Assessing the Effects of Green Surface Coatings on the Corrosion-Related Mechanical Attributes of Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation for the Samples
2.2. Testing Procedures
2.3. ABAQUS Procedures
3. Results and Discussion
- Tafel electrochemical behaviour.
- OCP values yielding a trend towards corrosion potential.
- EIS interfacial parameters.
- Strength and preservation of structure after exposure.
- Relative performance of materials.
3.1. Electrochemical Behaviour (Tafel Analysis)
3.2. Stability of Corrosion Potential (OCP Analysis)
3.3. Interfacial Resistance and Capacitance (EIS Analysis)
3.4. Mechanical Performance Post-Corrosion Exposure
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhang, W.; Wei, L.; Pu, L.; Liu, J.; Liu, H.; Li, Y.; Fan, J.; Ding, T.; Guo, Z. Alternating multilayer structural epoxy composite coating for corrosion protection of steel. Macromol. Mater. Eng. 2019, 304, 1900374. [Google Scholar] [CrossRef]
- Salim, R.; Ech-chihbi, E.; Fernine, Y.; Koudad, M.; Guo, L.; Berdimurodov, E.; Azam, M.; Rais, Z.; Taleb, M. Inhibition behaviour of new ecological corrosion inhibitors for mild steel, copper and aluminium in acidic environment: Theoretical and experimental investigation. J. Mol. Liq. 2024, 393, 123579. [Google Scholar] [CrossRef]
- Pezzato, L.; Akbarzadeh, S.; Settimi, A.G.; Moschin, E.; Moro, I.; Olivier, M.G.; Brunelli, K.; Dabalà, M. Corrosion and antifouling properties of copper-containing PEO coatings produced on steels. Surf. Coat. Technol. 2024, 482, 130631. [Google Scholar] [CrossRef]
- Jena, G.; George, R.P.; Philip, J. Fabrication of a robust graphene oxide-nano SiO2-polydimethylsiloxane composite coating on carbon steel for marine applications. Prog. Org. Coat. 2021, 161, 106462. [Google Scholar] [CrossRef]
- Winston, R.R.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- McFarland, M.L.; Provin, T.L.; Boellstorf, D.E. Drinking Water Problems: Corrosion; Agrilife Extension: College Station, TX, USA, 2011. [Google Scholar]
- Xu, R.; Xin, S.; Ni, Q.; Zeng, H.; Li, M. Initial corrosion characteristics of enamel coated carbon steel in hot tap water. Russ. J. Electrochem. 2021, 57, 636–643. [Google Scholar] [CrossRef]
- Ech-chihbi, E.; Salim, R.; Ouakki, M.; Koudad, M.; Guo, L.; Azam, M.; Benchat, N.; Rais, Z.; Taleb, M. Corrosion resistance assessment of copper, mild steel, and aluminium alloy 2024-T3 in acidic solution by a novel imidazothiazole derivative. Mater. Today Sustain. 2023, 24, 100524. [Google Scholar] [CrossRef]
- Cheng, T.; Huang, H.; Huang, G. Galvanic corrosion behaviour between ADC12 aluminium alloy and copper in 3.5 wt% NaCl solution. J. Electroanal. Chem. 2022, 927, 116984. [Google Scholar] [CrossRef]
- Baena, L.M.; Vásquez, F.A.; Calderón, J.A. Corrosion assessment of metals in bioethanol-gasoline blends using electrochemical impedance spectroscopy. Heliyon 2021, 7, e07585. [Google Scholar] [CrossRef]
- Policastro, S.A.; Anderson, R.M.; Hangarter, C.M. Analysis of galvanic corrosion current between an aluminium alloy and stainless-steel exposed to an equilibrated droplet electrolyte. J. Electrochem. Soc. 2021, 168, 041507. [Google Scholar] [CrossRef]
- Román, A.S.; Méndez, C.M.; Gervasi, C.A.; Rebak, R.B.; Ares, A.E. Corrosion resistance of aluminium-copper alloys with different grain structures. J. Mater. Eng. Perform. 2021, 30, 131–144. [Google Scholar] [CrossRef]
- Jandaghi, M.R.; Saboori, A.; Khalaj, G.; Shiran, M.K.G. Microstructural evolutions and its impact on the corrosion behaviour of explosively welded Al/Cu bimetal. Metals 2020, 10, 634. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Cheng, Y.; Zuo, X.; Wang, Y.; Yuan, X.; Huang, H. Effect of temperature on the corrosion behaviour and corrosion resistance of copper–aluminium laminated composite plate. Materials 2022, 15, 1621. [Google Scholar] [CrossRef]
- Xavier, J.R. Galvanic corrosion of copper/titanium in aircraft structures using a cyclic wet/dry corrosion test in marine environment by EIS and SECM techniques. SN Appl. Sci. 2020, 2, 1341. [Google Scholar] [CrossRef]
- Ehsani, A.; Heidari, A.A.; Sajedi, M. Graphene and graphene/polymer composites as the most efficient protective coatings for steel, aluminium and copper in corrosive media: A review of recent studies. Chem. Rec. 2020, 20, 467–493. [Google Scholar] [CrossRef] [PubMed]
- Abubakr, H.M.C.; Ghaith, M.E.; El-Sherif, A.A.; El-Deab, M.S. Influence of metal ions on cysteine as a corrosion inhibitor for low carbon steel in sulfuric acid: Polarisation, EIS, XPS, and DFT analysis. Int. J. Electrochem. Sci. 2024, 19, 100620. [Google Scholar] [CrossRef]
- Wang, N.; Bai, J.Y.; Wen, C.; Li, J.F.; Wang, X.G.; Tong, X.B.; Wang, J. Adaptability of sol-gel anti-corrosion coating on different metallic substrates. J. Phys. Conf. Ser. 2021, 2021, 012044. [Google Scholar] [CrossRef]
- Pezzato, L.; Settimi, A.G.; Fanchin, D.; Moschin, E.; Moro, I.; Dabalà, M. Effect of Cu addition on the corrosion and antifouling properties of PEO coated zinc-aluminised steel. Materials 2022, 15, 7895. [Google Scholar] [CrossRef]
- Ran, Q.; Shi, H.; Meng, H.; Zeng, S.P.; Wan, W.B.; Zhang, W.; Wen, Z.; Lang, X.Y.; Jiang, Q. Aluminium–copper alloy anode materials for high-energy aqueous aluminium batteries. Nat. Commun. 2022, 13, 576. [Google Scholar] [CrossRef]
- El Hawary, M.; Khachani, M.; Benhiba, F.; Kaichouh, G.; Warad, I.; Guenbour, A.; Zarrouk, A.; Bellaouchou, A. Investigation of the corrosion of stainless steel, copper and aluminium in sunflower biodiesel solution: Experimental and theoretical approaches. Chem. Data Collect. 2022, 40, 100870. [Google Scholar] [CrossRef]
- Othman, N.H.; Ismail, M.C.; Mustapha, M.; Sallih, N.; Kee, K.E.; Jaal, R.A. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Ansari, K.R.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coat. 2020, 147, 105741. [Google Scholar] [CrossRef]
- Parthipan, P.; Cheng, L.; Rajasekar, A. Glycyrrhiza glabra extract as an eco-friendly inhibitor for microbiologically influenced corrosion of API 5LX carbon steel in oil well produced water environments. J. Mol. Liq. 2021, 333, 115952. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Imidazole derivatives as corrosion inhibitors for copper: A DFT and reactive force field study. Corros. Sci. 2020, 171, 108724. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Arora, P.K.; Shrivastava, Y.; Kumar, H. Optimising FDM printing parameters for improved tensile properties in 3D printed ASTM D638 standard samples. Aust. J. Mech. Eng. 2023, 23, 277–290. [Google Scholar] [CrossRef]
- Smith, G.M.; Gildersleeve, E.J.; Luo, X.T.; Luzin, V.; Sampath, S. On the surface and system performance of thermally sprayed carbide coatings produced under controlled residual stresses. Surf. Coat. Technol. 2020, 387, 125536. [Google Scholar] [CrossRef]
- Nazarlou, Z.; Hosseini, S.F.; Seyed Dorraji, M.S.; Rasoulifard, M.H.; Aydemir, U. Ti3C2 MXene/polyaniline/montmorillonite nanostructures toward solvent-free powder coatings with enhanced corrosion resistance and mechanical properties. ACS Appl. Nano Mater. 2023, 6, 8804–8818. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2015.
- Hong, M.S.; Park, Y.; Kim, J.G.; Kim, K. Effect of incorporating MoS2 in organic coatings on the corrosion resistance of 316L stainless steel in a 3.5% NaCl solution. Coatings 2019, 9, 45. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Tao, J.; Chen, X.; Yang, L. Corrosion resistance and mechanical properties of coating/steel substrate system in acid rain environment. J. Constr. Steel Res. 2023, 201, 107740. [Google Scholar] [CrossRef]
- Almufarij, R.S.; El Sayed, H.A.F.; Mohamed, M.E. Eco-friendly approach for the construction of superhydrophobic coating on stainless steel metal based on biological metal–organic framework and its corrosion resistance performance. Materials 2023, 16, 4728. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Abdo, H.S.; Anis, A.; Al-Zahrani, S.M. Synergistic effect of nanoparticles: Enhanced mechanical and corrosion protection properties of epoxy coatings incorporated with SiO2 and ZrO2. Polymers 2023, 15, 3100. [Google Scholar] [CrossRef]
- Muresan, L.M. Nanocomposite coatings for anti-corrosion properties of metallic substrates. Materials 2023, 16, 5092. [Google Scholar] [CrossRef]
- Abdelfattah, K.B.; Abbas, M.A.; El-Garaihy, W.H.; Mohamed, A.M.A.; Salem, H.G. Corrosion and degradation behavior of MCSTE-Processed AZ31 magnesium alloy. Sci. Rep. 2025, 15, 4072. [Google Scholar] [CrossRef]
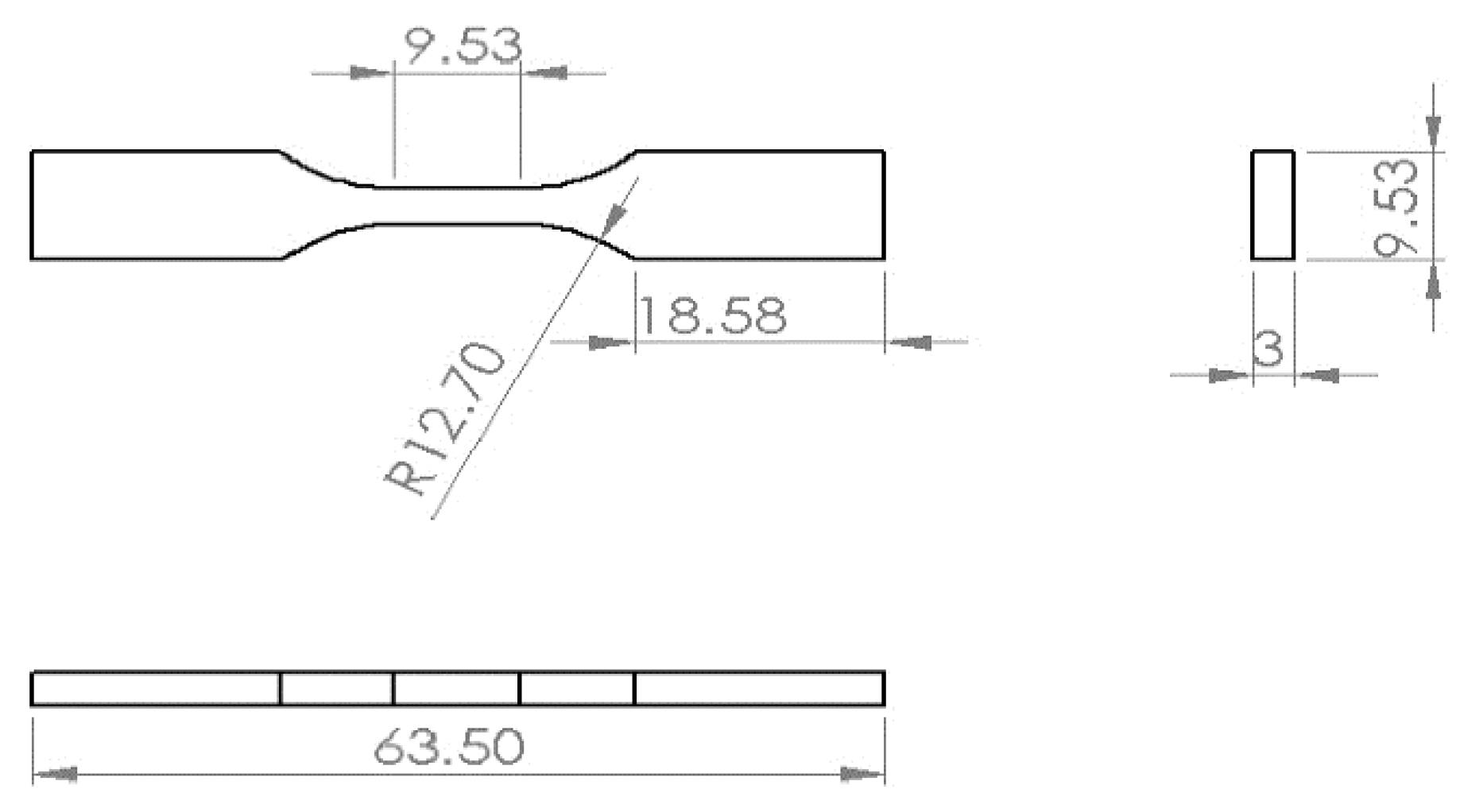
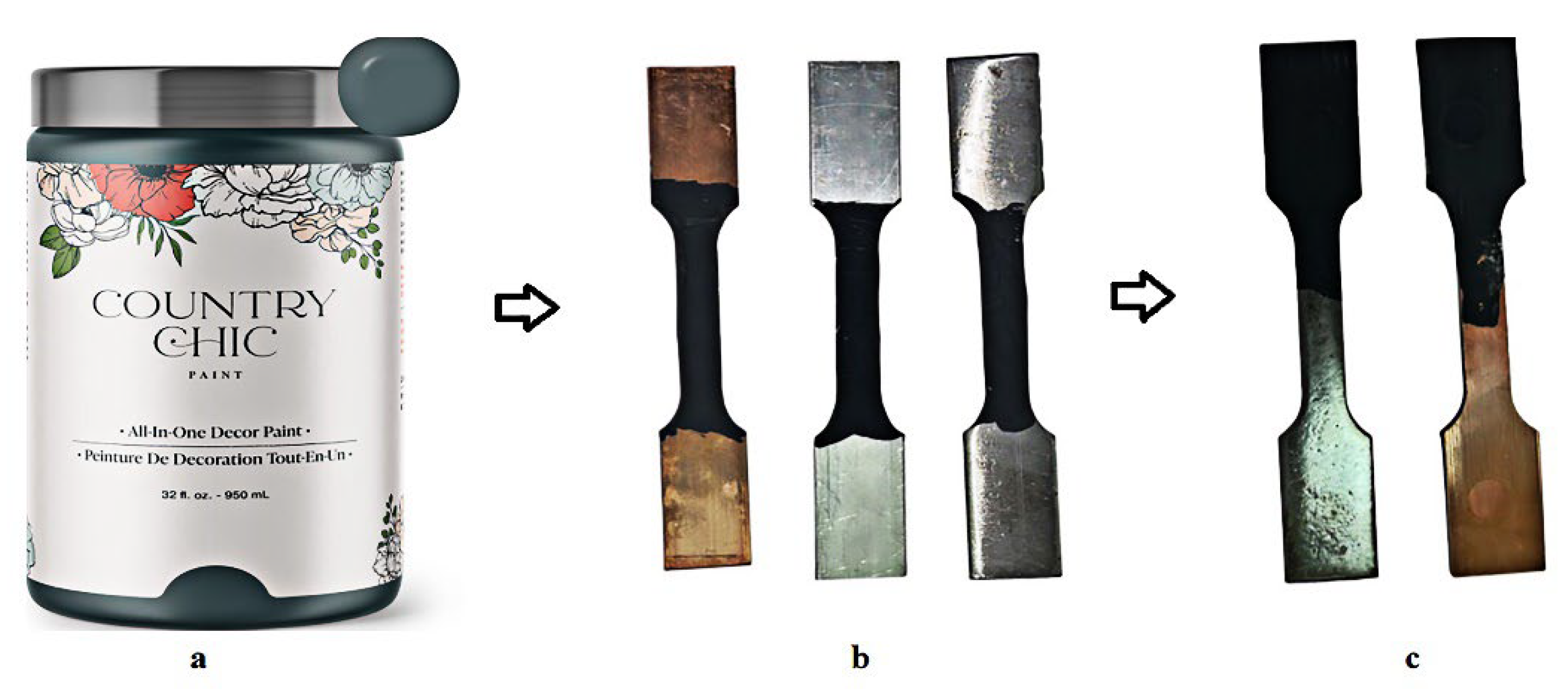




| Sample | Ecorr (mV) | Icorr (μA) | Βc (mV/dec) | Βa (mV/dec) | IE% | ||
|---|---|---|---|---|---|---|---|
| Carbon Steel | Uncoated | −0.46483 | 6.016 × 10−10 | 101.58 | 196.58 | 0% | 4.693 × 10−12 |
| Coated | −0.41265 | 7.5988 × 10−6 | 201.67 | 331.34 | 99.992% | 5.928 × 10−8 | |
| Copper | Uncoated | −0.26258 | 3.3087 × 10−5 | 230.55 | 110.77 | 0% | 2.589 × 10−7 |
| Coated | −0.25757 | 1.3035 × 10−6 | 199.33 | 218.10 | 96.06% | 1.02 × 10−8 | |
| Aluminium | Uncoated | −1.4847 | 8.5379 × 10−5 | 74.567 | 598.45 | 0% | 9.306 × 10−7 |
| Coated | −0.90329 | 8.92 × 10−9 | 174.99 | 367.64 | 99.98% | 9.7228 × 10−11 |
| Steel | Copper | Aluminium | ||||
|---|---|---|---|---|---|---|
| Uncoated | Coated | Uncoated | Coated | Uncoated | Coated | |
| Rs | 17.734 | 18.472 | 14.02 | 16.475 | 19.71 | 51.779 |
| Rp | 1638.8 | 2553.6 | 888.95 | 28,034 | 804.95 | 8.079 × 10−5 |
| CPE-T | 8.5142 × 10−4 | 5.4592 × 10−4 | 1.8058 × 10−3 | 3.98732 × 10−6 | 1.7335 × 10−5 | 8.5749 × 10−6 |
| CPE-P | 5.4844 × 10−1 | 5.9823 × 10−1 | 3.8353 × 10−3 | 7.7497 × 10−1 | 9.07872 × 10−1 | 8.1676 × 10−1 |
| Property | Steel | Copper | Aluminium | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Uncoated | Coated | Reference | Uncoated | Coated | Reference | Uncoated | Coated | Reference | |
| Max Load (kN) | 10.96 | 11.78 | 12.26 | 9.50 | 9.55 | 9.48 | 9.02 | 8.87 | 9.21 |
| Max Strength (MPa) | 384.39 | 413.19 | 430.04 | 333.37 | 334.94 | 332.60 | 316.39 | 311.32 | 323.22 |
| Yield Strength (MPa) | 299.35 | 337.73 | 338.55 | 310 | 318 | 319 | 270.86 | 274.38 | 278.30 |
| Elastic Modulus E (MPa) | 390 | 400 | 410 | 310 | 270 | 319 | 150 | 280 | 260 |
| Toughness (kN·mm) | 94.64 | 179.48 | 186.73 | 124.31 | 187.35 | 186.04 | 37.02 | 42.68 | 56.17 |
| Modulus of Toughness (MPa) | 133.74 | 143.78 | 258.59 | 99.40 | 67.27 | 67.13 | 20.46 | 23.59 | 31.04 |
| Resilience (kN·mm) | 5.39 | 11.78 | 10.18 | 5.80 | 6.25 | 6.20 | 1.65 | 2.42 | 2.63 |
| Modulus of Resilience (MPa) | 2.98 | 6.51 | 5.63 | 2.03 | 2.26 | 2.26 | 3.04 | 3.01 | 3.14 |
| Ductility (Elongation %) | 27.21 | 26.41 | 26.85 | 20.61 | 20.41 | 20.68 | 6.97 | 8.05 | 10.32 |
| Axial Stiffness (N/mm) | 175.04 | 179.53 | 184.02 | 139.13 | 121.18 | 143.17 | 67.32 | 125.67 | 116.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albadrani, M.A. Assessing the Effects of Green Surface Coatings on the Corrosion-Related Mechanical Attributes of Materials. Processes 2025, 13, 2576. https://doi.org/10.3390/pr13082576
Albadrani MA. Assessing the Effects of Green Surface Coatings on the Corrosion-Related Mechanical Attributes of Materials. Processes. 2025; 13(8):2576. https://doi.org/10.3390/pr13082576
Chicago/Turabian StyleAlbadrani, Mohammed A. 2025. "Assessing the Effects of Green Surface Coatings on the Corrosion-Related Mechanical Attributes of Materials" Processes 13, no. 8: 2576. https://doi.org/10.3390/pr13082576
APA StyleAlbadrani, M. A. (2025). Assessing the Effects of Green Surface Coatings on the Corrosion-Related Mechanical Attributes of Materials. Processes, 13(8), 2576. https://doi.org/10.3390/pr13082576






