1. Introduction
Jerusalem artichoke (JA) (
Helianthus tuberosus L.), also known as the tuberous sunflower, is a perennial plant belonging to the family
Asteraceae. It originates from North America, where it was cultivated by Indigenous peoples long before the arrival of Europeans. It was introduced to Europe in the early 17th century, initially as an ornamental and forage plant, and later as a vegetable recognized for its nutritional and medicinal properties [
1,
2,
3]. From an economic perspective, JA is a highly versatile crop. Its tubers contain significant amounts of inulin, a polysaccharide with prebiotic properties, rendering it particularly valuable to the dietary and pharmaceutical industries [
4,
5]. This plant is also utilized in the production of alcohol, biogas, and as animal fodder [
6,
7]. JA is characterized by its high tolerance to adverse soil and climatic conditions, allowing cultivation on marginal lands with challenging agricultural conditions [
8,
9,
10].
The biological and chemical properties of JA—including its high inulin content, dietary fiber, B vitamins, and mineral components—make its tubers a valuable dietary component for individuals with diabetes, obesity, or metabolic disorders [
11,
12]. Furthermore, compounds present in the tubers have demonstrated immunomodulatory and antioxidant activities, reinforcing their role in the prevention of lifestyle-related diseases [
13,
14]. In light of the growing interest in natural and functional food products, JA has been attracting increasing attention not only as a food crop but also as an industrial and bioenergy raw material [
6,
15].
Inulin is a natural polysaccharide, classified as a soluble dietary fiber and belonging to the group of fructans. Chemically, it is a mixture of oligomers and polymers of fructose residues terminated by a glucose unit, linked via β-(2→1) bonds [
16,
17,
18]. Its general chemical formula is GFn, where G denotes glucose, F denotes fructose, and n indicates the number of fructose units, which can range from 2 to over 60 depending on the source and extraction method [
19,
20]. Inulin naturally occurs in various plants such as chicory (
Cichorium intybus), Jerusalem artichoke (
Helianthus tuberosus), agave (
Agave spp.), and onion (
Allium cepa), serving as a storage carbohydrate [
21,
22,
23]. In terms of its physical properties, inulin is a white, odorless powder with a slightly sweet taste, readily soluble in hot water. Its solubility and functional properties—such as gelling, emulsifying, and water-holding capacity— have led to its wide use in the food industry as a texturizing agent and as a fat or sugar substitute [
17,
24,
25]. Inulin also exhibits cryoprotective and stabilizing effects in frozen products and improves the organoleptic properties and nutritional quality of food products [
26]. From a chemical standpoint, inulin is resistant to enzymatic hydrolysis in the human gastrointestinal tract; it is not digested in the stomach or small intestine but reaches the colon unchanged, where it is fermented by the gut microbiota [
16,
27]. The fermentation process yields short-chain fatty acids (SCFAs), such as acetic, propionic, and butyric acids, which exert health-promoting effects, including modulating lipid profiles, enhancing mineral absorption, and strengthening the intestinal barrier [
28,
29]. Due to these characteristics, inulin is recognized as a prebiotic, selectively stimulating the growth and activity of beneficial microorganisms [
30,
31]. The physicochemical and functional properties of inulin underpin its increasing importance in the food, pharmaceutical, and cosmetic industries, as well as in dietetics and medicine, particularly in the prevention and treatment of diet-related diseases [
32,
33,
34].
Drying is one of the most critical unit operations in the food, chemical, and pharmaceutical industries. Its primary goal is to extend shelf life, improve microbiological stability, and reduce transportation and storage costs [
35,
36,
37]. Among the various drying techniques, thermal methods—such as convective and convective–microwave drying—play a crucial role. These differ in their energy transfer mechanisms, processing times, and impacts on the quality of the final product [
38,
39].
Convective drying (hot-air drying) is a widely applied technique that relies on heat exchange between a heated medium (typically air) and the surface of the material. This process is technologically straightforward, requires relatively low capital investment, and allows for easy control of operating parameters. However, prolonged exposure of the material to high temperatures can result in the degradation of thermolabile components, deterioration of sensory qualities, and structural changes in the dried product [
40,
41,
42]. An alternative to conventional convective drying is the convective–microwave method, which combines hot-air heating with microwave energy. This approach excites polar water molecules throughout the material’s volume, thereby delivering thermal energy directly to the interior of the product. This significantly reduces drying time and minimizes the risk of surface overheating [
43]. The method supports higher product quality, better retention of bioactive compounds, and a more uniform moisture distribution [
38,
44]. Comparative analysis of the efficiency and product quality outcomes of both methods represents an important area of research, particularly in the context of optimizing drying processes while preserving the nutritional value, structural integrity, and sensory attributes of food products [
45,
46].
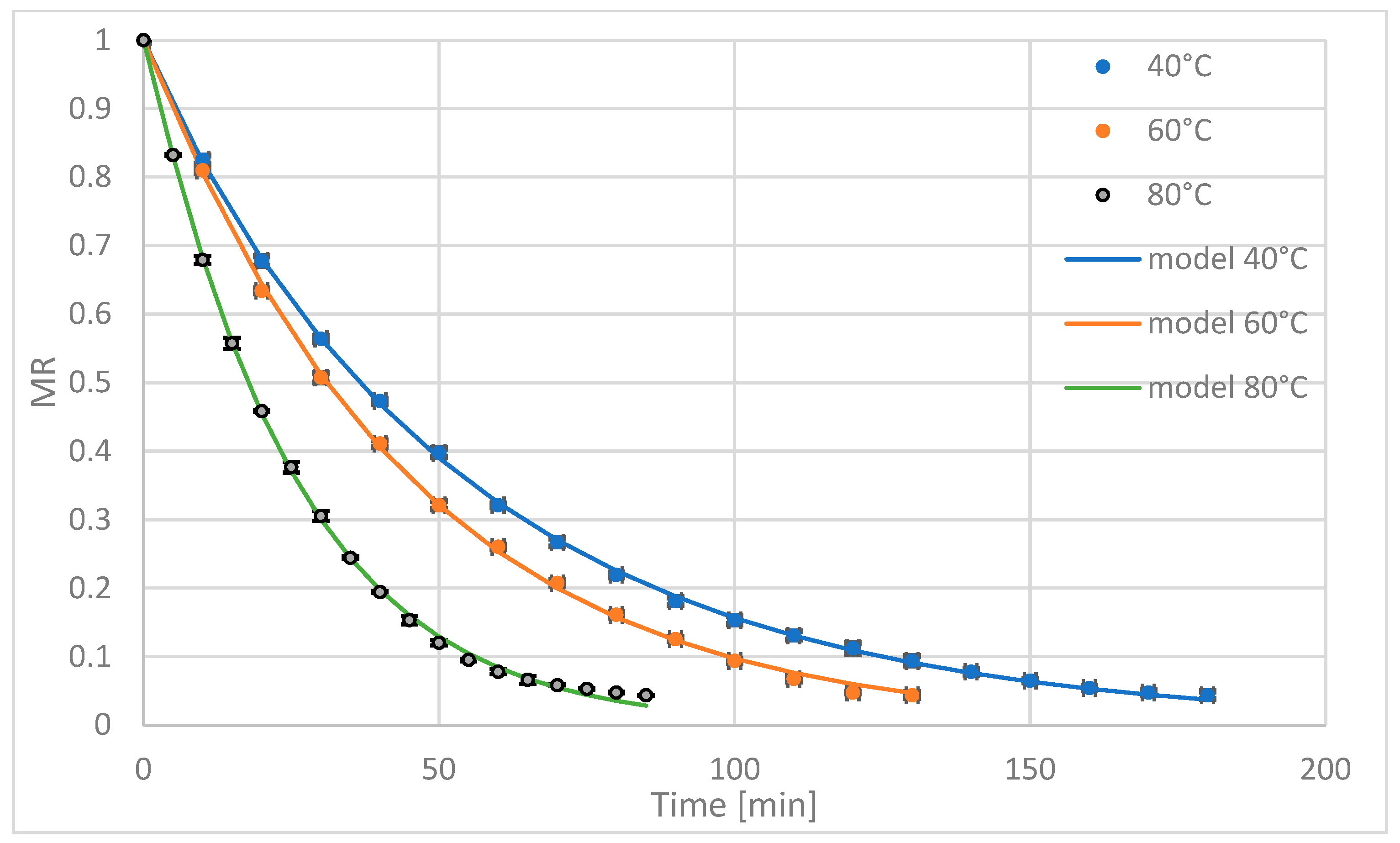
The aim of this study was to investigate the effect of convective drying (AD) and combined convective–microwave drying (AMD) on the drying kinetics and selected physicochemical properties of Jerusalem artichoke. The study hypothesized that AMD improves drying kinetics without compromising inulin content.
2. Materials and Methods
2.1. Material
The research material consisted of Jerusalem artichoke (Helianthus tuberosus L.) of the ‘Albik’ cultivar. The raw material was sourced from a local producer in the Lublin region, harvested in 2024. The tubers were cleaned and sliced into approximately 5 mm (±0.5 mm) thick pieces using a rotary slicer RCAM-300PRO (Royal Catering, Munich, Germany). The thickness of the slices was checked using an electronic caliper L400 (Varel, Hamburg, Germany). For both drying methods analyzed, the material was dried until a target final moisture content of 10% was achieved. Reducing the moisture content to 10% limits the growth of microorganisms and molds. According to industrial standards, a moisture content of ≤10% is preferred for many applications.
2.2. Drying Method
Convective and convective–microwave-drying processes were carried out using a dryer manufactured by PROMIS-TECH (Wroclaw, Poland). The airflow was directed through the bottom of the sample dish, perpendicularly to the material layer, at a velocity of 0.5 ± 0.05 m·s−1, measured directly beneath the dryer screen. The calibration process was conducted in accordance with the manufacturer’s recommendations at a temperature of 20 °C, 50% relative humidity, and atmospheric pressure. The dish containing the material was placed on a laboratory scale with a measurement accuracy of 0.1 g. During each weighing, the motor rotating the dish and the airflow were temporarily stopped. Mass measurements were taken every five minutes. Convective drying was performed at temperatures of 40 °C, 60 °C, and 80 °C. In the convective–microwave-drying process, the same temperatures were applied, supplemented with microwave power of 100 W per 100 g of raw material. The initial air humidity ranged between 50% and 60%. Temperature control was ensured by a heating element and a temperature probe located between the fan and the airflow speed control module. The device allowed continuous regulation of temperature, airflow velocity, and microwave power (at a frequency of 2450 MHz). Integrated computer software enabled real-time monitoring of drying parameters and data export to a spreadsheet. Each drying variant was repeated five times with drying continuing until a target final moisture content of 10% was achieved.
2.3. Modeling of Drying Curves
For both drying methods investigated, the water content (u
r—expressed in kg
H2O·kg
dm−1, where dm denotes dry matter) in the dried JA was determined at each measurement point using the following equation:
where
m—the mass of the material at a given measurement point [g],
ms—the dry matter content in JA [g].
The final mass of the sample (
mk), at which the desired final moisture content is achieved, was calculated using the following expression:
where
wp—initial moisture content of the raw material [%],
wk—assumed final moisture content of the dried material [%].
The drying kinetics were presented as the change in the reduced moisture content (
MR) as a function of drying time:
where
uτ—moisture content at a given measurement point [kgH2O·kgdm−1],
up—initial moisture content [kgH2O·kgdm−1],
ur—equilibrium moisture content [kgH2O·kgdm−1].
The equilibrium moisture content (ur) after both convective and convective–microwave drying is very low; therefore, it was assumed that ur is zero throughout the entire measurement range.
To describe the drying curves for convective and convective–microwave drying, six models most commonly used in the literature were applied (
Table 1).
2.4. Color Measurement
Color analysis was performed using the reflectance method with a spherical spectrophotometer X-Rite 8200 (X-Rite, Grand Rapids, MI, USA), equipped with a measurement aperture of 12.7 mm in diameter. Measurements were conducted using a D65 light source and a standard colorimetric observer with a 10° viewing angle. Before each measurement, the spectrophotometer was calibrated using a white standard. Color was determined in the CIELab* color space, in which three coordinates are obtained: L*, a*, and b*. The L* value represents lightness, ranging from 0 (black) to 100 (white). The a* coordinate indicates the color direction from green (negative values) to red (positive values), while the b* coordinate corresponds to the transition from blue (negative values) to yellow (positive values).
Based on the a* and b* coordinates, additional color parameters expressed in cylindrical coordinates were calculated—chroma (c) and hue angle (h)—according to the following formulas:
2.5. Total Phenolic Compounds Content
The total phenolic content (TPC) was determined using the Folin–Ciocalteu method [
36]. A 0.5 mL aliquot of the extract was mixed with 0.5 mL of water and 2 mL of Folin reagent (diluted 1:5 with water), and after 3 min, 10 mL of a 10% Na
2CO
3 solution was added. After 30 min, the absorbance of the mixture was measured at a wavelength of 725 nm using a UV Mini 1240 spectrophotometer (Shimadzu, Kyoto, Japan). The total phenolic content was expressed as gallic acid equivalents (GAE) per gram of dry matter.
2.6. Antiradical Activity
The antioxidant activity in scavenging free radicals was assessed using both ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) and DPPH (2,2-diphenyl-1-picrylhydrazyl) assays. The ABTS assay was performed based on the method proposed by Re et al. (1999) [
53], with minor adjustments. Specifically, the ABTS•
+ radical cation was generated by combining a 7 mM ABTS stock solution with 2.45 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 12 to 16 h. Prior to measurement, the radical solution was diluted with ethanol to achieve an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 0.1 mL of the test extract was added to 3.9 mL of the ABTS•
+ solution, and absorbance was recorded at 734 nm after 6 min of incubation. For the DPPH assay, the procedure outlined by Brand-Williams et al. (1995) [
54] was followed. A 0.1 mM methanolic solution of DPPH was prepared, and 0.1 mL of the extract was mixed with 3.9 mL of this solution. The reaction mixture was incubated in the dark at room temperature for 30 min, and the absorbance was measured at 517 nm.
In both methods, 50% methanolic extracts were employed. The extracts were obtained by homogenizing the dried plant material with 50% methanol (v/v), followed by filtration and storage at 4 °C until use. Trolox served as the reference antioxidant, and results were expressed as millimoles of Trolox equivalents per 100 g of dry matter (mmol Trolox/100 g d.m.).
2.7. Grinding of the Dried Samples
The grinding of the dried material was performed using a GRINDOMIX GM 200 (Retsch GmbH, Haan, Germany) knife mill (Retsch). The device was equipped with two stainless steel blades, each 1 mm thick, mounted on opposite sides of the shaft at different levels. A 100 g sample of the dried material was fed into the grinding chamber, and milling was performed at a shaft rotational speed of 7000 rpm. The process lasted for 30 s.
To eliminate the influence of final moisture content on grinding efficiency, all samples were conditioned for 48 h in a climate chamber under controlled conditions of 20 °C and 50% relative humidity prior to grinding. Each measurement was performed in five replicates.
2.7.1. Particle Size Distribution of the Dried Material and Mean Particle Size
The ground dried material was subjected to particle size distribution analysis by sieving the sample using a Retsch AS 200 (Retsch GmbH, Haan, Germany) vibratory sieve shaker. The device was equipped with a set of sieves with the following mesh sizes: 800 μm, 600 μm, 400 μm, 200 μm, and 100 μm. The sieving process of 20 g of dried material was carried out for 2 min at a vibration amplitude of 1.5 mm. The individual fractions were weighed, and their percentage share was determined. Measurements were conducted in five replicates.
Based on the particle size distribution, the mean particle size (ds) was determined [
55]:
where
hi—mean value of the class interval,
Pi—percentage share of the given class,
u—number of sieves used.
2.7.2. Eefficiency Indicators of Grinding
The measurements of electrical energy supplied to the knife mill were recorded as the power distribution over the duration of the grinding process (sampling interval of 0.5 s). Power measurements were performed using a digital multimeter M-4660-M (Conrad Electronic SE, Hirschau, Bavaria, Germany) with DIGISCOP v. 2.05 software, connected to a computer. A detailed description of the search methodology is provided by Hassoon et al. [
56].
The grinding efficiency index of the JA dried material was calculated as the ratio of the surface area generated by grinding to the energy consumed during the grinding process [
57].
2.8. Extraction and Isolation of Inulin
The extraction process of sugars, fructooligosaccharides (FOS: 1-kestose [GF2], nystose [GF3], and β-fructofuranosyl nystose [GF4]), and inulin was based on the methodology developed by Khuenpet et al. [
20] with certain modifications. Prior to extraction, the dried material was ground. A 100 g sample of dried material was subjected to two-step extraction with water at 70 °C for 30 min. The extraction was carried out at a water-to-dried material ratio of 20:1 (
w/
w). After extraction, the solution was centrifuged, heated to 80 °C, and alkalized using lime milk (Ca(OH)
2) for 30 min to achieve a pH of 11–12. The pH meter was calibrated using buffer solutions with pH values of 4.0, 7.0, and 10.0. Buffer control of the pH meter was performed using a buffer solution at pH 9.18, with the obtained results showing deviations not exceeding 0.1 pH units. Subsequently, the solutions were neutralized for approximately 30 min to reach a pH of 6.0–6.5. The obtained solution was then filtered. The resulting solutions were subjected to crystallization at 3–4 °C for 48 h. The inulin preparations were filtered under reduced pressure. The obtained preparations were then freeze-dried at 20 °C (the freeze-drier was equipped with a shelf heating system) under a drying chamber pressure of 100 Pa. The drying process lasted 24 h, with the final moisture content of the preparations not exceeding 3%. The determination of simple sugars, disaccharides, FOS, and inulin content was performed using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on a Dionex ICS-3000 (Dionex Corporation, Sunnyvale, CA, USA) system equipped with a Dionex CarboPac column and an electrochemical detector. Prior to analysis, samples were centrifuged at 5000 rpm for 10 min and filtered through a 0.45 µm filter. A volume of 20 µL of the solution was injected into the column. The mobile phase consisted of 30 mM NaOH, with a flow rate of 0.6 mL/min.
2.9. Statistical Analysis
A one-way analysis of variance (ANOVA) was performed. To determine the significance of differences between means, Tukey’s test was applied. All experiments and analyses were conducted in five replications. Drying kinetics regression analysis was carried out using nonlinear least squares estimation, with the coefficient of determination (R
2), root mean square error (RMSE), and the reduced chi-square value (χ
2) calculated. Additionally, the RMSE and χ
2 values were computed to assess the goodness of fit:
where
MRi,p—predicted value of the moisture ratio,
MRi,e—experimental value of the moisture ratio,
N—number of observations,
n—number of parameters in the model equation.
Furthermore, to compare the performance and parsimony of different drying models, the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) were also calculated. These information criteria help assess model adequacy by balancing the goodness of fit and the complexity of the model. Lower values of AIC and BIC indicate better model performance with fewer risks of overfitting.
The AIC and BIC were calculated using the following formulas:
where RSS—residual sum of squares:
Statistical analysis was performed using Statistica 13 software (StatSoft, Tulsa, OK, USA). All calculations were conducted at a significance level of α = 0.05. Means marked with different lowercase letters in the columns of the tables (in superscript) differ significantly (p < 0.05).
4. Conclusions
Among the drying methods analyzed, the Page model provided the best fit to the experimental data. Due to the shorter process duration, convective–microwave drying was the most advantageous. The shortest drying time was observed at the highest temperature within the tested range (AMD80 method). As the drying air temperature increased, values of lightness, chroma, and hue angle decreased. The powder obtained by convective–microwave drying exhibited higher lightness values and lower chroma and hue angle compared to convective drying. The powder color closest to the raw material was obtained after drying by the AD40 method, whereas the color coordinates after AMD80 drying differed the most from the raw material. The powder with the highest total polyphenol content and greatest antioxidant potential was obtained after AMD40 drying, while the poorest results were observed for the AMD80 method. Increasing drying temperature negatively affected indicators related to powder grinding. At a given temperature, these indicators were always better for powder obtained by convective–microwave drying. The drying process increased the content of glucose, fructose, sucrose, and fructooligosaccharides but decreased the inulin content. The highest inulin content in extracts and the final product was observed for the AD40 process (specifically 78.35 g·(100 gdm)−1 and 92.46 g·(100 gdm)−1, respectively). The lowest inulin content was found following the AMD80 method (72.2 g·(100 gdm)−1 and 91.33 g·(100 gdm)−1, respectively). Considering drying kinetics, the quality characteristics of the powder, and the concentrations of FOS and inulin, the most favorable drying process was convective–microwave drying at 40 °C.
Based on the conducted research, valuable insights were gained into the influence of drying methods and conditions on the fundamental quality characteristics of the dried material—factors crucial for the application of inulin powders in the food industry—as well as on the inulin content in these powders, which serve as important additives in functional foods. Given the growing interest in both inulin and fructooligosaccharides, it would be worthwhile to conduct similar investigations using freeze-drying. Future studies will aim to perform a comprehensive techno-economic analysis and evaluate the industrial implications of the examined drying methods, including energy consumption and quality trade-offs, to further support their practical implementation.