1. Introduction
1.1. Importance of Underground Hydrogen Storage (UHS)
Energy production from fossil fuels has greatly impacted global warming and climate change due to increasing anthropogenic CO
2 emissions. Achieving a successful transition into a low-carbon energy industry requires the adequate utilization of renewable energy resources. Currently, several regions have established long-term goals of achieving net-zero CO
2 emissions by 2050, and as a result, much attention has been given to exploring alternatives to replacing fossil fuel energies. Hydrogen is regarded as a clean energy source because hydrogen production has almost zero CO
2 emissions [
1,
2]. The low density of the substance, which indicates more volumetric capacity than other gases like CH
4, and lower temperatures to suit storage facilities, has caused storage problems. This has necessitated investigating substitutes such as underground hydrogen storage in porous media (UHSP), utilizing exhausted hydrocarbon reserves and saline aquifers. Many depleted shale basins throughout the United States have great volumetric capacity, fit for hydrogen storage [
3]. Large-scale, long-term storage is thought to be best accomplished by geological storage. Underground geological formations, including aquifers, rock caves, and depleted oil and gas reserves, can all have hydrogen.
The role of hydrogen storage is crucial in improving the stability of electricity systems, facilitating the integration of renewable energy sources, and promoting decarbonization initiatives. This solution offers sustained energy storage, bolsters energy security, and fuels both industries and transportation [
4]. Underground natural gas storage exhibits numerous parallels with underground hydrogen storage. From a geological perspective, underground space presents an ideal option for hydrogen storage. This hydrogen can serve as an energy carrier during periods of surplus energy production, allowing it to be released back into the electrical grid during peak demand, when its value is maximized [
5]. Different methods of storage for hydrogen are evaluated, including porous rocks such as depleted natural gas and oil deposits, aquifers, as well as artificial underground spaces like salt caverns and abandoned mine workings.
1.2. Role of Shale and Tight Reservoirs in Energy Storage
A good reservoir for a hydrogen storage system should possess appropriate porosity and permeability along with a strong caprock seal. Depth deposits, lithology of the site storage, storage capacity, geological tightness, recent experience, availability of structures, and current infrastructure define different techniques of geological storage development. Site selection depends critically on geological, technological, environmental, and financial aspects as well as on site location [
4]. Shale and tight reservoirs refer to types of rock formations that are less permeable compared to conventional reservoirs, which makes extracting hydrocarbons (like oil and gas) from them more challenging. Shale is a fine-grained sedimentary rock that often contains significant amounts of organic material. Over millions of years, this organic material can transform into hydrocarbons, making shale a common source rock for oil and gas. However, shale itself is typically low in permeability (the ability of fluids to flow through the rock), meaning oil and gas are not easily extracted without advanced techniques. Hydraulic fracturing (fracking) is commonly used to release hydrocarbons from shale reservoirs. Shale reservoirs, like the Bakken Formation or the Eagle Ford Shale, have become major sources of oil and gas production in the U.S. due to advances in drilling and fracking technologies.
Tight reservoirs are similarly low-permeability rock formations, often made up of sandstone, limestone, or shale that are compacted tightly, preventing oil or gas from flowing easily. These reservoirs are “tight” because the pores or fractures in the rock are so small and not well connected, making it difficult for hydrocarbons to move through them naturally. Nonetheless, the long-term storage of substantial quantities of hydrogen as an auxiliary energy source has proven to be a complex issue. To tackle this challenge, traditional underground geological storage options like depleted oil and gas reservoirs, aquifers, or caverns present viable possibilities. Primarily, depleted gas reservoirs are viewed as the most feasible option due to their technological advantages (significant storage capacity) and economic benefits. Furthermore, the depleted shale gas reservoirs present a promising option for hydrogen storage in conjunction with traditional storage reservoirs. Their tight porosity and permeability, extensive distribution, and elevated capillary pressures could either hinder the escape of hydrogen or considerably impede its movement, along with accommodating its substantial volume. Shale gas reservoirs represent complex heterogeneous porous materials, consisting of two primary components: organic matter (carbonaceous kerogen) and inorganic minerals [
6].
Figure 1 below shows the data for the various UHS types worldwide [
5].
This research employs a multidisciplinary approach, integrating geological, geochemical, and engineering viewpoints to provide a thorough understanding of UHS in tight and shale formations. The procedure starts with an evaluation of reservoir attributes, examining the storage potential of shale and tight reservoirs compared to conventional storage sites such as depleted gas fields and salt caverns. The analysis subsequently explores geomechanical and geochemical issues, scrutinizing elements like fracture dynamics, containment hazards, and hydrogen-induced changes in rock characteristics. Additionally, the investigation examines the dynamics of hydrogen flow within low-permeability formations, tackling the complexities related to injection and withdrawal cycles. Findings from experimental studies, numerical modeling, and field-scale applications are compiled to highlight significant limitations, technological progress, and optimal practices for enhancing hydrogen storage.
This research focuses on identifying key challenges that currently hinder the industrial use of underground hydrogen storage (UHS) in unconventional reservoirs, such as data gaps, scale-up difficulties, and long-term monitoring risks. It also examines key factors like sustainability, regulatory frameworks, and economic viability to ensure that any proposed storage solutions align with broader goals of energy security and environmental protection. Overall, this study aims to serve as a strategic guide, providing a clear and practical roadmap for advancing safe, efficient, and scalable hydrogen storage systems in shale and tight formations.
2. Geological and Reservoir Characteristics
2.1. Features of Shale and Tight Reservoirs
A significant challenge involves the geochemical reactions among rock, hydrogen, and formation brine, which may lead to mineral dissolution or precipitation and modifications in petrophysical properties, consequently impacting storage capacity and withdrawal efficiency. Carbonate reservoirs are often suitable for UHS owing to their pronounced hydrophilic wettability and specific rock–fluid interfacial tension properties. In the geological storage of hydrogen, in addition to the hydrogen solubility or trapping in the formation fluids, it is also essential to understand the hydrogen–mineral geochemical reactions in the subsurface condition and evaluate the implications on other parameters [
7].
Depth deposits, lithology of the site storage, storage capacity, geological tightness, recent experience, availability of structures, and current infrastructure define different techniques for developing geological storage. Site selection depends critically on geological, technological, environmental, and financial factors [
4]. Storage is significantly affected by hydrogen’s low density, small molecular size, and viscosity. A suitable reservoir for a hydrogen storage system should have appropriate permeability and porosity, along with a strong caprock seal. Porosity and permeability are fundamental properties for reservoir evaluation and production control. Additionally, total organic carbon content (TOC) is another key property for evaluating shale reservoirs. The higher the TOC, the greater the potential for hydrocarbon production [
8]. Therefore, porosity, permeability, and TOC are crucial parameters in characterizing shale gas reservoirs and assessing production potential. For conventional reservoirs, geologists usually establish this relationship based on petrophysical modeling. However, shale gas reservoirs are more complex due to low permeability and porosity. The major marine shale oil reservoirs in the U.S. are characterized by Type-II kerogen with relatively high TOC levels, generally over 3%, and relatively high maturity with Ro values of 0.6–1.5% [
9].
Table 1 [
9] below presents organic geochemical parameters of continental shale oil.
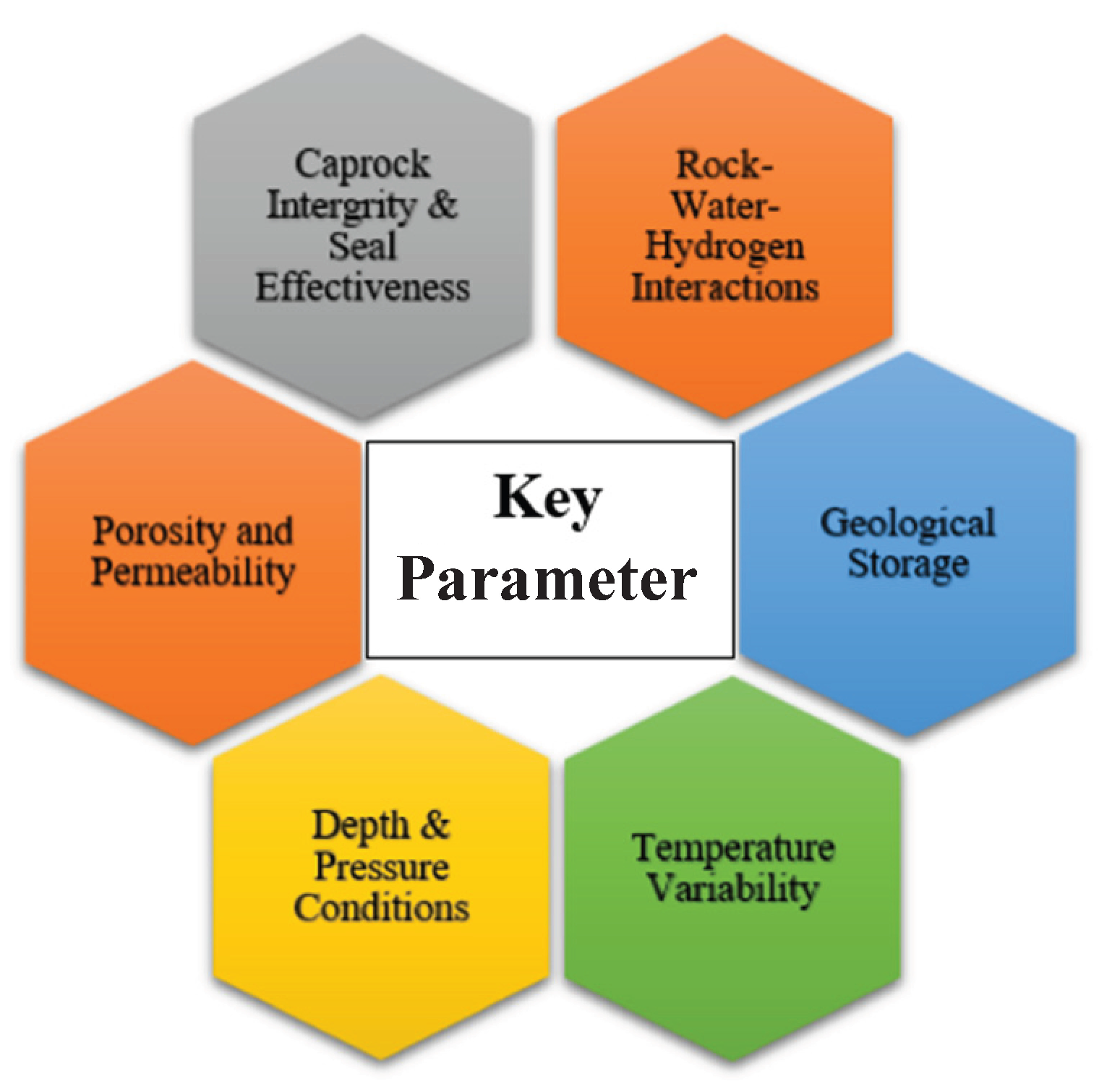
2.2. Essential Factors Affecting Hydrogen Storage Capability
Underground hydrogen storage (UHS) in geological formations is only viable when essential conditions are satisfied, as these determine the storage capacity, containment quality, and retrieval efficiency.
2.2.1. Porosity and Permeability
The amount of hydrogen that can be stored in a rock formation mainly depends on its porosity, which indicates more space for holding hydrogen. Permeability, however, influences how easily hydrogen can be injected into and extracted from the formation. In denser rocks with low permeability (10−8 ms−1), artificial stimulation techniques may be necessary to enhance flow and improve storage efficiency.
2.2.2. Caprock Integrity and Seal Effectiveness
A low permeability caprock, like shale or salt formations, plays a crucial role in inhibiting hydrogen migration and leakage. The integrity of the seal must endure cyclic injection and withdrawal without exhibiting any fractures.
2.2.3. Rock–Water–Hydrogen Interactions
The interaction of hydrogen with reservoir minerals and formation water has the potential to influence storage efficiency. Specific minerals, including iron oxides, can facilitate hydrogen consumption via redox reactions, which may hinder recoverability.
2.2.4. Depth and Pressure Conditions
Shallow formations, particularly those less than 500 m deep, are susceptible to hydrogen loss as a result of microbial activity. Deeper formations exceeding 1000 m offer enhanced pressure, which boosts hydrogen density, yet this also leads to increased operational expenses.
2.2.5. Temperature Variability
Elevated temperatures could potentially improve hydrogen diffusivity and lead to higher loss rates. Consistent temperature conditions in deep saline aquifers or depleted gas fields create an improved environment for storage.
2.2.6. Geological Storage Types and Suitability
Exhausted oil and gas reservoirs; thoroughly analyzed, with established infrastructure in place. Deep saline aquifers present significant storage potential; however, there is considerable uncertainty regarding their sealing capacity. Salt caverns demonstrate exceptional containment capabilities owing to their impermeable salt formations, making them a preferred choice for hydrogen storage.
Figure 2 is generated by the author.
2.3. Comparison with Conventional Storage Reservoirs
Geological formations with naturally high porosity and permeability, known as conventional storage reservoirs, are ideal for storing and recovering fluids, including hydrocarbons and gases like hydrogen. “The HyStorage Project in Germany demonstrated a 90% recovery rate of injected hydrogen in porous rock formations during its 2024 test phase, with no significant effects on reservoir performance or material corrosion. The Advanced Clean Energy Storage Facility in Utah, USA, is designed to store 100 metric tonnes of green hydrogen daily within salt caverns. Meanwhile, the Krummhörn Hydrogen Storage Facility in Germany, which began operations in 2024, has a total storage capacity of 500,000 normal cubic meters of green hydrogen. This work highlights advancements in underground hydrogen storage technologies, emphasizing impressive recovery rates, e.g., HyStorage’s 90%, scalability, e.g., HyStock’s 216 GWh capacity, and effective integration with renewable energy systems.” [
4]. Underground hydrogen storage involves confining high-pressure hydrogen (10.3 MPa and 20.6 MPa) within geological formations such as aquifers, caverns, abandoned mines, and depleted natural gas and oil reserves [
10]. Besides typical gas losses caused by microbial activity or mineral interactions in geological formations, hydrogen can also be lost through trapping in pore-scale capillaries.
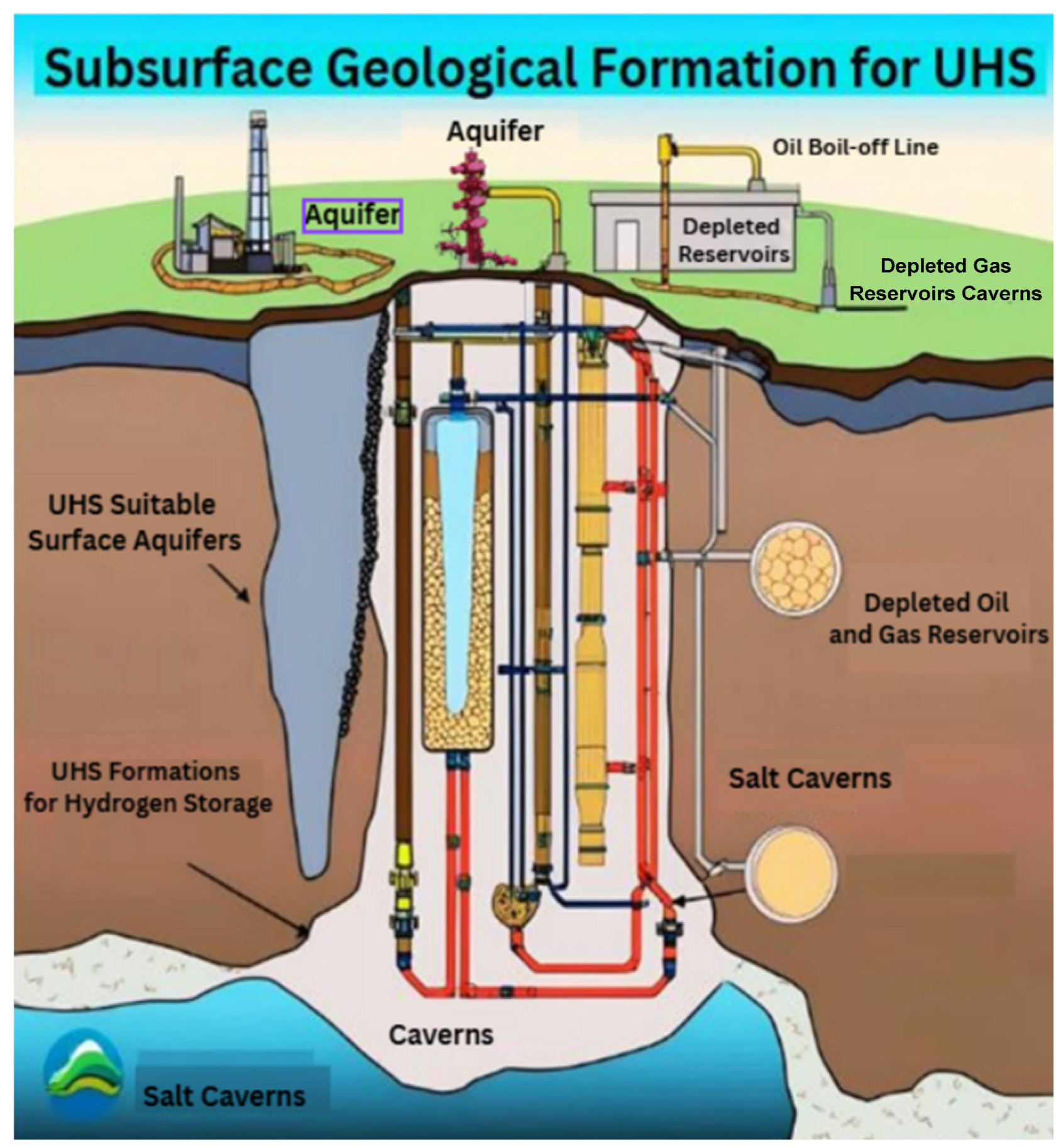
Figure 3 (generated by the author) below provides a clearer understanding of the different types of UHS.
2.3.1. Utilization of Depleted Oil and Gas Reservoirs for Storage
An impermeable caprock reinforces depleted oil and gas fields; aquifers on the sides and underneath them as well. The current hydrocarbon reserves show that caprock integrity is fully known. Consideration of elements like formation storage capacity, storage depth, and caprock formation thickness helps one choose a storage site [
11,
12]. Mostly by reversible trapping processes comprising structural/stratigraphic, capillary trapping, and solubility/dissolution in fluids, hydrogen is maintained in geological formations. UHS in depleted reservoirs must satisfy certain criteria if it is to operate consistently over a long period [
4]. Hydrogen present in oil fields could react with leftover oil to produce methane, thereby decreasing the level of stored gas.
On the other hand, hydrogen storage in natural gas fields has advantages as the leftover gas can act as a cushion, thereby maintaining the suitable pressure and guaranteeing enough deliverability [
10,
11]. In the presence of pollutants like methanogenic and sulfate-reducing bacteria, there can be significant hydrogen depletion. Particularly in depleted oil and gas reservoirs, the prospect of clathrate hydrate for hydrogen storage presents an interesting possibility for subterranean storage. There is already a lot of data about the location as comprehensive geological studies are carried out before the exploration and exploitation of a gas field. Maintaining low porosity and permeability is vital to prevent leakage; a hydraulic fracture threshold is advised to be below 10
−8 ms
−1. The location should show resistance to hydraulic fracturing, guaranteeing temperature stability between 4 °C and 80 °C, and simultaneously sustain a storage lifetime spanning 28–30 years [
13]. The described requirements are necessary to guarantee the efficient and safe usage of depleted reservoirs for long-term storage needs.
2.3.2. Storage in Aquifers
Aquifers, or groundwater reservoirs located in porous rock formations, have historically been used as safe natural gas storage locations. This characteristic makes them a viable alternative for hydrogen storage as well to transfer hydrogen efficiently through an aquifer, the overlying rock must have high porosity and permeability [
12]. Simultaneously, the existence of a low-permeability cap rock above the aquifer is required to prevent gas leakage. However, due to the unique chemical properties of hydrogen, research must focus on certain geological formations. In contrast to depleted oil and gas resources, iron or sulfur-rich aquifers may not be ideal for hydrogen storage. Furthermore, aquifers typically require around 80% cushion gas for stable storage, compared to just 50–60% in depleted fields [
4].
Thus, choosing an appropriate gas type for cushioning is important for aquifer storage projects. Given the physicochemical characteristics of hydrogen, major financing for subsurface infrastructure—including wells and injection systems—is needed to convert from subterranean gas storage to hydrogen storage in aquifers, hence improving its economic viability [
14]. While the storage development should have a porosity of more than 10%, a thickness of at least 300 m, and a vertical closure of at least 10 m, optimal storage depth varies from 200 to 2000 m. To keep reservoir integrity and storage performance, the discovery pressure should also fall between 2 and 8 MPa. UES’s effective application in saline aquifers depends on these criteria [
13].
2.3.3. Salt Caverns Storage
Salt caverns are pits produced in subterranean salt reservoirs by solution mining. Solution mining is the method of producing salt employing high-pressure water injection into subterranean salt reservoirs. Two forms of salt deposits allow caverns to develop: salt domes and bedded salts. Salt domes are homogenous, thick heaps of salt that make building a stable cavern for normal activities simple. Still, even with a well-designed cavern, if the depth is more than 6000 feet below the ground, excessive pressure and temperature might cause the salt to distort [
13]. The gas finds an impenetrable layer inside the cavern walls. From 100,000 to 1,000,000 m
3 [
10], the actual volume of salt caves might vary. Various strata of cavernous lithology have unique characteristics that affect sliding behavior, deformation, and creep rates along the bedding planes.
Reaching depths of 900 to 1100 m, the 60.5 km
2 Jintan salt mine in Jiangsu province, China, for large-scale hydrogen storage systems, offers a rather exciting alternative. Usually, the maximum pressure is kept between 75 and 85% of the original vertical beginning stress component [
4] to reduce structural loss resulting from salt hydraulic fracturing and possible collapse of the cemented well casing. With a 30 to 50-year expected storage lifetime, salt caverns provide a consistent and efficient option for subterranean hydrogen storage. Salt caverns are unique from other storage choices, including drained reservoirs and aquifers, each with their own set of advantages and drawbacks.
Table 2 (Source: Author) depicts the comparison with Unconventional storage reservoirs.
2.3.4. Comparative Assessment of UHS
A comprehensive understanding of underground hydrogen storage (UHS) necessitates both qualitative discussions and quantitative comparisons of essential reservoir properties. To improve the scientific rigor and practical relevance of this review,
Table 3 (Source: Author) presents a consolidated comparison of the main geological formations evaluated for UHS—Shale/Tight Reservoirs, Salt Caverns, and Saline Aquifers—across essential parameters such as porosity, permeability, storage capacity, injectivity, seal integrity, and risk factors.
3. Geomechanical Considerations
The geomechanical factors that primarily influence subsurface hydrogen storage include injection and production rates, wellbore shape, rock tensile strength, and the presence of fractures or faults [
15]. Geomechanical modeling examines the impacts of geomechanics, such as the potential for reservoir fractures, the stability of wellbores and cap rocks, and the reactivation of faults. The ongoing processes of gas input and withdrawal in hydrogen storage affect the effective stress due to variations in pore pressure, thereby jeopardizing the integrity of both the reservoir and the caprock. When hydrogen is repeatedly supplied and withdrawn, the pressure inside the reservoir varies. These pressure shifts might cause existing fissures or weak places in the rock to reopen, increasing the risk of leaks and gas loss [
4]. To determine how effectively a reservoir can manage this, consider how the rock reacts to stress. That includes understanding its strength, how far it can bend without breaking, and how it responds to various stresses. This type of knowledge is critical for ensuring the storage site’s stability and safety throughout hydrogen activities.
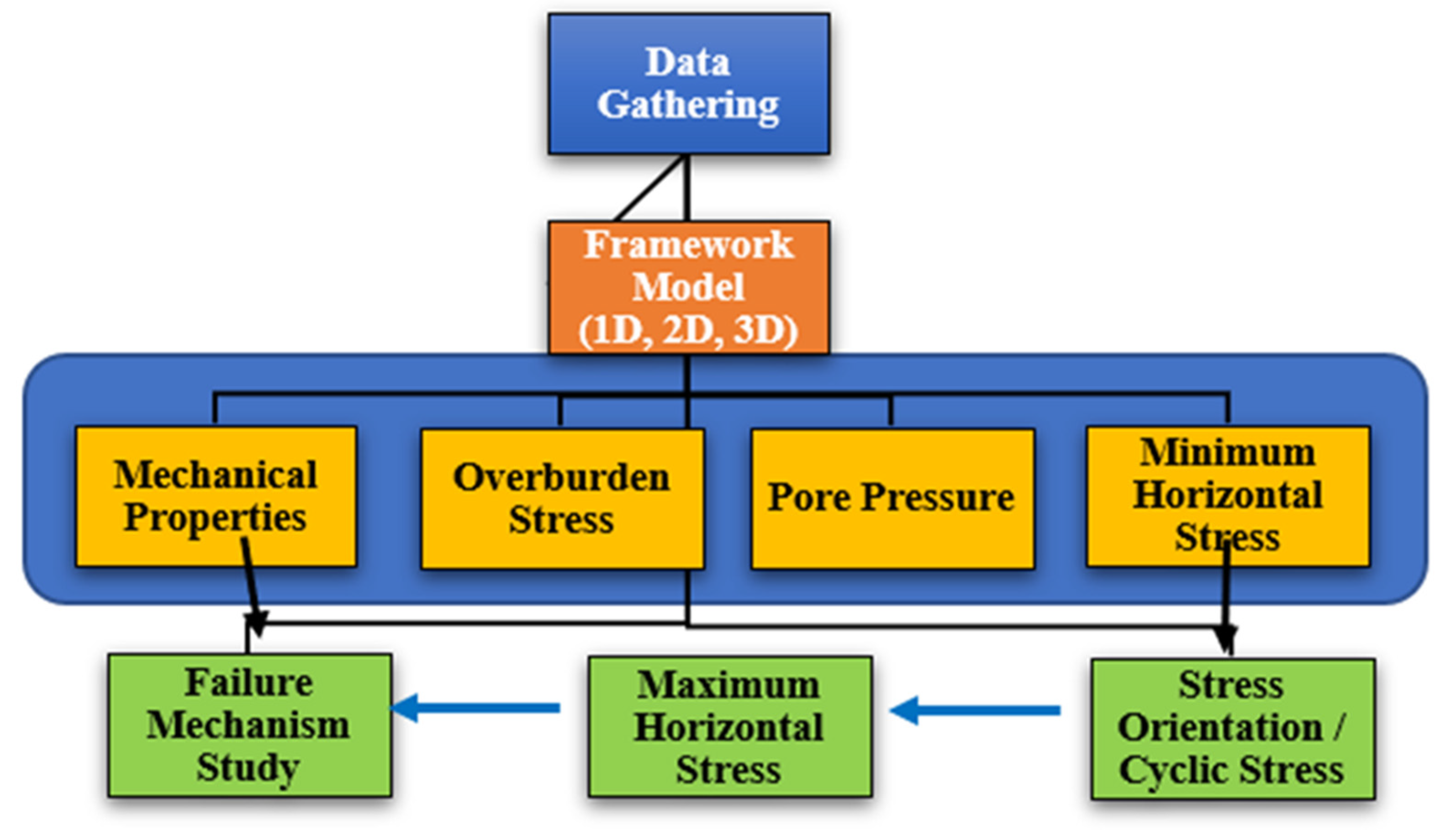
Figure 4, ref. [
16] illustration explaining the basics of geomechanical studies.
3.1. Stress Distribution and Reservoir Integrity
Especially in unconventional reservoirs, such as shale formations and depleted reservoirs, the viability of underground storage depends on the evaluation of stress distribution and reservoir integrity. Mechanical stability, permeability variations, and general integrity of containment are strongly influenced by the interaction of in situ forces with the reservoir rock. Three main stressors define the stress situation inside a reservoir, most importantly. The weight of the rock strata above determines vertical stress (σv). Tectonic forces, together with the surrounding geological setting, produce the maximum horizontal stress (σH). Determination of hydraulic fracturing behavior and fluid flow depends critically on the minimum horizontal stress (σh). The balance of these stresses determines the ability of a reservoir to safely store gases like hydrogen or hydrocarbons, therefore reducing the possibility for leakage or structural damage [
17].
When fluid input, withdrawal, or geomechanical disturbances change the stress distribution, reservoir rock fractures and cause rock failure that results in: Induced fractures raise permeability, but they also run a danger of accidental leaking. Shear failure results from applied stresses exceeding the intrinsic strength of the rock, therefore compromising structural integrity. Caprock integrity failure can result from the overburden rock losing its capacity to seal, therefore enabling trapped gas to move to shallower strata or perhaps escape to the surface.
Reservoir integrity depends on considerations for mechanical and hydraulic parameters being stable throughout storage operations. Geomechanical modeling is the simulation of stress changes brought on by injection and depletion cycles. Seismic monitoring is the identification of microseismic occurrences indicating cracks brought on by stress. Evaluating caprock’s sealing capability with an eye on its potential to stop fluid migration from the underlying formations.
3.2. Fracture Behavior and Containment Risks
Gases include hydrogen, natural gas, and CO
2 is stored underground; complex geomechanical mechanisms control their stability and confinement inside geological formations. The behavior of fractures, hazards related to containment, and the impact of cyclic loading on the stability of the storage system are essential elements determining the feasibility and safety of underground gas storage [
18]. The recognized hazards include probable declines in rock strength, ground subsidence or uplift, impaired well integrity, continuous hydrogen leaking, caprock seal efficacy lost, and seismic activity resulting from fault reactivation [
4].
By changing permeability, gas movement, and general confinement of stored gases, fractures in subsurface rocks can significantly impact subterranean storage. Natural geological events spanning millions of years or human operations, including hydraulic fracturing and gas injection, can also cause fractures [
19]. Different elements affect the formation and distribution of fractures: mechanical characteristics of the rock, current stress conditions, and fluid pressure applied during gas storage operations.
The injection of gas into a subsurface reservoir leads to an increase in pressure within the formation, which may result in the widening of pre-existing fractures or the formation of new fractures. This can improve the rock’s permeability, facilitating gas movement, which can be advantageous in certain scenarios. This is especially important for hydrogen storage, given that hydrogen molecules are smaller than those of methane or CO
2, which makes them more susceptible to diffusive leakage through microfractures [
18]. Studies of fracture behavior give great attention to the integrity of the caprock, which serves as the primary barrier preventing trapped gases from rising. Should the caprock show pre-existing flaws or endure notable pressure variations, its capacity to seal might be weakened, therefore allowing probable gas escape. Moreover, some fractured formations might experience shear failure, causing rock layers to slide along a fault line and hence compromise storage security [
20]. In regions with tectonic activity, the possibility of seismic events caused by fluid injection might aggravate containment issues, therefore emphasizing the need for careful geomechanical study before the choice of a storage site.
Comprehensive reservoir characterization is therefore required for the identification of fracture networks, assessment of caprock integrity, and prediction of fracture behavior under different storage circumstances, thereby addressing these difficulties. More knowledge of the evolution of pressure-induced cracks over time is made possible by advanced geomechanical modeling approaches, including microseismic monitoring and finite element simulations. By carefully controlling injection pressures and closely observing fracture development, operators may maximize gas storage and protect reservoir sustainability and safety over time.
3.3. Cyclic Loading Effects and Storage Stability
Geomechanical changes can be modeled more precisely to ensure reservoir integrity throughout UHS operations. During underground hydrogen storage, injection and withdrawal cycles cause reservoir pore pressure and temperature to vary, which can introduce significant risks during and after operations. Because both depleted reservoirs and salt caverns rely on wells for hydrogen injection and withdrawal, maintaining wellbore integrity is essential to prevent hydrogen leakage and ensure the safety and efficiency of the storage system [
4]. In underground gas storage systems, cyclic loading, the repetitive injection and evacuation of gas, causes alternate stress and strain on the reservoir rock [
21]. The mechanical stability of the storage development can be greatly influenced by these cycles, therefore causing reservoir compaction, changes in permeability, and structural collapse over time. Cyclic loading, the repetitive injection and removal of gas that results from underground gas storage activities, stresses and strains the reservoir rock alternately. The mechanical stability of the storage formation can be greatly altered by these cycles, therefore causing reservoir compaction, changes in permeability, and perhaps structural failure over time.
While withdrawal results in decompression and contraction, higher pressure during gas injection causes the rock to expand softly [
21]. Particularly in strata with high clay content or weakly cemented grains, constant cycles of expansion and contraction might compromise rock structural integrity. The fatigue impact might consolidate microcracks into bigger fractures over time, thereby altering the porosity and permeability of the storage development. Under extreme conditions, constant cyclic loading can cause permanent rock deformation, hence reducing storage efficiency and the effectiveness of following injections.
One major obstacle with cyclic loading is how it affects caprock integrity. The caprock acts as a barrier preventing gas migration; yet, constant pressurization might cause stress variations compromising its capacity to seal sufficiently. In rocks marked by naturally occurring fractures, these stress variations might progressively widen already present cracks, increasing the gas leakage risk. Cystic loading may cause fault reactivation in the presence of discontinuities such as faults in the caprock, therefore enabling the evacuation of trapped gases into the formations above. Apart from mechanical aspects, cyclic stress significantly influences the hydraulic features of the reservoir. Variations in pore pressure and rock compaction cause permeability variations with every injection cycle. Permeability hysteresis is a phenomenon whereby, following each withdrawal cycle, permeability does not entirely revert to its natural condition [
22,
23].
Moreover, utilizing core samples from possible storage locations, laboratory studies on cyclic stress on rock behavior in a controlled setting might provide crucial new perspectives. The results of cyclic loading studies have major ramifications for many worldwide subsurface storage projects. For example, salt caverns used for storing hydrogen are known to handle repeated stress quite well, mainly because they can naturally seal up small cracks over time. On the other hand, depleted oil and gas reservoirs—often reused for storing methane or hydrogen—can experience slow changes in how easily fluids move through them after several injection and withdrawal cycles [
23]. Through the integration of geomechanical analysis, real-time monitoring, and intelligent operational strategies, one can effectively address the impacts of these pressure cycles. This contributes to the stability and efficiency of underground storage systems for extended periods.
Table 4: ref. [
4] Comparison of Natural gas, CO
2, and H
2 Storage perspective in geomechanics [
22].
4. Geochemical Considerations
Hydrogen’s reactivity can produce dangerous gases that can compromise the wellbore, soil, and atmosphere. Geochemical interactions among reservoir fluids, cushion gas, hydrogen gas, and reservoir minerals may occur during UHS. Employing conversion into hydrogen sulfide (H
2S) and methane (CH
4), the processes cause hydrogen to be depleted. The geochemical reactions taking on in UHS might cause minerals to dissolve or precipitate, hence altering permeability and porosity. This modification could cause structural collapse or defects in the integrity of the cap rock [
13]. Geochemical processes include both biotic and abiotic interactions among a spectrum of reactions. The abiotic processes especially entail the interaction of non-living molecules such as hydrogen [
24], oil, gas, brine, rock minerals, and so on.
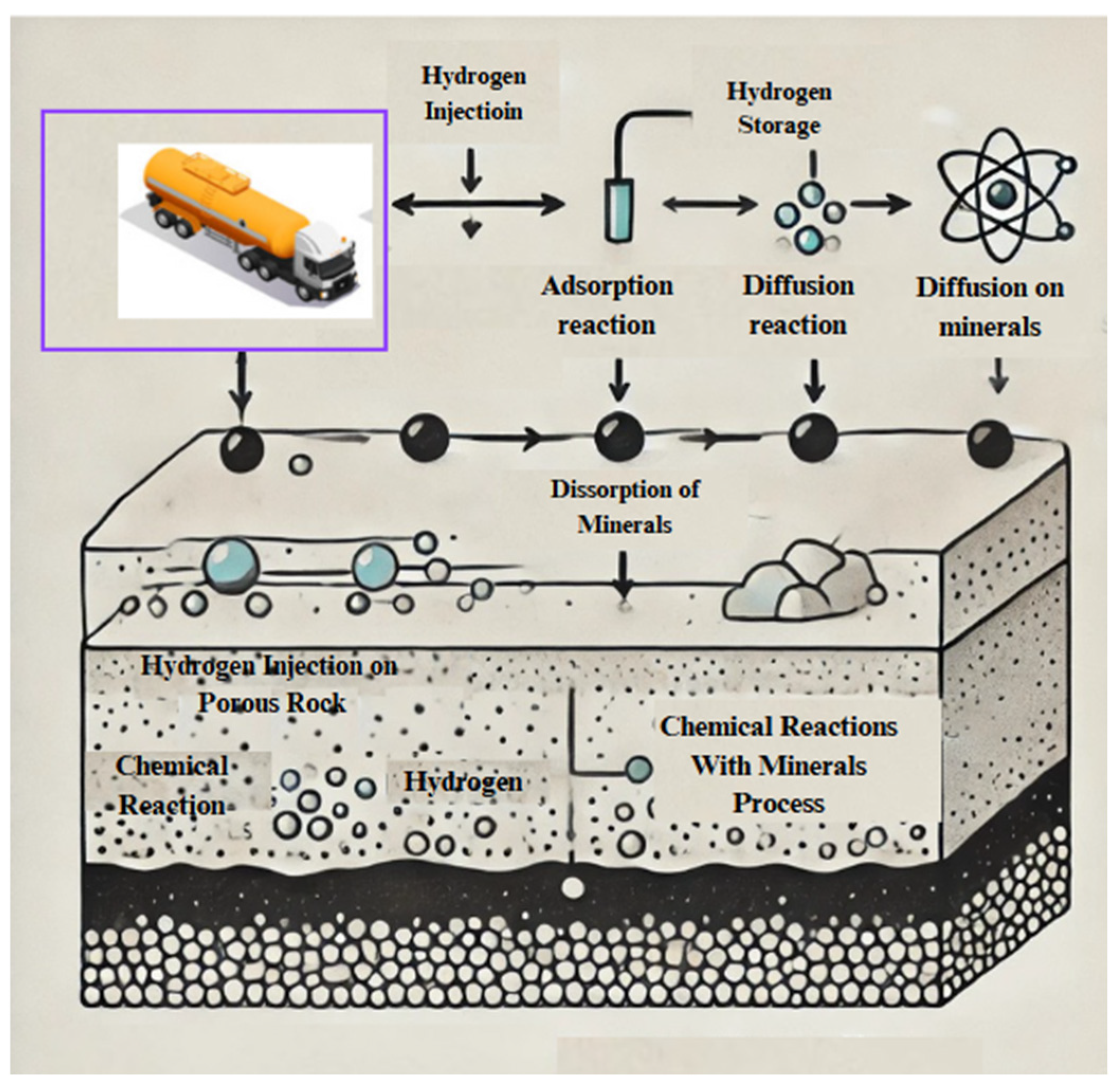
Figure 5 depicts interactions between hydrogen and geological minerals.
4.1. Hydrogen–Mineral Interactions
Evaluation of the feasibility and safety of subterranean hydrogen storage depends on interactions between hydrogen and geological materials. The interactions could compromise the efficiency of hydrogen containment [
25] or the integrity of storage reservoirs. Geochemical reactivity of hydrogen with sandstone formations has been investigated recently. The results imply that abiotic geochemical events in sandstone reservoirs have a minimal probability of causing hydrogen loss or degradation of reservoir integrity. This suggests that formations of sandstone might be appropriate for subsurface hydrogen storage [
26].
The presence of clay minerals in sandstone significantly influences hydrogen-sandstone geochemistry. Investigations into the influence of clay on hydrogen interactions in specific conditions revealed that the concentration of clay plays a crucial role in these interactions following extended exposure. Understanding the long-term behavior of hydrogen in these geological conditions requires a comprehensive awareness of its role.
Studies on the interactions between basalt, hydrogen, and water under geo-storage conditions have demonstrated that basaltic minerals exhibit minimal reactivity to hydrogen-water injections. The noted low reactivity suggests that basalt formations may serve as suitable candidates for hydrogen storage, offering a promising alternative to traditional sedimentary reservoirs [
27]. Investigations have been conducted on the interactions of hydrogen with particular minerals, such as kaolinite, muscovite, and α-quartz, utilizing ab initio methods. The results of these investigations reveal that hydrogen exhibits minimal interactions through van der Waals forces with these minerals, implying that under typical storage conditions, hydrogen is unlikely to participate in chemical reactions with them. While those defined by inert mineralogy, such as particular sandstones and basalts, seem more ideal for keeping hydrogen integrity over extended times, formations abounding in reactive minerals might provide challenges due to probable geochemical interactions.
4.2. Adsorption, Diffusion, and Wettability Mechanisms
The mechanics of adsorption, diffusion, and wettability are critical for maximizing subsurface hydrogen storage. These activities have a substantial impact on the efficiency and safety of hydrogen confinement in geological formations.
4.2.1. Adsorption Mechanism
The process of hydrogen adsorption on mineral surfaces significantly impacts storage capacity and retention dynamics. Physisorption, characterized by the influence of weak van der Waals forces, functions as the primary mechanism for hydrogen’s interaction with Earth minerals. Studies show that hydrogen molecules attach to mineral surfaces, potentially enhancing the effectiveness of subsurface hydrogen storage [
28]. A series of experiments and simulations have been conducted to investigate the potential effects of hydrogen adsorption and diffusion behavior on UHS. Conducted an in-depth analysis of the adsorption characteristics of high-pressure hydrogen across various minerals, demonstrating that the capacity for hydrogen adsorption is predominantly influenced by pore structure and specific surface area. This research highlights potential applications in the exploration of natural hydrogen and the development of underground hydrogen storage solutions [
29].
The presence of minerals like kaolinite in clay-rich formations can greatly influence hydrogen adsorption behavior. Thereby, the capacity of hydrogen storage for kaolinite slit is directly related to the increase in pressure. Investigations indicate that hydrogen molecules have the capacity to adsorb within the slit-like pores of kaolinite, thereby affecting the overall storage mechanisms in these porous media [
29].
4.2.2. Diffusion and Wettability Mechanisms
The movement of hydrogen through geological formations is crucial for evaluating leakage risks and understanding transport dynamics in storage reservoirs. Molecular dynamics simulations indicate that hydrogen self-diffusion coefficients rise with temperature in different mineral substrates, such as calcite, hematite, and quartz. The dependence of temperature highlights the significance of reservoir conditions in forecasting hydrogen mobility [
30].
In the context of hydrogen storage, the idea of wettability—that which describes the predisposition of a fluid to stick to a solid surface when additional immiscible fluids are present—is fundamental. Hydrogen trapping, migration, and possible leakage paths are strongly influenced by the wettability properties of reservoir rocks. Research in molecular dynamics has revealed that the wettability of quartz may be influenced by its surface chemistry and pressure conditions, therefore influencing hydrogen storage efficiency [
31]. Moreover, investigations on the wetting preferences of silica surfaces have provided important new perspectives on fluid displacement mechanisms during hydrogen injection. The findings emphasize the importance of comprehending mineral-fluid interactions to maintain the integrity and functionality of hydrogen storage systems [
32]. It is essential to meticulously evaluate factors like mineral composition, temperature, pressure, and surface chemistry to enhance storage capacity and reduce the risks linked to hydrogen migration or leakage. Ongoing investigation in these domains will improve our capacity to forecast and manage hydrogen dynamics within geological formations, thus facilitating the advancement of secure and effective energy storage options. Nonetheless, the investigation into hydrogen adsorption and diffusion characteristics under classical reservoir conditions (temperature < 150 °C and pressure < 50 MPa) remains constrained [
29].
4.3. Microbial and Bio-Chemical Reactions
Hydrogen has the potential to induce embrittlement in the auxiliary components of rock caverns, including compressors, pipes, and steel linings. Identifying significant shortcomings at each site is essential, especially regarding hydrogen utilization via homoacetogenesis, sulfate reduction, and methanogenesis [
4,
33]. Subsurface microorganisms have the potential to interact with stored hydrogen, resulting in a range of outcomes that could influence both storage efficiency and the integrity of the reservoir [
34]. In subsurface environments, specific microorganisms harness hydrogen as a source of energy. The consumption of microbes can lead to a decrease in stored hydrogen, consequently diminishing the efficiency of UHS operations. For instance, hydrogen-oxidizing bacteria may break down hydrogen, therefore reducing it within the storage reservoir. One of the main problems with UHS is sulfate-reducing bacteria hydrogen sulfide (H
2S). Among the various risks involved in the manufacturing of H
2S include toxicity, possible infrastructure damage, and the possibility of polluting stored hydrogen. The honesty of these systems could compromise the financial viability and safety of hydrogen storage projects [
33,
35]. For UHS, iron-rich strata offer a more appropriate choice as they suggest that the current storage facilities in rock cavernues might need changes. Steering clear of rocks like sulfide, carbonate, or sulfate is advised.
Initial results indicated that within a pH range of 6–7.5, sulfate reducers, homoacetogens, methanogens, and iron (III) reducing bacteria flourish. These bacteria thrive under salinity levels less than 60, less than 100, and less than 40 g/L
−1 in turn. Whereas iron (III) reducing bacteria thrive at 0–30 °C, sulfate reducers and homoacetogens flourish at 20–30 °C, methanogens like 30–40 °C [
35]. The variations in these interactions are influenced by the type of geological storage, with depleted hydrocarbon reservoirs and saline aquifers exhibiting a higher susceptibility to these effects compared to salt caverns. The study emphasizes the need for microbial activity; yet it does not provide quantitative information about the consequences of higher H
2 levels in saline and alkaline environments [
13].
For example, hydrogen may be metabolized by oxidizing hydrogen-consuming bacteria, hence reducing the storage reservoir. A key issue for UHS is how sulfate-reducing bacteria generate hydrogen sulfide (H
2S). H
2S generates several hazards, including toxicity, probable facility corrosion, and the possibility of contaminating stored hydrogen. The dependability of these procedures might endanger the financial feasibility and safety of hydrogen storage systems [
35].
Methanogenic archaea have the capability to transform hydrogen and carbon dioxide into methane, a process referred to as methanogenesis. This biochemical reaction not only causes hydrogen loss but also leads to the production of methane, which may be undesirable in the context of hydrogen storage. The presence of methane introduces challenges in the extraction and purity of the stored hydrogen. The activity of microorganisms can result in the development of biofilms in the porous structures of storage reservoirs. Biofilms can obstruct pore spaces, which in turn reduces the permeability of the reservoir. The decrease in permeability may impede the rates of hydrogen injection and withdrawal, thereby influencing the overall efficiency of the storage process [
36]. A thorough understanding of subsurface microbial ecology and biochemical processes is essential to grasp the interactions between microorganisms and stored hydrogen. It is essential to address these microbial and biochemical reactions for the effective execution of UHS projects.
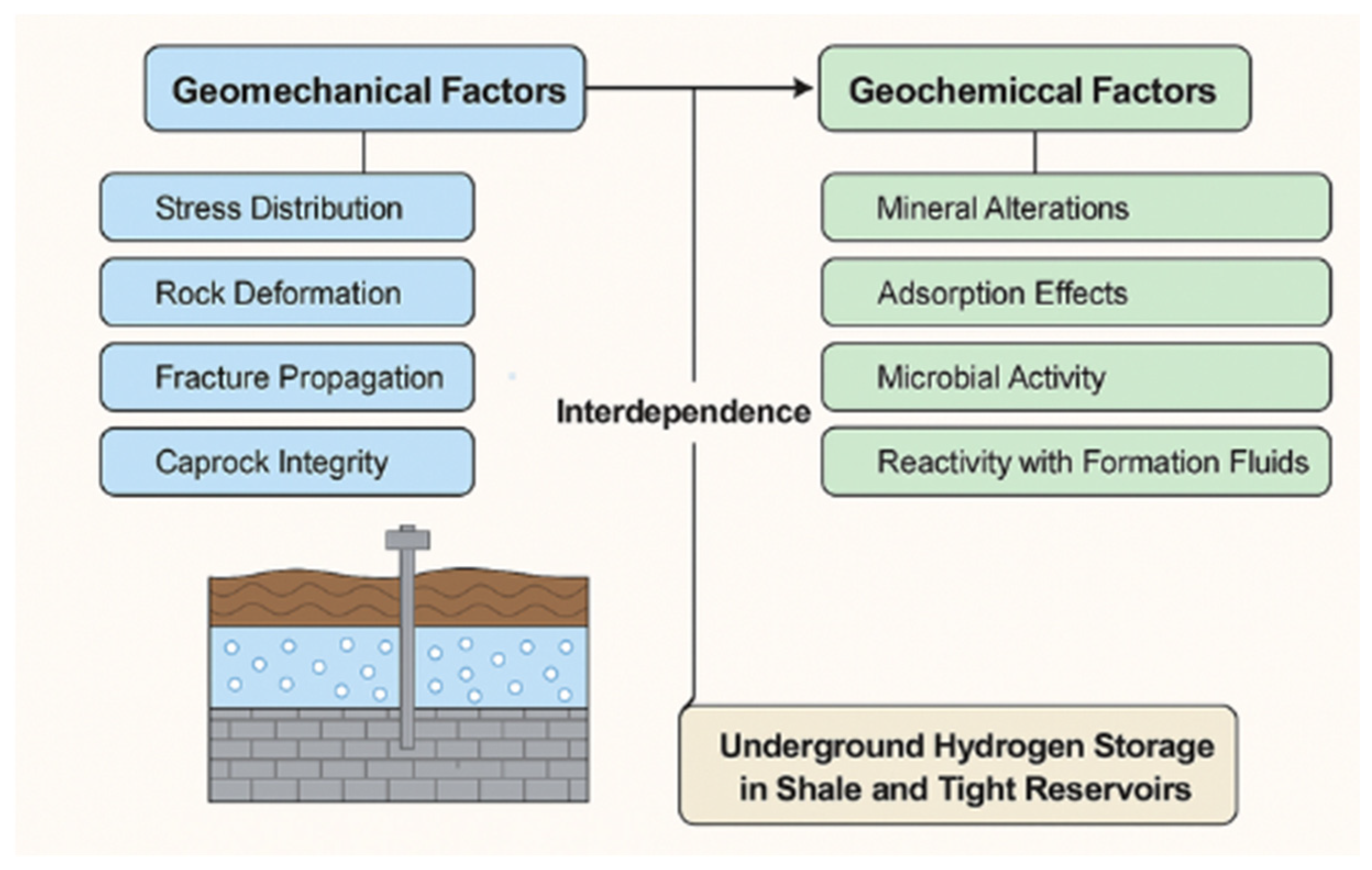
In conclusion,
Figure 6 indicates that the effective application of underground hydrogen storage (UHS) in shale and tight reservoirs is dependent on the delicate interaction of geomechanical and geochemical parameters. Stress distribution, fracture propagation, rock deformation, and caprock integrity are all geomechanical factors that influence the physical confinement and mechanical stability of a storage formation. Geochemical processes, such as mineral changes, hydrogen adsorption, microbial activity, and interactions with formation fluids, can all have a substantial impact on reservoir parameters like porosity and permeability. These chemical changes may degrade rock formations, modify stress regimes, or generate new fracture routes, whereas mechanical disturbances can expose reactive mineral surfaces, hastening geochemical processes. Additionally, microbial activity can cause hydrogen loss and jeopardize reservoir integrity. Recognizing and treating this linked behavior is crucial for the long-term safety, efficiency, and sustainability of hydrogen storage systems in unusual formations.
5. Storage Injectivity and Withdrawal Efficiency
The effectiveness of underground hydrogen storage is mainly influenced by two key aspects: the injectivity of the storage site and the efficiency of hydrogen retrieval. The efficacy of hydrogen injection and extraction from underground reservoirs is influenced by these factors, which in turn determine the feasibility of large-scale hydrogen storage [
37].
Storage injectivity is related to the efficiency of injecting hydrogen into a subterranean reservoir while guaranteeing maintained regulated pressure conditions. Reservoir permeability, pore throat diameters, fluid properties, and formation pressure mostly determine injectivity. Because their linked pore networks enable efficient hydrogen transport, high permeability formations like salt caverns and depleted gas reservoirs show better injectivity. Restricted pore connectivity and capillary pressures that hinder hydrogen migration cause the injectivity to be often greatly reduced in low permeability formations, like as shales or tight sandstones [
38]. One must give much thought to the integrity of the wellbore. Either poor well design, damaged casings, or cement degradation can cause hydrogen leaks during injection. Furthermore, explaining the drop in injectivity during numerous injection cycles might be chemical reaction degradation, fine migration, or gas-induced cracks in the formation. Advanced stimulation methods frequently employed include hydraulic fracturing, acidizing, and pressure cycling, which are designed to enhance permeability and, consequently, injectability.
On the other hand, withdrawal efficiency refers to how effectively stored hydrogen can be extracted from the reservoir for various applications. The management of reservoir pressure, mechanisms of hydrogen diffusion and retention, as well as capillary trapping, all influence the efficiency of extraction. Minimizing constraints on gas transport within reservoirs characterized by elevated porosity and permeability typically leads to enhanced extraction efficiency. Irregular flow patterns, such as gas fingering, can lead to ineffective extraction in fractured formations, resulting in the entrapment of residual hydrogen within the rock matrix [
39].
5.1. Hydrogen Movement in Low-Permeability Formations
The existing literature indicates that the intrinsic permeability of intact halite (pure rock salt) ranges from 10
−20 to 10
−22 m
2 [
40], demonstrating a notable sealing capacity, particularly for hydrogen. However, the presence of impurities can elevate salt permeability to a magnitude of 10−16 m
2. Calculations of permeability for hydrogen storage in salt caverns suggest that diffusion serves as the main mechanism in salt rocks exhibiting a permeability of under roughly 3 × 10
−21 m
2. The high level of permeability significantly minimizes the losses of hydrogen over time. Their observations indicate that the primary regions of potential leakage are the cavern roof and the wellbore cement [
15]. Shale gas is a form of unconventional natural gas that is generated and stored within high-carbon shale and its interlayer. Unlike conventional gas reservoirs, shale generally demonstrates permeability levels that align with the Nadarcy range. The permeability of the shale matrix ranges from 10
−9 to 10
−5 mD, while in the naturally occurring fractures, it spans from 10
−3 to 10
−1 mD. The typical porosity of shale reservoirs varies between 3% and 8.4% [
41].
The subsurface storage of hydrogen (H
2) in formations characterized by low permeability involves intricate interactions between geological attributes and fluid dynamics. The formations being analyzed, such as tight sandstones, shales, and specific carbonate reservoirs, demonstrate limited permeability, which constrains the efficiency of hydrogen injection, storage, and withdrawal operations. The behavior of hydrogen in these reservoirs is influenced by various transport mechanisms, such as Darcy and non-Darcy flow, molecular diffusion, adsorption, and capillary trapping. Grasping these mechanisms is essential for assessing storage viability and enhancing operational strategies. The storage of hydrogen in porous formations is greatly influenced by the inherent characteristics of the reservoir, such as porosity, permeability, wettability, and geomechanical stability. The small molecular size of hydrogen results in a higher diffusion rate compared to other gases like methane, which may elevate leakage risks and complicate the maintenance of long-term storage efficiency [
42].
Figure 7 illustrates the storage of hydrogen within a reservoir characterized by low permeability. Here Blue arrows indicate injection of hydraulic fracturing fluid to create fractures in the rock formation. The Red lines act as barriers or seals preventing upward or lateral migration of hydrogen. The Purple lines enable increased permeability for injection and withdrawal of hydrogen and the black dotted arrows indicates hydrogen being injected (or extracted) through these wells into the porous reservoir rock.
5.1.1. Mechanisms Governing Hydrogen Flow
A variety of transport and retention processes shape hydrogen’s behavior in low permeability rocks. The efficiency of hydrogen storage and recovery is highly influenced by the methods used. The basic element controlling hydrogen transport in porous materials is the dynamics controlled by pressure gradients. Darcy’s law exactly explains fluid flow in highly permeable rocks. In low permeable reservoirs, when pore throats are much narrower, Non-Darcy Flow becomes important. In these cases, Knudsen diffusion is quite important as gas molecules interact more often with the pore walls than with one another, therefore changing their transport properties [
37]. Furthermore, molecular diffusion influences the movement of hydrogen in tightly packed arrangements. The high diffusion coefficient of hydrogen molecules depends critically on their small size, which also helps them to flow efficiently via nanoscale pore structures. This function becomes somewhat crucial in situations where diffusive transport predominates over advective flow, and permeability is rather low. Fick’s diffusion depends much on concentration gradients, which also affects the hydrogen diffusing mechanism. Fick’s diffusion also influences surface diffusion, in which molecules travel to the surfaces of mineral grains inside the rock matrix.
The adsorption and retention inside the pore network are crucial determinants of hydrogen transport. The Van der Waals forces cause some of the gas molecules injected hydrogen into a reservoir to stick to mineral surfaces. This procedure is quite important in shales and clay-rich formations, as adsorption can greatly reduce the free hydrogen availability for extraction. The extent of adsorption is influenced by the mineral composition of the reservoir, with clay-rich deposits exhibiting greater hydrogen retention compared to those predominantly composed of quartz [
42]. The efficiency of hydrogen injection and withdrawal is fundamentally influenced by the interplay between capillarity, wettability, and adsorption. Capillary forces can trap hydrogen within pore spaces in a formation largely water-saturated, therefore limiting its flow. The conditions of wettability play a crucial role in determining the interaction of hydrogen with reservoir fluids and mineral surfaces, which in turn impacts its relative permeability and overall storage efficiency [
37]. Moreover, comprehending the impact of geomechanical factors on flow is essential for assessing reservoir performance throughout its entire lifespan. The processes of hydrogen injection and withdrawal result in fluctuations in reservoir pressure, which may influence the structural integrity of the rock. Low-permeability formations demonstrate a notable sensitivity to stress, suggesting that changes in pressure can lead to the closure of existing fractures, which in turn reduces permeability. Conversely, high-pressure injections may create new microfractures, potentially increasing permeability and enhancing injectivity. However, these changes necessitate careful supervision to avoid unintentional leakage paths that might compromise storage security [
43].
5.1.2. Exploring the Challenges of Hydrogen Storage in Low-Permeability Reservoirs
Low permeability formations for hydrogen storage pose various technological issues that must be addressed if effective storage and retrieval are to be made possible. One main problem found is insufficient injectivity. The limited permeability of the rock requires higher pressure gradients for hydrogen injection at reasonable flow rates, thereby possibly causing fractures and compromising reservoir integrity. This can improve permeability by creating fissures or, occasionally, cause compaction and a drop-in permeability [
42]. Important problems that need to be addressed include retention and entrapment. Among other things, trapping of hydrogen molecules, capillary pressures, adhesion to mineral surfaces, and molecular migration into nanopores can all lower effective withdrawal rates. This phenomenon raises operating expenses and reduces storage efficiency [
37].
Hydrogen increases the likelihood of leakage in low-permeability materials due to its relatively small molecular size. Even the smallest microfractures and overlooked defects that can serve as conduits may lead to gradual hydrogen leakage. Another factor being examined is geochemical interactions, as they could potentially undermine the storage integrity of the formation. Hydrogen’s interaction with minerals such as carbonates and clays can lead to the formation of secondary minerals, alterations in porosity, and potential reductions in permeability. The interactions enhance microbial activity, leading subterranean organisms to metabolize hydrogen [
43], which results in increased gas depletion and biofilm growth that could obstruct pore spaces.
5.2. Difficulties in Injection and Retrieval Cycles
A significant challenge to withdrawal efficiency is the retention of hydrogen caused by both adsorption and diffusion processes. The adsorption of hydrogen molecules onto mineral surfaces or their migration into smaller pore spaces within porous media may account for the observed decrease in gas recovery volume during successive withdrawal cycles. The relevance of this problem rises dramatically when capillary forces vary between rock strata in different forms, trapping of hydrogen. Furthermore, affecting the efficiency of withdrawal are the consequences of cyclic loading. Using cycles of injection and withdrawal will help to produce induced microfractures, lower permeability, or a change in pore structure deformation by altering the stress conditions in the reservoir. Among the notable challenges are changes in permeability, phase behavior, erosion of formations, and interactions between rocks and fluids [
42].
The primary challenges faced during hydrogen injection relate to formation damage, specifically the reduction in permeability resulting from changes in reservoir conditions. The damage to the formation results from a range of processes. The migration of small particles and the resulting obstruction of pore throats within the reservoir can lead to a decrease in injectivity over several cycles. This presents significant challenges for sandstone formations characterized by elevated clay content. Hydrogen-Induced Mineral Changes: significant effects of precipitation or dissolution may arise from the interactions between hydrogen and specific minerals. In formations abundant in carbonate, the dissolution process may enhance porosity while concurrently influencing the integrity of the rock matrix, thereby prompting inquiries about stability [
37]. Hydrogen effectively substitutes water in the injection process; however, capillary forces may lead to residual water being trapped in the pore spaces, thereby decreasing the effective pore volume available for gas storage [
42].
5.2.1. Pressure and Stress Changes Affecting Injectivity
The introduction of hydrogen into subterranean formations leads to notable fluctuations in pressure, potentially modifying the stress conditions within the reservoir. The alterations in stress levels can yield both beneficial and detrimental outcomes. Decrease in permeability as a result of heightened sensitivity to stress: numerous low-permeability reservoirs exhibit sensitivity to stress, indicating that elevated pressure during injection can lead to rock compression, which in turn decreases permeability and complicates future hydrogen extraction [
37]. Induced Fracturing and Potential Leakage: The process of high-pressure injection can generate new fractures, potentially enhancing injectivity while simultaneously posing risks of hydrogen leakage via unintended pathways. Rock Compaction and Creep: In salt caverns and soft rock formations, extended hydrogen storage may result in rock compaction over time, which can decrease available storage capacity and modify flow dynamics during retrieval [
42].
5.2.2. Retrieval Challenges and Loss of Hydrogen
Once hydrogen is stored, the efficient retrieval process becomes essential for maintaining economic viability. Nonetheless, multiple mechanisms contribute to hydrogen loss or reduced withdrawal efficiency. Adsorption and Retention in Reservoir Rock: The adherence of hydrogen to mineral surfaces occurs through van der Waals forces, which subsequently diminishes the volume of free gas that can be extracted. Reduced retrieval efficiency [
43] results from clearly higher adsorption rates shown by shale and clay-rich strata. Particularly in water-wet reservoirs, capillary trapping and residual saturation point to some of the injected hydrogen, perhaps becoming immobilized due to capillary forces. The presence of gas saturation restricts the quantity of hydrogen that can be harvested [
42]. Defensive Losses in Low-Permeability Formations: The diminutive molecular size of hydrogen facilitates its infiltration into microfractures and nanopores, complicating recovery efforts. The influence is particularly evident in formations characterized by notable micro-porosity [
37].
6. Experimental and Modeling Methodologies
The interactions among geochemical, geomechanical, and hydrodynamic factors play a significant role in UHS, a complex process that impacts the injection, storage, and generation of hydrogen gas. Anticipating UHS performance necessitates an understanding of these pathways. Geochemical factors are crucial for understanding and replicating the various processes associated with underground hydrogen storage (UHS). The models should accurately represent equilibrium processes along with precipitation and mineral dissolution reactions. The rates of these kinetic reactions are influenced by several factors, including temperature, pressure, and salinity [
44].
6.1. Experimental Investigations and Computational Modeling
Understanding hydrogen storage behavior in subterranean reservoirs necessitates both numerical simulations and laboratory experiments. These approaches aid in assessing critical parameters such as geomechanical stability, geochemical interactions, injectivity, and withdrawal efficiency. Combining computational and experimental methodologies yields valuable information for improving the long-term viability of subterranean hydrogen storage (UHS). To imitate the subsurface environment, laboratory studies are conducted under controlled conditions [
45,
46]. The evaluation of rock-hydrogen interactions is based on core flooding tests, permeability testing, and geochemical reactions. The primary experimental techniques are as follows:
Core Flooding Experiments: The experiments involve relative permeability, flow dynamics, and trapping mechanisms being analyzed by injecting hydrogen into rock cores. Their results provide important new perspectives on the behavior of hydrogen as it replaces other fluids and passes through low permeable formations [
47].
Geochemical Interaction Studies: The interactions of hydrogen with minerals present in reservoir rocks can lead to alterations in both porosity and permeability. The study of the mechanisms involved in mineral dissolution, precipitation, and microbial activity that can influence storage integrity is experimental.
Hydrogen Sorption and Diffusion Tests: Investigating adsorption and diffusion is essential for evaluating hydrogen retention within porous materials. The conducted experiments are instrumental in assessing possible losses resulting from capillary trapping or chemical reactions [
47].
Mechanical Integrity Tests: Cycles of repeated injection and withdrawal can lead to variations in stress, which may impact the stability of rock formations. Triaxial compression tests are employed to investigate rock deformation, fracture propagation, and fatigue under cyclic hydrogen injection conditions [
48].
Numerical simulations provide prediction models evaluating long-term performance in several operating environments, therefore enhancing laboratory research employing these models. Mathematical frameworks intended for geomechanical, geochemical, and fluid flow are used in the construction of the models.
Reservoir Simulation Models: The models provide simulations of hydrogen migration, pressure accumulation, and potential leakage hazards within subsurface reservoirs. Forecasting storage efficiency [
49] with real-world data gleaned from lab tests and field observations.
Geomechanical Modeling: Coupled hydro-mechanical models evaluate stress changes in the storage reservoir, therefore predicting probable fracture, fault reactivation, and subsidence brought about by the cyclic hydrogen injection and withdrawal.
Reactive Transport Models: This set of simulations combines fluid dynamics with geochemical processes, allowing for the forecasting of mineral dissolution, precipitation, and the impact of microbial activity on hydrogen stability.
Multiphase Flow Simulations: One of the subterranean reservoirs is hydrogen; it is a multiphase fluid coexisting with water or other gases. Computational fluid dynamics (CFD) models are fundamental instruments for exploring hydrogen distribution, phase behavior, and the effects of capillary entrapment.
Integrating laboratory findings with numerical simulations improves the precision of models for underground hydrogen storage. Data gathered from experiments serve to fine-tune simulation parameters, thereby minimizing uncertainties in forecasting long-term storage performance. The combination of both methods enhances decision-making in site selection, operational strategies, and risk assessment [
4].
Table 5 presents a comparison of the strengths and limitations associated with laboratory studies and numerical simulations [
5,
45,
50].
Software tools such as Eclipse (2025.1), DuMux (3.7), CMG-GEM (2024.10), TOUGH (3 or +), and COMSOL (6.3) are routinely used in underground hydrogen storage to replicate hydrogen flow and apply fluid flow concepts. Oil and gas reservoir modeling makes extensive use of SLB’s Eclipse program (100 and 300); although Eclipse 100 uses a black oil model, CMG-GEM and Eclipse 300 employ compositional models to control phase behavior variations in fluid compositions. Perfect for hydrogen and cushion gas injection, TOUGH, created to replicate gas injection and reactive transport [
4], is outstanding.
6.2. Field-Scale Applications and Case Studies
The shift towards extensive underground hydrogen storage is essential for incorporating hydrogen into energy frameworks and maintaining supply reliability. Field-scale applications offer crucial understanding of practical challenges and viability, enhancing laboratory experiments and numerical models. A number of case studies from around the globe have illustrated the capabilities of different geological formations, including salt caverns, depleted hydrocarbon reservoirs, and deep saline aquifers, for extensive hydrogen storage solutions.
6.2.1. Existing Field-Scale Hydrogen Storage Projects
Salt caverns are the most typically employed construction form for hydrogen storage, with their higher containment integrity and reduced leak risk. For instance, the Texas Gulf Coast Salt Dome Storage has been running since the 1970s, keeping significant volumes of hydrogen for use in the petrochemical and refining industries, especially [
50,
51]. In a similar vein, the Teesside hydrogen storage site in the UK has been utilized by industrial entities for many years, employing salt caverns for the efficient storage and distribution of hydrogen [
38]. On the other hand, for hydrogen storage, depleted gas reservoirs and saline aquifers are in the pilot state. Launched by Gasunie, the HyStock project in the Netherlands looks at issues related to geochemical interactions, microbiological activity, and the impacts of cyclic injection and withdrawal in a depleted gas reservoir [
52]. This pilot study seeks to evaluate cyclic injection and withdrawal processes, geochemical interactions, and microbial activity within authentic reservoir conditions. The focus is on closely observing pressure stability, ensuring caprock integrity, and adapting natural gas infrastructure for hydrogen applications. HyStock offers essential insights into the technical and regulatory obstacles linked to the repurposing of natural gas fields for hydrogen storage.
The Underground Sun Storage project by RAG Austria represents a pioneering pilot study that employs depleted gas reservoirs for the storage of hydrogen, performed under actual field conditions. The initiative is based in Upper Austria, showcasing the seasonal storage capabilities of renewable hydrogen generated through electrolysis. Primary areas of investigation encompass the examination of hydrogen interactions with reservoir rocks, the role of microbial consumption, and the implications of cyclic operational effects. Field tests have confirmed the secure storage and retrieval of hydrogen, offering a technical framework for integrating renewable energy with subsurface storage systems.
Finally, China has initiated multiple UHS demonstration projects centered on salt caverns and depleted gas reservoirs, demonstrating their dedication to hydrogen as a clean energy carrier. Notable efforts include pilot programs in the Jintan Salt Cavern, situated in Jiangsu province, focused on assessing high-pressure hydrogen storage. These initiatives investigate diffusion behavior, caprock sealing efficiency, and operational stability under deep subsurface conditions. The initiatives from China emphasize energy security and grid stability, providing scalable frameworks for the nationwide adoption of hydrogen technologies.
6.2.2. Obstacles in Practical Implementations
Although laboratory experiments and simulations establish essential understanding, practical applications encounter further intricacies. Significant obstacles consist of:
Hydrogen Containment and Losses: In contrast to natural gas, hydrogen possesses a smaller molecular size, which heightens the potential for diffusion and leakage through caprock and fault structures [
50].
Geochemical and Microbial Reactions: Hydrogen could interact with minerals and natural bacteria in deep reservoirs, leading to hydrogen consumption and raising questions about reservoir integrity [
5].
Cyclic Injection and Withdrawal Effects: Variations in pressure conditions during major activities might affect the mechanical integrity of the storage site, therefore influencing the permeability and maybe causing induced seismicity [
42].
Numerous extensive initiatives currently in progress are undergoing examination across Asia, North America, and Europe. Germany’s HyPSTER project is exploring hydrogen storage in salt caverns under practical conditions, while the H21 Leeds City Gate Project in the UK is examining the potential of utilizing natural gas infrastructure for hydrogen storage. These projects provide important new perspectives on UHS’s ongoing performance and financial viability. Understanding field-scale applications via case studies will help us to progress the development of safe, efficient, and scalable storage solutions, given the growing relevance of hydrogen in the energy transition. To increase operating efficiency, future research should focus on improving reservoir characterization, resolving hydrogen loss processes, and improving injection and withdrawal techniques.
7. Challenges and Knowledge Gaps
The security and stability of long-term storage rely heavily on the comprehensive enhancement of existing research gaps in underground hydrogen storage, particularly concerning geomechanical impacts, geochemical interactions, and the implementation of real-time leakage detection systems. The storage of hydrogen in underground formations involves distinct challenges, including chemical interactions, the need to maintain the integrity of confinement, and the risk of leaks occurring over prolonged durations.
(a) The integrity of the caprock and the potential for leakages are critical considerations. Due to hydrogen’s minuscule molecular size, it can navigate through existing cracks and porous formations, thereby heightening the risk of leaks [
42]. Additionally, the stress alterations induced by cyclic injection and withdrawal could lead to microfractures in the storage formation, consequently compromising containment as time progresses [
5]. (b) Geochemical and microbial reactions indicate that hydrogen can interact with reservoir minerals, leading to alterations in rock properties and possibly resulting in structural weakening. Interactions with iron-bearing minerals can lead to the generation of hydrogen sulfide (H
2S), a corrosive gas that poses a risk to wellbore integrity [
52]. Moreover, the role of microorganisms in storage reservoirs can lead to the utilization of hydrogen, converting it into methane or other byproducts, potentially reducing the efficiency of storage operations [
45]. (c) Induced Seismicity and Geomechanical Alterations; the ongoing cycle of hydrogen injection and withdrawal over prolonged durations leads to the persistent pressurization and depressurization of the storage formation. Changes in geomechanical stress brought about by variations in pressure may set off seismic events in geologically sensitive areas [
50]. (d) Pressure and gas flow tracking; identification of anomalies relies on constant downhole sensor pressure monitoring; tracer gases, including helium, enable to follow-up hydrogen transport [
38]. (e) Seismic and geophysical surveillance; microseismic monitoring identifies stress-induced fractures, whereas 4D seismic imaging offers insights into reservoir changes over time. (f) Geochemical and Microbial Activity Analysis: Systematic gas sampling and chromatography-based analysis facilitate the monitoring of gas composition variations, enabling the identification of potential reactions that could impact storage integrity [
5].
Although recent research has deepened our understanding of underground hydrogen storage (UHS), substantial discrepancies and limits remain. Laboratory experiments frequently employ non-uniform pressure and temperature settings, which make direct comparison difficult. Microbial investigations reveal wildly different hydrogen loss rates, with no standardized testing methodologies. Modeling methodologies usually reduce geological complexity, thereby underestimating leakage and mechanical dangers. Furthermore, field-scale data are confined to a few experimental projects, leaving long-term storage behavior and cyclic stability largely unknown. These limitations underscore the need for standardized techniques, longer-duration trials, and further field validations to improve the reliability of UHS evaluations.
8. Future Research Directions
The integration of artificial intelligence (AI) into monitoring systems significantly improves predictive modeling and data analysis, allowing a more proactive approach to risk management.
Especially for low-permeability formations, future research should focus on improving reservoir characterization techniques to gain a better knowledge of hydrogen migration and confinement behavior. Advanced sealing materials with more resistance to hydrogen diffusion will help to maintain storage system integrity and stop leakage, which is required for correct forecast of stress fluctuations and hazard mitigation including produced seismicity and caprock collapse are advances in geomechanical modeling and real-time monitoring systems. Identification of possible geochemical changes affecting reservoir stability depends on an analysis of hydrogen–mineral interactions.
Ultimately, it is essential to broaden pilot-scale field tests and case studies to confirm the results obtained from laboratory experiments and numerical simulations. Partnerships among academic institutions, industry players, and government bodies will be essential in developing standardized regulations and best practices for the safe storage of hydrogen at a commercial level.
9. Conclusions
This review has explored the geomechanical and geochemical factors influencing underground hydrogen storage (UHS), emphasizing its viability, challenges, and future advancements. The study examined stress distribution, fracture behavior, containment risks, adsorption-diffusion mechanisms, microbial interactions, and cyclic loading effects, all of which critically impact storage integrity and efficiency. The findings of our study emphasized the importance of low-permeability formations, for long-term containment despite presenting challenges due to restricted flow. Furthermore, advancements in numerical simulations and laboratory investigations will enhance our understandings of hydrogen dynamics in subsurface environments, thereby refining prediction models for injection and withdrawal processes.
This review is unique as it provides a comprehensive knowledge of subsurface hydrogen storage by combining geomechanical and geochemical points of view. This work distinguishes from other studies by combining reservoir mechanics with chemical interactions, therefore providing a holistic perspective required for the evolution of safe and efficient storage solutions. Emphasizing emergent research areas such as hydrogen–mineral interactions, microbial impacts, and the use of machine learning in reservoir models, this study lays the basis for future development in the topic. As the world moves to hydrogen-based solutions, constant research and technical developments will be necessary for the full realization of subterranean hydrogen storage potential. Cooperation among educational institutions, businesses, and regulatory authorities will help to influence future developments in sustainable, safe, and scalable hydrogen storage systems, thereby enabling their seamless integration into the future energy grid.