957 |
| 20 | 0.695 | 0.750 | 0.605 | 0.683 |
| 30 | 0.435 | 0.410 | 0.520 | 0.455 |
| 40 | 0.376 | 0.367 | 0.421 | 0.388 |
| 50 | 0.251 | 0.285 | 0.277 | 0.271 |
| 60 | 0.147 | 0.171 | 0.159 | 0.159 |
| 70 | 0.130 | 0.139 | 0.131 | 0.133 |
| 80 | 0.112 | 0.123 | 0.119 | 0.118 |
| Time, min | 1 | 2 | 3 | Average |
|---|---|---|---|---|
| 0 | 1.050 | 1.020 | 1.030 | 1.033 |
| 10 | 0.997 | 1.010 | 1.020 | 1.009 |
| 20 | 0.970 | 0.960 | 0.950 | 0.960 |
| 30 | 0.533 | 0.503 | 0.489 | 0.508 |
| 40 | 0.408 | 0.466 | 0.424 | 0.433 |
| 50 | 0.271 | 0.306 | 0.315 | 0.297 |
| 60 | 0.255 | 0.277 | 0.232 | 0.255 |
| 70 | 0.213 | 0.229 | 0.219 | 0.220 |
| 80 | 0.198 | 0.210 | 0.205 | 0.204 |
| Time, min | 1 | 2 | 3 | Average |
|---|---|---|---|---|
| 0 | 1.056 | 1.067 | 1.078 | 1.067 |
| 10 | 1.022 | 1.030 | 1.050 | 1.034 |
| 20 | 0.983 | 0.980 | 1.010 | 0.991 |
| 30 | 0.581 | 0.616 | 0.633 | 0.610 |
| 40 | 0.430 | 0.460 | 0.479 | 0.456 |
| 50 | 0.409 | 0.380 | 0.420 | 0.403 |
| 60 | 0.320 | 0.350 | 0.380 | 0.350 |
| 70 | 0.300 | 0.310 | 0.347 | 0.319 |
| 80 | 0.281 | 0.259 | 0.276 | 0.272 |
Appendix B
| Temperature H-6, °C | Complete Reaction Time, min | |||||
|---|---|---|---|---|---|---|
| Chemical Reaction (ChR) | Diffusion | Growth of Nuclei | ||||
| Through the Gas Film (DGF) | Through the Ash Layer (DAL) | GN-2 | GN-3 | GN-5 | ||
| 495 | 146 | 77 | 161 | 48 | 51 | 54 |
| 660 | 174 | 85 | 211 | 53 | 55 | 56 |
| 780 | 203 | 94 | 277 | 59 | 59 | 59 |
| Experimental Time, min | Estimated Time, min | ||
|---|---|---|---|
| TH-6 (780 °C) | TH-6 (660 °C) | TH-6 (495 °C) | |
| 0 | 0 | 0 | 0 |
| 10 | 9 | 8 | 8 |
| 20 | 12 | 12 | 21 |
| 30 | 35 | 36 | 34 |
| 40 | 49 | 43 | 39 |
| 50 | 55 | 58 | 50 |
| 60 | 62 | 64 | 66 |
| 70 | 67 | 69 | 71 |
| 80 | 74 | 72 | 74 |
| R2 | 0.9623 | 0.9576 | 0.9835 |
| Residual Error | 5.32 | 5.72 | 3.46 |
References
- Topak, A.; Toprak Döşlü, S. Electrochemical Tuning of Ni-Fe Catalysts Using Various Techniques for Efficient Hydrogen Evolution in Alkaline Media. Processes 2025, 13, 644. [Google Scholar] [CrossRef]
- Sang, P.; Luo, W.; Li, W.; Wang, C.; Chen, Y.; Zhou, L.; Ma, Z.; Wang, D.; Duan, Y. A Novel Martensitic Stainless Steel Material for CO2 Corrosion Environment. Processes 2024, 12, 2912. [Google Scholar] [CrossRef]
- Valencia, F.; Rabbani, M.; Fahimi, A.; Vahidi, E. Assessing the environmental burden of nickel sulfate for batteries: A life cycle perspective. Res. Conserv. Recycl. 2025, 215, 108130. [Google Scholar] [CrossRef]
- Lv, W.; Makuza, B.; Wang, F.; Marcuson, S.; Barati, M. A Review of Direct Reduction–Magnetic Separation Process for Ferronickel Production from Nickel Laterite. J. Sustain. Metall. 2025, 11, 3–28. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Q.; Bai, Z.; Yuan, S.; Li, Y.; Han, Y. Semi-industrial experiment on efficient preparation of nickel-iron alloy from laterite nickel ore: A suspension roasting pre-reduction electric furnace melting (SRPEF) process. J. Taiwan Inst. Chem. Eng. 2025, 173, 106164. [Google Scholar] [CrossRef]
- Zevgolis, E.N.; Daskalakis, K.A. Nickel Prod. The Nickel Production Methods from Laterites and the Greek Ferronickel Production among Them. Mater. Proc. 2022, 5, 104. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.; Wang, B.; Luo, J.; Peng, Z.; Chen, Y.; Li, G.; Rao, M. Sodium sulfate-assisted reductive roasting for enhanced Ni and Co recovery from limonitic laterite: Mechanism and pilot-scale rotary-kiln validation. Int. J. Miner. Metall. Mater. 2025. [Google Scholar] [CrossRef]
- Caron, M.H. Process of Recovering Values from Nickel and Cobalt-Nickel Ores. U.S. Patent 1 487 145, 18 March 1924. [Google Scholar]
- Angulo Palma, H.J.; Legrá, Á.L.; Urgellés, A.L.; Pedrera, C.H.; Gallegos, S.; Galleguillos, M.F.M.; Toro, N. Use of a mixture of coal and oil as an additive for selective reduction of lateritic ore by the Caron process. Hem. Ind. 2024, 78, 17–27. [Google Scholar] [CrossRef]
- Angulo Palma, H.J.; Legrá Legrá, A.; Lamorú Urgellés, A.; Gálvez, E.; Castillo, J. Post-combustion Effect on Nickel and Cobalt Extractions from the Caron Process. In Proceedings of the Fourth International Conference on Inventive Material Science Applications. Advances in Sustainability Science and Technology; Bindhu, V., Tavares, J.M.R.S., Ţălu, Ş., Eds.; Springer: Singapore, 2022; pp. 515–527. [Google Scholar] [CrossRef]
- Rojas Vargas, A.; Magaña Haynes, M.E.; Riverón, A.R. Lixiviación carbonato amoniacal: Estimación del níquel disuelto en el efluente de destilación. Rev. De Metal. 2019, 55, e149. [Google Scholar] [CrossRef]
- Canterford, J. Oxide Ores of Nickel—The Australian Situation. Miner. Procesing Extr. Metall. Rev. 1983, 1, 85–109. [Google Scholar] [CrossRef]
- Caron, M.H. Fundamental and practical factors in ammonia leaching of nickel and cobalt ores. JOM-J. Miner. Met. Mater. Soc. 1950, 2, 67–90. [Google Scholar] [CrossRef]
- De Graaf, J. The treatment of lateritic nickel ores—A further study of the Caron process and other possible improvements. Part I. Effect of reduction conditions. Hydrometallurgy 1979, 5, 47–65. [Google Scholar] [CrossRef]
- Chander, S.; Sharma, V. Reduction roasting/ammonia leaching of nickeliferous laterites. Hydrometallurgy 1981, 7, 315–327. [Google Scholar] [CrossRef]
- Chen, J.-h.; Jak, E.; Hayes, P. Investigation of the reduction roasting of saprolite ores in the Caron process: Microstructure evolution and phase transformations. Miner. Process. Extr. Metall. 2019, 130, 148–159. [Google Scholar] [CrossRef]
- Chen, J.; Jak, E.; Hayes, P. Investigation of the reduction roasting of saprolite ores in the Caron process: Reaction mechanisms and reduction kinetics. Miner. Process. Extr. Metall. 2019, 130, 425–432. [Google Scholar] [CrossRef]
- Angulo Palma, H.J. Sustitución Del Petróleo Aditivo Por Carbón Bituminoso En El Proceso De Tostación/Reducción De La Tecnología Caron. Ph.D. Thesis, Universidad de Oriente, Santiago de Compostela, Cuba, 11 April 2024. [Google Scholar]
- Mano, E.S.; Caner, L.; Petit, S.; Chaves, A.P.; Mexias, A.S. Ni-smectitic ore behaviour during the Caron process. Hydrometallurgy 2019, 186, 200–209. [Google Scholar] [CrossRef]
- Coello Velázquez, A.L.; Llorente Arce, L.; García, A. Efecto del petróleo aditivo en los indicadores energo-tecnológicos en los circuitos cerrados de molienda del mineral laterítico a nivel industrial. Min. Y Geol. 2020, 36, 316–327. [Google Scholar]
- Véliz Jardines, A.I.; Miranda López, J. Desarrollo de investigaciones sobre la tecnología Caron durante el procesamiento de las lateritas de baja ley de níquel y de los escombros lateríticos, clasificados como: Menas o minerales no industriales. Tecnol. Química 2022, 42, 361–383. [Google Scholar]
- Kawahara, M.; Toguri, J.; Bergman, R. Reducibility of laterite ores. Metall. Trans. B 1988, 19, 181–186. [Google Scholar] [CrossRef]
- Ramírez Pérez, I.M.; Ramírez Serrano, B. Efecto de la postcombustión sobre los principales índices técnico-económicos en un horno Herreshoff para la producción de níquel. Min. Y Geol. 2021, 37, 426–444. [Google Scholar]
- Rojas Vargas, A.; Sánchez Guillen, C.; Magaña Haynes, M.E.; Hernández Pedrera, C. Extracción potencial de níquel y cobalto con mineral laterítico de mina “Pinares de Mayarí” en la tecnología Caron. Parte II. Tecnol. Química 2022, 42, 405–419. [Google Scholar]
- Ilyas, S.; Kim, H.; Srivastava, R.R. Carbothermic Reduction Roasting of a Low-Grade Nickel Laterite Ore in the Modified Caron Process. In Ni-Co 2021: The 5th International Symposium on Nickel and Cobalt; Anderson, C., Goodall, G., Gostu, S., Gregurek, D., Lundström, M., Meskers, C., Nicol, S., Peuraniemi, E., Tesfaye, F., Tripathy, P.K., et al., Eds.; The Minerals, Metals & Materials Series; Springer: Berlin/Heidelberg, Germany, 2021; pp. 317–328. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H.; Ilyas, N.; Sattar, R. Extraction of nickel and cobalt from a laterite ore using the carbothermic reduction roasting-ammoniacal leaching process. Sep. Purif. Technol. 2020, 232, 115971. [Google Scholar] [CrossRef]
- De Alvarenga Oliveira, V.; Dos Santos, C.G.; De Albuquerque Brocchi, E. Assessing the influence of NaCl on the reduction of a siliceous laterite nickel ore under Caron process conditions. Metall. Mater. Trans. B 2019, 50, 1309–1321. [Google Scholar] [CrossRef]
- Valix, M.; Cheung, W. Effect of sulfur on the mineral phases of laterite ores at high temperature reduction. Miner. Eng. 2002, 15, 523–530. [Google Scholar] [CrossRef]
- Ramírez Mendoza, M. Modelación del proceso de postcombustión en un horno de reducción de níquel. Rev. De Metal. 2002, 38, 150–157. [Google Scholar] [CrossRef]
- Santana Lopez, E.; Montero Góngora, D.; Vega Arias, O.V. Identification of the Air Supply System for Combustion, with the Help of Artificial Neural Networks. Biomed. J. 2018, 10, 7714–7717. [Google Scholar] [CrossRef]
- Montero Góngora, D.; Columbié Navarro, Á.; Montero Laurencio, R.; Trujillo Codorniú, R.; Vázquez Seisdedos, L. Modelo NARX de la postcombustión en un horno de reducción de mineral laterítico. Rev. Ing. Electrónica Automática Y Comun. 2022, 43, 1–14. [Google Scholar]
- Angulo Palma, H.J.; Saldana, M.; Legrá, Á.L.; Urgellés, A.L.; Pedrera, C.H.; Gallegos, S.; Galleguillos, M.F.M.; Toro, N. Kinetic behaviour of the roasting/selective reduction process with the use of a mixture of bituminous coal and fuel oil as the additive. Hem. Ind. 2025, 79, 69–77. [Google Scholar] [CrossRef]
- Rhamdhani, M.; Hayes, P.; Jak, E. Nickel laterite Part 1–microstructure and phase characterisations during reduction roasting and leaching. Miner. Process. Extr. Metall. 2009, 118, 129–145. [Google Scholar] [CrossRef]
- Rhamdhani, M.; Chen, J.; Hidayat, T.; Jak, E.; Hayes, P. Advances in research on nickel production through the Caron process. In Proceedings of the European Metallurgical Conference 2009, Innsbruck, Austria, 28 June–1 July 2009; Harre, J., Ed.; GDMB: Clausthal-Zellerfeld, Germany, 2009; pp. 899–914, ISBN 978-3-940276-19-3. [Google Scholar]
- Cabrera, G.; Gómez, J.M.; Hernández, I.; Coto, O.; Cantero, D. Different strategies for recovering metals from Caron process residue. J. Hazard. Mater. 2011, 189, 836–842. [Google Scholar] [CrossRef]
- Rojas Vargas, A.; Magaaña Haynes, M.E.; del Toro Alvarez, D.; Angulo Palma, H.J.; Yasir AlJaberi, F.; Dawood Salman, A.; Mohsen Alardhi, S.; Mohammed, M.M.; Gabor’ Gyurika, I.; Ihsan Ali, O. Nickel Mining: Studying the Kinetics and Modeling of Extraction Process from Laterite Ore. South Afr. J. Chem. Eng. 2025, 53, 362–372. [Google Scholar] [CrossRef]
- Dung, N.Q.; Thuyet, N.M.; Oanh, N.T.H.; Viet, N.H. Fayalite formation under different heating techniques in iron ore composite briquette reduction. Can. Metall. Q. 2025, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Zhao, Y.; Luo, L.; Shen, L. Growth Behavior of Iron Grains During Reduction Roasting of Fayalite. J. Sustain. Met. 2024, 10, 2521–2533. [Google Scholar] [CrossRef]
- Shofi, A.; Iman Supriyatna, Y.; Budi Prasetyo, A. Selective Reduction of Southeast Sulawesi Nickel Laterite using Palm Kernel Shell Charcoal: Kinetic Studies with Addition of Na2SO4 and NaCl as Additives. Bull. Chem. React. Eng. Catal. 2020, 15, 501–513. [Google Scholar] [CrossRef]
- Castellanos Suárez, J. Cinética de la reducción de los minerales oxidados de níquel en Cuba. Min. Y Geol. 1984, 2, 197–222. [Google Scholar]
- Valix, M.; Cheung, W. Study of phase transformation of laterite ores at high temperature. Miner. Eng. 2002, 15, 607–612. [Google Scholar] [CrossRef]
- Marzoughi, O.; Anthony, W.; Rodrigues, F.; Elliott, R.; Peacey, J.; Pickles, C. Mechanism of carbothermic reduction of a sulfur-containing nickeliferous limonitic laterite ore. Miner. Process. Extr. Metall. 2020, 129, 267–281. [Google Scholar] [CrossRef]
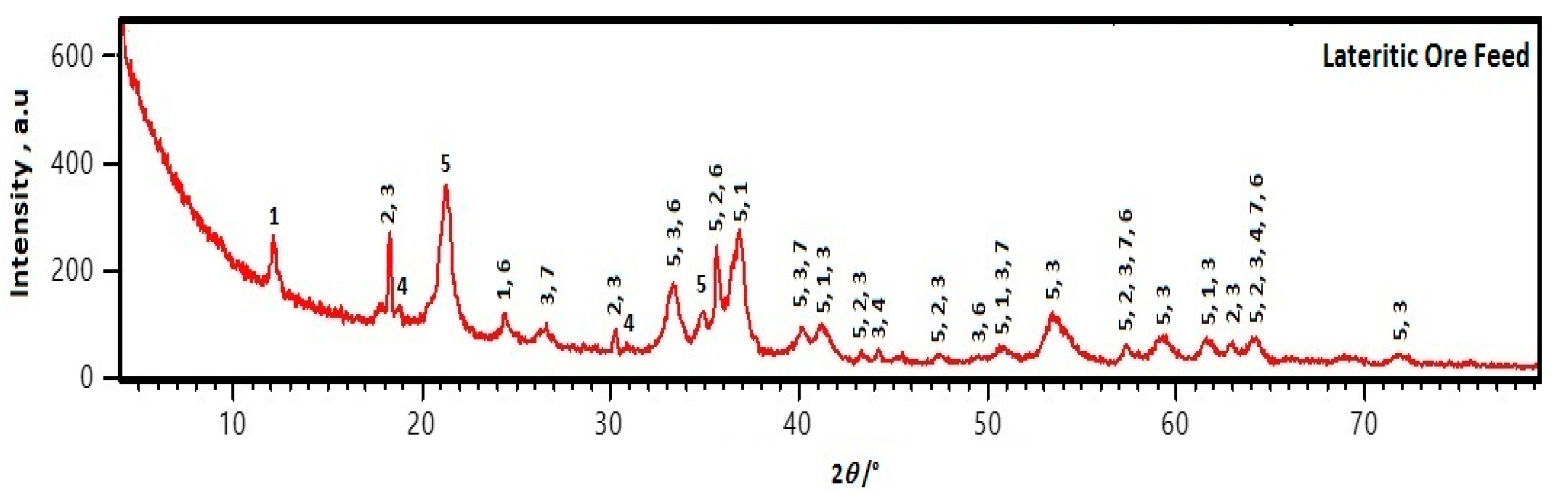
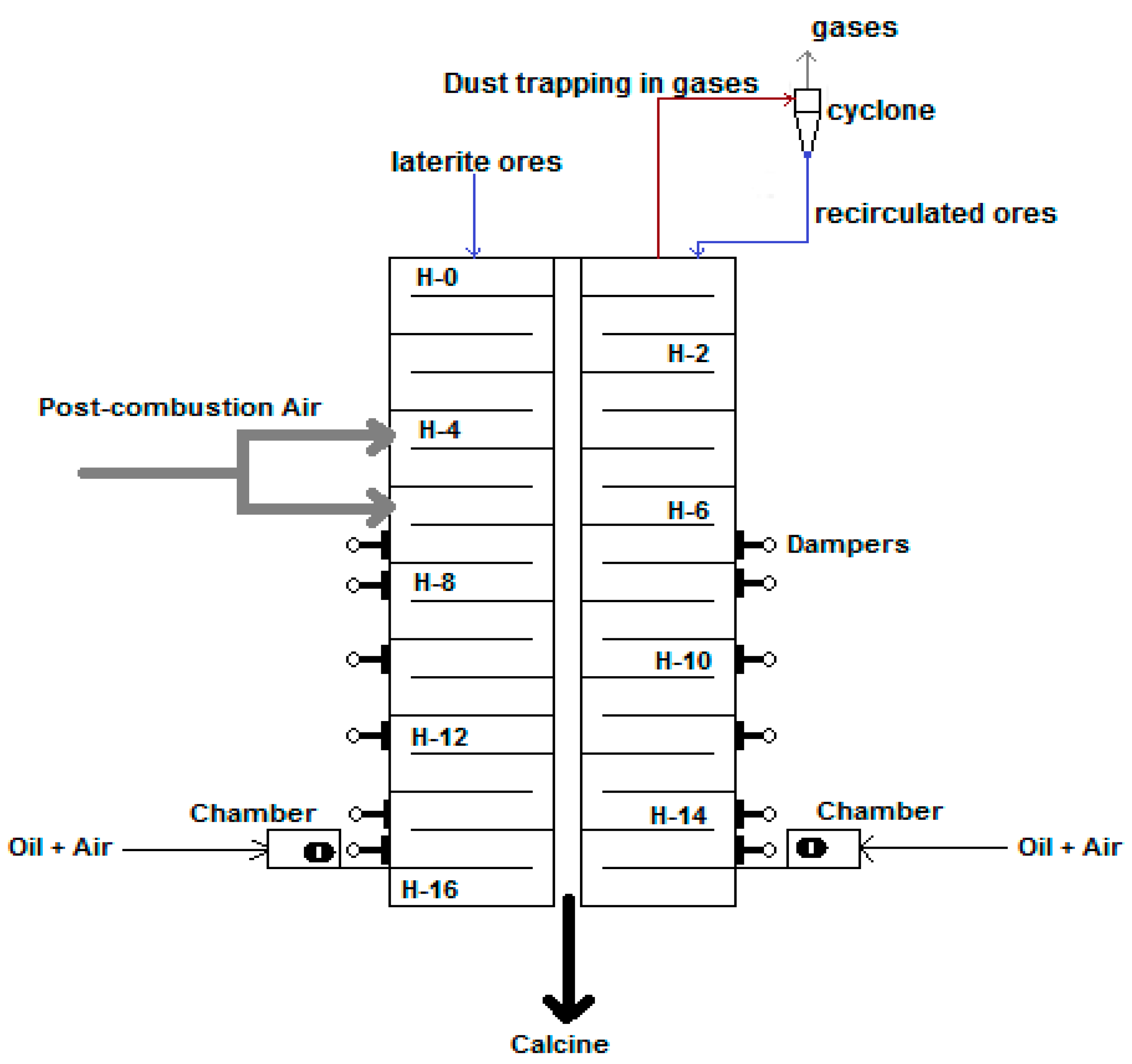
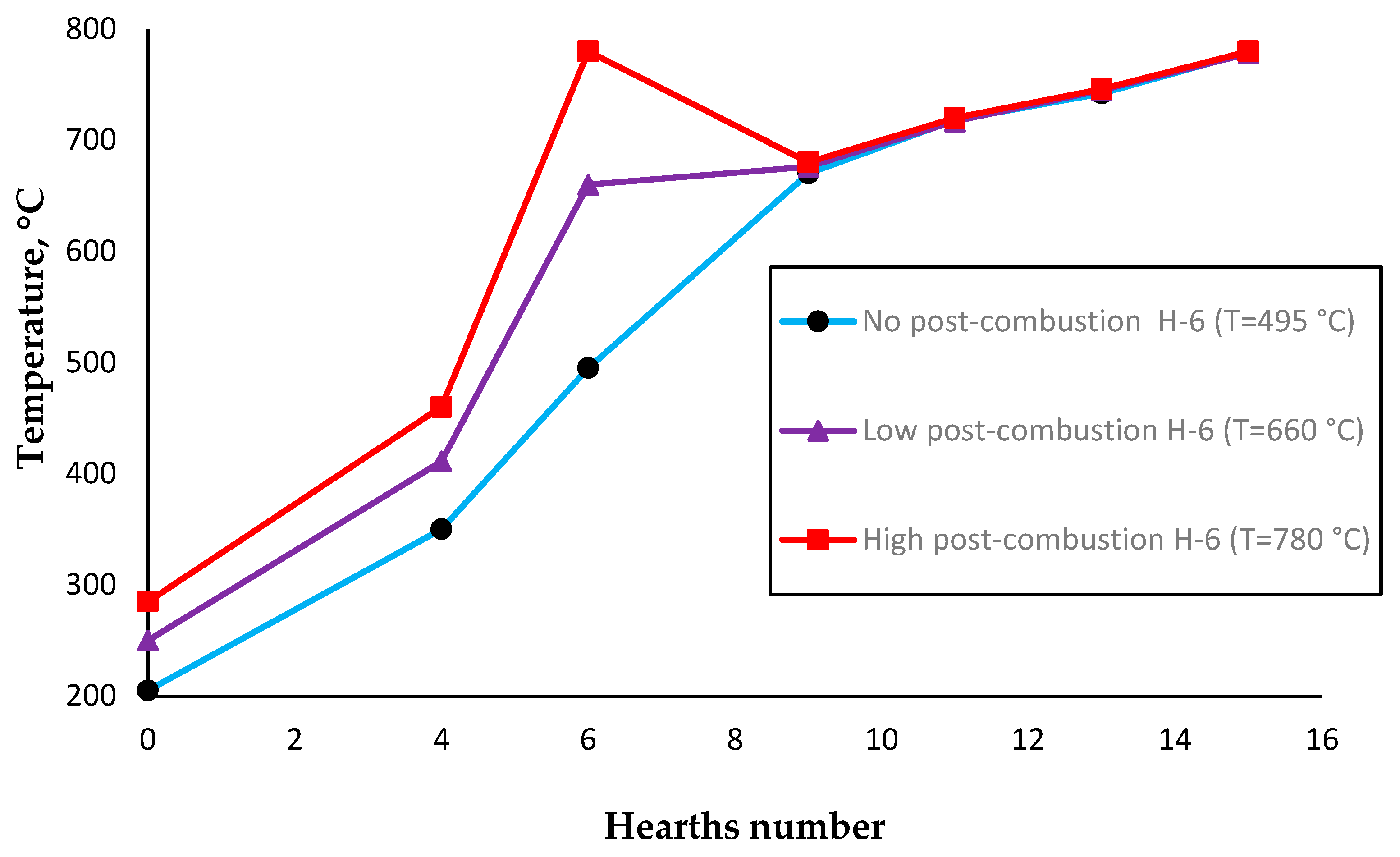

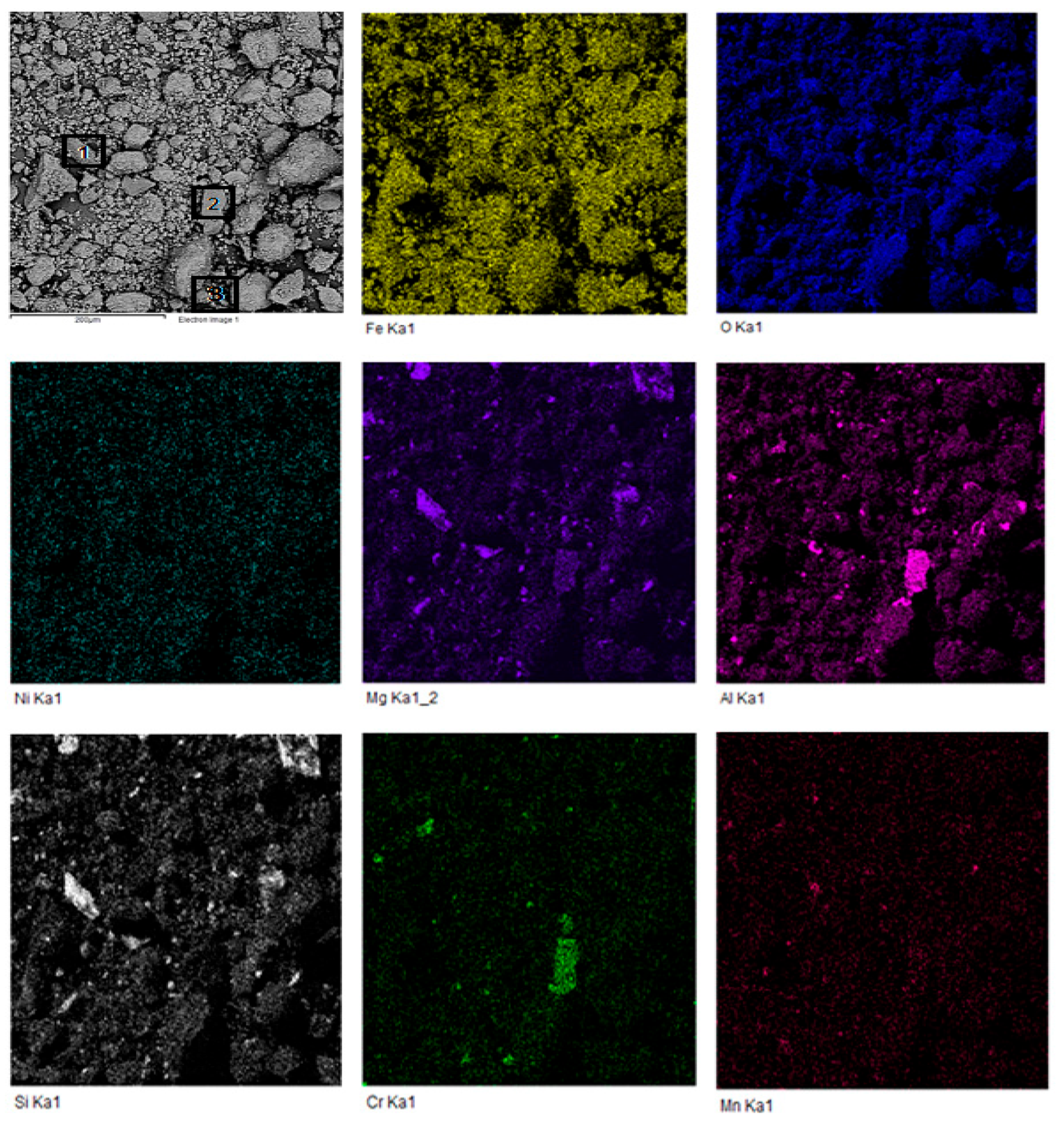


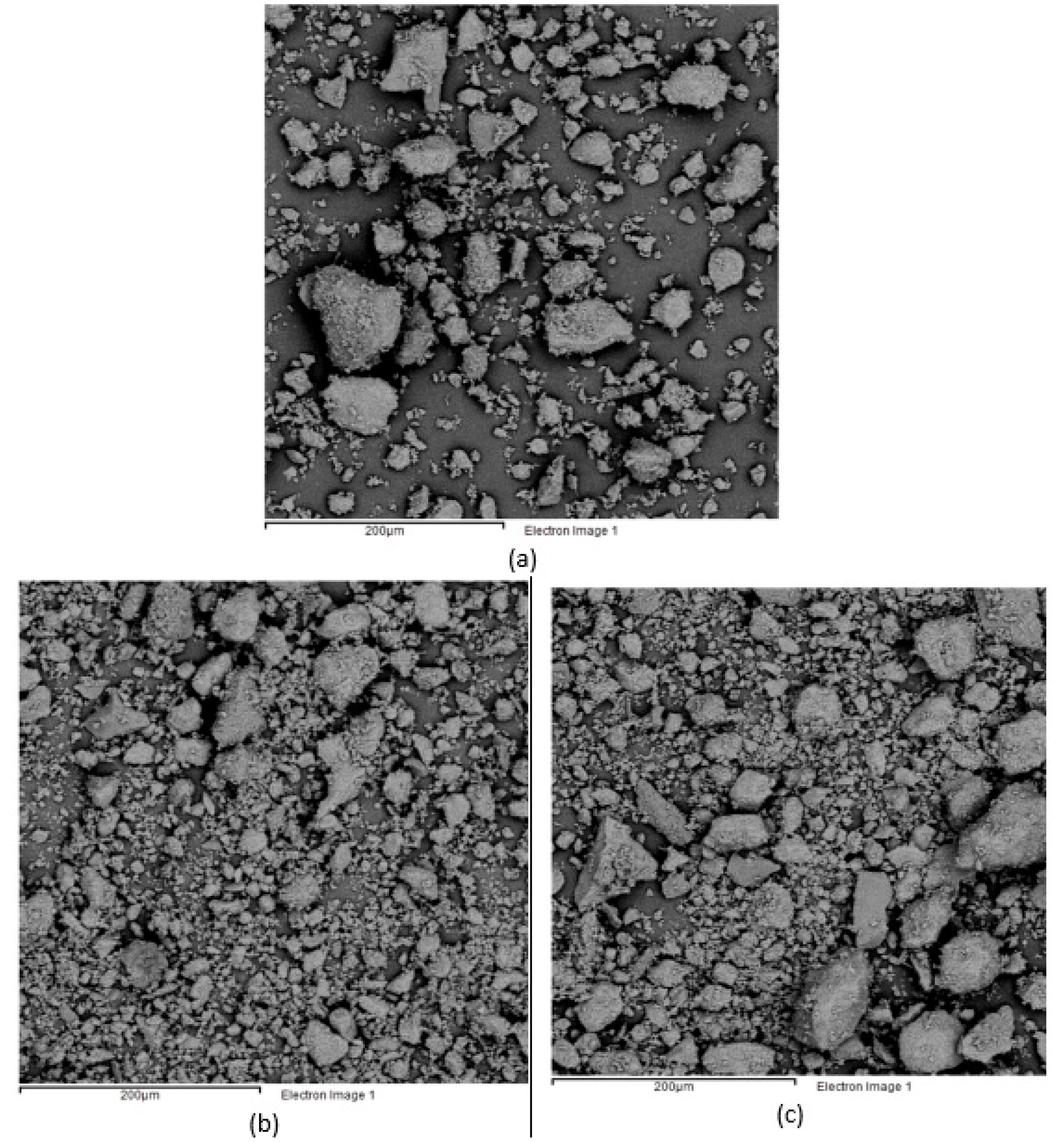
| Content, wt % | Particle Size, μm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 | 75 | 45 | <45 | |||||||||
| Ni | Co | Fe | MgO | SiO2 | Al2O3 | H2O | S | C | Contribution, % | |||
| 1.167 | 0.099 | 39.323 | 2.828 | 7.788 | 8.080 | 3.660 | 0.251 | 2.088 | 3.425 | 9.783 | 7.392 | 79.400 |
| Model Type | Mechanism | Equation |
|---|---|---|
| Unreacted core | Diffusion through the gas film (DGF) | |
| Diffusion through the ash layer (DAL) | ||
| Chemical reaction (ChR) | ||
| Nucleation | 2-D growth of nuclei (GN-2) | |
| 3-D growth of nuclei (GN-3) | ||
| 5-D growth of nuclei (GN-5) |
| Statistics | Content, wt % | ρ c, kg/m3 | MF d, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Co | Fe | Ni0 | Fe0 | Fe2+ | FeO | S | C | |||
| A a | 1.34 | 0.112 | 46.53 | 1.07 | 2.78 | 25.06 | 19.90 | 0.60 | 1.37 | 4.560 | 59.63 |
| σ b | 0.04 | 0.00 | 0.27 | 0.58 | 0.06 | 0.23 | 0.24 | 0.06 | 0.05 | 9.42 | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, H.J.A.; Toro, N.; Castillo, J.; Galleguillos Madrid, F.M.; Salazar, I.; Jamett, I.; Salinas-Rodríguez, E.; Saldana, M. New Evidence of the Effect of Post-Combustion During Roasting/Reduction of Nickel-Bearing Lateritic Ores. Processes 2025, 13, 2602. https://doi.org/10.3390/pr13082602
Palma HJA, Toro N, Castillo J, Galleguillos Madrid FM, Salazar I, Jamett I, Salinas-Rodríguez E, Saldana M. New Evidence of the Effect of Post-Combustion During Roasting/Reduction of Nickel-Bearing Lateritic Ores. Processes. 2025; 13(8):2602. https://doi.org/10.3390/pr13082602
Chicago/Turabian StylePalma, Hugo Javier Angulo, Norman Toro, Jonathan Castillo, Felipe M. Galleguillos Madrid, Iván Salazar, Ingrid Jamett, Eleazar Salinas-Rodríguez, and Manuel Saldana. 2025. "New Evidence of the Effect of Post-Combustion During Roasting/Reduction of Nickel-Bearing Lateritic Ores" Processes 13, no. 8: 2602. https://doi.org/10.3390/pr13082602
APA StylePalma, H. J. A., Toro, N., Castillo, J., Galleguillos Madrid, F. M., Salazar, I., Jamett, I., Salinas-Rodríguez, E., & Saldana, M. (2025). New Evidence of the Effect of Post-Combustion During Roasting/Reduction of Nickel-Bearing Lateritic Ores. Processes, 13(8), 2602. https://doi.org/10.3390/pr13082602










